Abstract
Background
Growth and differentiation factors 8 (GDF8) and 11 (GDF11) have attracted attention as targets for rejuvenating interventions. The biological activity of these proteins may be affected by circulating antagonists such as their respective prodomains, follistatin (FST315), WFIKKN1, and WFIKKN2. Reports of the relationship of GDF8 and GDF11 and their antagonists with aging and aging phenotypes such as skeletal muscle strength have been conflicting possibly because of difficulties in measuring these proteins and polypeptides.
Methods
Plasma GDF8 and GDF11 and their antagonists were measured using a multiplexed selected reaction monitoring assay and liquid chromatography–tandem mass spectrometry in 160 healthy adults aged 22–93 years. Quadriceps strength was measured by knee extensor torque using isokinetic dynamometry.
Results
Spearman correlations with age were the following: GDF11 prodomain (r = .30, p = .001), GDF11 mature protein (r = .23, p = .004), FST315 (r = .32, p < .0001), WFIKKN1 (r = −.21, p = 0.008), and WFIKKN2 (r = .18, p = .02). Independent of age, FST315 and WFIKKN1 were negatively associated with knee strength (p = .02, p = .03, respectively) in a multivariable model that included both GDF8 and GDF11 mature proteins.
Conclusions
When measured by an antibody-free selected reaction monitoring assay, GDF8, GDF11, and their antagonists are found in the circulation in the ng/mL range. In healthy adults, plasma GDF11 and antagonists FST315, WFIKKN1, and WFIKKN2 differed by age. Antagonists of GDF8 and GDF11, but not GDF8 and GDF11, were independently associated with skeletal muscle strength. Further work is needed to characterize the relationship of these protein and polypeptides with sarcopenia-related phenotypes such as physical function and walking disability.
Keywords: Aging, Follistatin, Growth and differentiation factor 8, Growth and differentiation factor 11, WFIKKN1, WFIKKN2
Growth and differentiation factor 11 (GDF11) was originally identified nearly two decades ago (1) and is closely related to growth and differentiation factor 8 (GDF8), also known as myostatin (2). GDF8 is a negative regulator of skeletal muscle growth and a focus of rejuvenation research because inhibition of myostatin can enhance skeletal muscle growth (2–4). The nature of the biological activity of GDF11 is still a matter of debate. It was suggested that GDF11 counteracts the effect of aging on skeletal muscle, heart, and brain (5,6), but other laboratories have not confirmed these observations and do not show a rejuvenating effect of GDF11 (7,8). In vivo administration of GDF11 in mice induced cardiac and skeletal muscle dysfunction and wasting (9,10) and upregulation of GDF15, a divergent member of the transforming growth factor-β superfamily that suppresses food intake through signaling of the GDF15 receptor in the brainstem (11).
GDF11 and GDF8 are released into the circulation in a similar manner. After cleavage from the signal peptide, intact GDF is cleaved by furin family proconvertases into prodomain and mature protein. Mature GDF11 and GDF8 proteins share approximately 90% sequence identity (7). The prodomain and mature protein dimers form a noncovalently bound latent complex in the circulation (2,12). The latent complex is activated through cleavage of the prodomain by BMP-1 and tolloid family metalloproteases (13). Both GDF8 and GDF11 bind activin type 2 receptors and ALK4/5/7 and induce the Smad 2/3 pathway (14,15). The GDF11/GDF8/activin pathway negatively regulates muscle size through Smad 2/3 (16).
Early studies that used antibodies (enzyme-linked immunosorbent assay [ELISA]) and aptamer assays (SOMAmers) reported that serum GDF11 concentrations decrease with age in mice (5,6). Egerman and colleagues (7) challenged the validity of these findings and showed that the antibodies and SOMAmers for GDF11 cross-reacted with the closely related homologue, GDF8. An ELISA for GDF8 used in an earlier study (16) was found to cross-react with GDF11 in a validation study (17). As SOMAmers and antibodies cannot reliably distinguish between the mature proteins of GDF8 and GDF11, a subsequent study reported that circulating “GDF11/8” declined with age in different animals (18).
There are six major proteins or polypeptides that bind with mature GDF8 and GDF11 in the circulation and block their action: the respective prodomains of GDF8 (3,7,12,13) and GDF11 (19), follistatin (FST315) (7,20), follistatin-related protein 3 (FSTL3) (21–23), WFIKKN1 (24), and WFIKKN2 (24). In characterizing the relationship of circulating GDF8 and GDF11 with aging phenotypes, the known circulating inhibitors are important because they alter the biological activity of GDF8 and GDF11. Protein complexes could block specific epitopes that are recognized by antibodies or aptamers, thus leading to underestimation of total circulating protein or polypeptide concentrations. Immunoaffinity-selected reaction monitoring (SRM) assays, which combine antibody capture of specific plasma proteins followed by liquid chromatography–tandem mass spectrometry (LC-MS/MS), have been developed for measurement of GDF8 (25,26) or both GDF8 and GDF11 (27).
Our specific aims were to examine the relationship of plasma GDF8 and GDF11 and their antagonists, as measured by a multiplexed SRM assay and LC-MS/MS (28), with age and with skeletal muscle strength in an extremely healthy cohort of adults across a wide age range.
Methods
Study Design and Study Subjects
The study participants consisted of 160 adults (80 men, 80 women), aged 22–93 years, who participated in the Baltimore Longitudinal Study of Aging (BLSA) or the Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing (GESTALT) study. The BLSA is a National Institutes of Health-supported prospective open cohort study of community-dwelling volunteers, largely from the Baltimore and Washington area, as described elsewhere (29). Participants are seen at the National Institute on Aging Clinical Research Unit, MedStar Harbor Hospital, Baltimore, Maryland, for follow-up visits every 1–4 years, with more frequent follow-up for older participants. They undergo 2.5 days of medical, physiological, and psychological examinations. The GESTALT study is an ongoing study of healthy adults across a wide age range in Baltimore, Maryland. The study will enroll 20 adults in each of five age groups (20–34, 35–49, 50–64, 65–79, and 80+ years) for a total of 100 participants.
Strict inclusion and exclusion criteria were made to select an extremely health group of adults (Supplementary Appendix 1). Physical activity was determined by a standardized questionnaire and was defined as high-energy activity in minutes per week. Written, informed consent was obtained from all participants. The protocol for this study was approved by the institutional review boards for the National Institute on Aging and the Johns Hopkins University School of Medicine.
Measurement of Skeletal Muscle Strength
Skeletal muscle strength was chosen as the main outcome measure of this study. It was measured in all participants. Body composition measurements using dual X-ray absorptometry were only available on a smaller subset of the study population. Maximum quadriceps strength was defined as the highest value of torque (peak torque) from either leg in up to three consecutive measurements of concentric knee extensor strength (Newtons per meter, Nm) using isokinetic dynamometry at an angular velocity of 0.52 rad/s (30°/s). Muscle strength was assessed using the Biodex Multi-Joint System PRO dynamometer (Biodex Medical System, Inc., Shirley, New York).
Measurement of Lean Body Mass
In BLSA participants, total body dual X-ray absorptometry was performed using the Prodigy Scanner (General Electric) and analyzed with version 10.51.006 software (General Electric). Appendicular lean mass, the sum of lean tissue in the arms and legs, derived from dual X-ray absorptometry measurements, was used as an approximation of muscle mass.
Collection of Plasma Samples
Blood was collected from the antecubital vein between 0700 and 0800 hours after an overnight fast in the National Institute on Aging Clinical Research Unit. Blood was immediately stored at 4°C, centrifuged within 4 hours, then immediately aliquoted and frozen at −80°C.
Measurement of GDF8, GDF11, and Their Antagonists Using Selected Reaction Monitoring and Liquid LC-MS/MS
Plasma samples were thawed on the day of analysis and centrifuged at 14,000g for 15 minutes at 4°C. Cleared fractions were manually transferred with a loading tip into a fresh 1.5 mL polypropylene tube, discarding insoluble aggregates and the upper layer of floating lipids. This procedure was sufficient to eliminate the confounding influence of lipids in downstream protein separation procedures. Alkylation, reduction, and digestion of plasma samples were conducted using a Biomek NXp station (Beckman Coulter) following a protocol established by our laboratory (30). After delipidation, 5 µL delipidated plasma was manually added into the reaction plate using reverse pipetting. Ninety-five microliter of 0.1% (w/v) RapiGest (Waters Co., Milford, Massachusetts) containing 100 mM Tris-HCl, pH 8.0, and 100 mM dithiothreitol were added and incubated at 55°C for 1 hour for denaturation and reduction. The reduced samples were then alkylated for 30 minutes at room temperature in the dark with iodoacetamide (50 mM final). After alkylation, 60 µL of 50 mM ammonium bicarbonate was added to the digests to achieve 460 μL digest volume. Trypsin/Lys-C Mix (Promega, Madison, Wisconsin) was added at an enzyme-to-substrate ratio of 1:50. Digestion was carried out for 18 hours at 37°C and terminated with 10% trifluoroacetic acid to a final concentration of 1%. All procedures were programmed and automatically performed unless otherwise mentioned. To achieve the best accuracy and precision, all the reagents were aspirated 5.5 mm from below the liquid surface of the reagent reservoir and dispensed 1.0 mm from below the liquid surface of the reaction well using override-tip technique. Acidified tryptic digests were cleaned up with 96-well SPE plate (Phenomenex, Torrance, California) according to the manufacturer’s instruction. A 96-well plate vacuum manifold (Waters) was used for all desalting procedures to provide more uniform peptide wash, retention, and elution. The elution reagents were evaporated to dryness and the residues were manually reconstituted with 100 µL of 0.1% formic acid containing a concentration-balanced mixture of synthesized heavy isotope-labeled peptides as internal standards for quantification. The specificity of the SRM assay for GDF8 and GDF11 has been verified through independent, blinded studies involving spiking of plasma samples with different concentrations of recombinant GDF8 and GDF11, respectively (R. D. Semba, September 2017).
We measured plasma GDF8 prodomain, GDF8 mature protein, GDF11 prodomain, GDF11 mature protein, FST315, FST303, WFIKKN1, and WFIKKN2 using an SRM assay and LC-MS/MS, as described in detail elsewhere (26). Although results are reported in ng/mL for each analyte based on standard curves using heavy isotope-labeled peptides, in strict terms, the SRM method provides a measure of relative abundance, not absolute quantification because of potential matrix effects (26). The mean intra- and inter-assay coefficients of variation, respectively, for the analytes were as follows: GDF8 prodomain (4.9% and 9.0%), GDF8 mature protein (7.1% and 15.9%), GDF11 prodomain (3.3% and 14.1%), GDF11 mature protein (5.1% and 10.7%), FST315 (1.0% and 6.4%), FST303 (2.9% and 13.8%), WFIKKN1 (4.3% and 5.2%), and WFIKKN2 (3.9% and 8.2%).
Measurement of GDF8 Using ELISA
To demonstrate how antagonist binding to GDF8 could affect GDF8 measurements, we processed samples using two different methods prior to ELISA. A simple random sample of 37 participants (19 women, 18 men) was selected from the 160 participants in the study. Plasma samples were prepared for ELISA (GDF8/myostatin Quantikine ELISA; R&D Systems, Minneapolis, Minnesota; catalog no. DGDF80) using the manufacturer’s updated instructions for preparation of samples both with “activation” (acid dissociation of antagonists binding with mature GDF8 protein) and without “activation” (31). In brief, to perform acid dissociation of binding by antagonists, plasma samples were incubated with 1 N HCl for 10 minutes at room temperature, after which 1.2 N NaOH/0.5 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid was added and mixed to the sample for pH normalization, as per the manufacturer’s instructions (31). Samples were run in duplicate with recombinant GDF8 standard (R&D Systems, catalog no. 788-G8/CF) on the same ELISA plates. Intra-assay precision and inter-assay precision were in the ranges achieved by the company accordingly: 1.8%–5.4% and 3.1%–6.0%, respectively.
Statistical Analysis
Characteristics of the sample population were summarized as mean (±standard deviation, SD) or number (percentage). Plasma protein and polypeptide concentrations were calculated in ng/mL. GDF8 results as measured by ELISA are reported as ng/mL. Scatterplots and Spearman correlations were used to explore the relationship between proteins or polypeptides with age and skeletal muscle strength. Spearman correlations were used to examine the relationships between plasma GDF8 as measured by SRM and ELISA, with and without “activation” of plasma samples. Sex differences were investigated stratifying the correlative analyses by gender. The Shapiro–Wilk test was used to assess normality. Spearman rank coefficients were used because of a nonnormal distribution of analytes. Multivariable linear regression models were used to examine the relationship between GDF8, GDF11, and their circulating antagonists with skeletal muscle strength. The level of significance used in this study was p < .05. All analyses were performed by SAS statistical package, version 9.3 (SAS Institute Inc., Cary, North Carolina) and R 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The demographic characteristics of the 160 study participants are shown in Table 1. Fifty-percent of the participants were women. The mean years of education were at the college graduate level. Mean body mass index, serum creatinine, and hemoglobin concentrations indicated a healthy group of adults, which was the goal of the strict inclusion and exclusion criteria of the study. There were no significant correlations between age and education, body mass index, serum creatinine, or hemoglobin (data not shown).
Table 1.
Demographic Characteristics of the 160 Study Participants
| Characteristic | Mean (SD) or % |
|---|---|
| Age, y | 57.2 (19.7) |
| Sex, % female | 50.0 |
| Race, % | |
| White | 71.1 |
| Black | 21.4 |
| Asian | 7.5 |
| Education, y | 16.8 (2.7) |
| Body mass index, kg/m2 | 26.6 (4.7) |
| Appendicular lean mass, kg† | 22.1 (5.3) |
| Physical activity, min/wk | 125.6 (227.2) |
| Serum creatinine, mg/dL | 0.9 (0.2) |
| Hemoglobin, g/dL | 13.9 (1.3) |
| Knee extension peak torque, Nm | 153.2 (61.5) |
Notes: BLSA = Baltimore Longitudinal Study of Aging; DXA = dual X-ray absorptometry.
†DXA measurements were available only in a subsample of 113 adults who were participants in the BLSA.
Mean (SD) plasma concentrations of proteins and polypeptides in ng/mL measured by SRM were as follows: GDF8 prodomain, 12.2 (9.0); GDF8 mature protein, 27.0 (7.1); GDF11 prodomain, 20.5 (9.7); GDF11 mature protein, 16.8 (8.9); FST315, 31.3 (8.5); FST303, 91.0 (96.8); WFIKKN1, 38.2 (10.0); WFIKKN2, 36.0 (16.7). Mean (SD) of plasma proteins and polypeptides measured by SRM are shown by sex-specific tertiles of age overall and by tertile of age, shown for each sex in Table 2.
Table 2.
Plasma Protein and Polypeptide Concentrations Measured in 160 Healthy Adults, by Sex-Specific Tertiles of Age
| Analyte† | All Participants | Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Middle | High | p‡ | Low | Middle | High | p‡ | Low | Middle | High | p‡ | |
| GDF8 prodomain | 11.1 (3.2) | 11.1 (3.7) | 14.3. (14.6) | .10 | 11.2 (3.1) | 11.4 (3.7) | 16.9 (20.2) | .15 | 11.1 (3.4) | 10.7 (3.7) | 11.7 (3.4) | .56 |
| GDF8 mature protein | 26.7 (7.9) | 28.4 (7.2) | 26.1 (6.1) | .21 | 27.4 (7.4) | 28.4 (7.4) | 26.4 (6.0) | .57 | 26.0 (8.3) | 28.4 (7.2) | 25.7 (6.2) | .35 |
| GDF11 prodomain | 17.3 (9.4) | 21.3 (8.9) | 23.3 (9.9) | .02 | 18.5 (10.4) | 19.9 (8.6) | 21.5 (9.9) | .62 | 15.9 (8.2) | 23.2 (9.2) | 25.0 (9.7) | .008 |
| GDF11 mature protein | 16.5 (12.4) | 15.2 (7.3) | 18.9 (5.2) | .09 | 15.7 (14.6) | 14.0 (6.4) | 17.6 (4.2) | .38 | 17.2 (10.0) | 16.4 (8.0) | 20.1 (5.9) | .22 |
| FST315 | 29.2 (7.1) | 28.9 (6.7) | 35.7 (9.6) | <.001 | 26.5 (5.5) | 28.5 (5.6) | 34.3 (6.3) | <.001 | 31.8 (7.6) | 29.3 (7.7) | 37.1 (12.0) | .01 |
| FST303 | 86.4 (78.2) | 79.4 (85.6) | 106.4 (120.3) | .35 | 100.3 (94.8) | 67.3 (57.9) | 126.7 (160.3) | .18 | 71.3 (53.0) | 92.5 (107.8) | 86.1 (54.8) | .60 |
| WFIKKN1 | 41.4 (9.1) | 35.8 (10.8) | 37.5 (9.3) | .01 | 41.7 (8.7) | 37.6 (11.1) | 36.2 (9.7) | .11 | 41.0 (9.8) | 33.9 (10.3) | 38.7 (8.9) | .03 |
| WFIKKN2 | 29.5 (11.8) | 42.0 (21.9) | 36.4 (12.0) | <.001 | 27.9 (10.8) | 37.7 (18.7) | 36.3 (11.3) | .03 | 31.1 (12.8) | 46.3 (24.3) | 36.5 (12.9) | .01 |
†Concentration in ng/mL, showing mean (SD).
‡Comparison across tertiles using analysis of variance (ANOVA).
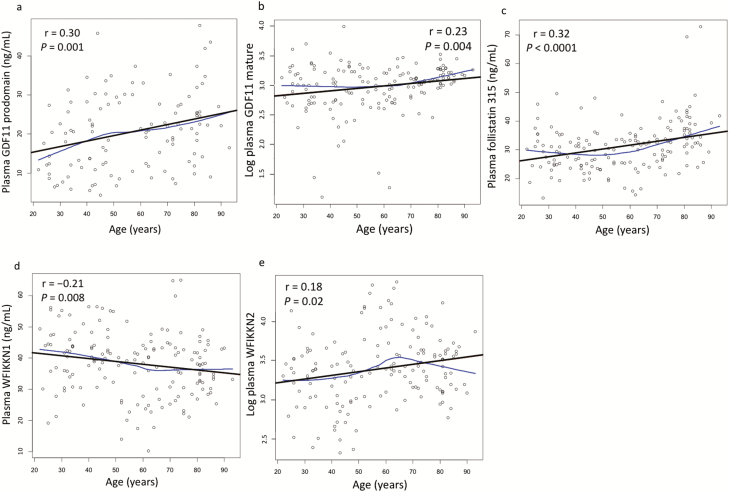
The Spearman correlations between proteins and polypeptides measured by SRM with age are shown in Table 3. There were positive correlations between age and GDF11 prodomain (p = .001) and FST315 (p <.0001), respectively. Plasma WFIKKN1 was negatively correlated with age (p = .008). GDF8 mature protein, GDF8 prodomain, GDF11 mature protein, and FST303 were not significantly correlated with age. Scatterplots of selected proteins and peptides with age are shown in Figure 1. The Spearman correlation between GDF8 mature protein and GDF11 mature proteins as measured by SRM was 0.125 (p = .12).
Table 3.
Spearman Correlations of Proteins and Polypeptides With Age in Healthy Adults
| Analyte | All Adults (n = 160) |
Men (n = 80) |
Women (n = 80) |
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| GDF8 prodomain | .05 | .52 | .01 | .93 | .09 | .39 |
| GDF8 mature protein | −.08 | .29 | −.09 | .38 | −.06 | .57 |
| GDF11 prodomain | .30 | .001 | .14 | .27 | .47 | .0004 |
| GDF11 mature protein | .23 | .004 | .29 | .009 | .18 | .10 |
| FST315 | .32 | <.0001 | .48 | <.0001 | .19 | .09 |
| FST303 | −.06 | .43 | −.17 | .14 | .07 | .55 |
| WFIKKN1 | −.21 | .008 | −.21 | .06 | −.19 | .08 |
| WFIKKN2 | .18 | .02 | .22 | .05 | .14 | .21 |
Figure 1.
Relationship of plasma (a) GDF11 prodomain, (b) GDF11 mature, (c) FST315, (d) WFIKKN1, and (e) WFIKKN2 with age in 160 healthy adults, with Lowess smoothing line (curved line) and linear regression line (bold straight line). Spearman correlations (r) and p-values are shown.
Multivariable linear regression models were used to examine the relationship of proteins and polypeptides with knee strength (Table 4). The Spearman correlation between knee strength and physical activity was r = .17 (p = .038); thus physical activity was included as a covariate in the multivariable models.
Table 4.
Multivariable Linear Regression Models of the Relationship Between Plasma GDF8 Mature Protein and Plasma GDF11 Mature Protein and Their Circulating Antagonists With Knee Strength in Healthy Adults
| Peptide or Protein | β | SE | p |
|---|---|---|---|
| (a) Multivariable models for GDF8 and GDF11, respectively, with knee strength, adjusted by age, sex, and physical activity | |||
| GDF8 mature | −.08 | 0.06 | .15 |
| GDF11 mature | .04 | 0.04 | .38 |
| (b) Multivariable model for GDF8 and circulating antagonists with knee strength, adjusted by age, sex, and physical activity | |||
| GDF8 mature | −.05 | 0.07 | .43 |
| GDF8 prodomain | .002 | 0.04 | .96 |
| FST315 | −.14 | 0.06 | .02 |
| FST303 | −.003 | 0.004 | .45 |
| WFIKKN1 | −.02 | 0.06 | .69 |
| WFIKKN2 | .03 | 0.04 | .37 |
| (c) Multivariable model for GDF11 and circulating antagonists with knee strength, adjusted by age, sex, and physical activity | |||
| GDF11 mature | .10 | 0.08 | .18 |
| GDF11 prodomain | .07 | 0.06 | .27 |
| FST315 | −.18 | 0.08 | .02 |
| FST303 | .005 | 0.01 | .45 |
| WFIKKN1 | −.18 | 0.07 | .02 |
| WFIKKN2 | .06 | 0.05 | .30 |
| (d) Multivariable model for GDF8 and GDF11 and their circulating antagonists with knee strength, adjusted by age, sex, and physical activity | |||
| GDF8 mature | −.10 | 0.08 | .22 |
| GDF8 prodomain | .001 | 0.05 | .98 |
| GDF11 mature | .10 | 0.07 | .19 |
| GDF11 prodomain | .09 | 0.06 | .18 |
| FST315 | −.18 | 0.07 | .02 |
| FST303 | .005 | 0.01 | .50 |
| WFIKKN1 | −.16 | 0.07 | .03 |
| WFIKKN2 | .08 | 0.06 | .18 |
First, we asked whether GDF8 or GDF11 mature proteins alone were associated with knee strength. In separate models adjusting for age, sex, and physical activity neither GDF8 nor GDF11 mature proteins were significantly associated with knee strength (Table 4a). Next, we asked whether the circulating antagonists were associated with knee strength. After adjusting for age, sex, and physical activity, FST315 was negatively associated with knee strength (p = .026) in a multivariable model that included GDF8 mature protein in the same model. In this model that included the antagonists, GDF8 mature protein was not significantly associated with knee strength (Table 4b). After adjusting for age, sex, and physical activity, FST315 (p = .016) and WFIKKN1 (p = .01) were negatively associated with knee strength in a multivariable model that included GDF11 mature protein. GDF11 mature protein was not significantly associated with knee strength in the same multivariable model (Table 4c). Finally, we asked whether GDF8, GDF11, or any of their circulating antagonists would be associated with knee strength in a final model that included all analytes. After adjusting for age, sex, and physical activity, FST315 and WFIKKN1 were negatively associated with knee strength (both ps = .02; Table 4d). It should be noted the multivariable models did not include covariates for comorbid conditions because these participants were selected using strict inclusion and exclusion criteria to represent extremely healthy aging (ie, they had no comorbidity or chronic diseases by study design).
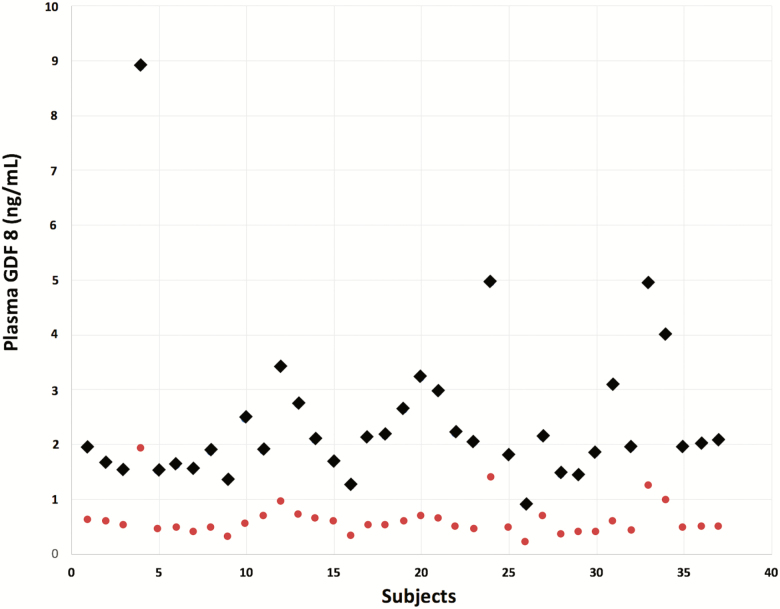
GDF8 was measured in a simple random subsample of 37 participants that consisted of 18 men, mean (SD) age 54.0 (18.0) years and 19 women, age 54.2 (21.6) years. There were no significant differences in race, education, body mass index, serum creatinine, hemoglobin, or knee extension peak torque between the random subsample and the entire sample of 160 participants (data not shown). Mean (SD) plasma concentrations of GDF8 measured with and without acid dissociation prior to ELISA measurement in these participants were 2.39 (1.43) and 0.62 (0.32) ng/mL, respectively (p < .0001). Acid dissociation consistently increased GDF8 measurements in all 37 participants (Figure 2). Spearman correlations of GDF8 mature protein as measured by ELISA with and without acid dissociation with GDF8 mature protein as measured by SRM were r = .09 (p = .59) and r = .27 (p = .11), respectively.
Figure 2.
Individual GDF8 measurements with acid dissociation (diamond) and without acid dissociation (circle) within the same 37 participants.
Discussion
This study shows that plasma GDF8 and GDF11 and their antagonists, as measured using antibody-free SRM and LC-MS/MS, are found at higher concentrations (ie, ng/mL range) in the circulation than previously described by immunoassays and immunoaffinity-SRM assays (7,17,18,25–27,32). This large difference in protein and polypeptide concentrations may account for some of the conflicting results regarding GDF8 and GDF11 that have previously been reported in the literature.
The large discrepancy between plasma concentrations of GDF8, GDF11, and their circulating antagonists, as measured using antibody-free SRM in this study, with previous measures using antibody-based approaches may be because GDF8 and GDF11 form binding complexes in the circulation with their respective prodomains, FST, FSTL3, WFIKKN1, and WFIKKN2 (20–24). Many of the epitopes that are recognized by antibodies to detect GDF8 or GDF11 may become inaccessible within these noncovalent protein binding complexes. To test this concept, we measured GDF8 concentrations using a commercial ELISA in plasma samples that had either been treated with acid to disrupt noncovalent binding complexes or were left untreated. Acid disruption of binding complexes led to a fourfold increase in plasma GDF8 concentrations detected using ELISA. However, it should be noted that the manufacturer of the ELISA kit warns that GDF8 antagonists such as FST and WFIKKN2 can interfere with measurements of GDF8 if their concentrations are more than10 ng/mL (31). It is likely that after acid disruption of protein binding complexes at pH 2 and then normalization of plasma to approximately pH 7 for antibody binding in the ELISA, circulating antagonists such as FST and WFIKKN2 can reform noncovalent binding complexes with GDF and still interfere with the detection of GDF8 using antibodies, leading to an underestimation of circulating GDF8. Plasma GDF8 as measured by ELISA, with or without acid dissociation, was not significantly correlated with plasma GDF8 measured by SRM. A potential explanation is that circulating antagonists interfere with measurement of plasma GDF8 using ELISA, as has been acknowledged by the manufacturer of the ELISA.
Other epidemiological studies have examined the relationship between CDF8 and sarcopenia. Ratkevicius and colleagues (33) showed that there were no significant differences in the concentrations of serum GDF8, measured using ELISA without acid dissociation, between young men without sarcopenia and older men with sarcopenia. Bergen and colleagues (25) showed that young men had significantly higher serum GDF8 concentrations, measured using immunoaffinity-SRM, compared with older men. In contrast, older women had 34% higher serum GDF8 concentrations compared with younger women (25).
Studies using immunoaffinity purification studies by Hill and colleagues (21,22) provide further support for the idea that protein binding complexes could affect the detection of GDF8 using antibody-based approaches such as ELISA or immunoaffinity-SRM assays (7,17,25–27,32). Immunoaffinity of GDF8 followed by LC-MS/MS revealed that most GDF8 isolated from plasma was bound to its prodomain. In addition, FSTL3 and WFIKKN2 were also bound to GDF8 (21,22). In the present study, we did not determine whether acid disruption of protein binding complexes affected the detection of GDF11 by ELISA. However, GDF8 and GDF11 mature proteins share approximately 90% sequence homology and are known to form protein complexes with the same circulating antagonists.
In healthy adults, the concentrations of GDF8 prodomain and mature protein did not change with age, whereas those of GDF11 prodomain and mature protein increased with age. Using an antibody-based approach, in which immunoaffinity purification using antibodies was conducted prior to LC-MS/MS, Schafer and colleagues (27) showed that GDF11 did not change with age. The concentrations of some of the antagonists of GDF8 and GDF11 show significant changes with age, such as FST315, WFIKKN1, and WFIKKN2. Previous reports of associations between GDF8 and GDF11 with age as measured using antibody- or aptamer-based approaches could reflect age-related changes in these antagonists binding to GDF8 and GDF11 mature proteins in the circulation rather than GDF8 or GDF11 themselves. FST303, the terminally cleaved form of FST in the circulation that has biological activity (34), is found in much higher concentrations than FST315, underscoring the potential importance of measuring both forms of FST in the circulation. A limitation of the present study is that FSTL3, an antagonist of both GDF8 and GDF11, was not included among the analytes in the SRM assay (28). The optimization of proteotypic peptides for FSTL3 is currently being carried out and will be reported elsewhere. One advantage of the antibody-free SRM approach used to measure analytes in this study is that that assay requires a small volume of plasma (28). In the present study, the higher molar concentration of the GDF11 propeptide relative to the GDF11 mature protein could possibly be due to incomplete removal of a binding protein from the mature protein in processing prior to trypsin digestion or that there are differences in the half-lives of GDF11 propeptide versus mature protein in the circulation.
This suggests that plasma WFIKKN1 and WFIKKN2 have opposite relationships with age. In healthy adults, WFIKKN1 decreases and WFIKKN2 increases with age. Plasma GDF8, which has a negative effect on skeletal muscle growth, is inhibited by WFIKKN2 (24). Latent GDF8, which includes the GDF8 mature protein homodimer that is noncovalently bound to one GDF8 prodomain, can be controlled by either WFIKKN1 or WFIKKN2 (35). It is unclear why WFIKKN1 and WFIKKN2 would have the opposite relationships with age.
Neither GDF8 mature protein or GDF11 mature protein was significantly associated with knee strength in separate multivariable models that were adjusted for age and sex. In contrast, FST315 was negatively associated with knee strength in the separate models that included GDF8 mature protein and GDF11 mature protein. In the multivariable model that included GDF11 mature protein, WFIKKN1 was negatively associated with knee strength. These findings suggest that some of the circulating antagonists to GDF8 and GDF11 could be modulating their activity and relationships between GDF8 and GDF11 with their known receptors. GDF8 has been a focus of rejuvenation research because inhibition of myostatin can increase skeletal muscle growth in animal models (2). The present study does not show an association of higher levels of circulating inhibitors of GDF8 with greater skeletal muscle strength.
This study characterized GDF8, GDF11, and their circulating antagonists in a population of adults that was selected, with 39 inclusion and exclusion criteria, to be healthy and represent a wide spectrum of age. Significant relationships were found between these proteins and polypeptides with both age and skeletal muscle strength among healthy adults. Future studies are needed to characterize the relationship of circulating GDF8, GDF11, and their antagonists with aging phenotypes such as cardiovascular disease and sarcopenia among adults with the burden of chronic diseases found in the general population.
Funding
The National Institutes of Health (grants R56 AG052973, R01 EY024596, R01 AG027012, R01 AG057723), and the Intramural Research Program of the National Institute on Aging.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
No other persons besides the authors have made substantial contributions to this manuscript. Luigi Ferrucci is a member of the editorial board of the journal.
References
- 1. McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320 [DOI] [PubMed] [Google Scholar]
- 2. McPherron AC. Metabolic functions of myostatin and GDF11. Immunol Endocr Metab Agents Med Chem. 2010;10:217–231. doi: 10.2174/187152210793663810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- 4. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loffredo FS, Steinhauser ML, Jay SM, et al. . Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sinha M, Jang YC, Oh J, et al. . Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Egerman MA, Cadena SM, Gilbert JA, et al. . GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith SC, Zhang X, Zhang X, et al. . GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–932. doi: 10.1161/CIRCRESAHA.115.307527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zimmers TA, Jiang Y, Wang M, et al. . Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol. 2017;112:48. doi: 10.1007/s00395-017-0639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammers DW, Merscham-Banda M, Hsiao JY, Engst S, Hartman JJ, Sweeney HL. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol Med. 2017;9:531–544. doi: 10.15252/emmm.201607231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones JE, Cadena SM, Gong C, et al. . Supraphysiologic administration of GDF11 induces cachexia in part by upregulating GDF15. Cell Rep. 2018;22:3375. doi: 10.1016/j.celrep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 12. Thies RS, Chen T, Davies MV, et al. . GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors. 2001;18:251–259. [DOI] [PubMed] [Google Scholar]
- 13. Wolfman NM, McPherron AC, Pappano WN, et al. . Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho DM, Yeo CY, Whitman M. The role and regulation of GDF11 in Smad2 activation during tailbud formation in the Xenopus embryo. Mech Dev. 2010;127:485–495. doi: 10.1016/j.mod.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009 [DOI] [PubMed] [Google Scholar]
- 16. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodgers BD, Eldridge JA. Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and Brain. Endocrinology. 2015;156:3885–3888. doi: 10.1210/en.2015-1628 [DOI] [PubMed] [Google Scholar]
- 18. Poggioli T, Vujic A, Yang P, et al. . Circulating growth differentiation factor 11/8 levels decline with age. Circ Res. 2016;118:29–37. doi: 10.1161/CIRCRESAHA.115.307521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Zeng F, Mitchell AD, Kim YS, Wu Z, Yang J. Transgenic overexpression of bone morphogenetic protein 11 propeptide in skeleton enhances bone formation. Biochem Biophys Res Commun. 2011;416:289–292. doi: 10.1016/j.bbrc.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneyer AL, Sidis Y, Gulati A, Sun JL, Keutmann H, Krasney PA. Differential antagonism of activin, myostatin and growth and differentiation factor 11 by wild-type and mutant follistatin. Endocrinology. 2008;149:4589–4595. doi: 10.1210/en.2008-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill JJ, Davies MV, Pearson AA, et al. . The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200 [DOI] [PubMed] [Google Scholar]
- 22. Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366 [DOI] [PubMed] [Google Scholar]
- 23. Robertson RD, Mukherjee A. Synexpression group analyses identify new functions of FSTL3, a TGFβ ligand inhibitor. Biochem Biophys Res Commun. 2012;427:568–573. doi: 10.1016/j.bbrc.2012.09.098 [DOI] [PubMed] [Google Scholar]
- 24. Kondás K, Szláma G, Trexler M, Patthy L. Both WFIKKN1 and WFIKKN2 have high affinity for growth and differentiation factors 8 and 11. J Biol Chem. 2008;283:23677–23684. doi: 10.1074/jbc.M803025200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergen HR III, Farr JN, Vanderboom PM, et al. . Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle. 2015;5:21. doi: 10.1186/s13395-015-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palandra J, Quazi A, Fitz L, Rong H, Morris C, Neubert H. Quantitative measurements of GDF-8 using immunoaffinity LC-MS/MS. Proteomics Clin Appl. 2016;10:597–604. doi: 10.1002/prca.201500112 [DOI] [PubMed] [Google Scholar]
- 27. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. . Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215. doi: 10.1016/j.cmet.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Semba RD, Zhang P, Zhu M, et al. . A targeted proteomic assay for the measurement of plasma proteoforms related to human aging phenotypes. Proteomics. 2017;17. doi: 10.1002/pmic.201600232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shock NW, Greulich RC, Andres RA, et al. . Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Government Printing Office; 1984. [Google Scholar]
- 30. Zhu M, Zhang P, Geng-Spyropoulos M, Moaddel R, Semba RD, Ferrucci L. A robotic protocol for high-throughput processing of samples for selected reaction monitoring assays. Proteomics. 2017;17. doi: 10.1002/pmic.201600339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quantikine ELISA. GDF-8/Myostatin Immunoassay. Minneapolis, MN: R&D Systems; 2016. 14pp. https://resources.rndsystems.com/pdfs/datasheets/dgdf80.pdf. Accessed February 7, 2017. [Google Scholar]
- 32. Walker RG, Angerman EB, Kattamuri C, Lee YS, Lee SJ, Thompson TB. Alternative binding modes identified for growth and differentiation factor-associated serum protein (GASP) family antagonism of myostatin. J Biol Chem. 2015;290:7506–7516. doi: 10.1074/jbc.M114.624130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratkevicius A, Joyson A, Selmer I, et al. . Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol A Biol Sci Med Sci. 2011;66:620–626. doi: 10.1093/gerona/glr025 [DOI] [PubMed] [Google Scholar]
- 34. Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147:3586–3597. doi: 10.1210/en.2006-0089 [DOI] [PubMed] [Google Scholar]
- 35. Szláma G, Trexler M, Patthy L. Latent myostatin has significant activity and this activity is controlled more efficiently by WFIKKN1 than by WFIKKN2. FEBS J. 2013;280:3822–3839. doi: 10.1111/febs.12377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.