Land cover change, of which urbanization is a major driver, remains the greatest threat to terrestrial biodiversity. More than half of all people now live in cities spread across 3% of the global terrestrial surface, and this population is predicted to rise to 68% by 2050 (1). Growth in urban land area is concomitantly forecast to triple between 2000 and 2030, to 1.2 million square kilometers (2). The growth of cities—anthropogenic biomes—provides particular challenges for biodiversity. Conservation ecologists are now increasingly interested in uncovering the life history attributes, ecological processes, and species-specific behaviors that dictate the structure of these novel urban organismal assemblages (3). In PNAS, Martin and Bonier (4) employ a global dataset of species interactions and proceed to erect and test three alternative hypotheses in which behavioral dominance might directly or indirectly influence the occurrence and distribution of urban species.
Cities represent a recent and dramatic shift from the historical habitats within which species evolved. The constraints on resource availability and reduction in both habitat diversity and structural complexity tend to lead to the simplification, homogenization, and reorganization of biotic communities in urban areas (5). Given their globally ubiquitous nature, relative ease of field identification, and sampling cost effectiveness, birds have been the taxon of choice for many urban biodiversity studies. Most historical research has focused on patterns of species occupancy in cities in biomes as diverse as the Arctic (6) and the Amazon (7), but such work on patterns is now giving way to progress on understanding processes (8). We now know that urban species tend to have broader environmental tolerance and increased behavioral flexibility, often reflected in larger brain sizes and even altered endocrine responses, and that these selection pressures drive further changes in animal phenotypes and genotypes (9). Competition between species may also limit species occurrence in cities but has historically received less detailed examination, given shortfalls in knowledge of the likely outcomes of species interactions.
Constraints on coexistence among competing species may regulate community structure via interspecific resource competition if resources are limited, as may often be the case within anthropogenic landscapes (10). Such competitive dominance exists when environmental conditions favor one species over another, leading to higher fitness of competitively superior species (11). However, this competitive dominance does not always equate to social dominance (i.e., dominance arising from consistent aggressive interspecific interactions) because more-aggressive species may still be outcompeted by their subordinates. However, social dominance can lead to competitive exclusion and, hence, narrower realized niches for subordinate species (12). Examining the role of competitive interactions in structuring communities is complicated by the need to assemble a large database of individual aggressive interactions, and such behaviors are rarely observed under field conditions. However, it has proved possible to assemble such databases, either by trawling through the academic literature looking for documented interactions (13) or, recently, through massively crowd-sourced, protocol-driven data collection by citizen scientists (14), opening the door to more nuanced studies of the effects of animal behavior on organismal assemblages.
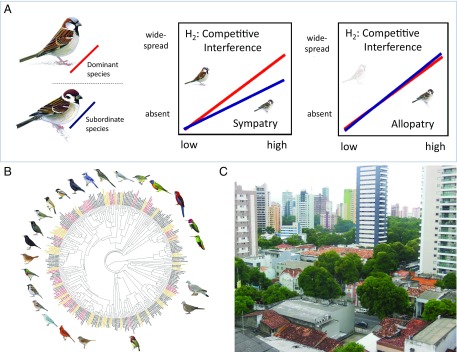
In PNAS, Martin and Bonier (4) propose three hypotheses with which to examine the impact of species interactions on species codistributions, considering that cities might represent either an opportunity for some species or, more frequently, a challenge. Their first hypothesis, the “subordinate tolerance hypothesis,” posits that subordinate species may be more successful in cities, given their exclusion from preferred resources and habitats by dominant species, in effect predisposing them to success in such highly disturbed environments often characterized by low resource availability and high predation pressure. Contrarily, Martin and Bonier’s “competitive interference hypothesis” (Fig. 1A) expects the monopolization of urban habitats by behaviorally dominant species. These are expected to suppress the abundance of, or entirely exclude, cooccurring subordinate species with similar ecologies in sympatry that might otherwise thrive in cities. These subordinate species might occupy urban niches in the absence of their competitors, although the authors note that such ecological filtering might also lead to a failure of subordinate species to accrue adaptations to urban environments at all. Lastly, the “dominant advantage hypothesis” recognizes that the aggression associated with behavioral dominance may be linked to other traits, such as disturbance tolerance, neophilia, and boldness, signaling phenotypic plasticity (12), which may predispose dominant species to urban adaptation and, hence, success in occupying cities.
Fig. 1.
Understanding interspecific dominance competition in urban landscapes. (A) Competitive interference hypothesis of Martin and Bonier (4) posits that behaviorally dominant species (e.g., the house sparrow, Passer domesticus) will suppress the abundance of, or entirely exclude, cooccurring subordinate species (e.g., the tree sparrow, Passer montanus) in sympatry that might otherwise thrive in cities. Adapted with permission from ref. 4; sparrow images courtesy of RSPB Images. (B) Species pairs included in the study represent a broad swathe of the avian tree of life. Adapted with permission from ref. 4. (C) Belém, Brazil, is an example of a city in a developing country that still retains wildlife habitat, potentially facilitating species coexistence.
Martin and Bonier (4) set out to test these alternative hypotheses by determining how behavioral dominance might either directly or indirectly influence species occurrence in a global sample of breeding birds in 492 large cities for which inferred dominance relationships among closely related species have been published (Fig. 1B). City-level breeding-bird status was solicited by regional expert elicitation, with respondents assigning scores of species status in cities from “absent” to “widespread,” giving the authors an index of the degree to which cities represent either an opportunity or a challenge for bird species. In recognizing the urban–rural continuum and that many cities may include substantial natural areas, they informed their multiple respondents per city to assign urban bird species status away from such natural-habitat enclaves. Their species interaction data came from a survey of the literature, with the caveats that some relationships are better established than others.
The authors initially uncovered evidence supporting their competitive interference hypothesis (Fig. 1A), with urban-adapted subordinate species proving to be less widespread in cities compared with closely related dominant species in sympatry. This indicates that direct competitive interactions may preclude subordinate-species occupancy of cities. However, they also found that this relationship belied substantial geographic variation in responses, with support for the competitive interference hypothesis in Europe, North America, and Australia, but not in Africa, South America, and Asia. To understand why this might be the case, they used Bayesian generalized linear mixed models to explore the role of variation in latitude, climate, economic development, human population size, phylogeny, and sampling biases in agreement with their hypotheses. Of these potential predictors, only the level of economic development proved significant, suggesting that economic development may intensify the impacts of competition on subordinate species, leading to a reduction in avian biodiversity in cities.
The study of Martin and Bonier (4) does not stretch to unpacking how a rather crude metric such as economic development acts in favor of dominant species by exacerbating the consequences of competition among closely related species of birds in developed countries. However, they do speculate about three possible pathways. The first is the potential for more marked spatiotemporal clumping of resources in developed countries, especially human handouts at waste treatment facilities. Second, there may be reduced control of resources (including habitat) in cities in developing countries, leading to higher habitat structural complexity and greater resource availability. Third, all species in developing countries may exhibit higher mortality rates, leading to a reduction in population sizes of dominant species and diminishing opportunities for their competitive exclusion of subordinate species from urban habitats. These pathways are, of course, not mutually exclusive, and given the broad functional and phylogenetic diversity of the species in the study, the drivers of community collapse in different groups may be highly idiosyncratic. These patterns are amenable to more detailed future analyses that look at species trait distributions that may influence urban persistence (15), differ between developed and developing countries, and covary with latitude. Understanding these relationships might also shine a light on another important caveat of the results of Martin and Bonier (4): they are unable to rule out the potential effect of dominant species restricting subordinates from preferred habitats outside of cities, leading to secondary effects on their distribution within cities.
Martin and Bonier (4) focus only on dyadic interactions between congeners—a comparative approach that permits phylogenetic and spatial breadth—yet interactions between species extend beyond species pairs, and interspecific competitive dominance research now seeks to quantify more complex patterns such as dominance hierarchies (14). Network theory analyses may reveal mathematical intransitivities, such as the rock–paper–scissors relationship in behavioral ecology (11). In this instance, despite pairwise competitive advantages, no single species can dominate and exclude all others in species-rich communities. However, environmental filters operating in urban areas may break these complex relationships if some species become locally extinct due to a loss of critical resources. Their local extinction may enable others to monopolize resources and exclude remaining functionally similar heterospecifics. Such impacts might be most pronounced in the tropics, where species packing is highest (16), and act to reduce species richness in the future as these cities develop. Tropical bird communities are also characterized by a high prevalence of obligate and facultative mixed-flock foraging species for which interspecific interactions are characterized by social mutualisms (17). Understanding how this codependence may act to dampen the impacts of competition would be a novel research priority. Also not to be ignored is the seasonal influx of migrant species into tropical and subtropical cities; the ranges of 92% of bird species intersect the tropics at some part of their life cycle (16). This intense seasonal pulse of disruptive competition from nonbreeding species might also account for geographic differences in the relationships between breeding dominant and subordinate species. Detailed field experiments are needed to provide direct evidence for the causal role of competition in such circumstances (8, 9), including the role of invasive species (18), which could be uncovered through a combination of manipulative and space-for-time swap studies.
The models of Martin and Bonier (4) provide insight into the effects of competition in structuring avian assemblages and pose a challenge for policy makers in such complex socioecological systems as cities (3). The loss of avian biodiversity services mediated by competitive interactions among species has potentially far-reaching implications for key ecosystem processes such as control of phytophagous and/or disease-vectoring insects and for seed dispersal, and these losses mirror those seen in the simplification of rural landscapes (10). There is an urgent need to improve urban wildlife habitat in the interstitial spaces between human infrastructure to ameliorate biodiversity loss driven by changes in species interactions, which are apparently even more problematic in developed countries than in developing countries (Fig. 1C). Bolder targets (19) and cost-effective strategies (3, 20) are required to drive this restoration forward in urban areas.
Footnotes
The author declares no conflict of interest.
See companion article on page E11495.
References
- 1.United Nations Department of Economic and Social Affairs, Population Division 2018 World Urbanization Prospects 2018. Available at https://population.un.org/wup/. Accessed October 28, 2018.
- 2.Seto KC, Güneralp B, Hutyra LR. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA. 2012;109:16083–16088. doi: 10.1073/pnas.1211658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm NB, et al. Global change and the ecology of cities. Science. 2008;319:756–760. doi: 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- 4.Martin PR, Bonier F. Species interactions limit the occurrence of urban-adapted birds in cities. Proc Natl Acad Sci USA. 2018;115:E11495–E11504. doi: 10.1073/pnas.1809317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sol D, González-Lagos C, Moreira D, Maspons J, Lapiedra O. Urbanisation tolerance and the loss of avian diversity. Ecol Lett. 2014;17:942–950. doi: 10.1111/ele.12297. [DOI] [PubMed] [Google Scholar]
- 6.Staniforth RJ. Effects of urbanization on bird populations in the Canadian Central Arctic. Arctic. 2002;55:87–93. [Google Scholar]
- 7.Lees AC, Moura NG. Taxonomic, phylogenetic and functional diversity of an urban Amazonian avifauna. Urban Ecosyst. 2017;20:1019–1025. [Google Scholar]
- 8.Lowry H, Lill A, Wong BB. Behavioural responses of wildlife to urban environments. Biol Rev Camb Philos Soc. 2013;88:537–549. doi: 10.1111/brv.12012. [DOI] [PubMed] [Google Scholar]
- 9.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 2006;21:186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Bregman TP, et al. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology. 2015;96:2692–2704. doi: 10.1890/14-1731.1. [DOI] [PubMed] [Google Scholar]
- 11.Zamudio KR, Sinervo B. Polygyny, mate-guarding, and posthumous fertilization as alternative male mating strategies. Proc Natl Acad Sci USA. 2000;97:14427–14432. doi: 10.1073/pnas.011544998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morse DH. Niche breadth as a function of social dominance. Am Nat. 1974;108:818–830. [Google Scholar]
- 13.Freshwater C, Ghalambor CK, Martin PR. Repeated patterns of trait divergence between closely related dominant and subordinate bird species. Ecology. 2014;95:2334–2345. doi: 10.1890/13-2016.1. [DOI] [PubMed] [Google Scholar]
- 14.Miller ET, et al. Fighting over food unites the birds of North America in a continental dominance hierarchy. Behav Ecol. 2017;28:1454–1463. [Google Scholar]
- 15.Piano E, et al. Urbanization drives community shifts towards thermophilic and dispersive species at local and landscape scales. Glob Change Biol. 2017;23:2554–2564. doi: 10.1111/gcb.13606. [DOI] [PubMed] [Google Scholar]
- 16.Barlow J, et al. The future of hyperdiverse tropical ecosystems. Nature. 2018;559:517–526. doi: 10.1038/s41586-018-0301-1. [DOI] [PubMed] [Google Scholar]
- 17.Mokross K, Ryder TB, Côrtes MC, Wolfe JD, Stouffer PC. Decay of interspecific avian flock networks along a disturbance gradient in Amazonia. Proc Biol Sci. 2013;281:20132599. doi: 10.1098/rspb.2013.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira PF, Lourenço R, Mota PG. Behavioural dominance of the invasive red-billed leiothrix (Leiothrix lutea) over European native passerine-birds in a feeding context. Behaviour. 2018;155:55–67. [Google Scholar]
- 19.Lees AC, Bowler P. Water: A drought plan for biodiversity. Nature. 2015;521:289. doi: 10.1038/521289e. [DOI] [PubMed] [Google Scholar]
- 20.Standish RJ, Hobbs RJ, Miller JR. Improving city life: Options for ecological restoration in urban landscapes and how these might influence interactions between people and nature. Landsc Ecol. 2013;28:1213–1221. [Google Scholar]