Abstract
Background:
Left atrial (LA) remodeling is a predictor of cardiovascular disease (CVD). We performed measurement of the LA function index (LAFI), a composite measure of LA structure and function, in a community-based cohort and here report the distribution and cross-sectional correlates of LAFI.
Methods:
In 1,719 Framingham Offspring Study participants (54% women, mean age 66 ± 9 years), we derived LAFI from the LA emptying fraction, left ventricular (LV) outflow tract velocity time integral, and indexed maximal LA volume. We used multivariable linear regression to assess the clinical and echocardiographic correlates of LAFI adjusting for age, sex, anthropometric measurements, and CVD risk factors.
Results:
The average LAFI was 35.2 ± 12.1. Overall, LAFI declined with advancing age (β = −0.27, P < .001). LAFI was significantly higher (37.5 ± 11.6) in a subgroup of participants free of CVD and CVD risk factors compared with those with either of these conditions (34.5 ± 12.2). In multivariable models, LAFI was inversely related to antihypertensive use (β = −1.26, P = .038), prevalent atrial fibrillation (β = −4.46, P = .001), heart failure (β = −5.86, P = .008), and coronary artery disease (β = −2.01, P = .046). In models adjusting for echocardiographic variables, LAFI was directly related to LV ejection fraction (β = −14.84, P < .001) and inversely related to LV volume (β = −7.03, P < .001).
Conclusions:
LAFI was inversely associated with antihypertensive use and prevalent CVD and was related to established echocardiographic traits of LV remodeling. Our results offer normative ranges for LAFI in a white community-based sample and suggest that LAFI represents a marker of pathological atrial remodeling.
Keywords: Left atrial function, Left atrial function index, Cardiovascular diseases, Atrial fibrillation, Epidemiology, Echocardiography
Adverse left atrial (LA) remodeling is associated with increased risk of cardiovascular disease (CVD) and CVD-specific and all-cause mortality.1–4 Increased LA volume and abnormalities in phasic function are examples of echocardiographic traits that capture aspects of adverse structural and functional LA remodeling and have prognostic significance, particularly as predictors of incident or recurrent atrial fibrillation, heart failure, and cerebrovascular accident.1–4 Although LA volume is the recommended LA echocardiographic trait for clinical practice,5 its prognostic value diminishes when ventricular systolic and diastolic function are considered concomitantly.6–8
A subtle decline in LA function, as detected by impaired LA phasic function (atrial reservoir phase, passive atrial emptying, and atrial systole), is associated with incident and recurrent CVD, adjusting for ventricular function, but such measures are not routinely collected as part of a standard echocardiographic examination.3,5,9–14 Left atrial function index (LAFI) is a composite measure of LA structure and function that combines information about atrial reservoir function as well as LA size, body habitus, and left ventricular (LV) function (as measured by stroke volume).15 LAFI might characterize LA remodeling better than currently used volumetric echocardiographic measures. In select populations with CVD, a lower LAFI is associated with an increased risk of incident heart failure, cerebrovascular events, atrial fibrillation recurrence, and all-cause mortality.16–19 In this retrospective investigation, we describe the distribution of LAFI and the cross-sectional clinical and echocardiographic correlates of LAFI in a community-based sample.
MATERIALS AND METHODS
Study Sample
The design and sampling of the Framingham Offspring study were published previously.20 Briefly, starting in 1971, the children of the original Framingham Heart Study cohort were enrolled and evaluated approximately every 4–8 years. A total of 2,888 participants underwent transthoracic echocardiography with digital image acquisition during examination cycle 8 (2005–8).21 Participants who were in atrial fibrillation at the time of their echocardiographic examination, who had significant mitral regurgitation on echocardiogram, and who had inadequate atrial images were excluded. We also created a subgroup free of CVD/CVD risk factors (n = 415) within the general study sample for analysis. This included nonobese participants, free of prevalent hypertension, diabetes, atrial fibrillation, coronary heart disease, cerebrovascular accident, transient ischemic attack, or heart failure. Participants who had one or more of these conditions were included in the subgroup with CVD/CVD risk factors. Laboratory parameters and echocardiographic measures were not considered for creation of CVD/CVD risk factors subgroups. Development of the general study sample and various subgroups is depicted in Supplemental Figure 1 (available at www.onlinejase.com).
The study protocol was approved by the Boston University Medical Center Institutional Review Board, the University of California, San Francisco, School of Medicine Review Board, and the University of Massachusetts Medical School Review Board, and all participants provided written informed consent.
Left Atrial Volumetric Assessment
We performed offline analysis of echocardiographic images from 1,795 participants with LA imaging of sufficient quality to enable LA volumetric measurement from apical two and four-chamber views. Prior studies have demonstrated that the presence of atrial fibrillation rhythm at the time of echocardiogram affects LA contractile function and significantly decreases LAFI.19 We therefore excluded the participants who were in atrial fibrillation at the time of echocardiogram (n = 40). Since atrial fibrillation is strongly associated with LA remodeling, the participants with history of atrial fibrillation who were in sinus rhythm during the echocardiogram were included in the current study to assess the ability of LAFI to capture LA remodeling associated with atrial fibrillation. Participants with moderate or higher degrees of mitral regurgitation on the echocardiogram (n = 36, quantified using color Doppler by the maximum systolic proximal mitral regurgitation jet height)22 were also excluded, leading to the final sample size of 1,719. The baseline characteristics of included and excluded participants are presented in Supplemental Table 1 (available at www.onlinejase.com). Excluded participants had significantly higher body mass index (29 kg/m2 vs 28 kg/m2 for included participants; P = .001), use of antihypertensive medications (57% vs 52% in included participants; P = .002), prevalence of atrial fibrillation (10% vs 5% in included participants; P < .001), and heart failure (4% vs 2% in the included participants; P = .003). Other clinical and demographic variables were similarly distributed between the included and excluded participants.
Two sonographers performed LA volume measurement. Serial quality control iterations were performed to maximize the inter-and intraobserver correlation. During each of these iterations, the maximal and minimal LA volumes were measured for 20 randomly selected participants by both sonographers. Sonographers were trained between serial iterations, and the interobserver coefficients of variation between the sonographers measured during the final quality control iteration for maximum (LAmax) and minimum LA volume (LAmin) measurement were 2.6% and 3.8%, respectively. Intraobserver coefficients of variation, derived similarly by remeasuring the LAmax and LAmin for 20 randomly selected participants, for LAmax and LAmin measurements were 3.4% and 4.4%, respectively. We could not calculate the inter- and intraobserver variability for LAFI because none of the observers made all the measurements required for calculation of LAFI in our study (LA volumes and LVOT-VTI). It is probable that the variability in LAFI calculation might be compounded due to variability in the individual components.
After completion of the final iteration of quality control, the sonographers reviewed the quality of echocardiographic images for all the Framingham Offspring examination 8 participants and converted the saved images into a digital format. Left atrial images were deemed “inadequate” if any of the following were present: (1) endocardial borders were not well visualized, (2) posterior wall of LA was not visualized (i.e., LA foreshortening was present), or (3) recorded cardiac cycles contained a premature beat. LA volumes deemed inadequate by one sonographer were then reviewed by the other sonographer or an investigator (D.D.M.) to confirm. Outlier values (mean ± 3 * SD) were investigated and r-measured.
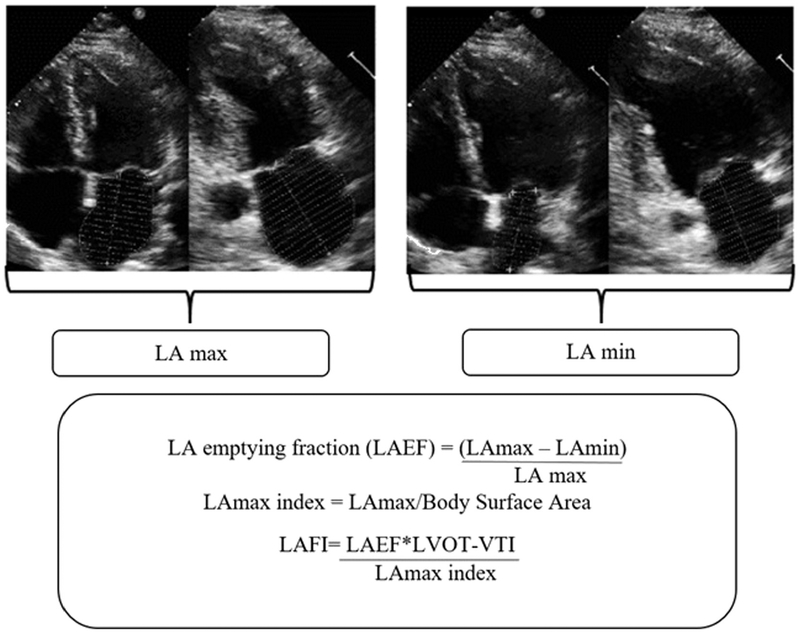
The LAmax and LAmin were obtained using the recommended Simpson’s biplane summation of disks method on a Digisonics DigiView System Software (ver. 3.7.9.3, Digisonics, Houston, TX).5 The original images were optimized for the volumetric LV and valvular assessment. The LAmax and LAmin used in this analysis were determined by averaging LAmax and LAmin measurements from the apical two- and four-chamber views. The LA emptying fraction (LAEF) was calculated as ([LAmax – LAmin]/LAmax) × 100. The LAmax index was calculated by dividing LAmax by the body surface area. The LAFI was calculated using a previously validated formula (Figure 1)15:
Figure 1.
Methodology for calculation of LAFI. The LAmax and LAmin volumes were measured by using Simpson’s biplane method (from apical four-chamber and two-chamber views). The LAEF was calculated as percentage of LA emptying with reference as LA-max. LAmax was linearly indexed to body surface area. In clinical practice, LVOT-VTI is measured by tracing the pulsed wave Doppler tracing obtained from LVOT. For our study, LVOT-VTI was derived by dividing LV stroke volume by LVOT area. LAFI was calculated by multiplying LAEF and LVOT-VTI and then dividing the product by the LAmax index.
Since LAFI is a derived measure dependent upon the measurement of LA volumes and LV outflow tract (LVOT) velocity-time integral (VTI), we could not calculate the interobserver variability for it because no two observers made all the measurements required for calculation of LAFI.
Echocardiographic Covariates and Definitions
M-mode and two-dimensional echocardiographic images were acquired using a standard protocol in parasternal and apical views as previously described.21 Leading-edge technology was used to make M-mode measurements, and all final measures were derived using the average of measurements over three or more cardiac cycles. M-mode measurements used for the current study included enddiastolic LV septal wall thickness (SWT), posterior wall thickness (PWT), LV end-diastolic diameter (LVEDD), and LV end-systolic diameter (LVESD). Left ventricular mass was calculated using a previously validated formula: 0.8 [1.04 (LVEDD + SWT + PWT)3 − (LVEDD)3] + 0.6 g.23 LV end-diastolic (LVEDV) and end-systolic volumes (LVESV) were calculated in apical two-chamber view using Simpson’s method. The LV ejection fraction (LVEF) was defined as ([LVEDV – LVESV]/LVEDV) ×100, and stroke volume was calculated as LVEDV - LVESV. The LVOT diameter was measured in parasternal long-axis view.24 LVOT pulsed wave Doppler tracings were not available for analyses, and the LVOT-VTI was derived by dividing the stroke volume by LVOT area (3.14 × (LVOT diameter/2)2). Since we excluded the participants with significant mitral regurgitation, the value of LVOT-VTI derived using stroke volume should be similar to the pulsed wave Doppler derived value.
Ascertainment of Cardiovascular Risk Factors and CVD Definitions
At each Framingham Heart Study Offspring examination, participants undergo a detailed medical history and physical examination, including measurement of systolic and diastolic blood pressure by a study physician and weight and height by technicians. Venous sampling is performed after an overnight fasting. Participants also undergo laboratory evaluation for CVD risk factors, including fasting blood glucose, serum creatinine, and blood cholesterol concentrations. Standard enzymatic methods are used for measurement of serum total cholesterol, high-density lipoprotein, and serum creatinine.25,26
Participants with systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or who were taking antihypertensive medications were categorized to have hypertension.25 Diabetes was defined as fasting plasma glucose ≥126 mg/dL and/or treatment with medications for diabetes. Criteria for defining atrial fibrillation, coronary heart disease, cerebrovascular accident, transient ischemic attack, and heart failure in the Framingham Offspring Study have been described elsewhere.20 Participants were considered to be current smokers if they reported smoking one or more cigarettes on a daily basis during the year preceding their heart study examination. Body mass index was calculated as the weight in kilograms divided by the square of height in meters. Obesity was defined as a body mass index ≥30 kg/m2.27 Estimated glomerular filtration rate was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation using contemporaneously measured serum creatinine.28
Statistical Analyses
Baseline participant characteristics are presented as numbers and percentages for categorical variables and as mean ± SD for continuous variables. Baseline participant characteristics were compared between the subgroup free of CVD/CVD risk factors and the subgroup with CVD/CVD risk factors using t-test for continuous variables and χ2 test for the categorical variables. We performed natural logarithmic transformation for variables observed to have a skewed distribution. Distribution histograms are presented to depict the variation of LAFI with age and by sex. Scatter plots for depicting the association of LAFI with LA volumes are presented. Separate histograms are presented for the subgroup free of CVD/CVD risk factors.
To assess associations of different variables with LAFI, stepwise multivariable linear regression models were used, forcing age and sex into each model. Two separate regression models were created: one model for LAFI in relation to clinical and demographic correlates and a second model for LAFI in relation to echocardiographic correlates. The clinical and demographic regression model consisted of candidate variables selected based on prior data demonstrating their associations with LA remodeling or LAFI.29 Eligible clinical and demographic variables included age, sex, current smoking status, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, diabetes mellitus, prevalent atrial fibrillation, prevalent heart failure, prevalent cerebrovascular accident and/or transient ischemic attack, prevalent coronary heart disease, estimated glomerular filtration rate less than 60 mL/min/1.73 m2, and ratio of serum total cholesterol to high-density lipoprotein cholesterol. The echocardiographic regression model, in addition to age and sex, included LVEF, LVEDV, and LV mass.18 We also tested for effect modification of LAFI with age and sex for each model.
In secondary analysis, we studied the association of LAFI with clinical and demographic factors and with echocardiographic variables among the subgroup of participants who had normal LA size based on consensus criteria (LAmax index <34 mL/m2, n = 1,293; Supplemental Figure 1, available at www.onlinejase.com).5 Two separate regression models were used, as detailed above: the clinical and demographic model and the echocardiographic model.
A P value of <.05 in two-tailed tests was considered statistically significant. All statistical analyses were performed using SAS (v9.4, SAS Institute Inc., Cary, NC) and SPSS (IBM SPSS version 24, Chicago, IL) software.
Results
The baseline demographic, clinical, and laboratory characteristics of the general study sample, subgroup free of CVD/CVD risk factors, and subgroup with CVD/CVD risk factors are presented in Table 1. Overall, the study participants were middle-aged and older adults, and 54% were women. Two-thirds of the participants had hypertension. Five percent had a history of atrial fibrillation (Table 1) but were not in atrial fibrillation at the time of the echocardiogram. The subgroup free of CVD/CVD risk factors had lower average age, and a significantly higher proportion were women compared to the subgroup with CVD/CVD risk factors. The echocardiographic characteristics of the study sample and various subgroups are presented in Table 2. The mean LAFI was 35.2 ± 12.1 for the overall sample and was significantly higher among the subgroup free of CVD/CVD risk factors when compared with the subgroup with CVD/CVD risk factors (37.5 ± 11.6 for CVD/CVD risk factor-free group vs 34.5 ± 12.2 for subgroup with CVD/CVD risk factors; P < .001).
Table 1.
Baseline characteristics of study participants, subgroup free of CVD/CVD risk factors, and subgroup with CVD/CVD risk factors
| Variable | All participants (N =1,719) | Subgroup free of CVD/CVD risk factors (n = 415) | Subgroup with CVD/CVD risk factors (n = 1,304) | P value |
|---|---|---|---|---|
| Age (years) | 66 ± 9 | 63 ± 9 | 67 ± 9 | <.001 |
| Women | 928 (54%) | 271 (65%) | 657 (50%) | <.001 |
| Body mass index (kg/m2) | 28 ± 5 | 24 ± 3 | 29 ± 5 | <.001 |
| Current smoker | 167 (10%) | 55 (13%) | 112 (9%) | .005 |
| Systolic blood pressure (mm Hg) | 128 ± 17 | 118 ± 11 | 131 ± 17 | <.001 |
| Diastolic blood pressure (mm Hg) | 73 ± 10 | 71 ± 8 | 74 ± 11 | <.001 |
| Antihypertensive medication use | 880 (51%) | – | 880 (67%) | – |
| Diabetes mellitus | 257 (15%) | – | 257 (20%) | – |
| Prevalent atrial fibrillation* | 89 (5%) | – | 89 (7%) | – |
| Prevalent heart failure | 32 (2%) | – | 32 (3%) | – |
| Prevalent CVA or TIA | 64 (4%) | – | 64 (5%) | – |
| Prevalent coronary artery disease | 175 (10%) | – | 175 (13%) | – |
| Estimated glomerular filtration rate less than 60 mL/min/1.73 m2 | 275 (16%) | 29 (7%) | 275 (16%) | <.001 |
| Total/HDL cholesterol ratio | 3.47 ± 1.05 | 3.29 ± 0.98 | 3.52 ± 1.06 | <.001 |
CVA, Cerebrovascular accident; TIA, transient ischemic attack; HDL, high density lipoprotein.
Continuous variables are presented as means ± SD and categorical variables as n (%). A dash (—) signifies exclusion criteria. P values are presented for comparison between the subgroup free of CVD/CVD risk factors and subgroup with CVD/CVD risk factors. Continuous variables were compared using independent samples t-test. Categorical variables were compared using the χ2 test.
Participants who were in atrial fibrillation at the time of echocardiogram were excluded.
Table 2.
Baseline echocardiographic characteristics of study participants, subgroup free of CVD/CVD risk factors, and subgroup with CVD/CVD risk factors
| Variable | All participants (N = 1,719) | Subgroup free of CVD/CVD risk factors (n = 415) | Subgroup with CVD/CVD risk factors (n = 1,304) | P value |
|---|---|---|---|---|
| LAFI | 35.2 ± 12.1 | 37.5 ± 11.6 | 34.5 ± 12.2 | <.001 |
| LA end-systolic volume (LAmax, mL) | 55 ± 17 | 49 ± 13 | 57 ± 17 | <.001 |
| LA end-diastolic volume (LAmin, mL) | 29 ± 11 | 25 ± 8 | 31 ± 11 | <.001 |
| LAEF(%) | 48 ± 7 | 49 ± 7 | 47 ± 8 | <.001 |
| LA end-systolic volume index (mL/m2) | 30 ± 8 | 28 ± 7 | 30 ± 9 | <.001 |
| LVOT - VTI (cm) | 20 ± 4 | 20 ± 3 | 21 ± 4 | .020 |
| LVEF (%) | 62 ± 18 | 63 ± 15 | 61 ± 18 | .08 |
| LVEDV (mL) | 94 ± 37 | 88 ± 30 | 96 ± 38 | <.001 |
| LV mass (gm) | 153 ± 68 | 138 ± 52 | 158 ± 72 | <.001 |
P values are presented for comparison between the subgroup free of CVD/CVD risk factors and subgroup with CVD/CVD risk factors.
Sex- and Age-Related Distributions of LAFI in the General Sample and Subgroup Free of CVD/CVD Risk Factors
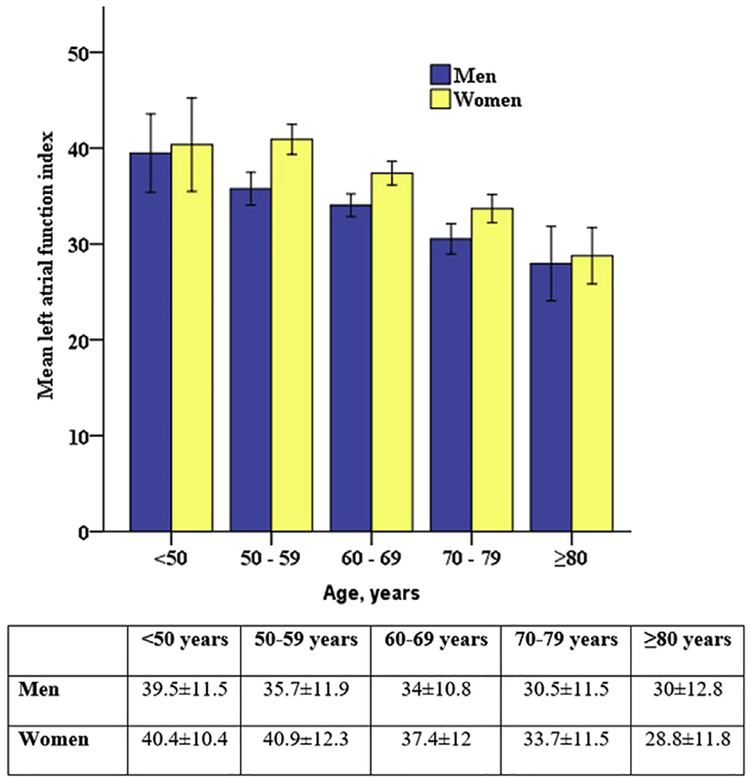
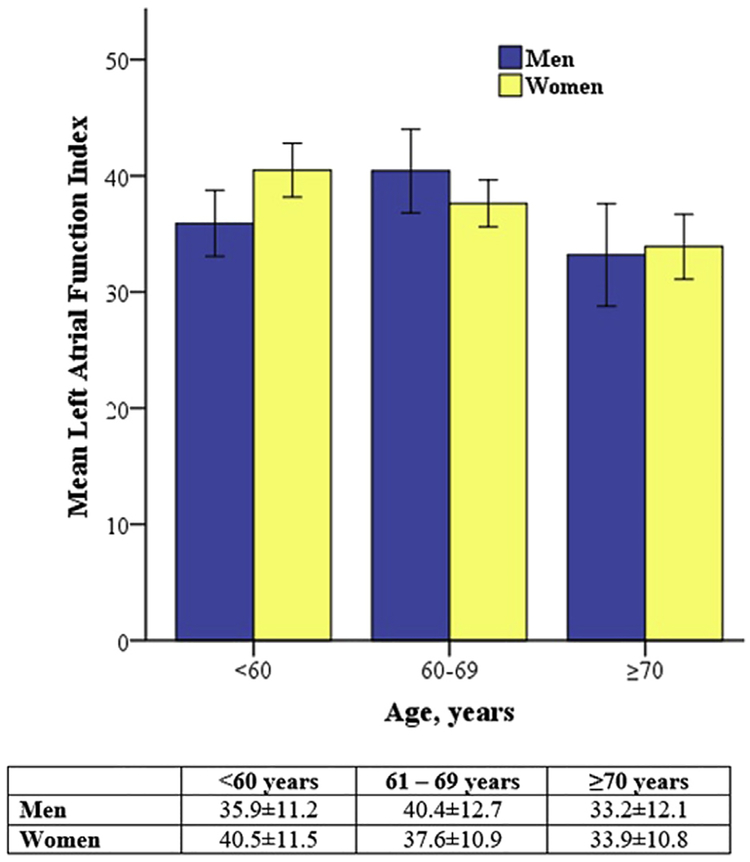
In our general study sample, LAFI was significantly higher in women as compared with men in all age categories (Figure 2). In the subgroup free of CVD/CVD risk factors, a higher LAFI in women was not consistent in all age categories, and the association with female sex with LAFI in this subgroup was not statistically significant (P = .26). In this subgroup, LAFI showed similar inverse association with age as the general sample (β = −0.25, P < .001; Figure 3).
Figure 2.
Distribution of the LAFI by age and sex. Women had significantly higher LAFI compared with men. With age, mean LAFI was lower in both men and women. Error bars represent 2 standard errors of mean. Age group- and sex-specific mean ± SD values are also presented.
Figure 3.
Distribution of LAFI with age and sex in the subgroup free of CVD/CVD risk factors. This subsample was free of hyper-tension, diabetes, obesity, atrial fibrillation, congestive heart failure, cerebrovascular disease, and coronary heart disease. Although mean LAFI was lower with age, there was no statistically significant association with sex in this subgroup. Error bars represent 2 standard errors of mean. Age group- and sex-specific mean 6 SD values are also presented.
We further explored the associations of the various components of LAFI with age and sex in the general sample. Age correlated directly with LAmax index (Spearman’s rho = 0.22, P < .001) and inversely with LAEF (Spearman’s rho = −0.20, P < .001). There was no significant correlation of age and LVOT-VTI (P = .52). Women had significantly higher LVOT-VTI (21 cm in women vs 20 cm in men; P < .001) and lower LAmax index (29 mL/m2 in women vs 30 mL/m2 in men; P = .004). LAEF was similar between women and men (48% in both men and women; P = .84).
Clinical and Demographic Correlates of LAFI
In a stepwise multivariable regression model incorporating clinical and demographic variables, LAFI was positively associated with female sex and was inversely associated with age, antihypertensive medication use, history of atrial fibrillation, prevalent heart failure, and coronary artery disease (Table 3). Regression coefficients (β) presented in Table 3 reflect the magnitude of change in LAFI by the presence of an associated categorical variable. For example, mean LAFI was 4.46 points lower in the participants with history of atrial fibrillation than in those without a history of atrial fibrillation. Regression coefficients for continuous variables reflect the change in LAFI per unit change in the associated variable. For example, LAFI decreased by 0.27 units for every year increase in age (Table 3). Partial correlation coefficients are also presented to reflect the comparative strength of association for LAFI with various covariates in each of the regression models. In the subgroup of the participants with normal LAmax size (LAmax index <34 mL/m2), LAFI was positively associated with female sex and was inversely associated with age, current smoking status, and atrial fibrillation in the clinical and demographic model (Supplemental Table 2, available at www.onlinejase.com). There was no significant interaction of covariates with age and sex in these regression models.
Table 3.
Clinical and demographic correlates of LAFI in stepwise linear regression model
| Variables | Estimated β (SE) | Partial correlation coefficient | P values |
|---|---|---|---|
| Age | −0.27 (0.03) | −0.20 | <.001 |
| Women (vs men) | 3.06 (0.57) | 0.13 | <.001 |
| Antihypertensive medication use (vs no use) | −1.26(0.61) | −0.08 | .038 |
| History of atrial fibrillation (vs no atrial fibrillation) | −4.46 (1.38) | −0.07 | .001 |
| Prevalent heart failure (vs no heart failure) | −5.86 (2.20) | −0.05 | .008 |
| Prevalent coronary artery disease (vs no coronary artery disease) | −2.01 (1.01) | −0.05 | .046 |
SE, Standard error.
Only variables with significant association with LAFI are listed above. Other variables tested were current smoking status, systolic blood pressure, diastolic blood pressure, diabetes mellitus, prevalent cerebrovascular accident and/or transient ischemic attack, estimated glomerular filtration rate less than 60 mL/min/1.73 m2, and ratio of total cholesterol to high-density lipoprotein. Age and sex were forced into the model.
Echocardiographic Correlates of LAFI
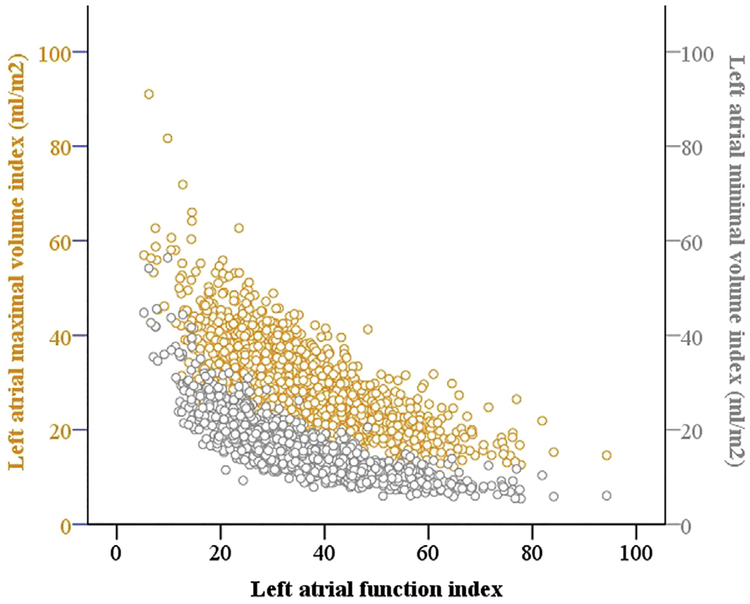
In a stepwise, multivariable regression model examining LAFI in relation to echocardiographic traits, LAFI was significantly and positively related to LVEF but inversely related to LVEDV (Table 4). These relations were maintained in the subgroup of the participants with normal LA size (Supplemental Table 3, available at www.onlinejase.com). There was no significant interaction of covariates with age and sex in these regression models. Figure 4 depicts the distribution of LAFI in relation to LAmax index and LAmin index.
Table 4.
Echocardiographic correlates of LAFI in stepwise linear regression model
| Variables | Estimated β (SE) | Partial correlation coefficient | P values |
|---|---|---|---|
| Age | −0.35 (0.03) | −0.26 | <.001 |
| Women (vs men) | 0.38 (0.73) | 0.01 | .60 |
| LVEF (ln) | 14.84 (2.77) | 0.14 | <.001 |
| LVEDV (ln) | −7.03 (1.33) | −0.14 | <.001 |
ln, Natural logarithmic transformation of the variable; SE, standard error.
Only variables with significant association with LAFI are listed above. Other variable tested was LV mass. Age and sex were forced into the model.
Figure 4.
Distribution of LAFI with LAmax and LAmin indexes.
DISCUSSION
In our retrospective cross-sectional analysis of a community-based sample, we describe the distribution and correlates of LAFI. In the general study sample and among persons with normal LA size, LAFI was higher in women and decreased with age. LAFI had an inverse association with antihypertensive medication use, atrial fibrillation, heart failure, and coronary artery disease. LAFI was positively associated with markers of positive cardiac remodeling (LVEF) but was inversely associated with markers of adverse cardiac remodeling (LVEDV).
The LAFI as a Composite Index of LA Remodeling
Left atrial mechanical function can be assessed during the various phases of LA filling (reservoir phase) and emptying (passive atrial emptying and atrial systole).3 Reservoir function of LA can be measured using volumetric (LAEF or LA emptying volume) or strain-based indices. Compensatory changes in LAEF or LA emptying volume might occur with change in LA size30 and/or LV systolic function.31 LAFI, when compared with other volumetric indices of LA reservoir function, attempts to isolate atrial remodeling from ventricular function by adjusting for LV stroke volume (LVOT-VTI).15 Moreover inclusion of LAmax index in the formula makes it a comprehensive measure to detect both functional and structural LA remodeling.
LAFI was first described by Thomas et al. in 2007 in a sample of 72 participants with atrial fibrillation who underwent electrical cardioversion.15 In this study, LAFI improved immediately with achievement of sinus rhythm after cardioversion and continued to improve further in the follow-up period as LA reservoir function improved. Since this original report, LAFI has been tested in the Heart and Soul Study (longitudinal study of the participants with stable coronary artery disease)16,17 and select CVD-based samples18,19,32 as a predictor of incident stroke, heart failure, atrial fibrillation recurrence, and all-cause mortality. However, LAFI has not been adopted widely in clinical or research settings likely because of the lack of normative and prognostic data in population-based samples. Ours is the first study to describe the distribution, normative values, and cross-sectional associations of LAFI in a population-based sample.
LAFI can be calculated using widely available two-dimensional echocardiography. The applicability of the other measures of LA function, such as magnetic resonance imaging-based volumetric assessment and echocardiography or magnetic resonance imaging-based LA strain assessment, is currently limited by the cost, imaging time, and other logistical challenges that prevent widespread use of these imaging modalities. In a recent study, we demonstrated that calculation of LAFI adds less than a minute to the standard echocardiography interpretation for the experienced operators.19 This is when LAmax, body surface area, and LVOT-VTI are presented to the operators. Thus, LAFI can be measured using standard two-dimensional echocardiograms in a time-efficient manner, potentially making it a useful tool for detection of LA remodeling.
Association of Age with LAFI
The LAmax index increases with advancing age, likely in relation to the increase in the burden of age-related comorbidities such as hypertension7 and CVD.6 We observed a similar direct correlation of the LAmax index with age. Similar to the LAmax index, the effect of aging on LAEF is also mediated through age-associated comorbidities.33,34 We observed that LAEF had an inverse correlation with age. We did not find a significant correlation of age with LVOT-VTI. By including two separate measures of LA remodeling with opposite directionality (LAmax index and LAEF) as a ratio, LAFI should be more sensitive in detecting subtle LA remodeling than either of these measures. We observed a consistent inverse association of LAFI with higher mean ages, despite inclusion of age-related comorbidities as covariates in the regression models with clinical and echocardiographic variables. A similar inverse association was seen with age in the subgroup free of CVD/CVD risk factors suggesting that LAFI can detect the LA remodeling seen in a healthy aging population.
Association of Sex with LAFI
We observed that women had higher LAFI than men in the general sample, likely related to lower average LAmax index and higher mean LVOT-VTI.35 The association of higher LAFI with female sex, however, was not consistent across all regression models. In the subgroup of free of CVD risk factors and CVD, the association of sex with LAFI was not statistically significant. Similarly, in the echocardiographic regression models, the association with female sex was not statistically significant. Likewise, Gupta et al. did not find sex to be an independent correlate of LAEF or LAmax index in multivariable regression models adjusted for the Framingham-CVD risk score variables, LV wall thickness, and indexed LVEDV in the Dallas Heart Study participants.13 Taken together, these findings suggest that female sex is associated with LAFI (and LA remodeling), but these associations may be driven by lower prevalence of adverse LV remodeling in females.
Association of Hypertension with LAFI
Hypertension is an established contributor to LA structural remodeling and dysfunction.36,37 In a prior investigation, Goncalves et al. did not find an association of LAEF with hypertension in the participants of the Atherosclerosis Risk in Community study.33 On the other hand, LA volumes (LAmax index and LAmin index) were significantly increased in the participants with hypertension in that study.33 Prior investigators have postulated that LA enlargement from systemic hyper-tension can lead to a Frank Starling response with preservation of LAEF. Thus, in the early stages of LA remodeling seen with hypertension, LAEF might not be impaired, even though LA size is increased. The LAFI formula indexes LAEF for LA size (LAmax index). We observed that LAFI had an inverse association with antihypertensive medication use. Antihypertensive medication use is a marker of both severity and chronicity of hypertension. Our findings suggest that LAFI, a composite index of LA reservoir function and size, may detect subtle forms of atrial remodeling in patients with hypertension.
Association of CVD with LAFI
LA remodeling is associated with several forms of CVD, including heart failure, atrial fibrillation, coronary artery disease, and cerebrovascular accident.4,16,17 Gupta et al. evaluated LA reservoir function (measured as LAEF) using two-dimensional echocardiography in 971 participants with atrial fibrillation enrolled in the ENGAGE-TIMI 48 trial and reported that reservoir LA function was lower than controls even in the participants with normal LA size.38 We observed an inverse association of LAFI with atrial fibrillation both in the overall sample as well as in the participants without structural LA remodeling (LAmax index <34 mL/m2). Thomas et al.15 and Nagase et al.32 have previously reported that LAFI improves significantly immediately after cardioversion or catheter ablation in participants with atrial fibrillation and continues to serially improve further during the follow-up as LA undergoes reverse remodeling after restoration of sinus rhythm. Taken together, these findings support the ability of LAFI to capture LA remodeling seen in the patients with atrial fibrillation, even in the presence of normal LA size.
Similarly, we noted an inverse association of LAFI with prevalent heart failure and a strong, direct association between LAFI and LV systolic function (Table 4), potentially indicating a relation between atrial mechanical dysfunction and ventricular dysfunction.18,33,39 Prior studies have reported the association of lower LAFI with an increased risk of incident heart failure in participants with coronary artery disease17 and with increased risk of mortality in participants with established heart failure.18 Taken together, these findings suggest that LAFI is able to detect prognostically significant LA remodeling in the patients with heart failure. Similarly, we observed an inverse association of LAFI with coronary artery disease. This association might be mediated by direct atrial ischemia/infarction or through pressure/volume loading of LA due to LV ischemia/infarction. Prior studies have reported that LA remodeling (as measured by lower LAFI) in participants with stable coronary artery disease is predictive of incident CVD.16,17
Echocardiographic Correlates of LAFI
LAFI showed positive association with LVEF (indicator of positive cardiac remodeling) and an inverse association with LVEDV (indicator of adverse cardiac remodeling). Our findings are consistent with prior studies that evaluated LA function in community-based and CVD-based cohorts.11,13,18,33 In the participants of the Dallas Heart Study, Gupta et al. found only a weak correlation of LAmax index with LAEF.13 Whereas LAEF is an indicator of functional LA remodeling, LAmax index reflects structural remodeling. By incorporation of both LAEF and LAmax index in its formula, LAFI, in addition to LVOT-VTI as a measure of stroke volume, might be a stronger and more comprehensive indicator of LA remodeling.
Limitations
Our results should be considered in the context of the strengths and limitations of our investigation. We describe LAFI, a composite measure of LA reservoir function and structure, in a large community-based sample that was unselected for CVD risk. Our study, however, has several limitations. First, our study comprised middle-aged to older adults of European ancestry. Thus, the applicability of our results to other races/ethnicities will need further exploration in multiethnic samples with appropriate echocardiographic measurements. Second, our study is cross-sectional, and thus we cannot infer causal relations or exclude residual confounding. In addition, the association of LAFI with clinical and echocardiographic covariates may be bidirectional, rendering any inferences challenging. Third, although we adjusted for LV mass, ejection fraction, and volumes in our echocardiographic regression models, the echocardiographic measures of diastolic function were not available and it is plausible that LV diastolic function could at least in part mediate the association of LAFI with age. Fourth, LV volumes (LVEDV and LVESV) required for calculation of stroke volume were measured in the apical two-chamber view only. The measurements from the apical four-chamber view were not available for the current investigation. Fifth, LVOT-VTI for our study was derived by dividing stroke volume by the LVOT area. In clinical practice, LVOT-VTI is measured by tracing the recording obtained by pulsed wave Doppler. Since the presence of significant mitral regurgitation would make the LVOT-VTI derivation using stroke volume invalid, we excluded the participants with significant mitral regurgitation from our current study.
CONCLUSION
In our community-based cross-sectional analysis, LAFI declined with advancing age in both men and women. LAFI was higher in women, however, this association was most likely driven by lower prevalence of adverse LVremodeling in females. LAFI was inversely related to anti-hypertensive medication use, history of atrial fibrillation, and prevalent heart failure. Moreover, LAFI associated inversely with currently established echocardiographic indicators of LV remodeling. Impaired LA mechanical function predicts incident CVD independent of LA size.3,10,13,14 Our findings may form the basis for future studies to evaluate the prognostic role of LAFI for predicting CVDs and mortality.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms. Shuxia Fan, RDVS, and Ms. Ewa Osypiuk, RDCS, for their help with data collection, measurement of LA volumes, and leadership of this initiative. We are also grateful to the echocardiography laboratory technicians for data collection during examination 8. We also thank Dr. Daniel Levy for his support and the participants of the Framingham Offspring Study for their time and dedication toward this study.
S.C. is supported in part by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01-HL131532 and R01-HL134168). G.F.M. is owner of Cardiovascular Engineering, a company that develops and manufactures devices to measure vascular stiffness; serves as a consultant to and receives honoraria from Novartis, Merck, Servier, and Philips Healthcare; and is funded by research grants from Novartis and the National Institutes of Health. D.D.M. is funded by grants from National Heart, Lung, and Blood Institute of the National Institutes of Health (KL2RR031981, 5R01HL126911–02, 1R15HL121761–01A1, and 1UH2TR000921–02), Biotronik, Philips, and Otsuka Pharmaceuticals. He is also an equity holder in Mobile Biosense and ATRIA.
The study was funded by grants HHSN268201500001I (to R.S.V.), N01-HC 25195 (to R.S.V.), 1R01HL128914 (to E.J.B.), and 2R01 HL092577 (to E.J.B.) from the National Institutes of Health and National Heart, Lung, and Blood Institute. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the article for publication.
Abbreviations
- CVD
Cardiovascular disease
- LA
Left atrial
- LAFI
Left atrial function index
- LAmax
Maximum left atrial volume
- LAmin
Minimum left atrial volume
- LV
Left ventricular
- LVEDD
Left ventricular end-diastolic diameter
- LVEDV
Left ventricular enddiastolic volume
- LVEF
Left ventricular ejection fraction
- LVESD
Left ventricular endsystolic diameter
- LVESV
Left ventricular endsystolic volume
- LVOT
Left ventricular outflow tract
- PWT
Posterior wall thickness
- SWT
Septal wall thickness
- VTI
Velocity-time integral
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.echo.2017.05.013.
Contributor Information
Mayank Sardana, Cardiology Division, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Gregory Nah, Cardiology Division, Department of Medicine, University of California, San Francisco, San Francisco, California.
Connie W. Tsao, Cardiovascular Division, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts.
Adedotun A. Ogunsua, Cardiology Division, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Eric Vittinghoff, Cardiology Division, Department of Medicine, University of California, San Francisco, San Francisco, California.
Randell C. Thomas, Cardiology Division, Department of Medicine, University of California, San Francisco, San Francisco, California.
Susan Cheng, Boston University’s and National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham; and Section of Preventive Medicine and Epidemiology and Cardiovascular Medicine, Department of Medicine, and Department of Epidemiology, Boston University Schools of Medicine and Public Health, Boston, Massachusetts; Cardiology Division, Department of Medicine, Brigham and Women’s Hospital, Massachusetts.
Aditya Vaze, Cardiology Division, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Jayashri R. Aragam, Cardiology Division, Department of Medicine, Brigham and Women’s Hospital, Massachusetts; Veterans Administration Medical Center, West Roxbury, and Harvard Medical School, Boston, Massachusetts.
Gary F. Mitchell, Boston University’s and National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham; and Section of Preventive Medicine and Epidemiology and Cardiovascular Medicine, Department of Medicine, and Department of Epidemiology, Boston University Schools of Medicine and Public Health, Boston, Massachusetts; Cardiovascular Engineering, Norwood, Massachusetts.
Emelia J. Benjamin, Boston University’s and National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham; and Section of Preventive Medicine and Epidemiology and Cardiovascular Medicine, Department of Medicine, and Department of Epidemiology, Boston University Schools of Medicine and Public Health, Boston, Massachusetts.
Ramachandran S. Vasan, Boston University’s and National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham; and Section of Preventive Medicine and Epidemiology and Cardiovascular Medicine, Department of Medicine, and Department of Epidemiology, Boston University Schools of Medicine and Public Health, Boston, Massachusetts.
Gerard P. Aurigemma, Cardiology Division, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Nelson B. Schiller, Cardiology Division, Department of Medicine, University of California, San Francisco, San Francisco, California.
David D. McManus, Cardiology Division, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Nisha I. Parikh, Cardiology Division, Department of Medicine, University of California, San Francisco, San Francisco, California.
REFERENCES
- 1.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, et al. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol 2003;42:1199–205. [DOI] [PubMed] [Google Scholar]
- 2.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 2003;107:2207–12. [DOI] [PubMed] [Google Scholar]
- 3.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 1995;92:835–41. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echo-cardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 6.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging 2009;2:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation 2010;121: 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90:1284–9. [DOI] [PubMed] [Google Scholar]
- 9.Vieira MJ, Teixeira R, Goncalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr 2014;27:463–78. [DOI] [PubMed] [Google Scholar]
- 10.Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ, et al. Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol 2003;91:1079–83. [DOI] [PubMed] [Google Scholar]
- 11.Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014;16:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blume GG, McLeod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, et al. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 2011;12:421–30. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J 2013;34:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, et al. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol 2008;101:1626–9. [DOI] [PubMed] [Google Scholar]
- 15.Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr 2008; 9:356–62. [DOI] [PubMed] [Google Scholar]
- 16.Wong JM, Welles CC, Azarbal F, Whooley MA, Schiller NB, Turakhia MP. Relation of left atrial dysfunction to ischemic stroke in patients with coronary heart disease (from the Heart and Soul Study). Am J Cardiol 2014;113:1679–84. [DOI] [PubMed] [Google Scholar]
- 17.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol 2012;59:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sargento L, Vicente Simoes A, Longo S, Lousada N, Palma Dos Reis R. Left atrial function index predicts long-term survival in stable outpatients with systolic heart failure. Eur Heart J Cardiovasc Imaging 2017;18:119–27. [DOI] [PubMed] [Google Scholar]
- 19.Sardana M, Ogunsua AA, Spring M, Shaikh A, Asamoah O, Stokken G, et al. Association of left atrial function index with late atrial fibrillation recurrence after catheter ablation. J Cardiovasc Electrophysiol 2016;27: 1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010;121:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, et al. Distinct aspects of left ventricular mechanical function are differentially associated with cardiovascular outcomes and all-cause mortality in the community. J Am Heart Assoc 2015;4:e002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delling FN, Rong J, Larson MG, Lehman B, Fuller D, Osypiuk E, et al. Evolution of mitral valve prolapse: insights from the Framingham Heart Study. Circulation 2016;133:1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22: 1–23. quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- 26.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study). Am J Cardiol 2006;97:83–9. [DOI] [PubMed] [Google Scholar]
- 31.Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999;100:427–36. [DOI] [PubMed] [Google Scholar]
- 32.Nagase T, Kato R, Nakano S, Shiki Y, Tanaka S, Ikeda Y, et al. Prediction of improvement in left atrial function index after catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2015;44:151–60. [DOI] [PubMed] [Google Scholar]
- 33.Goncalves A, Hung CL, Claggett B, Nochioka K, Cheng S, Kitzman DW, et al. Left atrial structure and function across the spectrum of cardiovascular risk in the elderly: the Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging 2016;9:e004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol 2002;40:1630–5. [DOI] [PubMed] [Google Scholar]
- 35.Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation 2006;113:1597–604. [DOI] [PubMed] [Google Scholar]
- 36.Soullier C, Niamkey JT, Ricci JE, Messner-Pellenc P, Brunet X, Schuster I. Hypertensive patients with left ventricular hypertrophy have global left atrial dysfunction and impaired atrio-ventricular coupling. J Hypertens 2016;34:1615–20. [DOI] [PubMed] [Google Scholar]
- 37.Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging 2009;2:93–9. [DOI] [PubMed] [Google Scholar]
- 38.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI48. Eur Heart J 2014;35:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brecht A, Oertelt-Prigione S, Seeland U, Rucke M, Hattasch R, Wagelohner T, et al. Left atrial function in preclinical diastolic dysfunction: two-dimensional speckle-tracking echocardiography-derived results from the BEFRI Trial. J Am Soc Echocardiogr 2016;29:750–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.