Abstract
Background
Biologic age may better reflect an individual’s rate of aging than chronologic age.
Methods
We conducted a transcriptome-wide association study with biologic age estimated with clinical biomarkers, which included: systolic blood pressure, forced expiratory volume at 1 second (FEV1), total cholesterol, fasting glucose, C-reactive protein, and serum creatinine. We assessed the association between the difference between biologic age and chronologic age (∆age) and gene expression in whole blood measured using the Affymetrix Human Exon 1.0st Array.
Results
Our discovery sample included 2,163 participants from the Framingham Offspring cohort (mean age 67 ± 9 years, 55% women). A total of 481 genes were significantly associated with ∆age (p < 2.8 × 10−6). Among them, 415 genes were validated (p < .05/481 = 1.0 × 10−4) in 2,946 participants from the Framingham Third Generation cohort (mean age 46 ± 9 years, 53% women). Many of the significant genes were involved in the ubiquitin-mediated proteolysis pathway. The replication in 414 Rotterdam Study participants (mean age 59 ± 8, 52% women) found 104 of 415 validated genes reached nominal significance (p < .05).
Conclusion
We identified and validated 415 genes associated with ∆age in a community-based cohort. Future functional characterization of the biologic age-related gene network may identify targets to test for interventions to delay aging in older adults.
Keywords: Gene expression, Biologic aging, Epidemiology
Adults aged 65 years and older are the fastest growing demographic in the United States, and are projected to grow to 74 million or nearly 21% of the U.S. population by 2030 (1). Advancing age is the major risk factor for multiple chronic conditions comprising leading causes of death in this age group, including cardiovascular disease, type 2 diabetes, cancer, and Alzheimer’s disease (2,3). There is growing interest in identifying interventions that target aging and aging-related morbidity with the goal of increasing the number of years spent in good health, which in turn would have major economic implications (4). A challenge in designing intervention studies focused on aging is defining the study outcome(s). One potential approach is to define relevant measures of biologic age (5).
Biologic age is a measure of individuals’ predicted age based on biomarkers. Biologic age can be estimated using clinical biomarkers readily available in physicians’ offices. Even in young healthy adults, biologic age varies among persons of the same chronologic age (6) and in older adults biologic age predicts mortality better than chronologic age (7). Biologic age has been proposed as a measure of physical resilience, with opportunities to further our understanding of how genetics, health behaviors, the environment, and psychosocial factors influence individuals’ ability to recover from stressors (8). Broadening knowledge of the biologic mechanisms promoting accelerated aging or low physical resilience may lead to effective interventions including drug therapies to delay aging that may have important clinical and public health implications.
Biologic age estimated from clinical biomarkers in our community-based sample of older adults was associated with overall mortality and age-related disease even after accounting for risk factors (9). The objective of the present study was to assess the association of genome-wide gene expression with biologic age in participants from the community-based Framingham Heart Study to identify genes and molecular mechanisms underlying the regulation of human aging.
Methods
Study Sample
The Framingham Heart Study Offspring cohort participants were enrolled in 1971 to 1975, and have been examined every 4–8 years since. Research examinations consist of a physician administered medical history, resting blood pressure, laboratory assessments, various noninvasive cardiovascular measures, and lung function testing. Offspring participants eligible for the present study attended examination 8 (2005–2008, n = 3,021) and provided blood samples for gene expression assessment. Participants were excluded if data were missing to construct the measure of biologic age (n = 471) or gene expression data were not available (n = 387) leaving a final study sample of 2,163. The study was approved by the Boston University Medical Center Institutional Review Board, and all the participants provided written informed consent.
Clinical Biologic Age
We estimated biologic age using a subset of six clinical markers that have been used in prior reports and that represent different physiologic systems: systolic blood pressure, forced expiratory volume at 1 second (FEV1), total cholesterol, fasting glucose, C-reactive protein, and serum creatinine. Systolic blood pressure was computed from two physician obtained seated measurements. Routine blood tests were performed on fasting morning samples. FEV1 was collected by trained technicians according to American Thoracic Society standards using a Collins Comprehensive Pulmonary Laboratory system (Collins 2000 Plus).
We used the Klemera and Doubal method (10) to compute biologic age because this algorithm showed better performance than other methods for computing biologic age in both estimation precision (10) and predictive ability (7). We defined Δage as biologic age minus chronologic age so that individuals with Δage>0 are biologically “older” than their chronologic age, whereas individuals with Δage<0 are “younger” biologically than their chronologic age. Clinical Δage was not correlated with chronologic age, and was associated with mortality and incident age-related disease in our sample (9).
RNA Extraction and Gene Expression Profiling
Gene expression profiling of Framingham Heart Study participants has been previously described (11,12). RNA was extracted from fasting whole blood samples using PAXgene blood tubes (PreAnalytiX, Hombrechtikon, Switzerland) according to the manufacturer’s standard operating procedures. Total RNA was then reverse-transcribed into cDNA and amplified using the WT-Ovation Pico RNA Amplification System (NuGEN, San Carlos, CA). The amplified cDNA was hybridized to the Affymetrix Human Exon 1.0 ST Array (Affymetrix, Inc., Santa Clara, CA). We used Robust Multi-array Average (13) to summarize signal intensities. The annotation for each transcript was obtained from Affymetrix NetAffx Analysis Center (Release 31). A total of 17,873 distinct RefSeq transcripts (17,562 unique genes) were included for downstream analysis.
Given that whole blood contains a variety of cell types, and the gene expression from whole blood could be affected by the proportion of different cell types, we adjusted for the proportion of different cell types. Cell counts were measured in 2,181 participants from the Third Generation cohort, including white blood cells, red blood cells, platelets, neutrophils, lymphocytes, monocytes, eosinophils, and basophils. The cell counts for the remaining participants were imputed from the measured cell counts based on the gene expression data (12). The percentages of each imputed cell type were then normalized, where the negative predicted values were set to 0 and the sum of the percentages for all cell types was set as 100%.
Statistical Analysis
Linear mixed effects models were used to test associations between clinical ∆age and gene expression, with clinical ∆age as the exposure variable and gene expression as the dependent measure. Family relatedness was treated as a random variance-covariance factor in the models. Our primary models were adjusted for age, sex, cell counts, and technical covariates. Our secondary models were additionally adjusted for body mass index and smoking status as concerns about whether biologic aging may reflect obesity have been raised (14). All the analyses were performed using the “kinship” R package (www.r-project.org/). We used the Bonferroni correction to account for multiple testing; significant transcripts were defined as those with p < .05/17,873 = 2.8 × 10−6.
Validation Sample
We conducted validation of the discovery results in the Framingham Third Generation (Gen 3) cohort participants. Gen 3 participants with at least one parent in the Offspring cohort were enrolled in 2002 and have undergone examinations every 6–8 years. Participants who attended examination 2 (2008–2011), provided a blood sample for RNA extraction and gene expression profiling (n = 3,180) (12) and had all components measured at exam 2 to construct biologic age (n = 2,946) were eligible. The Gen 3 participants were on average 20 years younger than the Offspring participants and due to the family relation between the two cohorts are not considered a completely independent sample.
Replication Sample
The Rotterdam Study 3 conducted peripheral blood gene expression profiling using PAXgene tubes to isolate and stabilize RNA and the Illumina HumanHT-12 v4 Expression BeadChip Kit; 15,639 unique genes were available after exclusions. Biological age was constructed using the Klemera-Doubal method with the same six clinical biomarkers used in the Framingham Offspring. Linear mixed effects models were used to text each probe (outcome) in the microarray data with ∆age (exposure) as in the Framingham Heart Study Offspring cohort sample. Due to differences in the array technologies between the two studies, not all significant transcripts/genes in Framingham were available to test for replication in Rotterdam.
Pathway Analysis
We examined the enrichment of genes related to clinical ∆age in biological pathways. KEGG pathways were queried, and Fisher’s exact test was used. The pathway analysis was performed using WebGestalt (15), a web-based pathway analysis tool. The default minimum pathway size 5 was used because pathways of small size are unlikely to be significant. Significant pathways were defined as those with false discovery rate (16) less than 0.05.
Network Analysis
We performed network analysis to examine the interaction between biological age-related genes using a dense module searching strategy (17). Gene interactions were obtained from the PINA database v2.0 (18) (nonhuman interactions were excluded). Each studied gene was assigned a score to indicate its association with clinical ∆age, which was equivalent to the absolute value of Wald test statistic from the association test. The most significant genes were defined as the seed genes. For each of the seed genes, we created a module that initially contained only the gene itself. Then we tried to expand the module by including neighboring genes that were previously reported to interact with the gene already in the module. The overall score (19) of the module is defined as , where k is the number of genes in the module, and gi is the score of the gene i. A neighboring gene would be added into the module only if its addition would increase the overall module score. The searching stopped if no gene could be added to the module. Such process repeated for each of the seed genes, and the resulting modules were merged to build an interaction subnetwork.
Enrichment Clinical ∆age-Related Genes in Methylation Genes
DNA methylation is an important mechanism to regulate gene expression. We thus examined if there were any CpG sites within clinical ∆age-related genes that also were associated with clinical ∆age. The Framingham DNA methylation data have been previously described (20,21). In brief, fasting peripheral whole blood was collected during the Offspring cohort examination 8. Genomic DNA was extracted from the blood and bisulfite-treated, which was then hybridized to the Infinium HumanMethylation450 BeadChip (Illumina, San Diego, CA) (22). The R package “DASEN” (23) was used to normalize the raw data and correct for the background noise. More than 400,000 CpG sites remained after quality filters, and were used for the association test with clinical ∆age. We defined significant CpG sites if they were associated with clinical ∆age with p < .05/N, where N was the number of CpG sites within the tested genes. A gene was defined as significant methylation if they contained at least one significant CpG site.
Results
Our discovery cohort included 2,163 Framingham Heart Study Offspring cohort participants (55% women, mean age 67 years). Descriptive characteristics of the participants are provided in Table 1.
Table 1.
Clinical Characteristics of the Discovery and Replication Samples
| Characteristicsa | Framingham Offspring N = 2,163 |
Framingham Gen 3 N = 2,946 |
Rotterdam Study N = 414 |
|---|---|---|---|
| Women, n (%) | 1,198 (55) | 1,564 (53) | 215 (52) |
| Age, year | 67 ± 9 | 47 ± 9 | 59 ± 8 |
| Clinical biological age, year | 67 ± 11 | 47 ± 12 | 59 ± 10 |
| ∆Age, year | 0 ± 6.2 | 0 ± 8.1 | 0 ± 6.3 |
| Clinical Variables in Biologic Age | |||
| Systolic blood pressure, mm Hg | 133 ± 18 | 118 ± 15 | 132 ± 19 |
| Forced Expiratory Volume at 1 s (L) | 2.6 ± 0.8 | 3.6 ± 0.8 | 3.2 ± 0.9 |
| Total cholesterol, mg/dL | 186 ± 37 | 187 ± 35 | 212 ± 39 |
| Glucose, mg/dL | 107 ± 22 | 96 ± 18 | 101 ± 16 |
| C-reactive protein, mg/dL | 3.2 ± 6.4 | 2.6 ± 4.1 | 2.3 ± 3.1 |
| Creatinine, mg/100 mL | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.2 |
| BMI kg/m2 | 28.4 ± 5.3 | 28.0 ± 5.9 | 27.8 ± 4.5 |
| Current smoking, n (%) | 167 (8) | 297 (10) | 98 (24) |
| Diabetes mellitus, n (%) | 292 (14) | 137 (5) | 35 (8) |
| Hypertension treatment, n (%) | 1037 (48) | 483 (16) | 2 (0.5) |
| Lipid treatment, n (%) | 926 (43) | 462 (16) | 102 (25) |
| Cardiovascular disease, n (%) | 160 (7) | 28 (1) | 32 (8) |
| Cancer, n (%) | 344 (16) | 131 (4) | 36 (9) |
Note: ∆Age = Clinical biologic age – chronologic age.
aCharacteristics are represented by mean ± SD or n (%).
Association of Clinical ∆age With Gene Expression
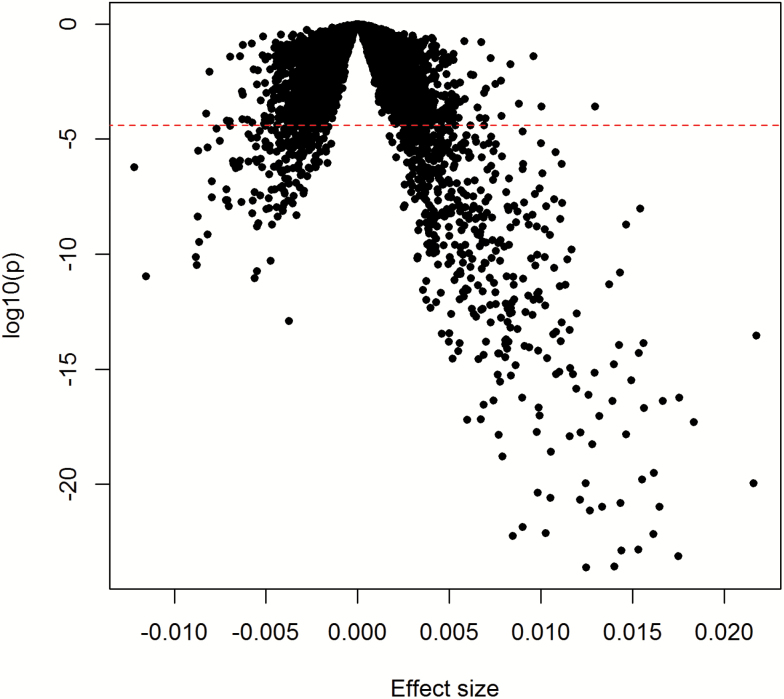
We identified 481 genes significantly associated with clinical ∆age at p < 2.8 × 10−6. In Figure 1, we display summary effect sizes and p-values of all studied genes. A larger number of genes were positively associated (383) than were negatively associated (98) with clinical ∆age (Supplementary Table 1). The 25 most significant genes were all positively associated with clinical ∆age (Table 2). The most significant gene was WNK1 (p = 2.4 × 10−24), which plays an important role in the regulation of electrolyte homeostasis, cell signaling, survival, and proliferation, and may be a key regulator of blood pressure. The most significant negatively associated gene was ABCG1 (p = 1.3 × 10−13), which encodes ATP Binding Cassette Subfamily G Member 1 and is involved in macrophage cholesterol and phospholipid transport.
Figure 1.
Volcano plot of association results from primary analyses. Each dot represents one gene. The x-axis represents the beta estimation (effect) of each gene, whereas the y-axis represents the log10(P). Positive effects represent that the genes were positively associated with clinical ∆age, whereas negative effects represent that the genes were negatively associated with clinical ∆age. The red dash line indicates p < .05/17,873 = 2.8 × 10−6.
Table 2.
Top 25 Genes Associated With Clinical ∆age
| Gene | Discovery Cohort: Framingham Offspring | Validation Cohort: Framingham Gen 3 | Replication Cohort: Rotterdam Study | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Betaa | SE b | p value | Betaa | SE b | p value | Betaa | SEb | p value | |
| WNK1 | 0.0125 | 0.0012 | 2.4E−24 | 0.0091 | 0.0008 | 1.3E−30 | 0.0093 | 0.0089 | .30 |
| NEDD4L | 0.0140 | 0.0014 | 2.7E−24 | 0.0089 | 0.0008 | 4.9E−27 | |||
| SPTB | 0.0175 | 0.0017 | 7.6E−24 | 0.0141 | 0.0010 | 3.0E−40 | |||
| PBX1 | 0.0144 | 0.0014 | 1.4E−23 | 0.0101 | 0.0009 | 1.7E−28 | 0.0102 | 0.0057 | .08 |
| SLC25A37 | 0.0153 | 0.0015 | 1.4E−23 | 0.0108 | 0.0010 | 3.5E−28 | 0.0362 | 0.0164 | .03 |
| CUL4A | 0.0085 | 0.0008 | 5.6E−23 | 0.0053 | 0.0005 | 9.2E−22 | 0.0080 | 0.0047 | .09 |
| IGF2BP2 | 0.0161 | 0.0016 | 6.8E−23 | 0.0127 | 0.0011 | 1.6E-30 | 0.0442 | 0.0197 | .03 |
| ZER1 | 0.0103 | 0.0010 | 7.8E−23 | 0.0071 | 0.0007 | 1.4E−23 | 0.0028 | 0.0054 | .60 |
| CCNI | 0.0090 | 0.0009 | 1.4E−22 | 0.0064 | 0.0006 | 2.3E−24 | 0.0082 | 0.0080 | .31 |
| ANK1 | 0.0127 | 0.0013 | 7.4E−22 | 0.0101 | 0.0008 | 1.9E−36 | 0.0103 | 0.0066 | .12 |
| CTNNAL1 | 0.0165 | 0.0017 | 1.1E−21 | 0.0098 | 0.0011 | 2.2E−19 | 0.0164 | 0.0072 | .02 |
| MICAL2 | 0.0133 | 0.0014 | 1.1E−21 | 0.0099 | 0.0009 | 1.9E−28 | 0.0025 | 0.0088 | .77 |
| RNF10 | 0.0143 | 0.0015 | 1.6E−21 | 0.0126 | 0.0009 | 2.7E−39 | 0.0148 | 0.0186 | .43 |
| UBE2O | 0.0121 | 0.0013 | 2.1E−21 | 0.0096 | 0.0008 | 5.2E−35 | |||
| MXI1 | 0.0105 | 0.0011 | 2.5E−21 | 0.0079 | 0.0007 | 5.4E−30 | 0.0230 | 0.0071 | .001 |
| C17orf39 | 0.0098 | 0.0010 | 4.4E−21 | 0.0078 | 0.0006 | 3.8E−33 | 0.0088 | 0.0061 | .15 |
| ALAS2 | 0.0216 | 0.0023 | 1.1E−20 | 0.0203 | 0.0016 | 8.0E−37 | 0.0883 | 0.0274 | .001 |
| PNP | 0.0124 | 0.0013 | 1.1E−20 | 0.0093 | 0.0008 | 7.9E−30 | |||
| EPB42 | 0.0155 | 0.0017 | 1.6E−20 | 0.0142 | 0.0010 | 1.7E−41 | 0.0409 | 0.0269 | .13 |
| ARHGEF12 | 0.0162 | 0.0017 | 3.2E−20 | 0.0123 | 0.0011 | 9.6E−28 | 0.0094 | 0.0055 | .09 |
| FAM20B | 0.0079 | 0.0009 | 1.6E−19 | 0.0046 | 0.0006 | 1.6E−16 | 0.0018 | 0.0064 | .78 |
| PIP5K1B | 0.0105 | 0.0012 | 2.6E−19 | 0.0049 | 0.0007 | 8.5E−11 | 0.0088 | 0.0050 | .08 |
| EIF2AK1 | 0.0128 | 0.0014 | 5.6E−19 | 0.0107 | 0.0010 | 1.4E−28 | 0.0290 | 0.0135 | .03 |
| DCAF12 | 0.0116 | 0.0013 | 1.2E−18 | 0.0111 | 0.0009 | 6.3E−35 | |||
| STAU1 | 0.0077 | 0.0009 | 1.4E−18 | 0.0052 | 0.0006 | 4.0E−18 | 0.0112 | 0.0061 | .07 |
Note: aBeta is in units of one standard deviation change in gene expression per year of clinical ∆age. bSE = Standard error.
We also performed a secondary analysis by additionally adjusting for body mass index and smoking status. As shown in Supplementary Figure 1, the results were highly correlated with the primary model (R2 = .86), suggesting that BMI and smoking had marginal effects on the association between gene expression and clinical ∆age.
To examine the effects of treatments for hypertension, diabetes and lipid disorders, we performed a sensitivity analysis by additionally adjusting for lipid-lowering, antidiabetic or blood-pressure-lowering medications. As shown in the Supplementary Figure 2, the results were highly correlated with the primary model (R2 = .89).
Validation in Framingham Gen 3 Participants
All of the top results in Table 2 were directionally consistent and highly significant in the Framingham Heart Study Gen 3 cohort participants. Among the 481 genes, 415 were directionally consistent and significant after Bonferroni correction (p < .05/481 = 1.0 × 10−4, Supplementary Table 1). The effect estimates between the discovery cohort and validation cohort were highly correlated R2 = .77 despite the difference in age in the two cohorts (Supplementary Figure 3a).
Replication in the Rotterdam Study
We attempted replication of our significant findings in the Rotterdam Study, which included 414 participants with estimated clinical biologic age and gene expression data. The Rotterdam participants were younger (mean age 59 years), and reported more current smoking compared to the Framingham Offspring participants (Table 1). Importantly, the Rotterdam Study used a different gene expression platform, so that only 366 of 415 validated significant genes were present in their gene expression platform. Results were available for 20 of the top 25 genes (Table 2), all were directionally consistent and 6 of the 20 genes reached a nominal significance level (p < .05), including MXI1, ALAS2, CTNNAL1, IGF2BP2, SLC25A37, and EIF2AK1. Among the full set of 366 genes available for testing, 104 genes were both directionally consistent and nominally significant (p < .05, Supplementary Table 1, Supplementary Figure 3b), and 5 genes were still significant after adjusting for multiple testing using the Bonferroni method (p < .05/366 = 1.4 × 10−4).
Pathway Analysis
We also examined the enrichment of 415 validated genes in biological pathways. As shown in Supplementary Table 2, only a single pathway, ubiquitin-mediated proteolysis, was significantly enriched with genes associated with clinical ∆age after adjusting for multiple testing (false discovery rate < 0.05). None of the remaining pathways were significant.
Network Analysis
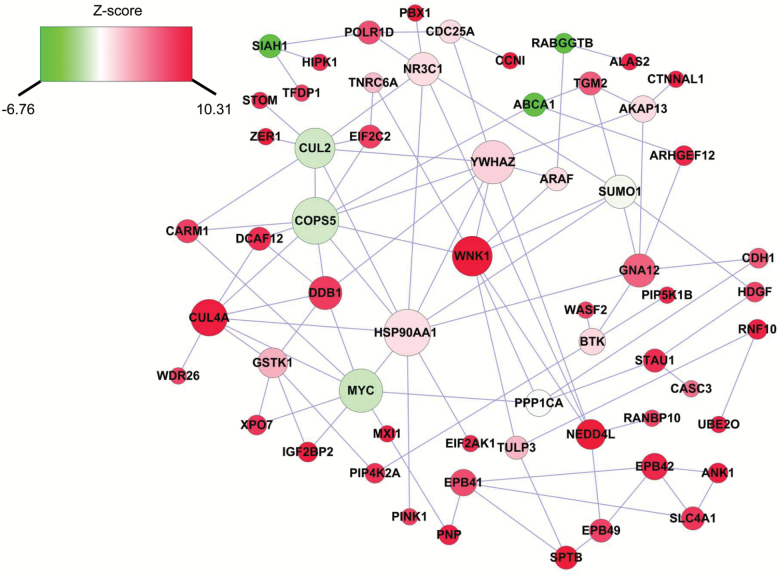
The genes associated with clinical ∆age might interact with each other to create a complex interaction network. We thus performed network analysis and constructed a clinical biologic age related subnetwork. Given that the large number of significant genes associated with clinical ∆age, the network analysis used the top 25 genes as the seed genes, and investigated their interactions with neighboring genes, which previously showed good performance to identify important interactions related to inflammatory biological age (24). As shown in Figure 2, the subnetwork was comprised of 60 nodes and 98 edges, whereas each node represents one gene, and each edge represents the interaction between genes. Interestingly, the most significant gene, WNK1, is one of the pivotal nodes in the network, which interacts with 8 other genes in the subnetwork, including the second significant gene NEDD4L. Previous studies found that WNK1 and NEDD4L were both involved in the regulation of renal sodium absorption and blood pressure (25), suggesting that the interaction between WNK1 and NEDD4L might play an important role in biological age.
Figure 2.
Clinical ∆age-related subnetwork derived from protein-protein interaction. Each node represents one gene, whereas each edge represents the interaction between two genes. The nodes were colored to represent their association with clinical ∆age: red color represents genes that were positively associated with clinical ∆age, whereas green color represents genes that were negatively associated with clinical ∆age. The node size is proportional to the number of edges that the node connects to.
Overlap with Genes Associated with Chronological Age
We also examined the overlap between genes associated with clinical ∆age and those associated with chronological age. Our previous study identified 1,497 genes associated with chronological age (26). Among them, 38 (2.5%) were also associated with clinical ∆age. A lack of overlap between genes associated with clinical ∆age and chronological age (p = .53) may suggest that clinical biomarker changes occur with age rather than cause aging.
Enrichment of Clinical ∆age-Related Genes in Methylation Genes
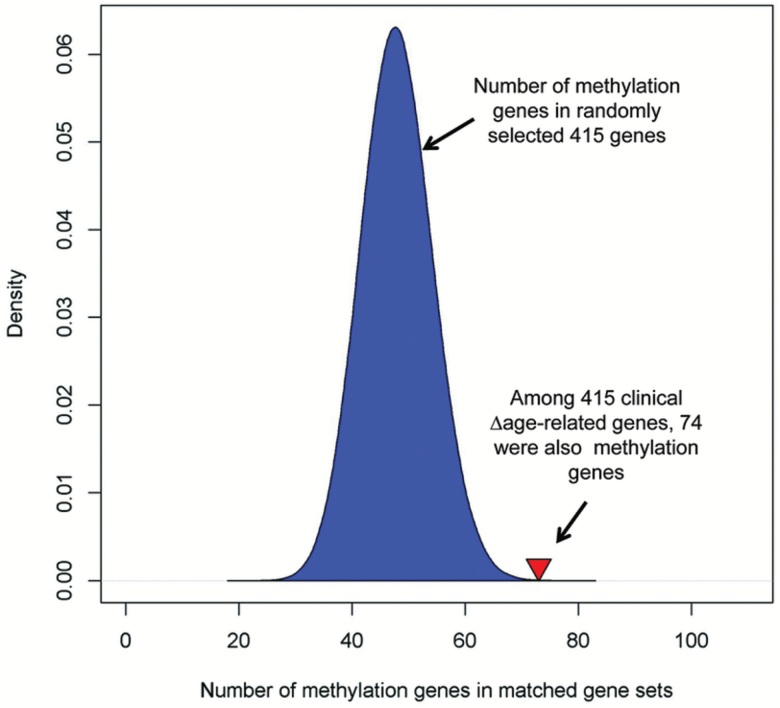
We then examined if clinical ∆age-related genes harbored CpG sites that also were associated with clinical ∆age. We found 74 out of 415 validated clinical ∆age-related genes harbored at least one CpG site in which methylation was associated with clincial ∆age (defined as methylated genes). Among these 74 genes, the most significant CpG sites within 58 genes (78.4%) were negatively associated with clinical ∆age. We then performed a random permutation to assess the significance of overlap by creating one million gene sets, each containing 415 randomly selected genes. Figure 3 shows the distribution of methylation genes in these randomly matched gene sets. On average, each randomly matched gene set contained 48 methylation genes, and only 74 among one million gene sets contained equal or more methylation genes than that of clinical ∆age-related genes, representing an empirical p-value of 7.4 × 10−5.
Figure 3.
Enrichment of clinical ∆age-related genes with corresponding differences in methylation. Among 415 validated genes whose expression was associated with clinical ∆age, 74 genes contained at least one CpG where methylation was associated with clinical ∆age (defined as methylated genes). In contrast, each randomly matched gene set contained a mean of 48 methylation genes. The blue area represents the distribution of randomly matched gene set, and the red triangle indicates the number of methylation genes among clinical ∆age-related genes.
Discussion
In the community-based Framingham Offspring cohort, we investigated the association of gene expression with a measure of clinical biologic age in over 2,000 older adults. We identified and validated 415 genes, among which the majority demonstrate increased expression with greater clinical ∆age. In the Rotterdam Study, 104 of the 415 validated genes were directionally consistent and nominally significant. In addition, most of the 74 genes contained CpG sites that were negatively associated with clinical ∆age, suggesting DNA methylation might be an important mechanism to regulate clinical biological age.
The strongest association for biologic age was in WNK1, a gene that encodes a protein that regulates blood pressure through control of sodium and chloride ion transport. Variants in WNK1 are linked to ambulatory blood pressures in general population samples (27) and to blood pressure severity in hypertensive families (28). Interestingly, WNK1 is associated with increased risk for ischemic stroke even after accounting for blood pressure suggesting additional biologic mechanisms beyond blood pressure regulation explain the genetic association (29). WNK1 is also involved in the human central nervous system with GABA signaling as well as other physiologic processes including epithelial transport, cell volume hemostasis, immune function, and cell migration (30). In mouse models, WNK1 mutants are embryonic lethal due to heart and vascular developmental defects (31). Thus, WNK1 may contribute to age-related disease and aging through multiple mechanisms.
ABCG1 had the strongest negative association, meaning that higher expression was associated with lower clinical biological age. ABCG1 gene is involved in macrophage and phospholipid transport and may regulate lipid hemostasis (32). In animal models, the endothelial cholesterol efflux pathways mediated by ABCG1 are protective from atherosclerosis and associated with suppression of endothelial inflammation (33). Longevity in humans is associated with higher numbers of protective alleles related to cardiovascular risk factors including lipid traits and blood pressure, supporting our findings (34).
The ubiquitin-mediated proteolysis biologic pathway was the only highlighted biologic pathway enriched with genes associated with clinical ∆age. Genes in this pathway include DDB1 and CUL4A, a family of ubiquitin ligases involved in DNA repair and degradation of DNA damage-response proteins. Defects in DDB1 cause xeroderma pigmentosum complementation group E (XPE), an autosomal recessive disorder with hallmarks of early onset cancer and photosensitivity. DCAF4 (DDB1 and CUL4-associated factor 4) is associated with leukocyte telomere length (35) a known measure of aging. DCAF4 interacts with DDB1 and CUL4; DDB1 modulates the transcription factor E2F1, which, in turn, regulates cell proliferation and telomerase. The CUL4-DDB1 complex has been reported to be involved in regulation of other key biologic processes such as stem cell differentiation and maintenance (36) and cullin-based ubiquitin ligases play a role in antiviral defense (37).
Some but not all of our findings may have been related to the chosen components of the clinical biologic age construct, which included systolic blood pressure and total cholesterol. For example, our strongest positive (WNK1) and negative (ABCG1) discovery findings are genes implicated in the regulation of blood pressure and lipids respectively. However, WNK1 has roles in biologic processes that extend beyond blood pressure regulation. Importantly, clinical biologic age was associated with overall mortality, cardiovascular and cancer death, and incident cardiovascular disease and cancer events in our prior work (9). A history of parental longevity, an endophenotype of aging, is associated with favorable cardiovascular risk factors, and diminished risk not only of cardiovascular disease but also of cancer and death (38–41). Importantly, genome-wide association study of parental longevity identified loci involved in inflammation, lipid metabolism, and cardiovascular diseases (42).
Our study has several limitations. Participants were middle-age to older adults of European descent; therefore, our findings may not be generalizable to premature mortality and other racial/ethnic groups. The clinical biological age was calculated from Offspring participants (mean age 67 ± 9), which might reflect changes in biomarkers only at older ages. Therefore, we also computed clinical biologic age in a combined sample of Offspring and Gen participants across a wide age range. As shown in Supplementary Figure 4, the ∆age using Offspring only (original calculation) and ∆age using the combined Offspring and Gen 3 participants (which had a much wider age range) was highly correlated (R2 = .92, p < 2.2 × 10–16), suggesting limited effect on clinical biological age calculation due to differences in age of study sample. Our clinical biological age measure included six clinical biomarkers, a subset of previously reported measures of biologic age (7). It is possible that an estimate of biologic age with all the clinical biomarkers may identify additional genes. Furthermore, it is unclear whether use of all biomarkers in a composite measure rather than a subset would make identification of genes with individual biomarkers less likely. Gene expression was measured from whole blood, which contains a variety of cell types and could have cell specific responses. The cell counts were only measured in 2,181 participants, whereas cell counts for the remaining participants were imputed from gene expression data, which introduce some variation to the results. Our study was limited to association analyses, and we cannot infer causality between clinical ∆age and gene expression. The clinical biomarker signature occurring with aging is meaningful in predicting health outcomes (9), however, further work is needed to determine if the biomarker changes cause aging or are a result of increasing pathology with age. In addition, the clinical ∆age and gene expression was measured at one examination time point, therefore we cannot comment on longitudinal variation in the relations between gene expression and clinical ∆age, and the potential for misclassification by focusing on a single time point ascertainment of phenotypes. Finally, further replication of our results in additional independent samples is needed. The modest sample size and population and platform array differences in the replication cohort may have hindered our ability to more definitely replicate our findings in a second cohort. Our study also has strengths that merit comment including the community-based samples and routine ascertainment of the biologic age phenotypes and the RNA expression transcripts.
In conclusion, we took a hypothesis-free approach to study transcriptome-wide profiling with clinical biologic age in a large carefully characterized cohort and identified and validated 415 genes that were significantly associated with clinical biologic age in a large community-based cohort. Future functional characterization of the identified gene network may enable the development of preventive strategies or therapies for aging or age-related diseases enabling a longer healthspan.
Funding
This work was supported by the National Institutes of Health grants R56AG029451 (J.M.M.), 3U24AG051129 (J.M.M.), 5R01HL092577 (E.J.B.), and 5R01HL128914 (E.J.B.). Measurement of clinical biomarkers was funded through R01 HL64753 (E.J.B.) and R01 HL076784 (E.J.B.) and further supported by R01AG028321 (E.J.B.). This project was also supported by Boston University Digital Health Initiative, and the National Center for Advancing Translational Sciences, National Institutes of Health, through BU-CTSI Grant Number 1UL1TR001430. Framingham gene expression profiling was funded through the Division of Intramural Research (D.L.), National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contracts No. N01-HC-25195 and HHSN268201500001I) .
The generation and management of the Illumina 450K methylation array data (EWAS data) for the Rotterdam Study was executed by the Human Genotyping Facility of the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, the Netherlands. The EWAS data was funded by the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, and by the Netherlands Organization for Scientific Research (NWO; project number 184021007) and made available as a Rainbow Project (RP3; BIOS) of the Biobanking and Biomolecular Research Infrastructure Netherlands (BBMRI-NL). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. This Rotterdam work was done within the framework of the Biobank-Based Integrative Omics Studies (BIOS) Consortium funded by BBMRI-NL, a research infrastructure financed by the Dutch government (NWO 184.021.007).
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Conflict of Interest
J.M.M. serves on the editorial board of the Journals of Gerontology: Medical Sciences.
Supplementary Material
Acknowledgments
The authors are grateful to the study participants, the staff from the Framingham Heart Study and Rotterdam Study, and the participating general practitioners and pharmacists. The authors thank Mr. Michael Verbiest, Ms. Mila Jhamai, Ms. Sarah Higgins, and Mr. Marijn Verkerk for their help in creating the Rotterdam methylation database.
References
- 1. Colby SL, Ortman JM.. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. [Google Scholar]
- 2. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson NB, Hayes LD, Brown K, et al. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005–2013. MMWR Surveill Summ. 2014;63(suppl 4):3–27. [PubMed] [Google Scholar]
- 4. Espeland MA, Crimmins EM, Grossardt BR, et al. ; Multimorbidity Clinical Trials Consortium Clinical trials targeting aging and age-related multimorbidity. J Gerontol A Biol Sci Med Sci. 2017;72:355–361. doi: 10.1093/gerona/glw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niedernhofer LJ, Kirkland JL, Ladiges W. Molecular pathology endpoints useful for aging studies. Ageing Res Rev. 2017;35:241–249. doi: 10.1016/j.arr.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age?J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi: 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71:489–495. doi: 10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murabito JM, Zhao Q, Larson MG, et al. Measures of biologic age in a community sample predict mortality and age-related disease: the Framingham offspring study. J Gerontol A Biol Sci Med Sci. 2018;73:757–762. doi: 10.1093/gerona/glx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi: 10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 11. Lin H, Yin X, Lunetta KL, et al. Whole blood gene expression and atrial fibrillation: the Framingham Heart Study. PLoS One. 2014;9:e96794. doi: 10.1371/journal.pone.0096794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joehanes R, Zhang X, Huan T, et al. Integrated genome-wide analysis of expression quantitative trait loci aids interpretation of genomic association studies. Genome Biol. 2017;18:16. doi: 10.1186/s13059-016-1142-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249 [DOI] [PubMed] [Google Scholar]
- 14. Newman AB. Is the onset of obesity the same as aging?Proc Natl Acad Sci USA. 2015;112:E7163. doi: 10.1073/pnas.1515367112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41(Web Server issue):W77–W83. doi: 10.1093/nar/gkt439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 17. Jia P, Zheng S, Long J, Zheng W, Zhao Z. dmGWAS: dense module searching for genome-wide association studies in protein-protein interaction networks. Bioinformatics. 2011;27:95–102. doi: 10.1093/bioinformatics/btq615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cowley MJ, Pinese M, Kassahn KS, et al. PINA v2.0: mining interactome modules. Nucleic Acids Res. 2012;40(Database issue):D862–D865. doi: 10.1093/nar/gkr967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ideker T, Ozier O, Schwikowski B, Siegel AF. Discovering regulatory and signalling circuits in molecular interaction networks. Bioinformatics. 2002;18(suppl 1):S233–S240. doi:10.1093/bioinformatics/18.suppl_1.S233 [DOI] [PubMed] [Google Scholar]
- 20. Lin H, Yin X, Xie Z, et al. Methylome-wide association study of atrial fibrillation in Framingham Heart Study. Sci Rep. 2017;7:40377. doi: 10.1038/srep40377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ligthart S, Marzi C, Aslibekyan S, et al. ; WHI-EMPC Investigators; CHARGE epigenetics of Coronary Heart Disease DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016;17:255. doi: 10.1186/s13059-016-1119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bibikova M, Barnes B, Tsan C, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. doi: 10.1016/j.ygeno.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 23. Pidsley R, Y Wong CC, Volta M, Lunnon K, Mill J, Schalkwyk LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics. 2013;14:293. doi: 10.1186/1471-2164-14-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin H, Lunetta KL, Zhao Q, et al. Transcriptome-wide association study of inflammatory biologic age. Aging (Albany NY). 2017;9:2288–2301. doi: 10.18632/aging.101321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manunta P, Lavery G, Lanzani C, et al. Physiological interaction between alpha-adducin and WNK1-NEDD4L pathways on sodium-related blood pressure regulation. Hypertension. 2008;52:366–372. doi: 10.1161/HYPERTENSIONAHA.108.113977 [DOI] [PubMed] [Google Scholar]
- 26. Peters MJ, Joehanes R, Pilling LC, et al. ; NABEC/UKBEC Consortium The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi: 10.1038/ncomms9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tobin MD, Raleigh SM, Newhouse S, et al. Association of WNK1 gene polymorphisms and haplotypes with ambulatory blood pressure in the general population. Circulation. 2005;112:3423–3429. doi: 10.1161/CIRCULATIONAHA.105.555474 [DOI] [PubMed] [Google Scholar]
- 28. Newhouse SJ, Wallace C, Dobson R, et al. Haplotypes of the WNK1 gene associate with blood pressure variation in a severely hypertensive population from the British Genetics of Hypertension study. Hum Mol Genet. 2005;14:1805–1814. doi: 10.1093/hmg/ddi187 [DOI] [PubMed] [Google Scholar]
- 29. Ikram MA, Seshadri S, Bis JC, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 2017;25:285–299. doi: 10.1016/j.cmet.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 31. Xie J, Wu T, Xu K, Huang IK, Cleaver O, Huang CL. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol. 2009;175:1315–1327. doi: 10.2353/ajpath.2009.090094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klucken J, Buchler C, Orso E, et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA. 2000;97:817–822. doi:10.1073/pnas.97.2.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerterp M, Tsuchiya K, Tattersall IW, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in endothelial cells accelerates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016;36:1328–1337. doi: 10.1161/ATVBAHA.115.306670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilling LC, Atkins JL, Bowman K, et al. Human longevity is influenced by many genetic variants: evidence from 75,000 UK Biobank participants. Aging (Albany NY). 2016;8:547–560. doi: 10.18632/aging.100930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mangino M, Christiansen L, Stone R, et al. DCAF4, a novel gene associated with leucocyte telomere length. J Med Genet. 2015;52:157–162. doi: 10.1136/jmedgenet-2014-102681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao J, Buckley SM, Cimmino L, et al. The CUL4-DDB1 ubiquitin ligase complex controls adult and embryonic stem cell differentiation and homeostasis. eLife. 2015;4:e07539. doi:10.7554/eLife.07539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nekorchuk MD, Sharifi HJ, Furuya AK, Jellinger R, de Noronha CM. HIV relies on neddylation for ubiquitin ligase-mediated functions. Retrovirology. 2013;10:138. doi: 10.1186/1742-4690-10-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dutta A, Henley W, Robine JM, Langa KM, Wallace RB, Melzer D. Longer lived parents: protective associations with cancer incidence and overall mortality. J Gerontol A Biol Sci Med Sci. 2013;68:1409–1418. doi: 10.1093/gerona/glt061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terry DF, Evans JC, Pencina MJ, et al. Characteristics of Framingham offspring participants with long-lived parents. Arch Intern Med. 2007;167:438–444. doi: 10.1001/archinte.167.5.438 [DOI] [PubMed] [Google Scholar]
- 40. Terry DF, Wilcox MA, McCormick MA, et al. Lower all-cause, cardiovascular, and cancer mortality in centenarians’ offspring. J Am Geriatr Soc. 2004;52:2074–2076. doi: 10.1111/j.1532-5415.2004.52561.x [DOI] [PubMed] [Google Scholar]
- 41. Westendorp RG, van Heemst D, Rozing MP, et al. ; Leiden Longevity Study Group Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J Am Geriatr Soc. 2009;57:1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x [DOI] [PubMed] [Google Scholar]
- 42. Pilling LC, Kuo CL, Sicinski K, et al. Human longevity: 25 genetic loci associated in 389,166 UK Biobank participants. Aging (Albany NY). 2017;9:2504–2520. doi: 10.18632/aging.101334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.