Significance
Development of shale gas has changed the economics of global energy. However, methane sometimes escapes from shale gas wells into aquifers and the atmosphere. Although many researchers investigate leakage of this greenhouse gas, detection can be difficult where natural sources of methane are present. Here, methane was studied in water near gas wells previously cited for well integrity issues. We identify chemical clues that can distinguish methane migration related to shale gas development from methane that was preexisting. We also document a geological setting—the upper part of a large geological fold at shallow depth—that may be prone to methane migration. Knowledge gained from this study may lessen the chances for fugitive methane emissions into aquifers in the future.
Keywords: shale gas, water quality, methane, noble gases, hydraulic fracturing
Abstract
Extensive development of shale gas has generated some concerns about environmental impacts such as the migration of natural gas into water resources. We studied high gas concentrations in waters at a site near Marcellus Shale gas wells to determine the geological explanations and geochemical implications. The local geology may explain why methane has discharged for 7 years into groundwater, a stream, and the atmosphere. Gas may migrate easily near the gas wells in this location where the Marcellus Shale dips significantly, is shallow (∼1 km), and is more fractured. Methane and ethane concentrations in local water wells increased after gas development compared with predrilling concentrations reported in the region. Noble gas and isotopic evidence are consistent with the upward migration of gas from the Marcellus Formation in a free-gas phase. This upflow results in microbially mediated oxidation near the surface. Iron concentrations also increased following the increase of natural gas concentrations in domestic water wells. After several months, both iron and SO42− concentrations dropped. These observations are attributed to iron and SO42− reduction associated with newly elevated concentrations of methane. These temporal trends, as well as data from other areas with reported leaks, document a way to distinguish newly migrated methane from preexisting sources of gas. This study thus documents both geologically risky areas and geochemical signatures of iron and SO42− that could distinguish newly leaked methane from older methane sources in aquifers.
Recent advances in horizontal drilling and high-volume hydraulic fracturing have helped the United States produce significantly more natural gas during the last decade (1). At the same time, shale gas development has led to increased public concern over impacts on water resources in areas of gas production. As of the end of 2017, about 12,000 shale gas wells have been drilled in Pennsylvania in the Marcellus, the most productive shale gas play in the world (1). The most commonly reported water-quality impacts in Pennsylvania have been cases of natural gas migrating into water supplies from gas wells with construction issues (2). However, leaks can be difficult to detect in Pennsylvania because natural sources of methane (CH4), the predominant hydrocarbon in natural gas, are common (3–6). For example, CH4 concentrations are often elevated in the region’s groundwaters because CH4 is produced biologically (7). In addition, thermogenic CH4—CH4 produced at depth at higher temperatures—can migrate into aquifers through natural mechanisms that might include transport as a dissolved solute in waters accompanied by salts from formation brine or, perhaps, as a separate free phase (8–13). To add to the complexity of determining the source of gas in aquifers in the Marcellus and other shale plays, CH4 can travel for kilometers along boreholes, fractures, faults, and bedding-plane openings and through porous sandstones (14–18).
These observations point to the need for investigations into the importance of local geological features in allowing, causing, or accelerating gas migration after drilling and completion of shale gas wells. We also need better methods to detect leaks when they occur. Understanding the causes of migration is important because the gas is an explosion hazard, and it can eventually discharge from aquifers into the atmosphere (19), where it is a greenhouse gas.
Given that both natural and anthropogenically affected sources of CH4 occur in most gas-producing shale plays, definitive assessment of the CH4 source and its migration pathway can be difficult. Isotopic measurements have been used in many shale gas plays to determine if stray gas was produced by bacterial (biogenic) or by higher-temperature (thermogenic) processes (6, 7) and to delineate the migration pathway (14, 16). Water chemistry data have also been used to investigate the CH4 source (e.g., refs. 2, 11, 12, and 20).
In this study we address three questions: (i) Can we identify geological conditions that exacerbate the potential for CH4 migration from shale gas wells? (ii) What is the impact of newly elevated CH4 concentrations on aquifer and stream chemistry? (iii) What tracers distinguish human-induced migration of CH4 from naturally migrating CH4? To answer these questions, we revisit a field site in central Pennsylvania where high CH4 concentrations have been highlighted in seeps and groundwater near a small first-order stream named “Sugar Run” in Lycoming County (Figs. 1 and 2). Elevated CH4 concentrations have been reported near Sugar Run since 2010, and several researchers and the state regulator, the Pennsylvania Department of Environmental Protection (hereafter. “PA DEP”), have suggested the CH4 is related to nearby gas wells (17, www.depreportingservices.state.pa.us/ReportServer/Pages/ReportViewer.aspx?/Oil_Gas/OG_Compliance; refs. 17, 21, 22).
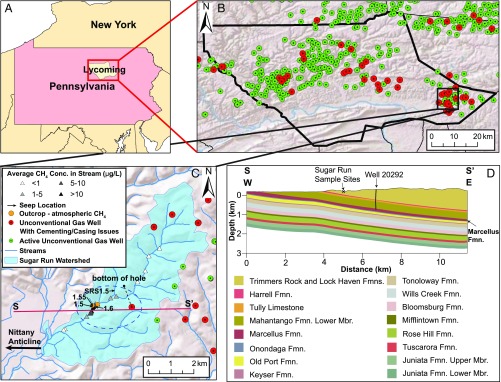
Fig. 1.
(A) Study area showing Lycoming County, Pennsylvania. (B) Expanded view showing all active unconventional gas wells that have (red) or have not (green) received well-integrity violations from the state regulator, the PA DEP (www.depreportingservices.state.pa.us/ReportServer/Pages/ReportViewer.aspx?/Oil_Gas/OG_Compliance) (also shown in C). (C) Expanded view of the Sugar Run watershed showing sample locations in streams (triangles) and bubbling seeps (arrows). Outcrop locations where CH4 was detected in the air near fractures are indicated by orange circles. Average dissolved CH4 concentrations in stream sites are shown as gray triangles (intensity of gray is contoured with respect to concentration as shown in the key and SI Appendix, Fig. S4). (D) Cross-section S–S′, defined in C, roughly follows the plunge of the Nittany Anticlinorium to the east. On this cross-section, well API #081-20292 intersects the Marcellus Formation at 997 m, and the depth of 0 represents sea level. The well pad where well API #081-20292 is drilled contains only that gas well. A lateral from that well follows the Marcellus Formation roughly perpendicular to the cross-section and is shown in C as a dashed line. Previously unreported data presented herein for seep, homeowner well, and air samples were sampled within the dotted circle in C. Fmn. Formation; Mbr, Member.
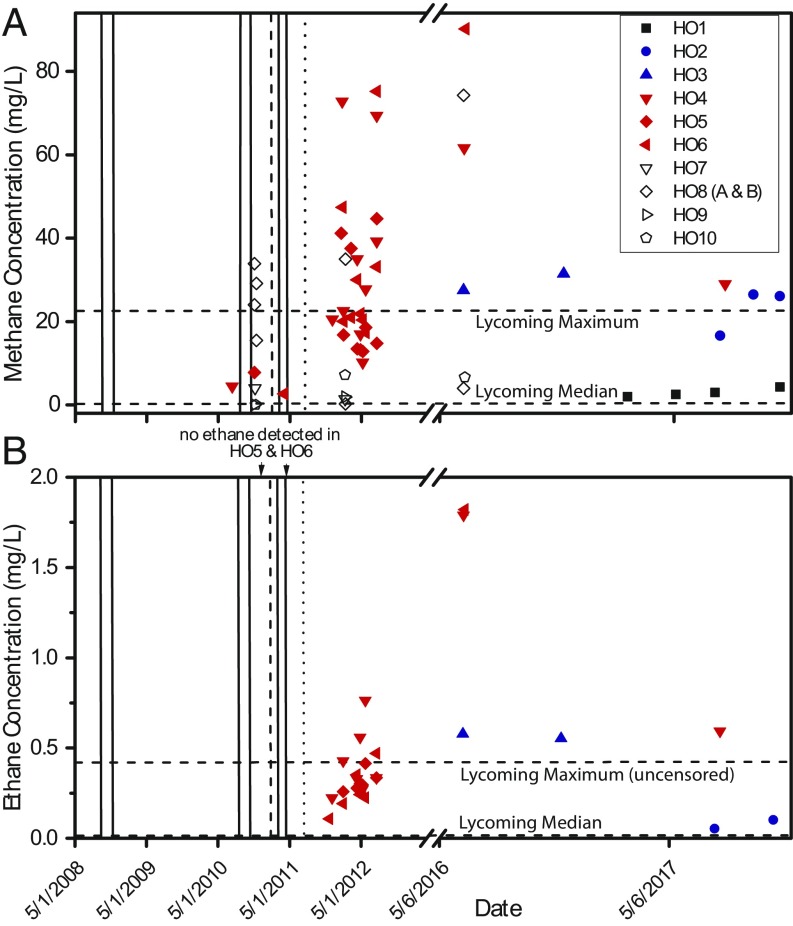
Fig. 2.
(A) A time series plot of dissolved CH4 concentrations from homeowner (HO) water wells 1–10 sampled in the study region near Sugar Run in Lycoming County, PA. Plotted data for water well HO8 include two water wells (A and B) sampled on the same property. Vertical solid lines indicate spud dates for unconventional gas wells cited for cementing/casing violations within a 5-km radius of seep 1.5. In addition, the dashed and dotted lines indicate the spud date and hydraulic fracturing date, respectively, for well API #081-20292. Samples indicated by open symbols are not discussed further and were reported by the gas company as part of the initial (38, 52) or subsequent (www.depgis.state.pa.us/emappa) investigations. Concentrations plotted after 26 October 2016 were sampled and measured by our team (Dataset S1). (B) A time series plot of dissolved C2H6 concentrations for wells HO6, HO5, HO4, HO3, and HO2 from data reported online (38), by the PA DEP (www.depgis.state.pa.us/emappa), or in this study. Horizontal dashed lines represent median and maximum concentrations for dissolved CH4 (n = 967) or C2H6 (n = 897) reported for Lycoming County samples outside the study region in Fig. 1C between 1995 and 2014. These samples were analyzed from published reports (33) or were collected as predrilling data by companies before gas wells were drilled and were released to the PA DEP and shared with Pennsylvania State University (34). Only 10 of 897 samples contained detectable C2H6. The line labeled “Lycoming Maximum (uncensored)” summarizes the highest predrilling concentration for samples with detected C2H6. For samples HO5 and HO6, indicated by arrows labeled “no ethane detected in HO5 & HO6” in B, data were censored but no reporting limits were indicated. C2H6 was not analyzed in HO4 before drilling commenced.
Sugar Run
The study region lies in an area near Hughesville, PA, where a high density of shale gas wells were drilled between 2008 and 2012 (Fig. 1). In the region, a high percentage of unconventional gas wells in a 13 × 13 km square centered around latitude 41.237783 and longitude −76.600508 have been cited by the PA DEP for issues related to cementing and casing (www.depreportingservices.state.pa.us/ReportServer/Pages/ReportViewer.aspx?/Oil_Gas/OG_Compliance). Specifically, 33.3% of the 101 spudded unconventional wells in this region have received one or more cementing- or casing-related violations. These frequencies are much higher than statewide estimates of violations (2, 23).
Sugar Run is a gaining stream, i.e., the stream gains water from groundwater along the flow path. We summarize previously unreported data for sites that were sampled ∼225 m upstream and downstream of a location (Fig. 1) where intermittent bubbling and upwelling seeps have been discussed in published literature since 2014 (17, 21, 22). Three of these seeps that upwell outside the wetted stream channel during normal conditions (labeled, based on their nearest stream sample location, as “seep 1.5,” “seep 1.55,” and “seep 1.6”) were sampled repeatedly (Fig. 1, SI Appendix, Figs. S1 and S2, and Dataset S1). Additionally, water samples were collected from four private water wells within the area marked by the dotted circle in Fig. 1C, and some measurements were made of CH4 concentrations in air (SI Appendix, Fig. S3). The water samples were compared with samples from a natural CH4 seep in Salt Spring State Park in Pennsylvania (SI Appendix).
Culpability for CH4 leakage into Sugar Run has not been established (24). One unconventional gas well [American Petroleum Institute (API) #081-20292] has been cited by the PA DEP for contaminating five homeowner water wells in the area with CH4 (Fig. 1) after drilling began on 12 February 2011 (SI Appendix, SI Text and Table S1). Within 4 km of well API #081-20292, 24 additional gas wells were drilled between 2008 and 2012, eight of which received citations for violations related to casing and cementing. These nine wells with citations are located within 5 km of the seeps studied at Sugar Run. Two additional gas wells that are situated just outside the 5-km radius were cited by the PA DEP for CH4 migration into seven homeowner wells in 2011 (25).
Heilweil et al. (21, 22) and Grieve et al. (17) reported water and hydrocarbon chemistry from Sugar Run, concluding that some samples of gases observed in stream and shallow groundwater in that area are consistent with a Marcellus origin. In this paper, we summarize previously unreported data for inorganic solutes, hydrocarbons, isotopes, noble gases, and limited atmospheric measurements in the Sugar Run area and discuss these data in context with previous Sugar Run data, regional groundwater data, the local geology, and the record of shale gas development.
Results
In this section, we summarize previously unreported water chemistry observations for Sugar Run in the context of the geologic setting.
Geological Observations.
Sugar Run is incising outcrops into the bedrock of the Trimmers Rock Formation (orange dots in Fig. 1C). The study area on the stream lies updip from the nine gas wells that have received integrity-related violations by the PA DEP (Fig. 1). Both the sample sites in Sugar Run and gas well API #081-20292 lie nearly on the axis of the Nittany Anticlinorium, a large east/west-trending, convex-up fold that plunges to the east under Sugar Run. The limbs of the anticline dip gently to the south and less gently to the north (Fig. 1 and SI Appendix, Fig. S5). Given this location, well API #081-20292 intersects the Marcellus Formation at a shallower depth (∼1,000 m deep) than most other Marcellus wells in state.
Field Observations.
Groundwater upwelling was identified by the presence of off-channel springs, orange sediments, an occasional rotten egg smell from hydrogen sulfide (H2S), or bubbling, all of which were reported by local residents to be new after drilling (SI Appendix, Fig. S1). Within 60 m of seep 1.55 and 5 m from stream location SR 2, we detected CH4 emitting along bedding planes and joints of all orientations in outcrops of the Trimmers Rock Formation (orange dots in Fig. 1C). CH4 in the air near the jointed outcrop near seep 1.55 (>9% by volume in air) was above the lower explosion limit (the lowest concentration in air necessary for combustion) for CH4 gas (i.e., 5% by volume) (26) on three occasions (SI Appendix, Fig. S3).
Dissolved Hydrocarbons.
CH4 concentrations in stream samples ranged from 0.0003 mg/L to 0.0766 mg/L and were highest at locations SR 1.5 and SR 1.55 (SI Appendix, Fig. S4 and Dataset S1), which are located near seeps 1.5 and 1.55, respectively. Consistent with this observation, seep concentrations (0.0001–8.6 mg/L) were generally larger than in the stream. CH4 was most concentrated in the seep that was most isolated from the stream channel (seep 1.6); in contrast, seeps 1.5 and 1.55 appeared to be more diluted by stream-water mixing.
At their highest, CH4 concentrations in four local homeowner wells HO1–HO4 (2.1–31.5 mg/L) were higher than the maxima measured in streams and seeps. Given that hydrocarbon concentrations in groundwater are known to vary with sampling technique (27), we emphasize the hydrocarbon analyses for our samples that were collected using the inverted-bottle technique. This method has also been used by the PA DEP and consultants hired by gas companies for collection of groundwater samples (5, 27). Hydrocarbon analyses for samples collected with different techniques are summarized in Dataset S1.
Concentrations of dissolved ethane (C2H6) in seeps and homeowner water wells, measured intermittently in this study, ranged from 0.005–0.060 mg/L and 0.051–0.595 mg/L, respectively. The molar ratio of CH4 (C1) to C2H6 (C2) ranged from 77 to 610 for seeps and homeowner water wells where C2H6 was measured (Dataset S1). Propane (C3H8) was investigated in one sample from each of two water wells (HO2 and HO4) and was detected in one of them (0.018 mg/L in well HO4).
For all samples in which dissolved gases were analyzed for δ13C-CH4, values ranged from −54.4‰ to +37.6‰. Higher δ13C-CH4 values were measured in seeps with lower CH4 concentration (SI Appendix, Fig. S6).
Inorganic Solute Chemistry in the Context of Hydrocarbons.
No trend in concentrations of CH4 versus sulfate (SO42−) was observed for stream water, but CH4 was generally more concentrated in well-water samples when SO42− was less concentrated. All well samples emitted the odor of H2S, and when a few of these were analyzed, dissolved H2S ranged from 0.16–3 mg/L (HO1 and HO4). Similar to the domestic well waters, seep 1.6 typically showed higher CH4 and lower SO42− concentrations. Such trends are expected based on thermodynamics alone, since CH4 can be used as an electron donor by SO42−-reducing bacteria (28).
Unlike the domestic water wells, H2S was not detected in any seep in the Sugar Run area. In addition, the concentrations of iron (Fe), arsenic (As), and manganese (Mn) were higher in seep 1.6 than reported in the homeowner water wells. In contrast to these elements that tend to occur at higher concentrations in anoxic environments, solutes that are associated with oxygenated waters (e.g., nitrate and uranium) were lower in seep 1.6 than in seeps 1.5 and 1.55 (Dataset S1). Furthermore, the concentrations of Fe, As, and CH4 in seep 1.6 vary together across seasons (SI Appendix, Fig. S7). Concentrations of CH4, Fe, Mn, and As in seeps 1.5 and 1.55 were also elevated and variable with time but were generally more diluted by stream waters, especially during higher water stages (Dataset S1). Although some of these dissolved species were elevated above the US Environmental Protection Agency (EPA) drinking water limits in seeps, concentrations in stream water were never observed at levels of concern for humans or ecosystems (Dataset S1). Lower stream concentrations are consistent with dilution with unimpacted waters from upstream.
Concentrations of strontium (Sr), barium (Ba), bromide (Br), and in some cases chloride (Cl) were also elevated in waters from the seeps compared with nearby surface water (Dataset S1). Like Fe and Mn, the highest Cl concentrations (range = 12.2–53.6 mg/L) were observed in seep 1.6. Finally, the measured 87Sr/86Sr ratio was significantly higher in seep 1.5 (0.71417) than in HO4 (0.71160), in seep 1.6 (0.71168) (Dataset S1), or in groundwater collected using a drive point piezometer near SR 1.5 (0.71141) that was reported previously (17).
Noble Gas Concentrations.
Water and gas samples were also collected in Sugar Run and in the salt spring at Salt Springs State Park, Montrose, PA, for noble gas analysis. These samples were collected with copper tubes using standard sampling techniques slightly modified as described in SI Appendix (29, 30). Measured ratios of 3He/4He, R, in water and gas samples (gas was sampled only from seep 1.55 and Salt Spring Park) are reported as R/Ra where Ra is the corresponding atmospheric value (i.e., 1.384 × 10−6) (SI Appendix, Tables S2–S4). R/Ra values for seep 1.55 and samples from Salt Spring Park are very low (0.0114 ± 0.0005–0.0165 ± 0.0006). In contrast, R/Ra ratios in all water samples from homeowner wells are slightly higher and are within the range of typical crustal values (∼0.02–0.05) (31). Isotopic ratios of almost all other noble gases are atmospheric within 2-σ except for 40Ar/36Ar ratios of some samples (SI Appendix, Table S4) (32).
Discussion
A major difficulty in identifying impacts on water quality in areas near shale gas development is distinguishing species that recently have contaminated waters from species that were present before development (2, 4). Such prior occurrence is common for CH4 and salt contaminants in the Appalachian Basin. For example, sodium (Na), Cl, Br, Ba, and Sr from deep brines and NaCl contamination from human sources are commonly observed in Pennsylvania groundwater along with naturally derived CH4 (e.g., refs. 8, 11–13, and 20). Part of the difficulty is the lack of adequate data documenting predevelopment water quality (2).
Here, we compare newly reported data and previously published data for the region near Sugar Run measured since 2010 with an estimate of background chemistry in the area that we refer to as the “Lycoming County groundwater dataset.” This dataset includes published data (33) and newly available predrill data (34) from Lycoming County (Figs. 1 and 2). Predrilling data are water-quality measurements made by commercial laboratories on samples collected by consultants hired by hydrocarbon-extraction companies before drilling oil or gas wells nearby (3, 18). Water-quality data in Sugar Run are also compared with waters reported to have been contaminated in other areas of Pennsylvania since shale gas drilling began in the state in 2004 (35). These other sites, referred to throughout as “presumably contaminated sites,” were deemed contaminated by government agencies after the drilling of nearby gas wells (SI Appendix).
CH4 and C2H6.
First, evidence is summarized as to why Sugar Run appears to have been contaminated by recent shale gas development.
CH4 concentrations in Sugar Run waters reach levels that are significantly higher than background concentrations for streams in Pennsylvania that do not align with geologic lineaments or do not have wetland inputs (21, 22, 34, 36). In addition, CH4 concentrations increased after drilling commenced in the area (Fig. 2). For instance, CH4 (referred to below as “C1”) and C2H6 (C2) concentrations for groundwater from wells HO4, HO5, and HO6 (located within the dotted circle in Fig. 1C) initially increased in 2011–2012 after drilling and are larger than the maximum concentrations (Fig. 2) reported for the 967 samples in the Lycoming County groundwater dataset that were collected between 1995 and 2014 (34). C1 and C2 concentrations for the three homeowner water wells have persisted well above predrilling measurements for more than 7 y (Fig. 2).
In addition, several lines of evidence are consistent with a thermogenic origin for the gas detected in Sugar Run after drilling. For example, C2H6, often detectable in thermogenic gas but only rarely detected in biogenic gas (37), was observed to increase in water wells HO4, HO5, and HO6 after gas-well drilling in the nearby region (Fig. 2). Decreases in C1/C2 ratios for HO4, HO5, and HO6 accompanied this increase in C2H6 (Dataset S1). Such decreases have been used to argue for gas from a more thermogenic source in some systems (35). C2H6 was also detected in water wells HO2 and HO3 and in seeps 1.5 and 1.6. (We did not try to measure C2H6 in stream water.) In contrast, for the compilation of groundwater data from Lycoming County, only 10 of the 897 samples from sites where C2H6 was analyzed showed detectable C2H6 (Fig. 2). Differing detection limits for C2H6 could also play some part in this latter discrepancy.
δ13C-CH4 in all samples near Sugar Run where isotopes were measured ranged from −54.4‰ to 37.6‰. Plots such as that in SI Appendix, Fig. S6 (7) indicate that one of the sources of the CH4 is a thermally mature thermogenic source that is influenced by seasonal mixing with biogenic CH4. Enriched δ13C-CH4 values for stream samples have been previously attributed to CH4 oxidation at this site (17, 21, 22). Indeed, Rayleigh fractionation of CH4 during oxidation is a reasonable explanation for the very high δ13C-CH4 values because biologic processes preferentially oxidize the lighter isotope (SI Appendix, Fig. S6).
The δ13C-CH4 values of the samples with largest CH4 concentrations (measured in seep 1.6) plotted in SI Appendix, Fig. S6 are assumed to approach that of the original unaltered endmember: −28.3‰. This value of δ13C-CH4, as well as values measured by Isotech Laboratories, Inc., for nearby homeowner wells HO2 (−29.81‰) and HO4 (−27.53‰), are similar to reported values for well API #081-20292, i.e., −28‰ to −29.5‰ (38). (We have not found isotopic values for other gas wells in the area.) Possible thermogenic sources consistent with these values include the Marcellus or Upper Devonian formations (39).
Noble Gases.
Noble gases dissolved in groundwater derive from the atmosphere, crust, and mantle (32), and relative contributions from these sources can be calculated based on a few assumptions (SI Appendix). For example, R/Ra ratios for He isotopes in our samples are much lower than the typical Mid-Ocean Ridge Basalt mantle value of ∼8 (40), negating the presence of mantle components. A comparison of R/Ra and He/Ne ratios (SI Appendix, Fig. S8) is also consistent with the absence of mantle He. These data thus differ from a previous study that reported minor mantle He for shallow groundwater in the Marcellus Shale footprint (13).
Likewise, crustal 40Ar (noted here as “40Ar*”) was detected in waters collected from homeowner well HO4 and in the gas from seep 1.55 (SI Appendix, Table S4). In addition, calculated 4He/40Ar* ratios of samples from HO4 range from 7.49 to 9.01, within the previously reported range (6.2–13.7) for samples of natural gas from the Marcellus Formation (41) and more than an order of magnitude lower than similar values from the shallower Upper Devonian Canadaway Formation (214.6–285.4) (41). These observations are consistent with the Marcellus Formation (and not Upper Devonian formations) being the source of both crustal noble gases and thermogenic CH4.
Noble gases also yield insight about the mechanism of transport. If Marcellus gas migrates as a solute in upwelling groundwater, 4He/CH4 and 20Ne/36Ar would fractionate and become altered (13, 14, 16) upon reaching the aquifer (SI Appendix). However, these two ratios in HO2 and HO4 are similar to that of Marcellus gas (SI Appendix, Fig. S9), an observation consistent with advective migration of CH4 in a free-gas phase. Solubility and mass balance arguments previously reported for CH4 and Cl also lead to the conclusion that CH4 is moving upward into Sugar Run as a free-gas phase (17).
Brine Salts and Migration Pathways.
Although we argue that the new influx of CH4 into Sugar Run occurs as free-gas–phase migration, gases might also be dissolved in waters that are moving upwards with salts from Appalachian Basin brines (ABB) because traces of salts that are consistent with these deep brines have been detected in Sugar Run. For example, on a plot of concentration ratios for Cl/Br versus concentration of Cl (SI Appendix, Fig. S10), waters from the stream, seeps, and homeowner wells mostly lie in the subfield representative of diluted ABB. In addition, 87Sr/86Sr values measured for groundwater near SR 1.5 (17), water well HO4, and seep 1.6 (Dataset S1) are consistent with the isotopic signature published for Middle Devonian formations such as the Marcellus, with 87Sr/86Sr ratios of 0.71000–0.71212 (8). In contrast, samples collected from the stream (17) and seep 1.5 (Dataset S1) yield 87Sr/86Sr ratios of 0.71342–0.71417, a signature more consistent with brines from formations above the Marcellus (SI Appendix, Fig. S11).
It is common to observe both brine salts (42) and CH4 in uncontaminated waters in Pennsylvania because thermogenic CH4 dissolved in such slightly saline waters moves naturally into aquifers in parts of the state (9). Thus, in most uncontaminated groundwater in Lycoming County, as well as elsewhere in the Appalachian Basin (e.g., West Virginia and New York), higher CH4 concentrations generally are observed in the presence of higher Cl concentrations (SI Appendix, Fig. S12). In comparison, most of the CH4-containing groundwater in water wells in the Sugar Run valley are low in Cl (all <14.4 mg/L). We infer that the relatively high-CH4 and high-Cl samples from Lycoming County, PA, West Virginia, and New York contain meteoric water mixed with naturally upflowing groundwater that contains dissolved CH4 and brine salts from natural sources (11, 12), while the high-CH4, lower-Cl groundwater entering Sugar Run is affected by natural gas from free-phase upflow (SI Appendix, Fig. S12).
Structural Characteristics.
In this section we explore whether Sugar Run may be particularly susceptible to gas migration because of fracture development.
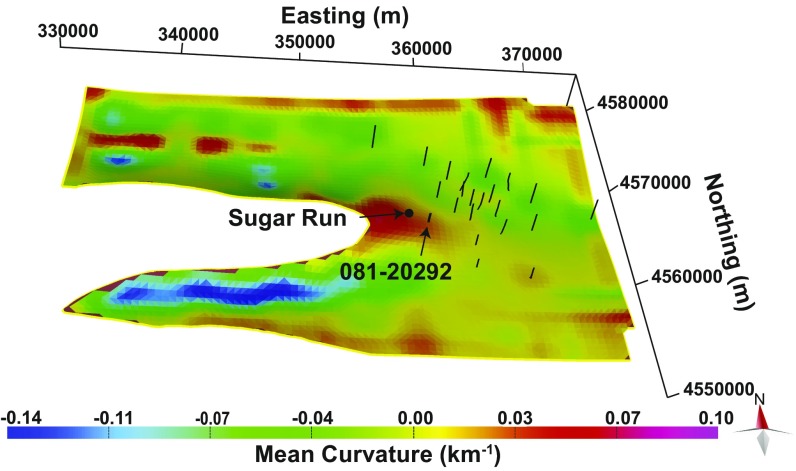
Vertical fractures (joints) in this area have been observed in several orientations (43, 44), including regionally extensive northwest- and north-northwest–striking joint sets as well as local joint sets that strike parallel or perpendicular to the axis of the anticline. If local joint sets form during folding, the intensity of this jointing usually correlates with fold curvature, i.e., is higher along the axis of the fold (45–48). The distribution of fracture intensity can be estimated using curvature analysis in such cases. Curvature is usually greatest along axes of anticlines and synclines (Fig. 3), although the exact relationship between jointing and curvature is difficult to predict (46).
Fig. 3.
Color contours showing the mean curvature, as defined by published algorithms (59), calculated for the top of the buried Marcellus Formation (smoothed using a 2-km moving-average window to minimize artifacts from modeling). Warmer colors indicate where the mean curvature is greatest and is likely to have caused the greatest density of vertical fractures that may allow the upflow of gas from depth. Curvature was calculated using Move’s surface geometry analysis tool. Labels show the locations of Sugar Run study area and the API #081-20292 well. The Marcellus Formation outcrops 3.3 km from the Sugar Run location point, i.e., where the white area cuts into the Sugar Run valley as shown in the figure.
Joints may enable CH4 migration in this area because the Marcellus Formation is very shallow (Fig. 1). The Marcellus Formation is closer to the surface under the seeps (0.6 km) than it is under any of the eight gas-well pads located within 5 km to the east. The depth to the Marcellus Formation is greater east of Sugar Run because the axis of the large fold comprising the Nittany Anticlinorium directly underlies Sugar Run (Fig. 1C and SI Appendix, Fig. S13) and plunges to the east. In fact, the Marcellus Formation comes to the surface about 3,400 m to the west of the study area along the anticline axis (shown as the curved edge of the white area in Fig. 3).
In addition to joints caused by folding in this area, joints also likely formed during unroofing. Such joints form only within 0.5 km of the surface as a rock unit is exhumed (49). This depth is much shallower than most of the Marcellus gas wells in Pennsylvania but is similar to the 0.6-km depth of the Marcellus Formation under the seep locations. Therefore, unroofing joints might also enable the migration of CH4 gas from the Marcellus Formation to the seeps. These joints could help CH4 migrate naturally or facilitate the migration of anthropogenically sourced CH4 in the event of gas-well leakage (50).
In addition to vertical migration, gas could be migrating updip along bedding planes and staircasing upward through bedding planes and joints. Updip gas migration has been shown in Pennsylvania to correlate with gas pressures above the saturation point, i.e., transport as a free-gas phase (14). Of the units overlying the Marcellus Formation, the Mahantango Formation is probably the most likely to accommodate layer-parallel gas migration, as a hydrogeologic study of the region has shown that it is more hydrologically productive than overlying units (51). The Mahantango Formation (Middle Devonian) lies ∼200 m below seep 1.5 (43, 51). Such a hypothetical path would move gas from the gas wells updip and along the axis of the anticline toward the seeps. Consistent with this, water wells in the study area with CH4 concentrations >0.11 mg/L are mostly west (updip) or northwest of gas wells (e.g., well API #081-20292), while CH4 concentrations are much lower in water wells to the south and east (downdip) (52).
Recently, other anticlines in Pennsylvania have also been shown to be associated with CH4-containing groundwater. Specifically, inspection of groundwater data has revealed that CH4 concentrations increase slightly near the Towanda Anticline to the northeast of Lycoming County in Bradford County, PA (18). Several cementing/casing-related violations were also issued by the PA DEP to shale gas wells along that anticline (www.depreportingservices.state.pa.us/ReportServer/Pages/ReportViewer.aspx?/Oil_Gas/OG_Compliance). That anticline is also associated with several large faults (18).
CH4 Impacts on Groundwater.
The evidence summarized so far is consistent with CH4 migration beneath Sugar Run since 2011. The most direct groundwater impacts are observed in wells HO4, HO5, and HO6. Here we focus on long-term groundwater impacts.
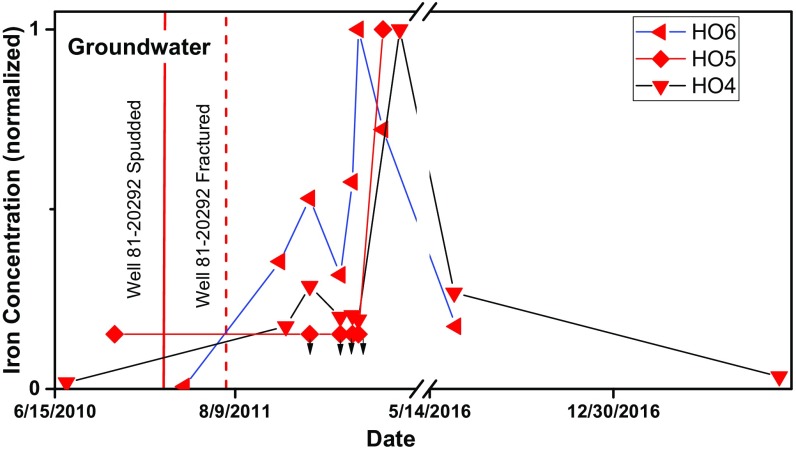
After CH4 concentrations increased in these three water wells, Fe concentrations increased and then decreased (Fig. 4). These observations are similar in some respects to observations published recently for a subsurface CH4 plume caused by a blowout at a gas well (53). Those authors argued that microorganisms catalyzed anaerobic oxidation of CH4 coupled with reduction of ferric oxides to produce soluble Fe(II) along the leading edge of the plume. A decrease in aqueous Fe, observed after the CH4 plume moved through, was attributed to the depletion of solid-phase ferric oxide minerals. Other reducible oxides such as Mn were similarly solubilized for a transient period. Transient spikes in Fe concentrations have also been observed in other water wells presumably affected by CH4 release from oil or gas activity (35, 54–57). Given the similarity between our observations and those reported in other research, we attribute the spikes in Fe concentrations (Fig. 4 and SI Appendix, Fig. S14) after initial increases in CH4 and C2H6 for wells HO4, HO5, and HO6 to reduction and mobilization of the metal because of anaerobic CH4 oxidation. Groundwater samples collected by the PA DEP and consultants are generally acidified but are not filtered, meaning the analysis includes dissolved and some suspended particulate Fe if it is present (42). In contrast, all of our samples analyzed for Fe were filtered before acidification (Dataset S1). Therefore, to allow comparison with the other data, the sample collected on 26 July 2017 and plotted on Fig. 4 was not filtered before analysis. The Fe concentration in that sample, reported in Dataset S1, was within a factor of 2 (0.02 mg/L) of its filtered counterpart sampled at the same time (0.01 mg/L).
Fig. 4.
Fe concentrations in groundwaters from wells HO4, HO5, and HO6 plotted versus time. Concentrations were normalized to the maximum values (0.58 mg/L, 0.14 mg/L, and 3.02 mg/L, respectively) (38). The HO4 sample collected on 18 July 2012 was slightly offset to avoid overlap. Downward arrows represent the reporting limit for censored analyses. For HO5, no reporting limit was indicated, so it is shown as an arrow at the concentration equivalent to the smallest reported concentration in that report. Data were derived from reports (www.depgis.state.pa.us/emappa; refs. 38 and 52), with one HO4 sample collected on 26 July 2017 from this study (see CH4 Impacts on Groundwater).
Based on our interpretation of SI Appendix, Fig. S14 and observations from the literature (28, 56), we might also expect to see CH4 oxidation coupled to SO42− reduction to sulfide. Indeed, high natural concentrations of CH4 are often observed with low SO42− in water supplies across the United States (e.g., refs. 20 and 58). Consistent with this, H2S was smelled or detected at wells HO1, HO2, HO3, and HO4 (Dataset S1). One reason for the observed drop in Fe concentrations (Fig. 4) might therefore also be that after the onset of SO42− reduction, Fe precipitated as one of several highly insoluble iron sulfide phases such as pyrite (28).
Distinguishing New CH4 from Preexisting CH4.
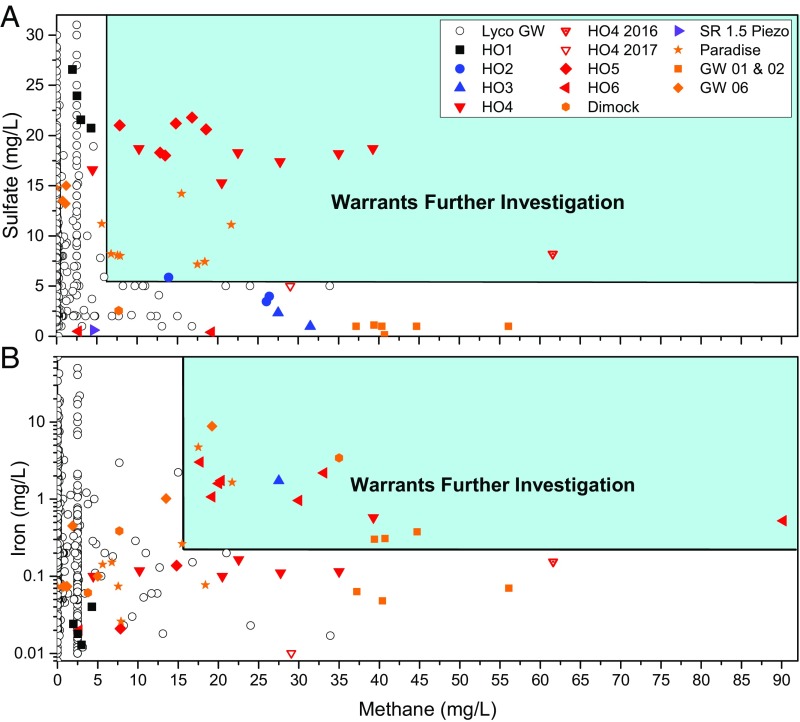
These observations suggest that onset of new CH4 contamination can sometimes be identified by a transient period of higher Fe and higher SO42− in groundwater. To test this, Fig. 5 shows plots of water-quality data from presumably uncontaminated, naturally equilibrated groundwater and presumably recently contaminated, nonequilibrated groundwater. Specifically, plots of SO42− versus CH4 (Fig. 5A) and Fe versus CH4 (Fig. 5B) are shown for (i) waters from our study area; (ii) the Lycoming County groundwater dataset, and (iii) published data from four separate presumably contaminated sites in northeastern Pennsylvania (35). For these last sites, locations were inferred using the maps in the report. The CH4-containing waters in the Lycoming County groundwater dataset were assumed to have long received influxes of naturally derived CH4. The waters from incidents in northeastern Pennsylvania were assumed to be contaminated by shale gas development as reviewed by the US EPA (35). These sites from incidents are shown in Fig. 5 and SI Appendix, Fig. S12 and are labeled as follows: (i) six wells along Paradise Road (labeled “GW 13,” “GW 18,” “GW 19,” “GW 20,” “GW 37,” and “GW 38,” following the EPA report) in Bradford County, PA (labeled “Paradise” in Fig. 5 and SI Appendix, Fig. S12); (ii) one well (GW 23 in the EPA report) near Dimock, PA (labeled “Dimock” in Fig. 5 and SI Appendix, Fig. S12); (iii) two wells near Granville Road and near the axis of the Towanda Anticline in Bradford County, PA (labeled “GW 01” and “GW 02”); and (iv) one well near Marshview Road in Bradford County, PA (labeled “GW 06”).
Fig. 5.
Graphs of groundwater chemistry from homeowner wells sampled in this study (see key for symbols labelling homeowner wells), the Lycoming County groundwater dataset (Lyco GW, empty black circles), and presumably contaminated sites in neighboring counties (orange symbols), as described in Distinguishing New CH4 from Preexisting CH4. (A) SO42− concentrations plotted versus CH4 concentrations show that most waters are high in either SO42− or CH4 but not both, as expected based on thermodynamic equilibrium. Waters from the Sugar Run area that are presumed to be experiencing a new CH4 influx (wells HO5 and HO4) plot in the upper right quadrant along with water from wells from the presumably contaminated sites. After several months, SO42− is inferred to be reduced, and waters no longer plot in the upper right quadrant (e.g., compare well HO4 in 2017 and in 2016). (B) Fe concentrations plotted versus CH4 concentrations show that, generally, Fe concentrations are low with elevated CH4 concentrations. Some wells from the Sugar Run study area and presumably contaminated sites contain high CH4 and Fe concentrations. “Lyco GW” refers to data provided by the PA DEP and from published reports (33) as described in Methods and Data Sources. The PA DEP data are also published online (34). Data for homeowner wells either were sampled in this study (Dataset S1) or have been published previously (www.depgis.state.pa.us/emappa; refs. 38 and 52).
In Fig. 5A, the waters with the highest SO42− concentrations at high CH4 concentrations and the waters with the highest CH4 concentrations at high SO42− concentrations in the Lycoming County groundwater dataset were used to plot lines to delineate what we infer to be natural, unperturbed waters. These data are assumed to represent groundwaters that are close to thermodynamic equilibrium because the CH4 concentrations in those samples are interpreted as long-standing, background concentrations. A similar procedure was followed for Fig. 5B to indicate highest Fe concentrations at high CH4 concentrations and highest CH4 concentrations at high Fe concentrations in the Lycoming County groundwater dataset. This approach identified data in the upper right quadrants of Fig. 5 as possible indicators of transient SO42− and Fe concentrations, respectively.
Strikingly, Fig. 5A shows that concentrations in Sugar Run water wells HO4 and HO5 shortly after the onset of drilling plot in the upper right quadrant along with data from the presumably contaminated water wells from other Pennsylvania incidents (35). These high-CH4 and high-SO42− samples stand out against predrilling groundwater from the rest of Lycoming County. For Fe, samples from water wells HO4 and HO6 also plot in the upper right quadrant of Fig. 5B along with several of the presumably contaminated water wells. High SO42− (>6 mg/L) and Fe (>0.3 mg/L) concentrations in waters with high CH4 concentrations may therefore be good indicators of recent contamination (Fig. 5).
Fig. 5 and SI Appendix, Fig. S14 also give indications of the duration of the inferred transient spike in SO42− and Fe. For example, concentrations in well HO4 of SO42− (predrilling concentration of 16.6 mg/L) sampled by the PA DEP on 14 June 2016 (8.21 mg/L) decreased to 5 mg/L on 26 July 2017 (this study), while elevated CH4 persisted (inverted triangles in Fig. 5A). If our interpretation is correct, the transient SO42− spike lasted at least 7 mo after the onset of CH4 migration (SI Appendix, Fig. S14). Likewise, Fe (concentration not detected in predrilling data) collected from HO4 and analyzed by the PA DEP on 14 June 2016 (0.154 mg/L) and on 26 July 2017 (0.01 mg/L) (this study) illustrates that the Fe transient spike may also last at least 7 mo when elevated CH4 persists (SI Appendix, Fig. S14).
Based on our interpretation of Fig. 5 and SI Appendix, Fig. S12, we suggest a possible protocol for quickly assessing groundwaters that may have been impacted by recent CH4 migration. If CH4 concentrations are greater than 10 mg/L and contain concentrations of Fe >0.3 mg/L and SO42− >6 mg/L, further investigations are warranted. Such further work would be especially warranted if chloride concentrations are <30 mg/L and the Ca/Na mass ratio is >0.52, because the waters would not look like natural brine salt-affected waters (SI Appendix, Fig. S12). Research is needed to test this protocol more broadly in the northeastern United States and elsewhere. Of course, geochemical characteristics that are consistent with one or more of these tests do not confirm that the water has been contaminated, and other measurements, e.g., isotopic studies, would be needed. Nevertheless, the protocol can identify sites where further testing or monitoring should be conducted.
In some waters, no SO42− is present in the aquifer before or after contamination, and such waters would plot as false negatives on Fig. 5A. In addition, if the duration of time since the onset of gas leaking is long enough, the new CH4 might exhaust the SO42− or Fe, providing another reason why some contaminated samples might plot as false negatives.
Conclusion
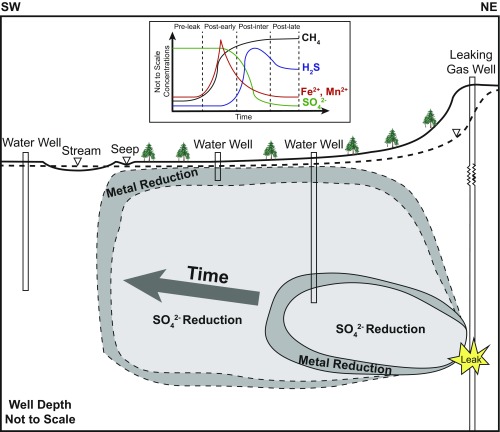
These observations of air and water chemistry at Sugar Run are best explained by a CH4 gas plume moving from depth into the aquifer over the last 7 y. During this period, one seep and one homeowner well that were measured contained gas and Sr that are isotopically like Marcellus fluids. At the upper part of the plume is a seasonal zone of oxidation of CH4 coupled to oxygen reduction (SI Appendix). At depth, CH4 oxidation is coupled to metal reduction or SO42− reduction (Fig. 6). The electron acceptors are likely used up in sequence from oxygen to metals to SO42−. Once the oxidants are depleted, CH4, C2H6, and other hydrocarbons pass through the system with less oxidation, allowing their concentrations to persist or increase (Fig. 2). The rate of hydrocarbon plume migration in the subsurface thus is affected by the availability of electron acceptors in the aquifer. With ongoing CH4 influx to the aquifer, some deleterious contaminants such as As can be mobilized.
Fig. 6.
Generalized schematic of an evolving CH4 plume released from a shale gas well. The Inset shows how water chemistry changes over time. The plot is patterned from data from homeowner well HO4 (Dataset S1). Two separate plumes are drawn for early and later time periods. The outer edge of each plume (dark gray) represents the zone of CH4 oxidation coupled to metal reduction. The inner section of each plume (light gray) represents the zone of CH4 oxidation coupled to SO42− reduction. Methane oxidation by oxygen or other species are not shown. Once electron acceptors are consumed, hydrocarbons flow unhindered from the subsurface to the surface, resulting in even higher concentrations and unaltered isotopic values if a leak continues. This diagram is not drawn to scale or to reflect the geology of Sugar Run.
Although not all water-quality data are released to the public in the Appalachian Basin, the rate of incidence of problems such as described in this paper appears to be relatively low compared with the number of shale gas wells that have been drilled (2, 3, 18). Nonetheless, it is important to study such presumably rare incidents for at least two reasons. First, problems that are understood can lead to better decisions. For example, drilling into a shale formation at a shallow depth along the axis of a large fold, such as described in this study area, may produce wells that intersect fractures that interconnect to form good pathways for upward-migrating contaminants. In addition, future research is needed to determine why some anticlines (e.g., the Nittany and Towanda anticlines) are associated with higher CH4 concentrations while others (the Rome and Wilmot anticlines in Bradford County) apparently are not (18). Such research could provide maps of areas where drilling should be precluded if CH4 migration is to be eliminated entirely or could lead to better management practices for drilling such areas.
Second, the migration of CH4 into aquifers can present explosion hazards—a well-known phenomenon—and also can change the redox state of the aquifer, a phenomenon that has not been as well documented. Such changes can feed consortia of bacteria that mobilize species that can degrade the aquifer, including the possibility of As mobilization. We have shown that the presence or absence of redox-active species in water samples with high CH4 concentration can be used to document recent, rather than long-duration natural, contamination by CH4. Specifically, the observation of high SO42− and high CH4 levels and/or high Fe and high CH4 levels in groundwater may indicate that a new source of CH4 has entered a groundwater system. Multiple lines of evidence are nonetheless necessary to make firm conclusions for any given site.
Methods and Data Sources
The geological setting of the 16.7-km2 watershed of Sugar Run (topographic slope of 10.4%) has been discussed previously along with stream measurements for campaigns in May, June, and November 2013 (22). During those periods the stream was dominated by baseflow, and discharge varied from 0.05–7.2 m3/s. No additional discharge measurements are reported here; however, the stream conditions were generally very similar to the previous report. In that study, modeling showed that the stream returns to baseflow conditions within 1.5 d of a storm event and that ∼170 m3/d of CH4-containing groundwater was entering the stream with a concentration of 3.2 mg/L in the segment above site 1.5 (17, 21, 22).
For samples reported here, the waters were analyzed for field parameters and different suites of inorganic ions, hydrocarbons [CH4 (C1) and in some cases C2H6 (C2) and C3H8 (C3)], isotopic signatures (including δ13C in CH4 and 87Sr/86Sr), and noble gases (SI Appendix). All noble gas samples discussed in this study were collected in copper tubes following a modified standard sampling protocol (29, 30), and these samples were analyzed at the Noble Gas Laboratory at University of Michigan (SI Appendix).
We compared our data for Sugar Run with data available online (www.depgis.state.pa.us/emappa), previously published data for the same area (17, 21, 22), and published reports of 41 groundwater analyses from eight water wells sampled during the Sugar Run investigation (38, 52). These Sugar Run data were compared with 892 analyses of predrilling groundwater in Lycoming County, mostly from private water wells sampled by consulting companies for the oil and gas companies and provided to us by the PA DEP [these values are published online (34)] and 75 groundwater analyses in Lycoming County sampled by the US Geological Survey (USGS) (33); together these comprise the Lycoming County groundwater data. These samples were analyzed at commercial laboratories or the USGS between 1995 and 2014 (34). No noble gas data were reported in the compiled dataset from the USGS, PA DEP, or gas companies.
Supplementary Material
Acknowledgments
We thank B. Lindsey of the USGS, the PA DEP, Z. Li, and A. Herman for providing or helping with predrilling data; the homeowners who provided access to private land in the Sugar Run valley; T. Sowers for analytical assistance for hydrocarbons; H. Ramirez, K. Jahn, L. Mateo, and G. Mount for field help; and Midland Valley for providing Move software through their academic software initiative. C. M. Hall of the Noble Gas Laboratory at University of Michigan codeveloped the sampling apparatus for noble gases described in SI Appendix. This work was funded by National Science Foundation IIS Award 1639150 (to S.L.B.) (Pennsylvania State University) and by a gift to Pennsylvania State University for the Pennsylvania State University General Electric Fund for the Center for Collaborative Research on Intelligent Natural Gas Supply Systems.
Footnotes
The authors declare no conflict of interest.
Data deposition: The water data have been uploaded to the Shale Network database as part of Consortium of Universities for the Advancement of Hydrologic Science, Inc. Hydrologic Information Systems (hiscentral.cuahsi.org/pub_network.aspx?n=228). The Lycoming County groundwater dataset is available on the Pennsylvania State University Data Commons (doi:10.18113/D35M2X).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809013115/-/DCSupplemental.
References
- 1.US Energy Information Administration 2018 Annual energy outlook, U.S. Energy Information Administration. Available at https://www.eia.gov/outlooks/aeo/. Accessed April 3, 2018.
- 2.Brantley SL, et al. Water resource impacts during unconventional shale gas development: The Pennsylvania experience. Int J Coal Geol. 2014;126:140–156. [Google Scholar]
- 3.Li Z, et al. Searching for anomalous methane in shallow groundwater near shale gas wells. J Contam Hydrol. 2016;195:23–30. doi: 10.1016/j.jconhyd.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Vidic RD, Brantley SL, Vandenbossche JM, Yoxtheimer D, Abad JD. Impact of shale gas development on regional water quality. Science. 2013;340:1235009. doi: 10.1126/science.1235009. [DOI] [PubMed] [Google Scholar]
- 5.Siegel DI, Azzolina NA, Smith BJ, Perry AE, Bothun RL. Methane concentrations in water wells unrelated to proximity to existing oil and gas wells in northeastern Pennsylvania. Environ Sci Technol. 2015;49:4106–4112. doi: 10.1021/es505775c. [DOI] [PubMed] [Google Scholar]
- 6.Baldassare FJ, McCaffrey MA, Harper JA. A geochemical context for stray gas investigations in the northern Appalachian Basin: Implications of analyses of natural gases from Neogene-through Devonian-age strata. Am Assoc Pet Geol Bull. 2014;98:341–372. [Google Scholar]
- 7.Whiticar MJ. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem Geol. 1999;161:291–314. [Google Scholar]
- 8.Warner NR, et al. Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proc Natl Acad Sci USA. 2012;109:11961–11966. doi: 10.1073/pnas.1121181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llewellyn GT. Evidence and mechanisms for Appalachian Basin brine migration into shallow aquifers in NE Pennsylvania, USA. Hydrogeol J. 2014;22:1055–1066. [Google Scholar]
- 10.Kayla CM, et al. Methane occurrence is associated with sodium-rich valley waters in domestic wells overlying the Marcellus shale in New York state. Water Resour Res. 2015;52:206–226. [Google Scholar]
- 11.Harkness JS, et al. The geochemistry of naturally occurring methane and saline groundwater in an area of unconventional shale gas development. Geochim Cosmochim Acta. 2017;208:302–334. [Google Scholar]
- 12.Kreuzer RL, et al. Structural and hydrogeological controls on hydrocarbon and brine migration into drinking water aquifers in southern New York. Ground Water. 2018;56:225–244. doi: 10.1111/gwat.12638. [DOI] [PubMed] [Google Scholar]
- 13.Darrah TH, et al. The evolution of Devonian hydrocarbon gases in shallow aquifers of the northern Appalachian Basin: Insights from integrating noble gas and hydrocarbon geochemistry. Geochim Cosmochim Acta. 2015;170:321–355. [Google Scholar]
- 14.Darrah TH, Vengosh A, Jackson RB, Warner NR, Poreda RJ. Noble gases identify the mechanisms of fugitive gas contamination in drinking-water wells overlying the Marcellus and Barnett Shales. Proc Natl Acad Sci USA. 2014;111:14076–14081. doi: 10.1073/pnas.1322107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llewellyn GT, et al. Evaluating a groundwater supply contamination incident attributed to Marcellus Shale gas development. Proc Natl Acad Sci USA. 2015;112:6325–6330. doi: 10.1073/pnas.1420279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen T, et al. Characterizing the noble gas isotopic composition of the Barnett Shale and Strawn Group and constraining the source of stray gas in the Trinity Aquifer, north-central Texas. Environ Sci Technol. 2017;51:6533–6541. doi: 10.1021/acs.est.6b06447. [DOI] [PubMed] [Google Scholar]
- 17.Grieve PL, et al. Using environmental tracers and modelling to identify natural and gas well-induced emissions of methane into streams. Appl Geochem. 2018;91:107–121. [Google Scholar]
- 18.Wen T, et al. Big groundwater data sets reveal possible rare contamination amid otherwise improved water quality for some analytes in a region of Marcellus Shale development. Environ Sci Technol. 2018;52:7149–7159. doi: 10.1021/acs.est.8b01123. [DOI] [PubMed] [Google Scholar]
- 19.Gorody AW. Factors affecting the variability of stray gas concentration and composition in groundwater. Environ Geosci. 2012;19:17–31. [Google Scholar]
- 20.Molofsky LJ, et al. Environmental factors associated with natural methane occurrence in the Appalachian Basin. Ground Water. 2016;54:656–668. doi: 10.1111/gwat.12401. [DOI] [PubMed] [Google Scholar]
- 21.Heilweil VM, et al. Stream measurements locate thermogenic methane fluxes in groundwater discharge in an area of shale-gas development. Environ Sci Technol. 2015;49:4057–4065. doi: 10.1021/es503882b. [DOI] [PubMed] [Google Scholar]
- 22.Heilweil V, Risser D, Conger R, Grieve P, Hynek S. 2014. Estimation of methane concentrations and loads in groundwater discharge to Sugar Run, Lycoming County, Pennsylvania. (US Geological Survey, Reston, VA), Open-File Report 2014–1126.
- 23.Ingraffea AR, Wells MT, Santoro RL, Shonkoff SBC. Assessment and risk analysis of casing and cement impairment in oil and gas wells in Pennsylvania, 2000-2012. Proc Natl Acad Sci USA. 2014;111:10955–10960. doi: 10.1073/pnas.1323422111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Pennsylvania Environmental Hearing Board 2017 Commonwealth of Pennsylvania Department of Environmental Protection vs Range Resources. Available at ehb.courtapps.com/public/document_shower_pub.php?csNameID=5093. Accessed May 1, 2018.
- 25.PA DEP 2015 DEP reaches penalty agreements with three natural gas exploration companies in the Northern Tier. Pennsylvania Department of Environmental Protection report. Available at https://www.media.pa.gov/Pages/DEP_details.aspx?newsid=487. Accessed March 1, 2018.
- 26.Zebataksis KW. 1965. Flammability characteristics of combustible gases and vapors (US Bureau of Mines, Washington, DC), Bulletin No. 627.
- 27.Molofsky LJ, et al. Effect of different sampling methodologies on measured methane concentrations in groundwater samples. Ground Water. 2016;54:669–680. doi: 10.1111/gwat.12415. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe AL, Wilkin RT. Evidence of sulfate-dependent anaerobic methane oxidation within an area impacted by coalbed methane-related gas migration. Environ Sci Technol. 2017;51:1901–1909. doi: 10.1021/acs.est.6b03709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen T, Castro MC, Hall CM, Pinti DL, Lohmann KC. Constraining groundwater flow in the glacial drift and Saginaw aquifers in the Michigan Basin through helium concentrations and isotopic ratios. Geofluids. 2015;16:3–25. [Google Scholar]
- 30.Wen T, et al. Methane sources and migration mechanisms in shallow groundwaters in Parker and Hood Counties, Texas–A heavy noble gas analysis. Environ Sci Technol. 2016;50:12012–12021. doi: 10.1021/acs.est.6b01494. [DOI] [PubMed] [Google Scholar]
- 31.Oxburgh E, O’Nions R, Hill R. Helium isotopes in sedimentary basins. Nature. 1986;324:632–635. [Google Scholar]
- 32.Ozima M, Podosek F. Noble Gas Geochemistry. Cambridge Univ Press; New York: 2002. [Google Scholar]
- 33.Gross E, Cravotta C., III 2016. Groundwater quality for 75 domestic wells in Lycoming County, Pennsylvania, 2014 (US Geological Survey, Reston, VA), Scientific Investigations Report 2016–5143, 74 p.
- 34.Brantley et al. 2018 Shale Network Database, Consortium for Universities for the Advancement of Hydrologic Sciences, Inc. Available at hiscentral.cuahsi.org/pub_network.aspx?n=228. Accessed October 3, 2018.
- 35.U.S. Environmental Protection Agency 2015 Retrospective case study in Northeastern Pennsylvania: Study of the potential impacts of hydraulic fracturing on drinking water resources. Office of Research and Development EPA/600/R-14/088. Available at https://www.epa.gov/hfstudy. Accessed January 1, 2017. [PubMed]
- 36.Wendt AK, et al. Scientist-nonscientist teams explore methane sources in streams near oil/gas development. J Contemp Water Res Educ. 2018;164:80–111. [Google Scholar]
- 37.Schoell M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim Cosmochim Acta. 1980;44:649–661. [Google Scholar]
- 38.Range Resources–Appalachia LLC 2013 Green Valley Rd Water Well Investigation Moreland TWP, Lycoming County, PA. Available at www.rangeresources.com/docs/default-source/lycoming/range-final-report-4-8-12.pdf?sfvrsn=2. Accessed November 7, 2017.
- 39.Reese SO, Negoba VV, Pelelpko S, Kosmer WJ, Beattie S. 2014. Groundwater and petroleum resources of Sullivan County, Pennsylvania (Department of Conservation and Natural Resources, Pennsylvania Geological Survey, Harrisburg, PA), Water Resource Report 71, Fourth Series.
- 40.Graham DW. Noble gas isotope geochemistry of mid-ocean ridge and ocean island basalts: Characterization of mantle source reservoirs. Rev Mineral Geochem. 2002;47:247–317. [Google Scholar]
- 41.Hunt AG, Darrah TH, Poreda RJ. Determining the source and genetic fingerprint of natural gases using noble gas geochemistry: A northern Appalachian Basin case study. Am Assoc Pet Geol Bull. 2012;96:1785–1811. [Google Scholar]
- 42.Siegel DI, Smith B, Perry E, Bothun R, Hollingsworth M. Pre-drilling water-quality data of groundwater prior to shale gas drilling in the Appalachian Basin: Analysis of the Chesapeake Energy Corporation dataset. Appl Geochem. 2015;63:37–57. [Google Scholar]
- 43.Faill RT. 1979. Geology and mineral resources of the Montoursville south and Muncy quadrangles and part of the Hughesville quadrangle, Lycoming, Northumberand, and Montour counties, Pennsylvania (Commonwealth of Pennsylvania, Department of Environmental Resources, Bureau of Topographic and Geologic Survey, Harrisburg, PA), no. Atlas 144ab.
- 44.Engelder T, Lash GG, Uzcátegui RS. Joint sets that enhance production from Middle and Upper Devonian gas shales of the Appalachian Basin. Am Assoc Pet Geol Bull. 2009;93:857–889. [Google Scholar]
- 45.Lisle R. Detection of zones of abnormal strains in structures using Gaussian curvature analysis. Am Assoc Pet Geol Bull. 1994;78:1811–1819. [Google Scholar]
- 46.Schultz-Ela D, Yeh J. Predicting fracture permeability from bed curvature. Rock Mech. 1992;33:579–590. [Google Scholar]
- 47.Fischer MP, Wilkerson MS. Predicting the orientation of joints from fold shape: Results of pseudo-three-dimensional modeling and curvature analysis. Geology. 2000;28:15–18. [Google Scholar]
- 48.Hennings PH, Olson JE, Thompson LB. Combining outcrop data and three-dimensional structural models to characterize fractured reservoirs : An example from Wyoming. Am Assoc Pet Geol Bull. 2000;84:830–849. [Google Scholar]
- 49.Hancock PL, Engelder T. Neotectonic joints. Geol Soc Am Bull. 1989;101:1197–1208. [Google Scholar]
- 50.Bair ES, Freeman DC, Senko JM. 2010 Subsurface gas invasion. Bainbridge Township, Geauga County, Ohio (Ohio Department of Natural Resources, Division of Mineral Resources Management), Expert Panel Technical Report. Available at oilandgas.ohiodnr.gov/resources/investigations-reports-violations-reforms#THR. Accessed January 1, 2018.
- 51.Lloyd O, Carswell LD. 1981. Groundwater resources of the Williamsport region, Lycoming County, Pennsylvania (Pennsylvania Geological Survey, Harrisburg, PA), Water Resource Report 51, Fourth Series, p 69.
- 52.Range Resources–Appalachia LLC 2012 Green Valley Rd Water Well Investigation Moreland TWP, Lycoming County, PA. Available at www.rangeresources.com/docs/default-source/lycoming/appendix-iv-range-presentation-to-pa-dep-10-1-12.pdf?sfvrsn=2. Accessed June 29, 2017.
- 53.Schout G, Hartog N, Hassanizadeh SM, Griffioen J. Impact of an historic underground gas well blowout on the current methane chemistry in a shallow groundwater system. Proc Natl Acad Sci USA. 2018;115:296–301. doi: 10.1073/pnas.1711472115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alawattegama SK, et al. Well water contamination in a rural community in southwestern Pennsylvania near unconventional shale gas extraction. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015;50:516–528. doi: 10.1080/10934529.2015.992684. [DOI] [PubMed] [Google Scholar]
- 55.Amos RT, et al. Evidence for iron-mediated anaerobic methane oxidation in a crude oil-contaminated aquifer. Geobiology. 2012;10:506–517. doi: 10.1111/j.1472-4669.2012.00341.x. [DOI] [PubMed] [Google Scholar]
- 56.Van Stempvoort D, Maathuis H, Jaworski E, Mayer B, Rich K. Oxidation of fugitive methane in ground water linked to bacterial sulfate reduction. Ground Water. 2005;43:187–199. doi: 10.1111/j.1745-6584.2005.0005.x. [DOI] [PubMed] [Google Scholar]
- 57.Kelly WR, Matisoff G, Fisher JB. The effects of a gas well blow out on groundwater chemistry. Environ Geol Water Sci. 1985;7:205–213. [Google Scholar]
- 58.McMahon PB, Belitz K, Barlow JRB, Jurgens BC. Methane in aquifers used for public supply in the United States. Appl Geochem. 2017;84:337–347. [Google Scholar]
- 59.Bergbauer S, Pollard DD. How to calculate normal curvatures of sampled geological surfaces. J Struct Geol. 2003;25:277–289, and erratum (2003) 25:2167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.