Abstract
Background
Gait speed is an important measure of lower extremity physical performance in older adults and is predictive of disability and mortality. The biological pathways involved in the decline of lower extremity physical performance are not well understood. We used a targeted metabolomics approach to identify plasma metabolites predictive of change in gait speed over time.
Methods
Gait speed was measured at baseline and over median follow-up of 50.5 months in 504 adults, aged ≥50 years, who had two or more study visits in the Baltimore Longitudinal Study of Aging (BLSA). Plasma metabolites were measured using targeted mass spectrometry (AbsoluteIDQ p180 Kit, Biocrates).
Results
Of 148 plasma metabolites (amino acids, biogenic amines, hexoses, glycerophospholipids) measured, eight were significantly associated with gait speed at baseline, independent of age and sex: hexoses (r = −0.148, p < .001), [sphingomyelin (SM) 16:1 (r = −0.091, p = .0009), SM 18:0 (r = −0.085, p = .002), SM 18:1 (r = −0.128, p < .0001], phosphatidylcholine aa 32:3 (r = −0.088, p = .001), lysophosphatidylcholine (LPC) 17:0 (r = 0.083, p = .003), LPC 18:1 (r = 0.089, p = .001), and LPC 18:2 (r = 0.104, p < .0001). Adjusting for baseline age, sex, and chronic diseases, baseline plasma LPC 18:2 was an independent predictor of the rate of change of gait speed over subsequent follow-up (p = .003). No other plasma metabolites were significantly associated longitudinal changes of gait speed over time.
Conclusions
Low plasma LPC 18:2, which has previously been shown to predict impaired glucose tolerance, insulin resistance, type 2 diabetes, coronary artery disease, and memory impairment, is an independent predictor of decline in gait speed in older adults.
Keywords: Aging, Gait speed, Lipidomics, Lysophosphatidylcholine, Metabolomics, Sarcopenia
Due to demographic changes worldwide, the proportion of older adults in the population is rapidly increasing. Hence, preventing morbidity and disability in later life has become a public health priority. Walking is one of the most common forms of physical activity in older adults and is an essential capacity for independent living. Gait speed, also known as walking speed, is considered an important indicator of physical well-being in older adults. Slow gait speed is a strong independent predictor of physical disability (1), impaired cognition and dementia (2,3), nursing home admission, health care utilization, and mortality (4). Usual gait speed is widely used for the consensus definition of sarcopenia (5).
The biological pathways that lead to sarcopenia are not well understood but include skeletal muscle insulin resistance (6), mitochondrial dysfunction (7), increased inflammation and oxidative stress (8), endothelial dysfunction (9), defects in proteostasis (10), and impaired neuromuscular junction activity (11). Based upon the findings of pre-clinical and clinical studies, circulating proteins and metabolites such as myostatin (12), branched chain amino acids (13), and brain-derived neurotrophic factor (14) are considered biomarkers of sarcopenia. Metabolomics has been used to examine the relationship between serum metabolites and skeletal muscle mass in functionally limited older adults (15) and in adult women (16). Recent metabolomics studies show higher plasma ceramides are associated with lower gait speed (17), and amino acid metabolites are associated with walking disability (18). Relatively less is known about lipids compared with proteins and other metabolites, in part because they are difficult to measure and study due to their structural diversity and sheer number of discrete molecular species (19). Lipids are the dominant biologic molecules in human plasma. Mass spectrometry-based platforms now allow the quantification of hundreds of lipids in human plasma (20). Lysophosphatidylcholine (LPC), a major class of glycerophospholipids in human plasma, are implicated in inflammation, insulin resistance, obesity, and type 2 diabetes (21). LPC appear to protect human skeletal muscle cells from lipotoxicity through activation of peroxisome proliferator-activated receptor (PPAR)δ, a known pathway involved in aging and inflammation (22). The relationship between circulating LPC and physical performance in older adults has not been characterized.
We used a targeted metabolomics approach to identify circulating metabolites that were associated with a decline in gait speed in older adults. Our aim was to gain insight into potential biological pathways involved in sarcopenia. To address this aim, we measured plasma metabolites in a well characterized cohort of older adults.
Subjects and Methods
The study subjects consisted of 504 participants, aged ≥50 years, in the Baltimore Longitudinal Study of Aging (BLSA) who were seen between January 2006 and December 2008 (“baseline”) and had at least two or more follow-up visits after baseline up to June 2014. The study design was aimed at studying the cross-sectional association of plasma metabolites with walking speed and to identify baseline metabolites that predicted differential decline of gait speed over follow-up. Participants were assessed at an in-patient study clinic for follow-up visits every 1–4 years, with more frequent follow-up for older participants. They underwent 2.5 days of medical, physiological, and psychological exams. Gait speed was measured over a 6-month course. The participants asked to walk at their usual pace. The time to complete the walk was converted into gait speed (m/s). The better performance of two trials was used for the analysis. The study protocol was approved by the institutional review boards of the National Institute of Environmental Health Science (NIH, North Carolina) and the Johns Hopkins School of Medicine Institutional Review Board. The study protocol was conducted in accordance with the 1964 Helsinki Declaration. At every visit, after the scope, procedures, and related risk were explained to participants, they consented to participate in the study and signed an informed consent document.
Measurement of Serum Metabolites
Blood was collected from participants who stayed overnight at the NIA Clinical Research Unit, Medstar Harbor Hospital in Baltimore, MD. Blood samples were drawn from the antecubital vein between 07:00 and 08:00 h after an overnight fast. Participants were not allowed to smoke, engage in physical activity, or take medications before the blood sample was collected. Blood samples were immediately stored at 4°C, centrifuged within 4 h, then immediately aliquoted and frozen at −80°C. The collection of ethylenediaminetetraacetic acid plasma in the BLSA is consistent with guidelines for biomarker studies (23). Plasma metabolites were measured using liquid chromatography tandem mass spectrometry (LC-MS/MS). Metabolites were extracted and concentrations are measured using the AbsoluteIDQ p180 kit (Biocrates Life Sciences AG, Innsbruck, Austria) following the manufacturers protocol for a 5500 QTrap (Sciex, Framingham, MA) mass spectrometer equipped with an electrospray ionization source, a Shimadzu CBM-20A command module, LC-20AB pump, and a Shimadzu SIL-20AC-HT autosampler, a CTO-10Ac column oven heater, and running with Analyst 1.5.2 software, as described elsewhere (13). Briefly, 10 μL of plasma was pipetted onto a 96-well Biocrates kit. The samples were dried at room temperature (RT) for 30 min. 50 µL of 5% PITC reagent was added and incubated for 20 min and the plate was dried under nitrogen for 1 h. 300 µL of 5 mM ammonium acetate in methanol was added and incubated at RT on a shaker (450 rpm) for 30 min. The plate was centrifuged at 500×g for 2 min and labeled; 50 µL of each sample was transferred to a 96-deep well LC plate, and 10 uL of each sample was transferred to the 96 deep well FIA plate. To the LC plate, 450 µl of 40% methanol (in HPLC grade water) was added. To the FIA plate, 490 µL of FIA running solvent was added. 10 µL was injected onto the Eclipse XDB C18, 3.5 μm, 3.0 × 100 mm with a Phenomenex C18 Security Guard Cartridge, 3.0 mm ID. The mobile phase consisted of solvent A (water containing 0.2% formic acid) and solvent B (acetonitrile containing 0.2% formic acid), with the following gradient: 0–0.5 min 0% B, 5.5 min: 95% B; 6.5min: 95% B; 7.0 min: 0% B; 9.5 min: 0% B. LC plate evaluation of the samples was carried out using the MetIDQ software. The FIA plate was run with 20 µL injection directly onto the MS at a flow of 30 µL/min with water/acetonitrile (1:1) containing 0.2% formic acid as the mobile phase, with the following flow rate program: 0–1.6 min: 30 µL/min; 2.4 min: 200 µL/min; 2.80 min: 200 µL/min and 3.00 min: 30 µL/min. Concentrations were calculated using the Analyst/MetIDQ software and reported in µmol/L. The kit potentially measures 188 metabolites, but 40 metabolites were either completely below the limit of detection (most acylcarnitines and very long chain LPCs) or were excluded from the analysis because they were below the limit of detection in more than 10% of subjects. After exclusions, quantitative information on 148 metabolites, including 25 amino acids, 11 biogenic amines, 1 hexoses, 10 sphingolipids, 7 acylcarnitines, and 94 glycerophospholipids (lyso-, diacyl-, and acyl-alkyl phosphatidylcholines) were available for the analysis. Glycerophospholipids are differentiated on the basis of ester and ether bonds in the glycerol moiety. Diacyl or “aa” indicates that fatty acids are bound with ester bonds at the sn-1 and sn-2 positions on the glycerol backbone. Acyl-alkyl or “ae” indicates that the fatty acid at the sn-1 position is bound with an ether bond. The total number of carbon atoms and double bonds in fatty acid chains is represented by “C x:y”, where x denotes the number of carbons and y denotes the number of double bonds. The MS spectra were evaluated using Analyst/MetIDQ (Biocrates) software. Human plasma samples spiked with standard metabolites were used to monitor the reproducibility of the assay. The inter-assay and intra-assay coefficients of variation ranged from 5 to 15% for nearly all analytes.
Statistical Analysis
Spearman correlations were used to examine the relationship between plasma metabolite concentrations and usual gait speed at baseline, adjusting for age and sex. Bivariate and multivariable linear regression models were used to examine the relationship between plasma metabolite concentrations at baseline with the slope of gait speed over follow-up. Multivariable models of metabolites and slope of gait speed were adjusted for covariates such as age, anemia, and chronic diseases that were significantly associated with gait speed in bivariate analyses. A false discovery rate (FDR) approach was used to correct for multiple testing (24,25). All reported associations passed the FDR cut-off of 5%.
Results
Characteristics of the 504 participants are shown in Table 1. At baseline, the mean (SD) gait speed was 1.15 (0.23) m/s. The mean (SD) decline of gait speed from baseline to the last visit was 0.024 (0.203) m/s. The cross-sectional relationship between plasma metabolites and gait speed at baseline was examined using Spearman correlations adjusted for age and sex. There were eight plasma metabolites that were significantly associated with gait speed at baseline: hexoses (r = −0.148, p < .0001), sphingomyelin (SM) 16:1 (r = −0.091, p = .0009), SM 18:0 (r = −0.085, p = .002), SM 18:1 (r = −0.128, p < .0001), phosphatidylcholine (PC) aa 32:3 (r = −0.088, p = .001), LPC 17:0 (r = 0.083, p = .003), LPC 18:1 (r = 0.089, p = .001), and LPC 18:2 (r = 0.104, p < .0001). There were 1,350 visits with a median follow-up time of 50.5 months. The overall slope of gait speed over follow-up was −0.009. Bivariate associations between demographic and disease variables and slope of gait speed over follow-up are shown in Table 2. Age, anemia, hypertension, coronary artery disease, congestive heart failure, peripheral artery disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease, Parkinson’s disease, lower extremity joint disease, and chronic kidney disease at baseline were significantly associated with slope of gait speed over follow-up. Multivariable models of plasma metabolites at baseline and slope of gait speed over follow-up are shown in Table 3. There were 12 plasma metabolites that were significantly associated with slope of gait speed over follow-up. The regression coefficients in the multivariable longitudinal models indicate changes in slope associated with one standard deviation increase in the plasma metabolite. In multivariable model 1, adjusting for age and sex, PC aa 32:3, PC ae 38:3, creatinine, and LPC 18:2 were significantly associated with a steeper decline of gait speed over follow-up. Only LPC 18:2 was significantly associated with slope of gait speed after the analysis was adjusted for age, sex, anemia, and chronic diseases.
Table 1.
Selected Baseline Characteristics of the 504 Study Participants From the Baltimore Longitudinal Study of Aging
| Characteristic | Mean (SD) or % |
|---|---|
| Age, years | 70.7 (9.9) |
| Female sex, % | 49.0 |
| Current smoking, % | 2.2 |
| Body mass index, kg/m2 | 26.7 (4.0) |
| Anemia, % | 20.0 |
| Depression, % | 10.1 |
| Hypertension, % | 61.7 |
| Coronary artery disease, % | 8.3 |
| Congestive heart failure, % | 7.9 |
| Peripheral artery disease, % | 3.6 |
| Stroke, % | 15.1 |
| Diabetes mellitus, % | 11.5 |
| Chronic obstructive pulmonary disease, % | 16.1 |
| Parkinson’s disease, % | 2.2 |
| Lower extremity joint disease, % | 61.7 |
| Cancer, % | 12.7 |
| Chronic kidney disease, % | 40.7 |
Table 2.
Bivariate Relationship Between Demographic and Disease Variables with Decline of Usual Gait Speed
| Variable | Beta | SE | p |
|---|---|---|---|
| Age, years | −0.0038 | 0.0003 | <.0001 |
| Sex | 0.0033 | 0.0066 | .62 |
| Current smoking | 0.0173 | 0.0228 | .45 |
| Body mass index, kg/m2 | −0.0019 | 0.0008 | .11 |
| Anemia | −0.0323 | 0.0082 | <.0001 |
| Depression | −0.0039 | 0.0110 | .72 |
| Hypertension | −0.0387 | 0.0066 | <.0001 |
| Coronary artery disease | −0.0252 | 0.0120 | .04 |
| Congestive heart failure | −0.0437 | 0.0121 | .004 |
| Peripheral artery disease | −0.0830 | 0.0175 | <.0001 |
| Stroke | −0.0390 | 0.0091 | <.0001 |
| Diabetes mellitus | −0.0281 | 0.0103 | .007 |
| Chronic obstructive pulmonary disease | −0.0259 | 0.0090 | .004 |
| Parkinson’s disease | −0.0825 | 0.0224 | .003 |
| Lower extremity joint disease | −0.0282 | 0.0067 | <.0001 |
| Cancer | −0.0022 | 0.0100 | .82 |
| Chronic kidney disease | −0.0364 | 0.0066 | <.0001 |
Table 3.
Multivariable Models of Plasma Metabolites at Baseline and Slope of Usual Gait Speed Over Follow-Up
| Variablea | Model 1 Adjusted for Age, Sex |
Model 2 Adjusted for Age, Sex, Anemia, Chronic Diseasesb |
||||
|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | |
| Phosphatidylcholine aa 32:3 | −0.01 | 0.003 | .0006 | −0.007 | 0.003 | .01 |
| Phosphatidylcholine aa 42:1 | −0.003 | 0.003 | .25 | −0.002 | 0.003 | .50 |
| Phosphatidylcholine ae 34:1 | −0.002 | 0.003 | .49 | −0.002 | 0.003 | .48 |
| Phosphatidylcholine ae 38:3 | −0.006 | 0.003 | .03 | −0.005 | 0.003 | .07 |
| Phosphatidylcholine ae 42:5 | −0.003 | 0.003 | .30 | −0.002 | 0.003 | .48 |
| Citrulline | −0.002 | 0.003 | .48 | 0.004 | 0.003 | .16 |
| Alanine | −0.005 | 0.003 | .04 | −0.004 | 0.003 | .17 |
| Phenylalanine | −0.006 | 0.003 | .06 | −0.002 | 0.003 | .53 |
| Creatinine | −0.005 | 0.003 | .05 | −0.004 | 0.003 | .16 |
| Kynurenine | −0.005 | 0.003 | .08 | −0.003 | 0.003 | .26 |
| Lysophosphatidylcholine 18:2 | 0.009 | 0.001 | .001 | 0.008 | 0.003 | .003 |
| Lysophosphatidylcholine 20:4 | 0.006 | 0.003 | .04 | −0.006 | 0.003 | .03 |
Notes: aThe betas in the multivariable models indicate the change in slope associated with 1 standard deviation increase in the log transformed metabolite (in micromoles/L): PC aa 32:3 (0.33), PC aa 42:1 (0.39), PC ae 34:1 (0.31), PC ae 38:3 (0.43), PC 42:5 (0.28), citrulline (0.40), alanine (0.33), phenylalanine (0.22), creatinine (0.31), kynurenine (0.44), LPC 18:2 (0.44), LPC 20:4 (0.43).
bHypertension, coronary artery disease, congestive heart failure, peripheral artery disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease, Parkinson’s disease, lower extremity joint disease, and chronic kidney disease.
Discussion
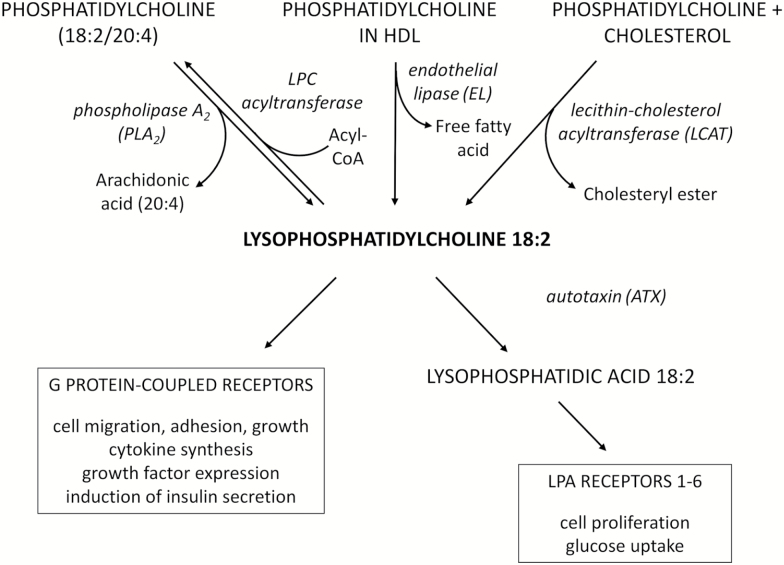
This targeted metabolomics study shows that low plasma LPC 18:2 is a strong, significant correlate of gait speed and a significant independent predictor of future accelerated gait speed decline in older adults. These findings are robust to the confounding effects of chronic disease are unlikely to be due to correction for multiple comparisons. The chemical structure of LPC consists of a glycerol backbone, a hydrophilic polar choline headgroup, and a hydrophobic acyl side chain, which in the case of LPC 18:2 is linoleic acid. LPC are a major class of lipids in plasma and circulate in the 100 to 300 micromolar concentration range. The most abundant LPC in human plasma is 16:0 followed, in order, by 18:2, 18:0/18:1, 20:4, and other minor LPC species (26,27). LPC is transported in plasma primarily by albumin. Alpha-1 acid glycoprotein, a major acute phase protein, is also a transporter of LPC (28). Plasma LPC is generated by phospholipase A2 (PLA2) from phosphatidylcholine that is membrane-bound or constitute the polar surface of lipoproteins (Figure 1). The activity of endothelial lipase (EL), including phospholipase A1 (PLA1), on HDL can generate LPC 18:2 and other LPC species (29). The dominant position of 18:2 in human plasma is in sn-2 (30), thus the activity of PLA1 specifically cleaves phospholipid at the sn-1 position, generating the sn-2 18:2 LPC. Plasma LPC can also originate from phosphatidylcholine during the formation of cholesteryl esters. LPC serves as a ligand for specific G protein-coupled signaling receptors (31,32). LPC is converted to lysophosphatidic acid (LPA) by autotaxin (ATX), an adipokine (Figure 1) (33). LPA has specific receptors involved in growth and differentiation (34).
Figure 1.
Lipid pathways involving LPC 18:2. Phosphatidylcholine in plasma membranes and lipoproteins is converted to LPC 18:2 by phospholipase A2. LPC 18:2 can be converted back to phosphatidylcholine by lipophosphatidylcholine (LPC) transferase. Endothelial lipase can convert phosphatidylcholine in HDL to LPC 18:2. LPC 18:2 can also be generated in the synthesis of cholesteryl esters from phosphatidylcholine and cholesterol. LPC 18:2 has specific activity as a ligand for G protein-coupled receptors or can be converted by autotaxin to lysophosphatidic acid (LPA) 18:2. LPA 18:2 can potentially interact with LPA receptors 1–6.
The biological mechanisms by which LPC 18:2 could be related to gait speed, although still hypothetical, are probably related to skeletal muscle function or brain function, since muscle and brain control are the two main factors affecting lower extremity performance. Previous studies have found LPC concentrations in both plasma and skeletal muscle were associated with insulin resistance, independent of overweight and obesity (35). As noted previously, skeletal muscle insulin resistance is implicated in the pathogenesis of sarcopenia (6). LPC plays a role in inflammation (31), which has been associated both cross-sectionally and longitudinally with sarcopenia (8). LPC is a specific ligand for G protein-coupled signaling receptors. LPA can potentially interact with LPA receptors (Figure 1). LPC and LPA have been shown to have both pro- and anti-atherogenic activity from in-vitro studies and animal models (31,34). There are also conflicting data on the effects of LPA on glucose metabolism and insulin resistance from in-vitro studies and animal models (36,37). Furthermore, in vitro studies suggest that the activity of different LPC in various biological processes is determined by acyl chain length and saturation. For example, LPC species such as LPC 18:2 differ in their effects on endothelium-dependent vasorelaxation (38,39), production of cyclooxygenase (COX)-2 in vascular endothelial cells (40,41), and prostacyclin production in human aortic endothelial cells (42,43).
Interestingly, LPA 18:2 is in the biosynthesis pathway for cardiolipin, a unique dimeric phospholipid that is specific to mitochondria and an essential constituent of mitochondrial membranes (44). Cardiolipin plays an essential role in mitochondrial bioenergetics because it shapes the curvature of the mitochondrial cristae, which affect the assembly and function of the electron transportation chain complexes (45), and modulates mitochondrial metabolic signaling (46). The four acyl chains in cardiolipin in normal human skeletal muscle are linoleic acid (18:2), however, changes in acyl chain composition has been observed in many diseases and can adversely affect mitochondrial function (46).
Our findings are consistent with previous research in which LPC 18:2 was associated with age and aging-related phenotypes in both cross-sectional and prospective studies. In a large population of healthy adults in France, plasma LPC 18:2 concentrations decreased with age and were lower in males (27). Plasma LPC 18:2 was negatively correlated with insulin resistance index (HOMA-IR) (47). Plasma LPC concentrations were reduced in obese adults and those with type 2 diabetes (39). Plasma LPC 18:2 was significantly lower in patients with Alzheimer’s disease and mild cognitive impairment compared with healthy controls (41,43). In a study of 4,297 adults in the population-based Cooperative Health Research in the Region of Augsburg (KORA) cohort, adults with low serum LPC 18:2 had greater risk of developing impaired glucose tolerance over seven years of follow-up (48). In the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study, elevated serum LPC 18:2 was associated with a lower risk of developing type 2 diabetes (49). Low plasma LPC 18:2 was also an independent predictor of incident type 2 diabetes in a cohort of Finnish men (50). In a non-targeted metabolomics study involving more than 3,600 adults from three population-based studies, independent of cardiovascular risk factors, circulating LPC 18:2 predicted incident coronary heart disease (Hazards Ratio 0:81 per standard deviation, p < .001) (51). In a non-targeted metabolomics study of 4,092 adults, LPC 18:2 was an independent predictor of incident coronary artery disease (52). In three independent prospective cohort studies [KORA S4, KORA S2, and the Age, Gene/Environment Susceptibility Reykjavik – Risk Evaluation for Infarct Estimates (AGES-REFINE) studies], three metabolites (LPC 18:2, LPC 17:0, and arginine) improved the predictive value of the Framingham risk score for myocardial infarction (53). In the prediction of myocardial infarction in KORA S4, KORA S2, and AGES-REFINE cohorts, addition of LPC 18:2, LPC 17:0, and arginine in multivariable analyses removed the association between high sensitivity C-reactive protein and incident myocardial infarction, indicating a potential link of these metabolites with systemic inflammation (53). Low plasma LPC 18:2 was an independent predictor of memory impairment in older adults (54).
The strengths of this study are a well-characterized longitudinal aging cohort with standardized collection of fasting plasma samples and measurements of gait speed. An FDR approach and stringent q-value were used to account for multiple comparisons. The limitations of this study include that the findings from this study cannot necessarily be generalized to other study populations due to social and cultural differences. An important limitation is that the BLSA is a convenience sample of adults, not a population-based study, and participants tend to be healthier, more educated, and have better socioeconomic status than the general population. We cannot exclude the possibility that there may be some unmeasured, residual confounding present after adjusting for the available covariates, and that the study may have been limited in power to detect associations between plasma metabolites with lower effect size with gait speed. The overall decline of gait speed in this population was relatively small, which may have precluded the identification of other metabolites that could be associated with larger range of functional decline in older adults. A targeted metabolomics platform, which measures a specific set of amino acids, biogenic amines, acylcarnitines, and glycerophospholipids, was used for this discovery phase study. There are many metabolites beyond this focused set that require further exploration. Further studies will be needed to corroborate these findings in other populations.
The present study shows that gait speed is another aging-related phenotype that is independently predicted by low LPC 18:2. Further work is needed to gain insight into the biological mechanisms that could explain the relationship of LPC 18:2 with diverse phenotypes such as insulin resistance, type 2 diabetes, coronary artery disease, and memory impairment. In regard to skeletal muscle, the relationships of LPC 18:2, LPA 18:2, to the biosynthetic pathway of cardiolipin (18:2)4 is a potential mechanism that should be addressed in future studies.
Funding
The National Institutes of Health R01 AG27012, R01 EY024596, R56 AG052973, P30 AG021334 Johns Hopkins University Older Americans Independence Center, and the Intramural Branch of the National Institute on Aging, Baltimore, Maryland.
Conflict of interest
L.F. serves on the editorial board of JGMS. The remaining authors declare no conflicts of interest.
References
- 1. Perera S, Patel KV, Rosano C et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71:63–71. doi:10.1093/gerona/glv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor ME, Lasschuit DA, Lord SR et al. Slow gait speed is associated with executive function decline in older people with mild to moderate dementia: a one year longitudinal study. Arch Gerontol Geriatr. 2017;73:148–153. doi:10.1016/j.archger.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 3. Kuate-Tegueu C, Avila-Funes JA, Simo N et al. Association of gait speed, psychomotor speed, and dementia. J Alzheimers Dis. 2017;60:585–592. doi:10.3233/JAD-170267 [DOI] [PubMed] [Google Scholar]
- 4. Studenski S, Perera S, Patel K et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi:10.1186/s40200-017-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229:R67–R81. doi:10.1530/JOE-15-0533 [DOI] [PubMed] [Google Scholar]
- 7. Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8:349–369. doi:10.1002/jcsm.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol. 2017;233:R15–R42. doi:10.1530/JOE-16-0598 [DOI] [PubMed] [Google Scholar]
- 9. Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis. 2013;23 (Suppl 1):S44–S50. doi:10.1016/j.numecd.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi:10.1146/annurev-biochem-060614-033955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci. 2014;6:208. doi:10.3389/fnagi.2014.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McPherron AC. Metabolic functions of myostatin and GDF11. Immunol Endocr Metab Agents Med Chem. 2010;10:217–231. doi:10.2174/187152210793663810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moaddel R, Fabbri E, Khadeer MA et al. Plasma biomarkers of poor muscle quality in older men and women from the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2016;71:1266–1272. doi:10.1093/gerona/glw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suire CN, Eitan E, Shaffer NC et al. Walking speed decline in older adults is associated with elevated pro-BDNF in plasma extracellular vesicles. Exp Gerontol. 2017;98:209–216. doi:10.1016/j.exger.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lustgarten MS, Price LL, Chale A, Phillips EM, Fielding RA. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J Gerontol A Biol Sci Med Sci. 2014;69:717–724. doi:10.1093/gerona/glt152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korostishevsky M, Steves CJ, Malkin I, Spector T, Williams FM, Livshits G. Genomics and metabolomics of muscular mass in a community-based sample of UK females. Eur J Hum Genet. 2016;24:277–283. doi:10.1038/ejhg.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wennberg AMV, Schafer MJ, LeBrasseur NK et al. Plasma sphingolipids are associated with gait parameters in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2017;00(00):1–6. doi:10.1093/gerona/glx139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy RA, Moore S, Playdon M et al. Metabolites associated with risk of developing mobility disability in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2018;74:73–80. doi:10.1093/gerona/glx233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812–1823. doi:10.1056/NEJMra1104901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quehenberger O, Armando AM, Brown AH et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi:10.1194/jlr.M009449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drzazga A, Sowińska A, Koziołkiewicz M. Lysophosphatidylcholine and lysophosphatidylinosiol–novel promissing signaling molecules and their possible therapeutic activity. Acta Pol Pharm. 2014;71:887–899. [PubMed] [Google Scholar]
- 22. Klingler C, Zhao X, Adhikary T et al. Lysophosphatidylcholines activate PPARδ and protect human skeletal muscle cells from lipotoxicity. Biochim Biophys Acta. 2016;1861:1980–1992. doi:10.1016/j.bbalip.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 23. Tuck MK, Chan DW, Chia D et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi:10.1021/pr800545q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Storey JD. A direct approach to false discovery rates. J Roy Stat Soc Ser B. 2002;64:479–498. [Google Scholar]
- 25. Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J Roy Stat Soc Ser B. 2004;66:187–205. [Google Scholar]
- 26. Ojala PJ, Hirvonen TE, Hermansson M, Somerharju P, Parkkinen J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J Leukoc Biol. 2007;82:1501–1509. doi:10.1189/jlb.0507292 [DOI] [PubMed] [Google Scholar]
- 27. Trabado S, Al-Salameh A, Croixmarie V et al. The human plasma-metabolome: reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS One. 2017;12:e0173615. doi:10.1371/journal.pone.0173615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ojala PJ, Hermansson M, Tolvanen M et al. Identification of alpha-1 acid glycoprotein as a lysophospholipid binding protein: a complementary role to albumin in the scavenging of lysophosphatidylcholine. Biochemistry. 2006;45:14021–14031. doi:10.1021/bi061657l [DOI] [PubMed] [Google Scholar]
- 29. Gauster M, Rechberger G, Sovic A et al. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005;46:1517–1525. doi:10.1194/jlr.M500054-JLR200 [DOI] [PubMed] [Google Scholar]
- 30. Marai L, Kuksis A. Molecular species of lecithins from erythrocytes and plasma of man. J Lipid Res. 1969;10:141–152. [PubMed] [Google Scholar]
- 31. Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208:10–18. doi:10.1016/j.atherosclerosis.2009.05.029 [DOI] [PubMed] [Google Scholar]
- 32. Grzelczyk A, Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data – new insight into their function. Biochimie. 2013;95:667–679. doi:10.1016/j.biochi.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 33. Ferry G, Tellier E, Try A et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–18169. doi:10.1074/jbc.M301158200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aikawa S, Hashimoto T, Kano K, Aoki J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem. 2015;157:81–89. doi:10.1093/jb/mvu077 [DOI] [PubMed] [Google Scholar]
- 35. Tonks KT, Coster AC, Christopher MJ et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity. 2016;24:908–916. doi:10.1002/oby.21448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yea K, Kim J, Lim S et al. Lysophosphatidic acid regulates blood glucose by stimulating myotube and adipocyte glucose uptake. J Mol Med (Berl). 2008;86:211–220. doi:10.1007/s00109-007-0269-z [DOI] [PubMed] [Google Scholar]
- 37. Rancoule C, Attané C, Grès S et al. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia. 2013;56:1394–1402. doi:10.1007/s00125-013-2891-3 [DOI] [PubMed] [Google Scholar]
- 38. Rao SP, Riederer M, Lechleitner M et al. Acyl chain-dependent effect of lysophosphatidylcholine on endothelium-dependent vasorelaxation. PLoS One. 2013;8:e65155. doi:10.1371/journal.pone.0065155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barber MN, Risis S, Yang C et al. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012;7:e41456. doi:10.1371/journal.pone.0041456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brkić L, Riederer M, Graier WF, Malli R, Frank S. Acyl chain-dependent effect of lysophosphatidylcholine on cyclooxygenase (COX)-2 expression in endothelial cells. Atherosclerosis. 2012;224:348–354. doi:10.1016/j.atherosclerosis.2012.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cui Y, Liu X, Wang M et al. Lysophosphatidylcholine and amide as metabolites for detecting alzheimer disease using ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry-based metabonomics. J Neuropathol Exp Neurol. 2014;73:954–963. doi:10.1097/NEN.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 42. Riederer M, Ojala PJ, Hrzenjak A et al. Acyl chain-dependent effect of lysophosphatidylcholine on endothelial prostacyclin production. J Lipid Res. 2010;51:2957–2966. doi:10.1194/jlr.M006536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Proitsi P, Kim M, Whiley L et al. Association of blood lipids with Alzheimer’s disease: a comprehensive lipidomics analysis. Alzheimers Dement. 2017;13:140–151. doi:10.1016/j.jalz.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 44. Schlame M, Greenberg ML. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim Biophys Acta. 2017;1862:3–7. doi:10.1016/j.bbalip.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1837:408–417. doi:10.1016/j.bbabio.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 46. Dudek J. Role of Cardiolipin in mitochondrial signaling pathways. Front Cell Dev Biol. 2017;5:90. doi:10.3389/fcell.2017.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhong H, Fang C, Fan Y et al. Lipidomic profiling reveals distinct differences in plasma lipid composition in healthy, prediabetic, and type 2 diabetic individuals. Gigascience. 2017;6:1–12. doi:10.1093/gigascience/gix036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang-Sattler R, Yu Z, Herder C et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. doi:10.1038/msb.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Floegel A, Stefan N, Yu Z et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–648. doi:10.2337/db12-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suvitaival T, Bondia-Pons I, Yetukuri L et al. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism. 2017. doi:10.1016/j.metabol.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 51. Ganna A, Salihovic S, Sundström J et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014;10:e1004801. doi:10.1371/journal.pgen.1004801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang XZ, Zheng SX, Hou YM. A non-targeted liquid chromatographic-mass spectrometric metabolomics approach for association with coronary artery disease: an identification of biomarkers for depiction of underlying biological mechanisms. Med Sci Monit. 2017;23:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ward-Caviness CK, Xu T, Aspelund T et al. Improvement of myocardial infarction risk prediction via inflammation-associated metabolite biomarkers. Heart. 2017;103:1278–1285. doi:10.1136/heartjnl-2016-310789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mapstone M, Cheema AK, Fiandaca MS et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi:10.1038/nm.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]