Abstract
Preeclampsia is a pregnancy-specific condition manifested by new-onset maternal hypertension with systemic inflammation, including increased innate immune system complement activation. While exact pathophysiology is unknown, evidence suggests that inadequate spiral artery invasion and resulting utero-placental insufficiency is the initiating event. Cigarette smoking during pregnancy decreases the risk of preeclampsia. Nicotine, a major component of cigarettes, stimulates the efferent cholinergic anti-inflammatory pathway through peripherally expressed nicotinic acetylcholine receptors (nAChR) and is known to attenuate ischemia reperfusion injury in kidney and liver. Prior studies indicated that complement activation was critical for placental ischemia-induced hypertension in a rat model. Thus, it was hypothesized here that nicotine was responsible for the protective effect of cigarette smoking in preeclampsia and would attenuate placental ischemia-induced hypertension and systemic complement activation. The reduced utero-placental perfusion pressure (RUPP) model in the pregnant rat was employed to induce placental ischemia, resulting in complement activation, fetal resorptions, and hypertension. On gestation day (GD)-14, nicotine (1 mg/kg) or saline was administered via subcutaneous injection prior to RUPP surgery and daily through GD18. On GD19, placental ischemia significantly increased mean arterial pressure (MAP) in saline injected animals. However, the placental ischemia-induced increase in blood pressure was not evident in nicotine treated animals and nicotine treatment significantly increased MAP variability. Circulating C3a was measured as an indicator of complement activation and increased C3a in RUPP compared to Sham persisted with nicotine treatment, as did fetal resorptions. These data suggested to us that nicotine may contribute to the decreased risk of preeclampsia with cigarette smoking, but this protective effect was confounded by additional effects of nicotine on the cardiovascular system.
Keywords: Preeclampsia, hypertension, nicotine, complement, placental ischemia, smoking, gestational hypertension
Introduction
Hypertensive disorders in pregnancy affect ≈ 10% of pregnancies (Abaolos et al. 2013). As a subset of such pregnancy-specific disorders, preeclampsia is the leading cause of maternal and perinatal mortality and morbidity worldwide, affecting up to 4% of pregnancies (Ananth et al. 2013). Preeclampsia is chiefly characterized by new-onset maternal hypertension and proteinuria, though recent ACOG criteria recognize preeclampsia in the absence of proteinuria if systemic symptoms include thrombocytopenia, impaired liver, development of renal insuffi-ciency, pulmonary edema, or cerebral or visual disturbances (ACOG 2013; Ananth et al. 2013). Preeclampsia also increases the risk of subsequent hypertension in the mother and offspring (Alsnes et al. 2017; Behrens et al. 2017; Best et al. 2017). Although the precise mechanisms involved are incompletely understood, the predominant theory posits the initiating event of inadequate spiral artery invasion limiting utero-placental blood flow, reducing placental perfusion in the first half of pregnancy and resulting in symptoms of preeclampsia during the second half of pregnancy (Fisher 2015). In response to the ischemic event, systemic inflamma-tion and angiogenic imbalance occur, along with dysregulation of innate and adaptive immunity including the complement system (Lynch and Salmon 2010). Aside from placental delivery, effective treatments remain elusive.
Smoking during pregnancy decreases the risk of preeclampsia by up to 50% (England and Zhang 2007). Nicotine, a major constituent of cigarette smoke, is anti-inflammatory and causes immunosuppression through nicotinic acetylcholine receptors (nAChR) (Yang et al. 2014). These ligand-gated receptors are expressed by peripheral cells and activate the cholinergic anti-inflammatory pathway, an efferent neural network mediated by the vagus nerve, to modulate innate immune response (Tracey 2002; Bertrand et al. 2015). Stimulation of the α7-nAChR subunit alters cytokine synthesis, most notably macrophage secretion of tumor necrosis factor (TNF)-α (Wang et al. 2003). In normal pregnancy and placental development, endogenous acetylcholine binds nAChR to supplement placental vessel and syncytio-trophoblast develop-ment by regulating nutrient uptake, blood flow, and vascularization (Arias 2000; Lips et al. 2005). In the preeclamptic placenta α7-nAChR protein expression is increased at term (Kwon et al. 2007; Machaalani et al. 2015), potentially a response to hypoxia similar to that seen in endothelial and neuronal cells in vitro (Kwon et al. 2007). Also, acetylcholine levels in preeclamptic placenta are increased with defective acetylcholine release observed, possibly resulting in a compensatory increase in α7-nAChR (Kwon et al. 2007; Machaalani et al. 2015).
Nicotine activates the anti-inflammatory reflex through α7-nAChR binding and subsequent inhibition of pro-inflammatory cytokine production. Pretreatment with nicotine ameliorates renal and liver ischemia-reperfusion injury by suppressing cytokine-mediated peripheral inflammation (Yeboah et al. 2008; Park et al. 2013. The α7-nAChR-specific antago-nist methyllycaconitine prevents nicotinic suppression of cytokines in liver, further demonstrat-ing the potential utility of targeting α7-nAChR in ischemia reperfusion injury (Park et al. 2013).
The pro-inflammatory cytokine interleukin (IL)-6 has been implicated in placental ischemia-induced hypertension in the rat (Gadonski et al. 2006). In vitro studies in endothelial cells demonstrated that serum from pregnant women at term increases IL-6 production. In addition, anti angiogenic factors sFlt1 and sEng damage endothelial cells, mimicking endothelial dysfunction in preeclampsia. Nicotine inhibited IL-6 production and suppressed NF-κB activa-tion in vitro, demonstrating anti-inflammatory effects and providing a potential explanation for the protective effects of nicotine in preeclampsia (Mimura et al. 2010; Sharentuya et al. 2010). These in vitro data suggest that investigating the effect of nicotine in vivo in placental ischemia-induced hypertension is warranted.
The complement system is an innate immune amplification system with increased activation in preeclampsia compared to normal pregnancy (Lynch and Salmon 2010). The therapeutic utility of targeting the complement system to treat preeclampsia is supported by numerous studies demonstrating increased complement activation in preeclampsia compared to normal pregnancy, as well as by a case study demonstrating that treatment of severe preeclampsia with an antibody to complement component C5 prolonged the pregnancy by more than two weeks (Burwick and Feinberg 2013). In addition, our own research demonstrated that inhibition of complement activation following placental ischemia in the pregnant rat attenuates the subsequent hypertension (Lillegard et al. 2013). Thus, it was hypothesized here that the anti-inflammatory effects of nicotine will suppress complement activation and attenuate placental ischemia-induced hypertension. The established Reduced Utero-placental Perfusion Pressure (RUPP) model in the rat was employed to investigate effects of nicotine on placental ischemia-induced hypertension and to determine if the reduced risk of preeclampsia in smokers may be related to anti-inflammatory effects of nicotine.
Materials and methods
Reduced utero-placental perfusion pressure (RUPP) procedure
The reduced utero-placental perfusion pressure (RUPP) model was used to induce placental ischemia and stimulate high blood pressure in pregnancy as previously described (Crews et al. 2000; Alexander et al. 2001; Granger et al. 2006; Gilbert et al. 2007). Surgeries were performed under isoflurane anesthesia with pregnant Sprague Dawley dams (Charles River, Raleigh, NC) on gestation day (GD)-14 of the 21-day gestation period. A ventral midline incision was made and a sterile silver clip (0.203 mm inner diameter) placed around the aorta, just above the iliac bifurcation to reduce uterine perfusion by ≈ 40%. Precaution was taken to avoid damaging adjacent nerves. A silver clip (0.100 mm inner diameter) was placed at the ovarian ends of right and left uterine horns to reduce compensatory placental blood flow. Sham surgery differed only in the placement of clips and was used in control animals. On GD18, the left carotid artery was cannulated [under isoflurane anesthesia] and cannula patency was sustained with a 25% dextrose lock solution in sterile pyrogen-free saline. On GD19, mean arterial pressure (MAP) was measured in unanesthetized restrained animals via the carotid cannula for 2 hr prior to tissue collection as we previously described (Lillegard et al. 2013).
Following blood pressure measurements, animals were anesthetized with isoflurane, bled via the abdominal aorta for collection of serum and EDTA plasma, fetal and placental weights recorded and tissues collected and frozen in liquid nitrogen. Second to third order mesenteric arteries were also isolated at time of tissue collection and endothelial dependent (acetylcholine) and independent (sodium nitroprusside) relaxation assessed in arteries pre-contracted with the thromboxane mimetic U46619 as we previously described (Lillegard et al. 2014). Briefly, mesenteric arteries were mounted in DMT system baths (Model 610M, Danish Myo Technology, Aarhus, Denmark), normalized to a transmural pressure of 100 mm Hg, and the active contraction over baseline to 5.7 × 10−7 M U46619 determined. Fractional relaxation to half log increments of acetylcholine were then assessed. After washing, contraction with U46619 was repeated, followed by determination of fractional relaxation to sodium nitroprusside.
Nicotine administration
Nicotine (1 mg nicotine base/kg/day) or saline vehicle was administered daily via subcu-taneous injection beginning 0.5–3.0 hr prior to RUPP/Sham surgery, with the last dose delivered on GD18. This dose was chosen to mimic nicotine concentrations resulting from smoking 1–2 packs of cigarettes per day and has been used in studies of rat pregnancy (Hudson and Timiras 1972; Yang et al. 2014a,b). Nicotine ditartrate dihydrate (98%, Acros Organics, Morris Plains, NJ) was dissolved in normal saline solution to obtain a 3 mg/ml concentration of the salt, and the was pH adjusted to 7.5 with NaOH. Rats were randomly assigned to one of four experimental groups based on surgical procedure and drug treatment: (1) Sham surgery with normal saline vehicle (Sham Vehicle, n = 7); (2) RUPP surgery with normal saline vehicle (RUPP Vehicle, n = 7); (3) RUPP surgery with nicotine (RUPP Nicotine, n = 9); or, (4) Sham surgery with nicotine (Sham Nicotine, n = 8).
Complement measurement (C3a)
Complement activation product, C3a, was measured by Western immunoblot as previously described in Regal and Klos (2000), with modifications. NuPAGE Novex 10% Bis-Tris gels with MES SDS Running Buffer and secondary antibody IRDye 800CW Goat anti-rabbit IgG (H+L) diluted to 1:10,000, were used and imaged with LiCor Odyssey Fc. A standard pool of rat serum activated by yeast was used as a C3a standard and diluted appropri-ately to construct a 5-point standard curve on each gel. Based on 1 μL standard serum, relative amounts of C3a (expressed C3a units/μl) in samples were calculated by linear regression.
Statistical analysis
Data are expressed as mean ± SE of the mean and differences were defined as significant at p-values < 0.05. Likelihood ratio tests were used to determine if variances were equal. Two-way analysis of variance (ANOVA) was performed either assuming or not assuming equal variances as appropriate using JMP and SAS software (SAS Institute, Cary, NC). Data were log transformed to stabilize variances as needed. Four contrasts were evaluated for comparison of means: Sham Vehicle vs. RUPP Vehicle, RUPP Vehicle vs. RUPP Nicotine, Sham Vehicle vs. Sham Nicotine, and Sham Nicotine vs. RUPP Nicotine.
Results
Nicotine effect on placental ischemia-induced changes in MAP and mesenteric artery function
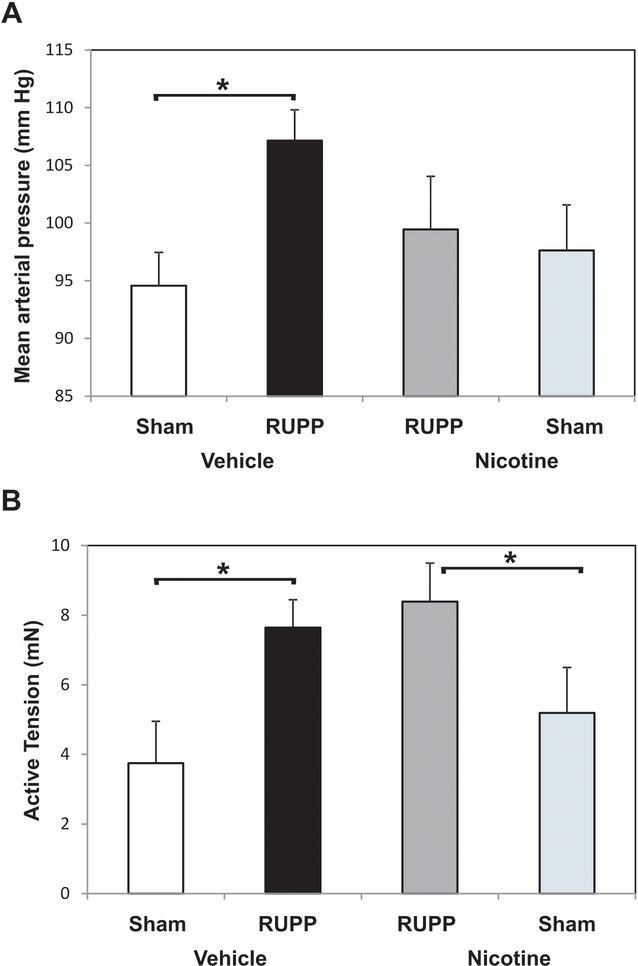
The variance of the MAP measurement in nicotine treated animals was statistically greater as determined by likelihood ratio test and thus, means were compared assuming a nicotine effect on variance. RUPP surgery significantly increased MAP in RUPP Vehicle vs. Sham Vehicle groups as we have previously reported (Lillegard et al. 2013, 2014; Regal 2015, 2016) (Figure 1A). Nicotine treatment (1 mg/kg/day) did not significantly reduce MAP follow-ing RUPP surgery when comparing RUPP Vehicle to RUPP Nicotine by a least-squares mean contrast in a two-way ANOVA. However, if one compares RUPP Nicotine to Sham Nicotine groups, the increase in blood pressure was not significant, indicating nicotine prevented placental ischemia-induced increases in blood pressure.
Figure 1.
Nicotine effect on MAP and contractile response of mesenteric arteries following placental ischemia. RUPP or Sham animals were treated with nicotine or saline vehicle GD14–18 and MAP and contractile responses determined on GD19. (A) MAP increased in RUPP Vehicle (n= 7) compared to Sham Vehicle (n=7), but RUPP Vehicle was not significantly greater than RUPP animals treated with 1 mg nicotine/kg/day (RUPP Nicotine, n = 9). RUPP Nicotine was not significantly different from Sham Nicotine (n = 8) treated animals. *p < 0.05. (B) RUPP surgery significantly increased the active tension generated in response to 0.57 μM U46619 comparing RUPP Vehicle (n = 6) to Sham Vehicle (n = 6). In nicotine-treated animals, the contractile response to U46 was significantly higher in RUPP (n = 8) vs. Sham (n = 5). *p < 0.05.
Previous studies in the RUPP model indicate that placental ischemia modestly attenuates acetylcholine-induced relaxation of mesenteric arteries (Lillegard et al. 2014). However, this endothelial dysfunction was not apparent in the aorta of RUPP animals (Lillegard et al. 2013). In the present study, acetylcholine-induced relaxation of mesenteric arteries was not significantly altered by placental ischemia (data not shown). However, placental ischemia resulted in a significantly greater increase in contractile response to 0.57 μM U46619 than in Sham animals (Figure 1B). In nicotine-treated animals, the contractile response to U46619 was significantly higher in the RUPP animals compared to in the Shams.
Effect of nicotine on fetal resorptions and pup weight
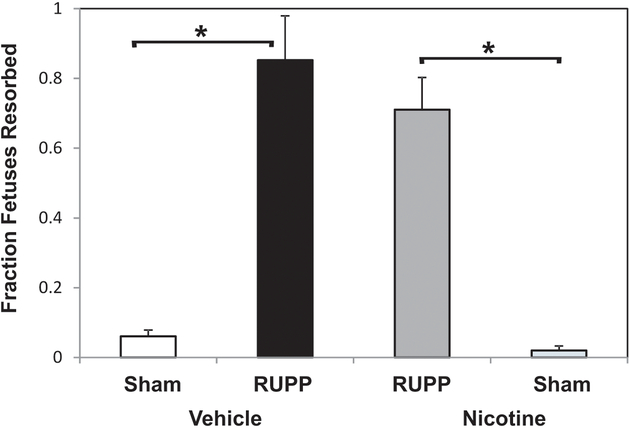
Placental insufficiency following RUPP surgery leads to fetal growth restriction and resorption in the rat (Lillegard et al. 2013,. 2014; Regal et al. 2015, 2016). The fraction of pups resorbed was significantly increased in the RUPP Vehicle vs. Sham Vehicle groups (Figure 2). Nicotine treatment did not affect RUPP-induced resorptions and the comparison of RUPP Nicotine to Sham Nicotine was significantly different (p < 0.05). In general, the RUPP procedure resulted in a reduction in fetal weight. In this study, average fetal weights tended to decrease in RUPP vs. Sham (2.21 ± 0.19 [RUPP Vehicle] vs. 2.27 ± 0.06 g [Sham Vehicle]), but did not reach statistical significance. An ANOVA approach indicated a significant effect of nicotine overall (p < 0.05) on fetal weight (RUPP Nicotine, 1.97 ± 0.06 g; Sham Nicotine, 2.17 ± 0.04) (data not shown). Placental weights were not significantly affected by the RUPP procedure or nicotine in this cohort.
Figure 2.
Nicotine does not alter the fraction of fetuses resorbed. In vehicle-treated animals, RUPP surgery (n = 7) increased pup resorptions compared to Sham (n = 7). This effect persisted with nicotine treatment in RUPP (n = 9) and Sham (n = 8). *p < 0.05.
Nicotine does not affect placental ischemia-induced complement activation
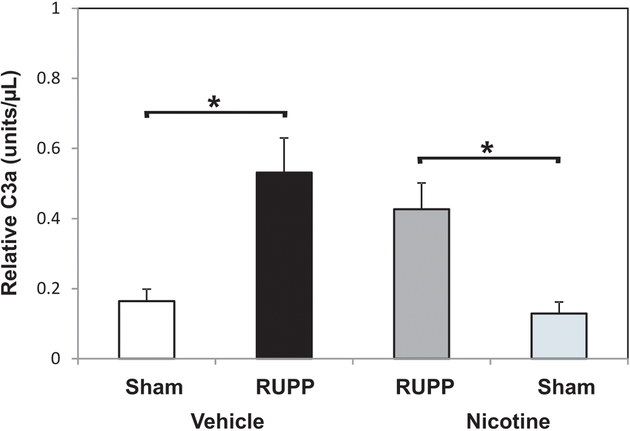
Excessive complement activation occurs after placental ischemia and was used as an indicator of inflammation. Complement component C3a was quantified to measure relative complement activation. The data show that nicotine treatment did not suppress the placental ischemia-induced increase in complement activation following RUPP surgery (Figure 3).
Figure 3.
Nicotine does not affect the placental ischemia-induced increase in circulating C3a. C3a serum concentration is increased in RUPP Vehicle (n = 7) vs. Sham Vehicle (n = 7). Treatment with 1 mg nicotine/kg/day over a 5-day period did not attenuate the RUPP (n = 8) increase in C3a compared to Sham (n = 8). *p < 0.05.
Changes in neutrophil levels are considered another indicator of inflammation. Previous studies had examined the importance of neutrophils in placental ischemia-induced hypertension and found that placental ischemia itself did not change the number of circulating neutrophils, but neutrophil depletion attenuated placental ischemia-induced hypertension (Regal et al. 2015). Here, nicotine treatment did not significantly alter neutrophil levels in the circulation (data not shown).
Discussion
The present studies demonstrate placental ischemia does not significantly increase blood pressure in the pregnant rat following nicotine treatment during mid to late gestation. The physiological significance of this observation is tempered by increased variance of the blood pressure measurement following nicotine treatment relative to the magnitude of increased blood pressure in the RUPP model, and the lack of a significant difference between RUPP animals treated with nicotine vs. saline. Potential use of nicotine to reduce the risk of preeclampsia may require careful targeting of the α7-nAChR peripherally to minimize widespread effects of nicotine on multiple nicotinic receptors and the nicotine effect on blood pressure. Thus, targeting of cholinergic anti-inflammatory pathways with α7-nAChR agonists may be an effective strategy to minimize preeclamptic symptoms prior to and following the onset of placental ischemia.
The experimental conclusions are limited since the comparison of RUPP Nicotine to RUPP Vehicle animals was not statistically significant. However, no significant increase in blood pressure was detected comparing RUPP Nicotine to Sham Nicotine. Our experimental design assumed that the variance of vehicle and nicotine treated animals would be the same and a 9% reduction in MAP (107 mm Hg → 98 mm Hg) would be detectable with 8 animals/treatment group. Clinical studies indicate that even a 5–10 mm Hg change in blood pressure can be significant (McMahon et al. 1990; Hong 2017). Nicotine treatment in RUPP animals decreased MAP to 99 and significantly increased the variance of the MAP measurement. Experiments were well controlled in that all rats received equivalent handling and injections, whether saline or nicotine.
The potentially favorable effect of nicotine on placental ischemia-induced hypertension is overshadowed by effects nicotine has on the cardiovascular system in general, and its ability to increase blood pressure itself. Nicotine can cause acute vasoconstriction (Mattila and Vartiainen 1964) and with acute and chronic nicotine injection in the rat results in increased blood pressure (Moon et al. 2014; Yagi et al. 2015). The increase in blood pressure is attributed to the ability of nicotine to activate nAchR on peripheral postganglionic sympathetic nerve endings and release of catecholamines from the adrenal medulla (Haass 1996). Nicotine is also implicated in increased blood pressure in humans (Cooke et al. 2015). In the current study, 1 mg nico-tine/kg/day did not significantly increase blood pressure, but clearly increased variance of the blood pressure measurement compared to vehicle treated. Even at 1 mg/kg/day, as others have reported (Becker et al. 1968), behavioral effects were evident shortly after nicotine injections. Blood pressure was measured 18–24 hr after the last nicotine treatment and behavioral effects were not evident at that time. Measurement of blood pressure in the current study was done in unanesthetized restrained animals through a carotid cannula. Care was taken to minimize disturbance of the animals and measurements always occurred in a quiet room between 6:30 and 9:00 AM for all groups. However, central effects of nicotine may accentuate excitability of the animals and the effects of external noise during that time.
Nicotine is clearly not the only component of cigarette smoke that may be responsible for the protective effect of smoking in preeclampsia. Smokeless tobacco containing nicotine is not protective for preeclampsia (England et al. 2003), suggesting that other combustible products of cigarette smoke may be important. One such component that has been considered is carbon monoxide (CO). End-tidal CO concentrations are decreased in women with preeclampsia, suggesting the inverse association between cigarette smoke and onset of the disease may be due to CO (Kreiser et al. 2004; England L and Zhang 2007). CO exhibits similar physiological properties to the potent vasodilator, nitric oxide (NO), including decrease in vascular tone and inhibition of inflammatory cascades (Otterbein et al. 2000; Zhang et al. 2001; Fujita et al. 2010). A recent study demonstrates that daily administration of the CO donor molecule, CORM-3, alleviates placental ischemia-induced hypertension in the rat through a mechanism independent of angiogenic pathways (George et al. 2017). Treatment with the CO donor molecule did not increase the variability of the blood pressure response to placental ischemia, suggesting that this is a more viable approach for affecting placental ischemia-induced hypertension than nicotine. However, the favorable effect of CO donor molecules on blood pressure does not improve fetal outcomes, similarly to nicotine. Nicotine has been demonstrated to suppress inflammation by inducing heme oxygenase-1 (HO-1), which degrades heme into biliverdin, ferrous iron, and CO (Ryter et al. 2006; Tsoyi et al. 2011; Park et al. 2013). Thus, for nicotine, some of the anti-inflammatory effects or favorable effects on placental ischemia-induced hypertension may be due to CO production.
Numerous mediators and systems have been implicated in the pathophysiology of preeclampsia; various animal models initiate pregnancy-induced hypertension with administra-tion of single mediators such as sFlt1, TNFα, or autoantibodies to the angiotensin receptor (LaMarca et al. 2016; Sones and Davisson 2016). However, the RUPP model does not presume involvement of a single mediator in preeclampsia and relies on inducing ischemia as the initiating event, with subsequent inflammation and angiogenic imbalance. RUPP is a well-established model of pregnancy-induced hypertension that does not incite characteristics of severe preeclampsia (Balta et al. 2011; Li et al. 2012). As with many other models of preeclampsia, the RUPP model does not address the origins of the placental defects that cause the ischemia, but models the response to ischemia in the second half of pregnancy.
The dose of nicotine employed was chosen after reviewing studies of others in the pregnant rat, as well as considering whether the dose was similar to human exposure. A dose of 1 mg nicotine/kg/day was chosen to mimic smoking 1–2 packs of cigarettes/day and has been established as a dose that does not cause developmental complications in pregnancy in the rat (Becker et al. 1968; Hudson and Timiras 1972). Nicotine administration via subcutaneous injection is minimally invasive and was chosen as a way to deliver daily doses of nicotine with minimal animal handling (Ali et al. 2015). The data here suggested to us that nicotine administration directly before and following placental ischemia did not affect complement activation or fetal resorptions, but prevented placental ischemia from increasing blood pressure. The favorable effect on blood pressure does not result in favorable fetal outcomes as evident by the significant negative effect of nicotine on fetal weight. Smoking is not protective for fetal outcomes and increases risk of preterm delivery and fetal growth restriction (England et al. 2003; Wong et al. 2015).
Complement activation and systemic inflammation are implicated in preeclampsia and we previously demonstrated that complement inhibition in the RUPP model attenuates associated hypertension, suggesting inhibition of complement activation may be a viable therapeutic strategy (Lillegard et al. 2013). The present data indicated that placental ischemia-induced hypertension did not occur in nicotine-treated animals even when an increase in C3a was still evident. These data suggested to us that the effects of nicotine on MAP are via a complement-independent mechanism.
Acute activation of the cholinergic anti-inflammatory pathway by cholinergic agonists and vagus nerve stimulation attenuates ischemia reperfusion-induced damage in the liver, kidney, and aorta (Bernik et al. 2002; Yeboah et al. 2008; Park et al. 2013). The α7 subunit is an essential component of the cholinergic anti-inflammatory mechanism responsible for these therapeutic effects (Wang et al. 2003; Yeboah et al. 2008; Park et al. 2013). When one considers ischemia in the placenta and hypertension as the endpoint, the effects of nicotine are more difficult to determine since nicotine itself impacts blood pressure and may acutely contribute to constriction of arteries. Evidence suggests that chronic nicotine treatment expedites the onset of hypertension in genetically pre-disposed rats and high doses (6 mg nicotine/kg/day) blunt maternal systemic and renal adaptation to pregnancy (Ferreira et al. 2016). Nicotine has also been implicated in the development of hypertension by affecting macrophage infiltration into the kidney (Harwani et al. 2016). As a vasoconstrictor and stimulator of sympathetic neurotransmis-sion, nicotine increases blood pressure acutely. The competing effects of nicotine as an anti-inflammatory and vasoconstrictor may obscure a robust and clear effect in eliminating hypertension in placental ischemia.
Conclusions
Placental ischemia is a consistent feature of preeclampsia leading to excess complement activation and hypertension. This study is the first to investigate the therapeutic potential of nicotine administration in a chronic placental ischemia model in the rat. We report that nicotine does not ameliorate complement and inflammatory cascades following placental ischemia, nor improve fetal outcomes, but it does mitigate the hypertensive response to placental ischemia.
Acknowledgements
The authors gratefully acknowledge Dr Ronald Regal, Department of Mathematics and Statistics, University of Minnesota Duluth for statistical consultation as well as Dr Lyle G Best for valuable discussions regarding the decreased risk of preeclampsia with smoking.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Abalos E, Cuesta C, Grosso A, Chou D, Say L. 2013. Global and regional estimates of pree-clampsia and eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol 170:1–7. [DOI] [PubMed] [Google Scholar]
- ACOG (American College of Obstetricians and Gynecologists). 2013. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy [Consensus Development Conference]. Obstet. Gynecol 122:1122–1131. [DOI] [PubMed] [Google Scholar]
- Alexander B, Kassab S, Miller M, Abram S, Reckelhoff J, Bennett W, Granger J. 2001. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide [Research Support, Non-U.S. Government Research Support, U.S. Gov’t, P.H.S.]. Hypertension 37:1191–1195. [DOI] [PubMed] [Google Scholar]
- Ali S, Hamed E, Ayuob N, Shaker A, Suliman M. 2015. Effects of different routes of nicotine administration on gastric morphology and hormonal secretion in rats. Exp. Physiol 100:881–895. [DOI] [PubMed] [Google Scholar]
- Alsnes I, Vatten L, Fraser A, Bjorngaard J, Rich-Edwards J, Romundstad P, Asvold B. 2017. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: Prospective and sibling studies in the HUNT Study (Nord-Trondelag Health Study) in Norway. Hypertension 69:591–598. [DOI] [PubMed] [Google Scholar]
- Ananth C, Keyes K, Wapner R. 2013. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. Br. Med. J 347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias H 2000. Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors. Neurochem. Intl 36:595–645. [DOI] [PubMed] [Google Scholar]
- Balta O, Boztosun A, Deveci K, Gulturk S, Ekici F, Kaya A, Cetin A, Cetin M. 2011. Reduced uterine perfusion pressure model is not successful to mimic severe preeclampsia. Placenta 32:675–680. [DOI] [PubMed] [Google Scholar]
- Becker R, Little C, King J. 1968. Experimental studies on nicotine absorption in rats during pregnancy. Effect of subcutaneous injection of small chronic doses upon mother, fetus, and neonate. Am. J. Obstet. Gynecol 100:957–968. [DOI] [PubMed] [Google Scholar]
- Behrens I, Basit S, Melbye M, Lykke J, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd H. 2017. Risk of post-pregnancy hypertension in women with a history of hypertensive dis-orders of pregnancy: nationwide cohort study. Br. Med. J 358:j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernik T, Friedman S, Ochani M, DiRaimo R, Susarla S, Czura C, Tracey K. 2002. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg 36:1231–1236. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Lee C, Flood D, Marger F, Donnelly-Roberts D. 2015. Therapeutic potential of α7 nicotinic acetylcholine receptors. Pharmacol. Rev 67:1025–1073. [DOI] [PubMed] [Google Scholar]
- Best L, Lunday L, Webster E, Falcon G, Beal J. 2017. Pre-eclampsia and risk of subsequent hypertension in an American Indian population. Hypertension Pregnancy 36:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwick R, and Feinberg B. 2013. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 34:201–203. [DOI] [PubMed] [Google Scholar]
- Cooke W, Pokhrel A, Dowling C, Fogt D, Rickards C. 2015. Acute inhalation of vaporized nicotine increases arterial pressure in young non-smokers: a pilot study. Clin. Auton. Res 25:267–270. [DOI] [PubMed] [Google Scholar]
- Crews JK, Herrington JN, Granger JP, Khalil RA. 2000. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35:367–372. [DOI] [PubMed] [Google Scholar]
- England L, and Zhang J. 2007. Smoking and risk of preeclampsia: A systematic review. Front. Biosci 12:2471–2483. [DOI] [PubMed] [Google Scholar]
- England L, Levine R, Mills J, Klebanoff M, Yu K, Cnattingius S. 2003. Adverse pregnancy outcomes in snuff users. Am. J. Obstet. Gynecol 189:939–943. [DOI] [PubMed] [Google Scholar]
- Ferreira V, Passos C, Maquigussa E, Pontes R, Bergamaschi C, Campos R, Boim M. 2016. Chronic nicotine exposure abolishes maternal systemic and renal adaptations to pregnancy in rats. PloS One 11:e0150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S 2015. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol 213:S115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D, Tanabe A, Sekijima T, Soen H, Narahara K, Yamashita Y, Terai Y, Kamegai H, Ohmichi M. 2010. Role of extracellular signal-regulated kinase and AKT cascades in regulat-ing hypoxia-induced angiogenic factors produced by a trophoblast-derived cell line. J. Endocrinol 206:131–140. [DOI] [PubMed] [Google Scholar]
- Gadonski G, LaMarca B, Sullivan E, Bennett W, Chandler D, Granger J. 2006. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of IL-6. Hypertension 48:711–716. [DOI] [PubMed] [Google Scholar]
- George E, Cockrell K, Arany M, Stec D, Rimoldi J, Gadepalli R, Granger J. 2017. Carbon monoxide releasing molecules blunt placental ischemia-induced hypertension. Am. J. Hyperten (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger J. 2007. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am. J. Hyperten 20:686–691. [DOI] [PubMed] [Google Scholar]
- Granger J, LaMarca B, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. 2006. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Meth. Mol. Med 122:383–392. [DOI] [PubMed] [Google Scholar]
- Haass M, and Kubler W. 1996. Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther 10:657–665. [DOI] [PubMed] [Google Scholar]
- Harwani S, Ratcliff J, Sutterwala F, Ballas Z, Meyerholz D, Chapleau M, Abboud F. 2016. Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circul. Res 119:1101–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K 2017. Blood pressure management for stroke prevention and in acute stroke. J. Stroke 19:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D, and Timiras P. 1972. Nicotine injection during gestation: Impairment of reproduction, fetal viability, and development. Biol. Reprod 7:247–253. [DOI] [PubMed] [Google Scholar]
- Kreiser D, Baum M, Seidman D, Fanaroff A, Shah D, Hendler I, Stevenson D, Schiff E, Druzin M. 2004. End-tidal carbon monoxide levels are lower in women with gestational hyper-tension and pre-eclampsia. J. Perinatol 24:213–217. [DOI] [PubMed] [Google Scholar]
- Kwon J, Kim Y, Kim S, Kang M, Maeng Y, Lee K, Park Y. 2007. Difference in the expression of α7 nicotinic receptors in the placenta in normal versus severe preeclampsia pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol 132:35–39. [DOI] [PubMed] [Google Scholar]
- LaMarca B, Cornelius D, Harmon A, Amaral L, Cunningham M, Faulkner J, Wallace K. 2016. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am. J. Physiol 311:R1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, LaMarca B, Reckelhoff J. 2012. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol 303:H1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard K, Johnson A, Lojovich S, Bauer A, Marsh H, Gilbert J, Regal J. 2013. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol. Immunol 56:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard K, Loeks-Johnson A, Opacich J, Peterson J, Bauer A, Elmquist B, Regal R, Gilbert J, Regal J. 2014. Differential effects of complement activation products C3a and C5a on cardiovascular function in hypertensive pregnant rats. J. Pharmacol. Exp. Ther 351:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips K, Bruggmann D, Pfeil U, Vollerthun R, Grando S, Kummer W. 2005. Nicotinic acetylcholine receptors in rat and human placenta. Placenta 26:735–746. [DOI] [PubMed] [Google Scholar]
- Lynch A, and Salmon J. 2010. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 31:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaalani R, Ghazavi E, David RV, Hinton T, Makris A, Hennessy A. 2015. Nicotinic acetylcholine receptors (nAChR) are increased in the pre-eclamptic placenta. Hypertension Pregnancy 34:227–240. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. 1990. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335:765–774. [DOI] [PubMed] [Google Scholar]
- Mattila M, and Vartiainen A. 1964. The vasoconstrictor action of some nornicotine derivatives. Ann. Med. Exp. Biol. Fenn 42:27–32. [PubMed] [Google Scholar]
- Mimura K, Tomimatsu T, Sharentuya N, Tskitishvili E, Kinugasa-Taniguchi Y, Kanagawa T, Kimura T. 2010. Nicotine restores endothelial dysfunction caused by excess sFlt1 and sEng in an in vitro model of preeclamptic vascular endothelium: A possible therapeutic role of nicotinic acetylcholine receptor (nAChR) agonists for preeclampsia. Am. J. Obstet. Gynecol 202:464–466. [DOI] [PubMed] [Google Scholar]
- Moon H, Kang P, Lee H, Min S, Seol G. 2014. Effects of 1,8-cineole on hypertension induced by chronic exposure to nicotine in rats. J. Pharm. Pharmacol 66:688–693. [DOI] [PubMed] [Google Scholar]
- Otterbein L, Bach F, Alam J, Soares M, Tao Lu H, Wysk M, Davis R, Flavell R, Choi AM. 2000. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med 6:422–428. [DOI] [PubMed] [Google Scholar]
- Park J, Kang J, Lee S. 2013. Activation of the cholinergic anti-inflammatory pathway by nicotine attenuates hepatic ischemia/reperfusion injury via heme oxygenase-1 induction. Eur. J. Pharmacol 707:61–70. [DOI] [PubMed] [Google Scholar]
- Regal J, and Klos A. 2000. Minor role of the C3a receptor in systemic anaphylaxis in the guinea pig. Immunopharmacology 46:15–28. [DOI] [PubMed] [Google Scholar]
- Regal J, Lillegard K, Bauer A, Elmquist B, Loeks-Johnson A, Gilbert J. 2015. Neutrophil depletion attenuates placental ischemia-induced hypertension in the rat. PloS One 10:e0132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal J, Strehlke M, Peterson J, Wing C, Parker J, Nieto N, Bemis L, Gilbert J, Fleming S. 2016. Role of IgM and angiotensin II Type I receptor autoantibodies in local complement activation in placental ischemia-induced hypertension in the rat. Mol. Immunol 78:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter S, Alam J, Choi A. 2006. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev 86:583–650. [DOI] [PubMed] [Google Scholar]
- Sharentuya N, Tomimatsu T, Mimura K, Tskitishvili E, Kinugasa-Taniguchi Y, Kanagawa T, Kimura T. 2010. Nicotine suppresses IL-6 production from vascular endothelial cells: A possible therapeutic role of nicotine for preeclampsia. Reprod. Sci 17:556–563. [DOI] [PubMed] [Google Scholar]
- Sones J, and Davisson R. 2016. Preeclampsia, of mice and women. Physiol. Genomics 48:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K 2002. The inflammatory reflex. Nature 420(6917):853–859. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Jang H, Kim J, Chang H, Lee Y, Pae H, Kim H, Seo H, Lee J, Chung H, et al. 2011. Stimulation of α7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxidants Redox Signal 14:2057–2070. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella C, Tanovic M, Susarla S, Li J, Wang H, Yang H, Ulloa L, et al. 2003. Nicotinic acetylcholine receptor α7 subunit is essential regulator of inflammation. Nature 421:384–388. [DOI] [PubMed] [Google Scholar]
- Wong M, Barra N, Alfaidy N, Hardy D, Holloway A. 2015. Adverse effects of perinatal nicotine exposure on reproductive outcomes. Reproduction 150:R185–193. [DOI] [PubMed] [Google Scholar]
- Yagi S, Tanida M, Satomi J. 2015. Possible role of afferent autonomic signals in abdominal organs in anorexic and cardiovascular responses to nicotine injection in rats. Neuroreport 26:445–449. [DOI] [PubMed] [Google Scholar]
- Yang J, Shi S, Shi L, Fang D, Liu H, Garfield RE. 2014a. Nicotine, an α7 nAChR agonist, reduces lipopolysaccharide-induced inflammatory responses and protects fetuses in pregnant rats. Am. J. Obstet. Gynecol 211:531–537. [DOI] [PubMed] [Google Scholar]
- Yang J, Shi S, Shi L, Liu H, Fang D, Garfield R. 2014b. Nicotine treatment prolongs gestation and inhibits cervical ripening in pregnant rats. Am. J. Obstet. Gynecol 210:71–77. [DOI] [PubMed] [Google Scholar]
- Yeboah M, Xue X, Duan B, Ochani M, Tracey K, Susin M, Metz C. 2008. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Intl 74:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Kaide J, Rodriguez-Mulero F, Abraham N, Nasjletti A. 2001. Vasoregulatory function of the heme-heme oxygenase-carbon monoxide system. Am. J. Hyperten 14:62S–67S. [DOI] [PubMed] [Google Scholar]