Significance
The treatment of genetic kidney disease is challenging, as this requires both the correction of the underlying gene defect and the delivery of the treatment. Here we show that by using antisense oligonucleotides, we can induce exon skipping of a mutated exon in CEP290, within renal epithelial cells derived from a patient with a ciliopathy syndrome called Joubert syndrome. This treatment rescues the truncated CEP290 protein to a near full-length protein and restores the ciliary phenotype. In a Cep290 murine model of Joubert syndrome, exon skipping is achievable with systemic treatment of an antisense oligonucleotide, which rescues both the ciliary and kidney disease phenotypes. This work paves the way toward personalized genetic therapies in patients with inherited kidney diseases.
Keywords: Joubert syndrome, Cep290, cystic kidney, antisense oligonucleotide therapy, ciliopathy
Abstract
Genetic treatments of renal ciliopathies leading to cystic kidney disease would provide a real advance in current therapies. Mutations in CEP290 underlie a ciliopathy called Joubert syndrome (JBTS). Human disease phenotypes include cerebral, retinal, and renal disease, which typically progresses to end stage renal failure (ESRF) within the first two decades of life. While currently incurable, there is often a period of years between diagnosis and ESRF that provides a potential window for therapeutic intervention. By studying patient biopsies, patient-derived kidney cells, and a mouse model, we identify abnormal elongation of primary cilia as a key pathophysiological feature of CEP290-associated JBTS and show that antisense oligonucleotide (ASO)-induced splicing of the mutated exon (41, G1890*) restores protein expression in patient cells. We demonstrate that ASO-induced splicing leading to exon skipping is tolerated, resulting in correct localization of CEP290 protein to the ciliary transition zone, and restoration of normal cilia length in patient kidney cells. Using a gene trap Cep290 mouse model of JBTS, we show that systemic ASO treatment can reduce the cystic burden of diseased kidneys in vivo. These findings indicate that ASO treatment may represent a promising therapeutic approach for kidney disease in CEP290-associated ciliopathy syndromes.
Joubert syndrome (JBTS) is an archetypal ciliopathy syndrome, characterized by multisystem involvement, including retinal dystrophy and degeneration, cerebellar vermis aplasia, and nephronophthisis. JBTS is incurable and nephronophthisis represents the major cause of pediatric renal failure (1). Studying the molecular genetics of JBTS has led to the discovery of numerous genes underlying this disorder, which all encode protein products associated with the primary cilium. CEP290 is the most common genetic cause of JBTS with a cerebello-retinal-renal phenotype (2). Mutations in CEP290 may give rise to additional phenotypes as part of a disease spectrum including Leber congenital amaurosis (LCA), Senior Loken syndrome, Meckel Gruber syndrome, and Bardet Biedl syndrome (3–6). The CEP290 gene has 54 exons that encode a protein located at the transition zone of the primary cilium where it is thought to play a gatekeeper role in the entry and exit of proteins from the ciliary axoneme (7). Ciliary signaling mechanisms such as the Sonic hedgehog (SHH) pathway have been shown to be disrupted in both mouse models of JBTS and primary patient renal epithelial cells; pharmacological manipulation of SHH signaling has been shown to ameliorate features of the disease, such as primary cilia defects (8, 9). However, such pharmacological interventions do not correct the primary lesion and, particularly in the case of modulation of SHH signaling, carry a risk of unwanted side effects.
Recent studies have suggested alternative therapeutic strategies for ciliopathies that directly correct the genetic lesion, in particular targeting CEP290 mutations in LCA (10). The most common cause of LCA is an intronic mutation that creates a splice donor site and a pseudoexon, disrupting the CEP290 transcript (11). Treatment with a splice-blocking antisense oligonucleotide (ASO) was able to restore the normal transcript in both patient cell lines and a mouse model of LCA (10). The majority of CEP290 mutations causing JBTS are within the coding sequence, which is more suited to gene replacement therapy. However, the size of the gene (54 exons) and its protein product (290 kDa) pose a considerable challenge for conventional, viral-based, gene replacement therapies, although lentiviral vector delivery of full-length CEP290 has been used successfully in patient fibroblast cells (12). Following a report of a patient with a mild LCA phenotype associated with nonsense-mediated alternative splicing of CEP290 (11), an extensive study of endogenous (wild-type) splicing revealed widespread, low-level alternative splicing that could be modeled to predict genetic pleiotropy associated with CEP290 mutations (13). Indeed, confirming this hypothesis, endogenous basal exon skipping and nonsense-associated altered splicing has been documented in patient fibroblasts with nonsense mutations in CEP290 with mild retinal phenotypes (14). These observations are reminiscent of the Duchenne/Becker muscular dystrophy paradigm (15), which responds to targeted exon skipping therapies (16, 17). Given that most of the CEP290 protein consists of repeated coiled-coil domains, often encoded by a single exon, CEP290 seems an ideal candidate for ASO-mediated exon skipping therapy.
Results
Clinical and Genetic Investigations.
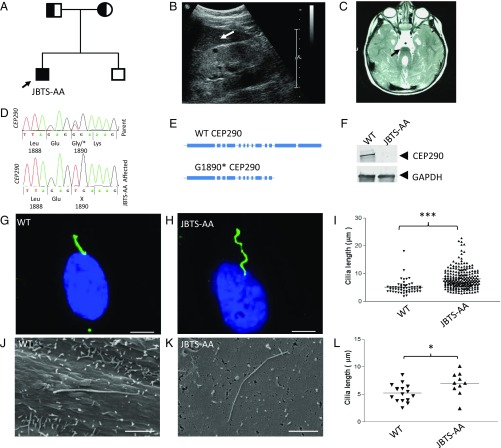
Here we describe a 14 y old boy (JBTS-AA) from consanguineous parents (Fig. 1A) affected with JBTS. He initially presented with congenital ptosis and visual failure secondary to an early onset retinal degeneration (SI Appendix, Fig. S1) and subsequently developed ataxia and significant renal impairment (Table 1). Clinical investigations revealed hyperechogenic kidneys showing cystic change and loss of corticomedullary differentiation (Fig. 1B), consistent with a diagnosis of nephronophthisis, and a “molar tooth” sign on brain MRI (Fig. 1C) which is due to cerebellar vermis aplasia and is the defining feature of JBTS. Molecular genetic investigations in JBTS-AA confirmed a homozygous nucleotide substitution leading to a stop codon (c.5668G > T; p.G1890*) in exon 41 of CEP290 (Fig. 1D). This mutation is predicted to lead to a truncated CEP290 protein lacking numerous C-terminal coiled-coil domains (Fig. 1E).
Fig. 1.
Clinical phenotype, molecular genetics, and primary cilia defects in human urine-derived renal epithelial cells from a Joubert syndrome patient. (A) Pedigree diagram showing single affected male, JBTS-AA (squares, males; circles, females). (B) Renal ultrasound scan of JBTS-AA showing minor cystic change within the kidney (arrowed) and loss of corticomedullary differentiation consistent with a diagnosis of nephronophthisis. (C) MRI scan of JBTS-AA showing a molar tooth sign (arrowed) with cerebellar vermis aplasia and elongated superior cerebellar peduncles. (D) Sequence chromatograms showing homozygous change in CEP290 c.5668G > T; p.G1890* segregating from parental DNA (father’s chromatogram is shown). (E) SMART Domain structure of WT CEP290 protein (2,479 amino acids) predicts multiple coiled-coil domains (show as blue bars). The truncated protein CEP290 G1890* results in loss of C-terminal coiled-coil domains. (F) Western blotting of protein from human urine-derived renal epithelial cells showing loss of full-length CEP290 protein in JBTS-AA. Blots were probed with a CEP290 antibody directed at the C terminus of CEP290, with GAPDH shown as a loading control. (G) Wild-type (WT) and (H) patient (JBTS-AA) hURECs imaged under high-power immunofluorescence using anti-ARL13B (green) to identify ciliary membrane. (Scale bar: 5 µm.) (I) Quantification of ciliary length in cells grown in serum-free medium for 48 h, measured by immunofluorescence imaging (WT, n = 55, JBTS-AA, n = 222; ***P < 0.0001, unpaired t test). (J) Wild-type (WT) and (K) patient (JBTS-AA) hURECs seen under scanning EM reveals abnormally long cilia in patient cells after 48 h serum starvation (Scale bar: 2 µm.). (L) Quantification of increase in cilia length (WT, n = 16, JBTS-AA, n = 10; *P < 0.05, unpaired t test).
Table 1.
Molecular and clinical characteristics of affected patient (JBTS-AA)
| Characteristics | JBTS-AA |
| Ethnicity and consanguinity | Pakistani, parents first cousins, segregation confirmed |
| Renal ultrasound scan findings | Increased echogenicity, corticomedullary cysts, loss of corticomedullary differentiation |
| Kidney function | Chronic kidney disease stage 3, creatinine 1.91 mg/dL (aged 14 y) |
| Ocular symptoms (age of onset, y) | Congenital ptosis (1 mo) Early onset retinal degeneration initially diagnosed with visual failure (2 mo) |
| Central nervous symptoms | Ataxia, cerebellar vermis aplasia/hypoplasia with “molar tooth sign” on brain MRI |
| Nucleotide alterations | Homozygous c.5668G > T (exon 41) |
| Alteration in coding sequence | Homozygous Gly1890* |
| Reference sequence | NM_025114 |
| SNP ID | rs137852832 |
| ExAC allele frequency | 0.0001432 |
Stop codon
Phenotyping Using Primary Renal Epithelial Cells.
To characterize the cellular and molecular consequences of this mutation, we derived primary, nontransformed human urine-derived renal epithelial cells (hURECs) from patient JBTS-AA and age/sex matched controls (WT). Western blotting, using a CEP290 C-terminal antibody, confirmed an almost complete absence of full-length CEP290 protein (Fig. 1F) from patient hURECs. Immunofluorescence analysis of cilia structure revealed elongated primary cilia on patient hURECs (JBTS-AA) compared with control (WT) cells (Fig. 1 G–I). This was confirmed by scanning electron microscopy (Fig. 1 J–L). In terms of percentage ciliation rates in hURECs, there was no difference between wild-type and JBTS-AA cells (SI Appendix, Fig. S2). We have previously described an elongated cilia phenotype in hURECs derived from an unrelated JBTS patient harboring CEP290 mutations (9), suggesting abnormally long primary cilia may be a common, renal feature of JBTS. To investigate this further, we carried out immunofluorescence analysis of cilia structure in two further (unrelated) JBTS patients with CEP290 mutations and kidney failure (SI Appendix, Table S1). Immunofluorescence staining of renal biopsies for acetylated tubulin and ARL13B revealed the presence of elongated and tortuous primary cilia in both patients compared with normal kidney (SI Appendix, Fig. S3) confirming that the elongated primary cilia found on patient hURECs accurately reflect the phenotype observed in vivo. It is intriguing to note that elongated cilia have also been found in a wide range of other ciliopathy models including: Bbs-4 null mice (18), jck mice (19), Meckel syndrome (20), Kif7 mutated cells (21), and JBTS secondary to KIAA0556 mutations (22), suggesting elongated primary cilia may be a widespread feature of ciliopathies in general and that correction of this phenotype attenuates cystic kidney disease (23).
Targeted Exon Skipping of CE290 Rescues Phenotype in Primary Renal Epithelial Cells.
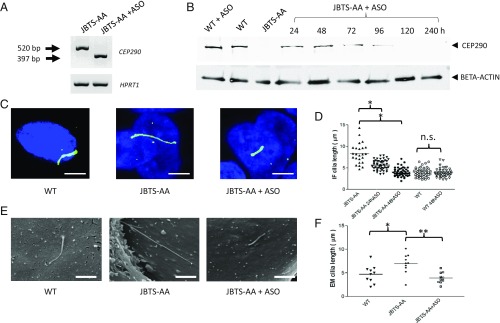
Exon 41 of CEP290 is 123 nucleotides in length and encodes a single, discrete, coiled-coil domain of the protein (SI Appendix, Fig. S4). Consequently, a splice-blocking ASO targeted to the splice donor site of exon 41 would be predicted to induce skipping of exon 41, while maintaining the correct reading frame, to produce a transcript encoding a near full-length protein of 2,438 amino acids (286 KDa) that contains a normal C terminus and is lacking only a single coiled-coil domain (SI Appendix, Fig. S4). To test the functionality of the splice blocking ASO, we carried out RT-PCR analysis of the CEP290 transcript from hURECs and confirmed efficient ASO-induced alternative splicing, skipping exon 41 (Fig. 2A). In the absence of any available N-terminal CEP290 antibodies, quantitative RT-PCR of the CEP290 transcript targeted to exons 6–7, upstream of the mutation in JBTS-AA, showed that CEP290 expression levels in JBTS-AA hURECs are reduced to ∼20% of wild-type levels, suggesting degradation of the mutant mRNA transcript; levels of the transcript are rescued following ASO-induced splicing (SI Appendix, Fig. S5A). Furthermore, we observed restoration of protein expression by Western blot in ASO-treated JBTS-AA hURECs, with near wild-type levels of protein detectable with the C-terminal CEP290 antibody at 24 h post treatment, which persisted for 96 h (Fig. 2B). In addition to the structural ciliary changes observed, JBTS-AA hURECS showed an increase in SHH levels (∼fourfold) indicating dysregulation of ciliary signaling. Transcript levels of SHH were, however, not fully rescued by ASO treatment (SI Appendix, Fig. S5B).
Fig. 2.
ASO-induced exon skipping restores CEP290 protein expression and rescues cilia defects in patient hURECs. (A) Gene expression analysis using RT-PCR of hURECs obtained from patient JBTS-AA. Lane 1 demonstrates CEP290 (predicted 520 bp) expression in untreated cells. Lane 2 shows RT-PCR products obtained after treatment with 1 μm ASO directed toward exon 41 splice-junction. ASO-mediated exon skipping leads to a shorter CEP290 mRNA transcript (predicted product size of 397 bp). (B) CEP290 exon 41 skipping induced by ASO of JBTS-AA hURECs demonstrates the rescue of CEP290 protein translation. WT hURECs protein lysates demonstrate the presence of full-length CEP290 protein (290 kDa), which was not adversely affected by ASO treatment. JBTS-AA hURECs protein lysates show a near absence of full-length protein. JBTS-AA ASO-treated hURECs show rescue of near full-length CEP290 protein translation at 24 h post treatment, which persists for 96 h. BETA-ACTIN (42 kDa) serves as loading control. (C) Immunofluorescence (IF) microscopy of wild-type hURECs, untreated JBTS-AA hURECs, and JBTS-AA hURECs treated with ASO to induce exon skipping of exon 41 rescues the phenotype of long cilia. DAPI- blue (Nuclei), Green - ARL13B (Ciliary membrane). (Scale bar: 5 μm.) (D) IF quantification of the cilia length in JBTS-AA hURECs followed by treatment with ASO. Cilia length was quantified after treatment with 1 μM ASO at 24 h and 48 h. The mean cilia length measured for untreated JBTS-AA cilia (n = 24) was 8.36 μm (detected using ARL13B antibodies). The mean cilia length after treatment with 1 μM ASO for 24 h (n = 50) was 4.56 μm and after treatment for 48 h (n = 50) was 4.02 μm. The mean cilia length of WT hURECs cilia (n = 49) before treatment was 3.96 μm and after treatment with 1 μM ASO for 48 h (n = 50) was 3.87 μm. Data are shown as scattergrams with mean shown. *P < 0.0001 determined by one-way ANOVA. (E) Scanning electron microscopy of hURECs cilia in WT, untreated JBTS-AA, and JBTS-AA treated with ASO and imaged at 48 h. (Scale bar: 2 μm.) (F) Electron microscopy (EM) quantification of the cilia length in WT, JBTS-AA, and JBS-AA+ ASO treated hURECs rescued by treatment with ASO. The mean cilia lengths were: WT hURECs 4.8 μm, JBTS-AA 6.74 μm, and JBTS-AA + ASO 3.78 μm. Data are shown as scattergrams with mean indicated.*P < 0.05 and **P < 0.01, one-way ANOVA. n = 10 cilia for each group. n.s., not significant.
Despite lacking a single coiled-coil domain encoded by exon 41, the near full-length CEP290 protein correctly localized to the transition zone in ASO-treated JBTS-AA hURECs (SI Appendix, Fig. S6); this provides evidence that the removal of exon 41 in CEP290 does not affect protein localization. Given the correct localization of the restored protein, lacking a single coiled-coil domain, we investigated whether this could ameliorate the phenotype of JBTS-AA hURECs. Primary cilia were analyzed using fluorescence microscopy directed at ARL13B and a significant reduction in the length of JBTS-AA primary cilia, equivalent to wild-type cilia was observed after 48 h (Fig. 2 C and D); this was also confirmed by scanning electron microscopy (Fig. 2 E and F). It is noteworthy that the reduction in cilia length is dramatic and significant even at 24 h, indicating a rapid response to ASO treatment. This extends our previous findings, that pharmacological treatments can reduce cilia length in JBTS hURECs (9), and provides evidence to suggest that this phenotype, which relies upon the continued presence of the mutation, is reversible. Importantly, ASO treatment of wild-type control hURECs had no effect on the length of the primary cilium, suggesting that the absence of the coiled-coil domain encoded by exon 41 can be tolerated.
Targeted Exon Skipping of Cep290 in Vivo Rescues Ciliary and Cystic Kidney Disease Phenotypes.
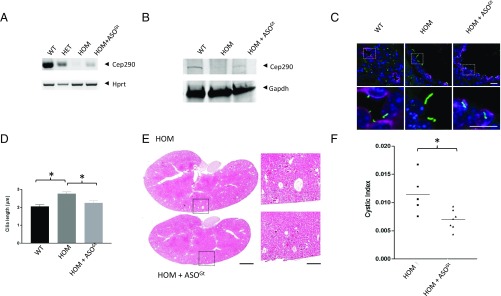
Having shown a positive effect of ASO treatment on primary, nontransformed patient kidney cells ex vivo, we wished to determine whether a similar strategy could be applied in vivo. The most faithful model of JBTS described to date is the Cep290Gt(CC0582)Wtsi mouse line (which we will refer to as Cep290Gt, previously reported as Cep290LacZ) that presents with a renal-retinal-brain phenotype caused by insertion of a “gene trap” within Cep290 after exon 25 (8). As the Cep290Gt mice were produced from embryonic stem (ES) cells containing an intronic splice acceptor/β-galactosidase/neomycin phosphotransferase (SA-IRES-βGEO) “gene trap” cassette (3,975 bp), we designed a new oligonucleotide to block the splice acceptor of the gene trap cassette (vivo-ASOGt) to restore full-length Cep290 transcript and protein (SI Appendix, Fig. S7). As proof of concept, treatment of immortalized kidney cells derived from homozygous Cep290Gt/Gt mice with vivo-ASOGt resulted in a modest restoration of correct splicing between exons 25 and 26, with a concomitant decrease in exon 25 splicing into SA-IRES-βGEO, and a return of a full-length Cep290 protein detected by Western blotting using a C-terminal Cep290 antibody (SI Appendix, Fig. S7). In contrast to ASO treatment of JBTS-AA hURECs, wild-type levels of transcript/protein were not achieved in the transformed mouse cell line following vivo-ASOGt treatment. RT-PCR analysis with a panel of primers located across the SA-IRES-βGEO cassette suggests that we do not see complete restoration of wild-type transcript levels (SI Appendix, Fig. S7), possibly due to the presence of cryptic splice sites within the SA-IRES-βGEO cassette (24, 25). However, given that CEP290 is not haploinsufficient and it has been reported that small fragments of CEP290 can ameliorate LCA (26), we reasoned that even subwild-type levels of protein may be beneficial. Therefore, we proceeded to investigate the potential effects of a modest restoration of full-length protein in vivo, in Cep290Gt/Gt mice. Systemic administration of vivo-ASOGt was carried out via a series of i.v. injections over 10 d. RT-PCR analysis and sequencing of whole kidney RNA revealed correctly spliced transcripts (Fig. 3A and SI Appendix, Fig. S8), consistent with splicing from exon 25 to exon 26, skipping SA-IRES-βGEO. Full-length Cep290 protein was detected by a Western blot of whole kidney extracts using C-terminal Cep290 antibody (Fig. 3B) indicating that vivo-ASOGt was capable of inducing skipping of SA-IRES-βGEO in kidney, in vivo. Whole murine kidney tissue protein extract was used to assess the response of ciliary signaling molecules. Compared with wild-type controls, Cep290Gt/Gt kidneys had increased Shh signaling, as demonstrated by a rise in the Gli3 activator to repressor ratio that was partially restored by treatment with vivo-ASOGt (SI Appendix, Fig. S5 C and D). Immunofluorescence analysis revealed an increase in the length of primary cilia (Arl13b staining) in cortical collecting ducts (aquaporin-2 staining) in homozygous Cep290Gt/Gt mice compared with wild-type littermates (Fig. 3 C and D), highlighting a further similarity between the Cep290Gt mouse model and JBTS patients with CEP290 mutations (SI Appendix, Fig. S2). This increase in cilia length of cortical collecting duct cells could be rescued by ∼50% by systemic vivo-ASOGt treatment, similar to the 24 h ASO treatment of JBTS-AA hURECs. Having shown full-length protein and a shortening of the abnormally long primary cilia following vivo-ASOGt treatment, we carried out quantitative assessment of cystic burden in vivo-ASOGt treated and untreated Cep290Gt/Gt kidneys. Remarkably, vivo-ASOGt treatment resulted in a striking and significant improvement in cystic index (Fig. 3 E and F) of 37%, indicating that measurable therapeutic benefit can be achieved in vivo by relatively modest levels of ASO-induced splice blocking.
Fig. 3.
In vivo systemic ASO treatment restores Cep290 protein expression and reduces cystic burden in a JBTS mouse model. (A) RT-PCR of Cep290 from murine kidney mRNA. RT-PCR products spanning exons 25–27 are shown from kidneys of wild-type (WT), heterozygous Cep290Gt/+ (HET), and homozygous Cep290Gt/Gt (HOM) mice. Almost no product is observed when the gene trap is present homozygously. Treatment with vivo-ASOGt of HOM mice increases the amount of wild-type Cep290 transcript. Hprt is shown as a control. (B) Western blot showing Cep290 protein isolated from WT, HOM, and HOM mice treated with vivo-ASOGt to induce skipping of the gene trap. The level of protein in homozygous animals increases in the presence of the ASOGt treatment. GAPDH (37 kDa) is shown as loading control. (C) Immunofluorescence imaging of renal primary cilia within cortical collecting ducts in WT, HOM, and HOM mice treated with vivo-ASOGt. Cilia are stained with Arl13b (green), collecting ducts are identified using aquaporin-2 (magenta), and nuclei are stained with DAPI (blue). Images are taken from samples with median values of their respective groups. (Scale bar: 10 μm.) (D) Quantification of mean primary cilia length from renal tissues of WT (n = 3), HOM (n = 4), and HOM + ASOGt treated mice (n = 7). Mean number of cilia measured in each animal = 97. (*P < 0.05, one-way ANOVA). (E) Hematoxylin and eosin staining of untreated HOM and vivo-ASOGt treated HOM mice. Images are taken from samples with median values of their respective groups. (Scale bar: 1 mm.) (F) Quantification of the cystic index of untreated HOM (n = 5) and vivo-ASOGt treated HOM mice (n = 7) at 42 d of age. The mean cystic index is reduced from 0.011 in untreated animals to 0.0069 in animals treated with vivo-ASOGt (*P < 0.05, unpaired t test).
Discussion
We have shown that in human renal epithelial cells from a patient with JBTS secondary to a nonsense mutation in exon 41 of CEP290, ASO-mediated exon skipping can ameliorate the ciliary phenotype and restore localization of a near full-length CEP290 protein to the base of the cilium. We have then validated this approach in vivo, by using a different ASO targeting strategy to promote alternate splicing of a murine gene trap insertion within intron 25 of Cep290, thereby rescuing, in part, full-length Cep290 transcript and protein to achieve a phenotypic rescue of renal ciliary length in vivo as well as the cystic kidney disease. The observed phenotypic changes in murine and human cells, as well as in mouse kidney tissue following treatment with two completely different ASOs indicate that the observed effects are specific, and result from the restoration of CEP290/Cep290.
Overall, these data have several important implications. Firstly, systemic delivery of ASOs can ameliorate kidney pathology in a whole animal model. In addition, ASOs can rescue disease phenotypes after disease has become established in both JBTS patient-derived cells and an animal model, confirming the indications from pharmacological treatments that the cystic kidney disease is reversible. Finally, ASO-mediated exon skipping is tolerated at the level of protein localization, as the removal of a coiled-coil domain does not result in the mislocalization of the CEP290 protein. Given that many CEP290 mutations cluster in the C-terminal coiled-coil-rich region and are potentially skippable (SI Appendix, Fig. S9), it is tempting to speculate that these mutations may also be amenable to ASO-mediated therapy.
One limitation of this study is that despite G1890* being one of the most common mutations, we have not extended our human studies to other skippable exons in CEP290. Here true personalized medicine approaches, using patient-derived cells, such as we have used with patient JBTS-AA, will be required to determine if exon skipping of alternate exons, which may contain more crucial functional domains than exon 41, are possible. The direct consequences of skipping exons within the CEP290 gene require testing in both ex vivo and in vivo systems. A Cep290 mouse model, mimicking the human G1890* mutation, would be required to determine fully the effects of ASO-mediated exon skipping in both kidney and extrarenal tissues. Given the ease of access for subretinal injections, to date there has been a focus on ASO-based therapies for human retinal disease, including CEP290-associated LCA (10, 27). As our work has shown, systemic delivery of ASOs is possible and seems to ameliorate kidney disease; this needs to be assessed more fully in renal tissues as well as retinal tissues for dosing regimens and timing of therapies. However, given the efficiency of ASO-mediated exon skipping observed in patient kidney cells, where the next available splice acceptor is used almost exclusively, this is likely to confirm that ASO treatments should be considered as a promising therapeutic approach. It seems likely, following on from the success of ASO-based therapy for targeting CEP290 retinal disease, that ASO-based therapy for CEP290-associated renal disease has true translational promise and may provide a genetic rescuing therapy for this severe ciliopathy syndrome.
In conclusion, we show that using ASO-mediated exon skipping rescues a ciliary defect in patient renal epithelial cells and can be used systemically to target CEP290 transcripts within kidney tissues, offering a therapeutic approach to treating inherited renal ciliopathies.
Materials and Methods
Study Approval.
Ethical approval was obtained from the National Research Ethics Service Committee North East (14/NE/1076), United Kingdom. All animal experiments were conducted according to protocols approved by the Animal Ethics Committee of Newcastle University and the Home Office, United Kingdom.
Statistics.
All data are shown as the mean ± SEM, unless otherwise stated and unpaired Student’s t test or one-way ANOVA followed by a Bonferroni corrected post hoc test when comparing two or more groups. A P value of less than 0.05 was considered statistically significant.
Clinical and DNA Sequencing.
Following informed and written consent, urine and blood samples were obtained from a 14-y-old boy with clinical JBTS with a retinal, renal, and cerebellar phenotype and a healthy gender- and age-matched control. All methods were performed in accordance with the relevant ethical guidelines and regulations.
Sequencing of Joubert syndrome genes was performed by UKGTN using an 18 Gene Panel (Oxford Regional Genetic Laboratory Service). Confirmation of mutations and segregation analysis was performed on other family members, following informed consent, using Sanger sequencing, using exon specific primers.
In Vitro Studies Using Human Kidney and hURECs.
Immunostaining of human kidney tissues from patients with CEP290 mutations was performed. In vitro studies following hUREC isolation (28), culture, and ASO treatments were performed, including immunostaining, Western blotting, and reverse transcription-PCR and quantitative RT-PCR. Electron microscopy studies were performed on hURECs. Please see SI Appendix for detailed materials and methods.
In Vivo ASO Treatment of Cep290Gt/Gt Animals.
In vivo studies were performed on 28-d-old Cep290Gt/Gt animals. Renal tissues were harvested and used for immunofluorescence studies, Western blotting, RNA extraction, and reverse transcription-PCR and quantitative RT-PCR. Please see SI Appendix for detailed materials and methods.
Supplementary Material
Acknowledgments
Thank you to the patient and family members who contributed to this study. This work is funded by The Medical Research Council (Award MR/M012212/1), a Kidney Research UK Post-doctoral fellowship (to S.A.R.) (Award PDF_003_20151124), and Northern Counties Kidney Research Fund. L.D. is funded by The Medical Research Council Discovery Medicine North Training Partnership.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809432115/-/DCSupplemental.
References
- 1.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 2.Valente EM, Brancati F, Boltshauser E, Dallapiccola B. Clinical utility gene card for: Joubert syndrome–update 2013. Eur J Hum Genet. 2013;21:1187. doi: 10.1038/ejhg.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baala L, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brancati F, et al. International JSRD Study Group CEP290 mutations are frequently identified in the oculo-renal form of Joubert syndrome-related disorders. Am J Hum Genet. 2007;81:104–113. doi: 10.1086/519026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitch CC, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–448, and correction (2008) 40:927. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 7.Betleja E, Cole DG. Ciliary trafficking: CEP290 guards a gated community. Curr Biol. 2010;20:R928–R931. doi: 10.1016/j.cub.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Hynes AM, et al. Murine Joubert syndrome reveals Hedgehog signaling defects as a potential therapeutic target for nephronophthisis. Proc Natl Acad Sci USA. 2014;111:9893–9898. doi: 10.1073/pnas.1322373111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava S, et al. A human patient-derived cellular model of Joubert syndrome reveals ciliary defects which can be rescued with targeted therapies. Hum Mol Genet. 2017;26:4657–4667. doi: 10.1093/hmg/ddx347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garanto A, et al. In vitro and in vivo rescue of aberrant splicing in CEP290-associated LCA by antisense oligonucleotide delivery. Hum Mol Genet. 2016;25:2552–2563. doi: 10.1093/hmg/ddw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Littink KW, et al. A novel nonsense mutation in CEP290 induces exon skipping and leads to a relatively mild retinal phenotype. Invest Ophthalmol Vis Sci. 2010;51:3646–3652. doi: 10.1167/iovs.09-5074. [DOI] [PubMed] [Google Scholar]
- 12.Burnight ER, et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21:662–672. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drivas TG, Wojno AP, Tucker BA, Stone EM, Bennett J. Basal exon skipping and genetic pleiotropy: A predictive model of disease pathogenesis. Sci Transl Med. 2015;7:291ra97. doi: 10.1126/scitranslmed.aaa5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barny I, et al. Basal exon skipping and nonsense-associated altered splicing allows bypassing complete CEP290 loss-of-function in individuals with unusually mild retinal disease. Hum Mol Genet. May 16, 2018 doi: 10.1093/hmg/ddy179. [DOI] [PubMed] [Google Scholar]
- 15.Klein CJ, et al. Somatic reversion/suppression in Duchenne muscular dystrophy (DMD): Evidence supporting a frame-restoring mechanism in rare dystrophin-positive fibers. Am J Hum Genet. 1992;50:950–959. [PMC free article] [PubMed] [Google Scholar]
- 16.van Deutekom JC, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 17.Kinali M, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: A single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokrzan EM, Lewis JS, Mykytyn K. Differences in renal tubule primary cilia length in a mouse model of Bardet-Biedl syndrome. Nephron Exp Nephrol. 2007;106:e88–e96. doi: 10.1159/000103021. [DOI] [PubMed] [Google Scholar]
- 19.Smith LA, et al. Development of polycystic kidney disease in juvenile cystic kidney mice: Insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol. 2006;17:2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- 20.Tammachote R, et al. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum Mol Genet. 2009;18:3311–3323. doi: 10.1093/hmg/ddp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He M, et al. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16:663–672. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders AA, et al. KIAA0556 is a novel ciliary basal body component mutated in Joubert syndrome. Genome Biol. 2015;16:293. doi: 10.1186/s13059-015-0858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husson H, et al. Reduction of ciliary length through pharmacologic or genetic inhibition of CDK5 attenuates polycystic kidney disease in a model of nephronophthisis. Hum Mol Genet. 2016;25:2245–2255. doi: 10.1093/hmg/ddw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roshon M, DeGregori JV, Ruley HE. Gene trap mutagenesis of hnRNP A2/B1: A cryptic 3′ splice site in the neomycin resistance gene allows continued expression of the disrupted cellular gene. BMC Genomics. 2003;4:2. doi: 10.1186/1471-2164-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anson DS, Limberis M. An improved beta-galactosidase reporter gene. J Biotechnol. 2004;108:17–30. doi: 10.1016/j.jbiotec.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Li L, Su Q, Gao G, Khanna H. Gene therapy using a miniCEP290 fragment delays photoreceptor degeneration in a mouse model of Leber congenital amaurosis. Hum Gene Ther. 2018;29:42–50. doi: 10.1089/hum.2017.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerard X, Garanto A, Rozet JM, Collin RW. Antisense oligonucleotide therapy for inherited retinal dystrophies. Adv Exp Med Biol. 2016;854:517–524. doi: 10.1007/978-3-319-17121-0_69. [DOI] [PubMed] [Google Scholar]
- 28.Ajzenberg H, et al. Non-invasive sources of cells with primary cilia from pediatric and adult patients. Cilia. 2015;4:8. doi: 10.1186/s13630-015-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.