Abstract
The purpose of this review is to describe the current state of the art in terms of available devices and indications for the transcatheter device closure of atrial septal defects (ASD) and patent foramen ovale (PFO) in children and young adults. Techniques for transcatheter device closure of ASD (TC-ASD) are well established and TC-ASD has a proven track record of efficacy and safety. Device erosion, a rare but potentially catastrophic adverse event of TC-ASD has raised questions about the relative safety of TC-ASD versus operative open heart surgical ASD closure (O-ASD). Despite a concerted effort to study the issue, there are no solutions that avoid the risk of erosions definitively when using the Amplatzer device. There is evidence that concern about erosions has changed practice in the United States, specifically that more patients are referred for O-ASD than in the past. Efforts to study these changes have also revealed more variation in TC-ASD practice than previously appreciated. New devices for ASD closure with properties that may reduce the risk of erosion are being developed and brought to market, but, in the meantime, cardiologists must continue to balance the risks and benefits of TC- and O-ASD closure. Recent studies demonstrating the superiority of PFO device closure over medical therapy for cryptogenic stroke is likely to lead to changes in practice for structural/interventional cardiologists. Previously, the overwhelming majority of ASD closure procedures were performed for right heart volume overload due to left to right shunt, but stroke prophylaxis may become a more significant part of the volume in pediatric/congenital catheterization laboratories. Care should be taken in extrapolating this data to children and younger adults, and important questions about patient selection remain unresolved.
Keywords: erosion, pediatric cardiology, transcatheter intervention, outcomes
Current Devices for TC-ASD:
With an incidence of 6 to 10 per 10,000 live births[1], ostium secundum atrial septal defects (ASD) are one of the most common forms of congenital heart disease. King and Mills developed the first device for transcatheter closure of ASD (TC-ASD) in 1976[2]. The history of device development for TC-ASD is a testament to innovation in the field of congenital/structural cardiology[3]. Over time, TC-ASD has become the predominant technique for closing most ASD; >80% of isolated ASD treated at primary pediatric hospitals in the United States are closed in the catheterization laboratory[9].
At the present time, two devices for TC-ASD are widely available in the United States (Figure 1): the Amplatzer Septal Occluder (ASO) (St. Jude Medical, St. Paul MN) (Figure 1A and Figure 2) and the Gore Cardioform device (W.L. Gore and Associates, Flagstaff, AZ) (Figure 1B). The ASO was the first device approved by the FDA for TC-ASD demonstrating safety and efficacy in a non-randomized IDE trial[4], which was reinforced in a subsequent multicenter registry study[5]. It has been in use long enough for several large single-center case series to report excellent medium and long-term outcomes[6–8]. In contemporary case series of US centers, the ASO is used in between 70–86% of cases [9,10].
Figure 1: Radiographic Appearance of Devices for transcatheter closure of ASD.
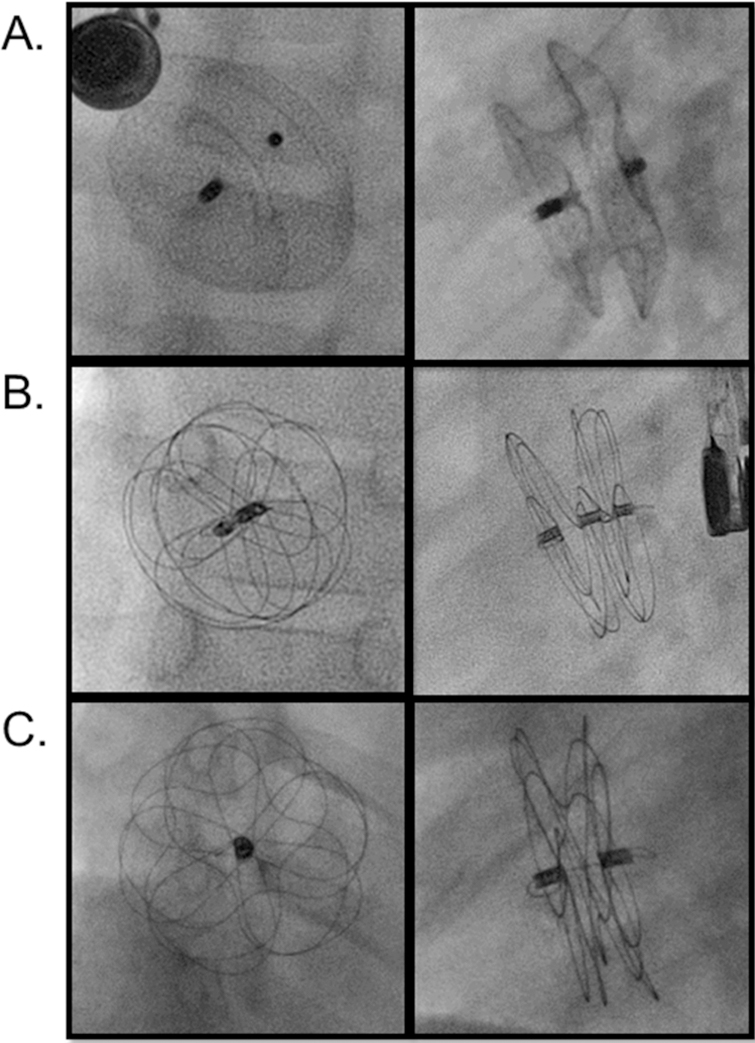
Digital acquisition images of A) Amplatzer septal occluder (anterior posterior and lateral projections),(Courtesy of Abbott, Inc., Abbott Park, IL; with permission.) B) Gore Cardioform device (en face and orthogonal views), C) Cardioform Atrial Septal Defect Occluder (en face and orthogonal views). (Courtesy of W.L. Gore and Associates, Flagstaff, AZ; with permission.)
Figure 2: Amplatzer Septal Occlusion Device.
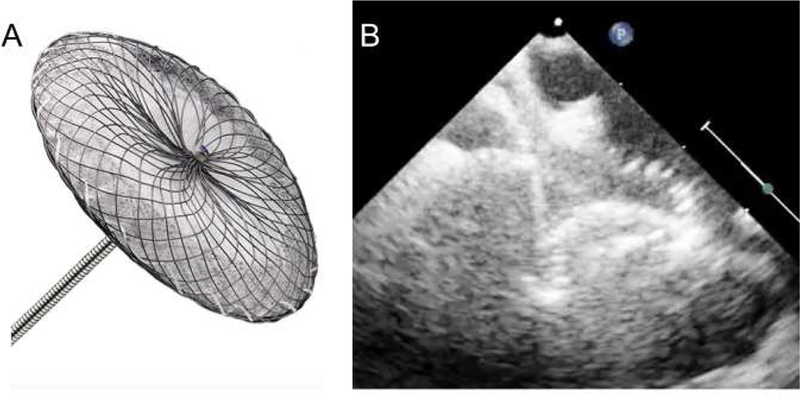
A) Amplatzer septal occluder (artist rendition – courtesy of Abbott), B) Echocardiographic appearance of the Amplatzer ASD device splayed around the aorta. ),(Courtesy of Abbott, Inc., Abbott Park, IL; with permission.)
The Gore Helex Septal Occluder (W.L. Gore and Associates, Flagstaff, AZ) has also demonstrated excellent safety and efficacy in both short[11–13] and middle term outcomes[14]. The Helex Septal Occluder has been replaced by the Gore Cardioform device and is no longer sold in the US. The Cardioform device has a Nitinol wire frame covered with an expanded tetrafluoroethylene membrane. Like the Helex before it, this device was not self-centering and limited to relatively small defects. The initial device trial and continuing access series are complete with manuscripts pending at this time. Gore has produced a second Cardioform device – the Cardioform Atrial Septal Defect Occluder (C-ASDO)-with a larger diameter central waist and an expanded range of diameters, both designed to facilitate closure of larger diameter ASD’s (Figure 1C and Figure 3). The newer C-ASDO device is currently undergoing an FDA Pivotal trial (Gore ASSURED Trial; ClinicalTrials.gov Identifier NCT02985684 ) in the United States. In a multicenter series from Canada, both Gore devices have demonstrated similar safety and efficacy to previous devices[15]. The Amplatzer multi-fenestrated septal occluder (“Cribriform” device) (St. Jude Medical, S t. Paul, MN) resembles the ASO device but has symmetric discs and a narrow waist, designed to allow it to cover the septum of patients with multiple defects.
Figure 3: Gore Cardioform ASD Device.
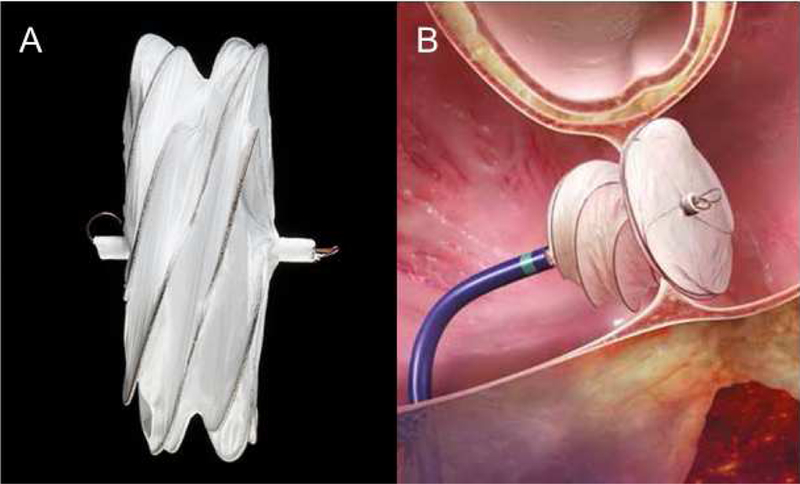
A) Picture of the Gore Cardioform ASD Device, B) Cartoon depiction of the Gore Cardioform Device in situ. (Courtesy of W.L. Gore and Associates, Flagstaff, AZ; with permission.)
Device Erosion of the Amplatzer Septal Occluder device:
Erosions of the device following TC-ASD with the Amplatzer device were first reported in a series of case reports in 2003 and 2004[16–18]. Alarm about erosions was particularly acute because of the perception that TC-ASD was technically straightforward and demonstrably safer than O-ASD, the catastrophic potential of erosions, and the fact that erosions appeared to occur unpredictably and as much as years after device implantation. Rapidly, a board of physicians was convened to review known cases of device erosion, identifying deficient anterior-superior or retro-aortic rim (along with device over-sizing) was identified as a risk factor present in all of their cases [19]. In 2012, a United States Food and Drug Administration Panel Review convened and was followed by revision of the manufacturers Indication for Use, labeling a retro-aortic rim <5mm in diameter as a relative contraindication to TC-ASD with an ASO device[20–22]. Concern for device erosion has persisted [23–27], but limitations in longitudinal follow-up of implanted devices has made it impossible to accurately measure the number of devices implanted, the total number of erosions, and the risk of erosion. Best estimates of risk are between 0.04 and 0.3% of device implants[19,21,23,24,28]. Most cases of erosion occur shortly after device implantation, but erosions have been reported as late as 8 years after initial placement[29,30].
The low overall rates of erosion and limited experience at individual centers has complicated identification of patient- and procedure-level risk factors for device erosion. Neither the first version of the IMPACT® [9] nor C3PO [10] contained data about post-discharge adverse events. Since that time, a prospective post-market surveillance study of the ASO was initiated. However, enrollment was stopped in December of 2016, and the results have yet to be published. In the first revision of the IMPACT® registry the capacity to include follow-up data for pre-specified interventions including TC-ASD was added. It remains to be seen whether centers are accurately reporting their longitudinal data in this voluntary database. Without manufacturer or registry follow-up, use of other large (non-clinical) observational data-sets (e.g. insurance claims data) may be necessary to obtain better estimates of erosion risk in the current era.
At present, it remains up to individual cardiologists to determine whether TC-ASD is the best option for ASD closure. There is not, as of yet, data to determine whether 1) anatomic variations (bare vs. small retro-aortic rim or concomitant deficient superior tissue rim) or 2) a combination of anatomy and choice of device (a patient with deficient retro-aortic or superior rim and a large or over-sized device) can provide predict superior risk stratification[19,28,31]. Though deficient retro-aortic rim has consistently been found in erosion cases, it is not sufficient to identify which patients are at risk for erosion. Subsequent research has demonstrated that the prevalence of deficient retro-aortic rim is between 40–60% of children referred for TC-ASD[8,9,32,33] and slightly lower in adult patients[34]. A recent case-control study using data from the Erosion Board’s collection and the ASO Post-Approval Study reiterated 1) that deficient retro-aortic and superior vena cava rims were present in a much higher proportion of cases than controls, 2) ASD were larger in diameter and larger in proportion to patient weight than in controls, and 3) several factors suggestive of device over-sizing (balloon size much larger than static defect size or device much larger than static defect size) were more common in cases and controls[28]. Operators are now cautious not to allow devices to indent the retroaorta or roof of the left atrium, but debate remains about “splaying” the ASO device around the aorta as shown in Figure 2. There continues to be more work to identify the factors or combination of factors that identify patients in which the risk of TC-ASD exceeds that of O-ASD.
It is important to reiterate that the relative clinical benefit of TC-ASD to O-ASD is well established. In head to head comparisons, TC-ASD has consistently demonstrated equivalent efficacy with excellent safety in comparison to O-ASD[4,35,36] with the added benefit of having significantly less discomfort, superior cosmetic results, and a shorter length of stay. Reevaluating these outcomes outside of clinical trials is challenging. There are relatively few contemporary series comparing the results of TC-ASD and O-ASD. Large multicenter series are necessary for comparisons because of systematic differences between patients undergoing O-ASD and TC-ASD and the need for statistical adjustment to account for confounding by indication. Contemporary multi-center series of TC-ASD have demonstrated the risk of in-hospital mortality is 0–0.015% [9,10,37]. In the same time period, data from the Society for Thoracic Surgeons Congenital Heart Surgeons database suggests that the risk of in-hospital mortality after O-ASD is between 0.3–0.9% even after adjusting for pre-operative risk factors[38]. Even adjusting for measurable differences in case-mix, studies have consistently demonstrated that peri-procedural morbidity (i.e. complications) is significantly higher after O-ASD as well[39], which is also reflected in a longer length of stay and higher hospital costs following O-ASD[39,40]. Even with uncertainty regarding what the risk of “real” current risk of erosion is (which is likely a function of both patient selection and device selection), the additional risk of erosion is unlikely to overcomes the relative benefits of TC-ASD over O-ASD.
However, in light of this concern it is important to determine whether concern regarding erosion is affecting practice. Analysis of clinical registry data demonstrated that patients with deficient retro-aortic rim were no less likely to receive ASO devices than those with larger retro-aortic rims[9]. At the same time, analysis of administrative data has allowed, for the first time, measurement of the tendency to pursue O-ASD and TC-ASD[9], demonstrating that prior to 2013, the proportion of TC-ASD was increasing, but that between 2013 and 2015 this trend reversed and the proportion of O-ASD increased slightly relative to TC-ASD (Figure 4). Though this trend may reflect the reasonable desire to avoid erosions, this trend in practice would potentially have risks. Though referring patients for O-ASD will inevitably reduce the risk for erosion, this practice only results in a net reduction in harm to patients if the benefit exceeds the inherently higher risks of O-ASD.
Figure 4: Estimated probability of TC-ASD vs. O-ASD 2007–2015.
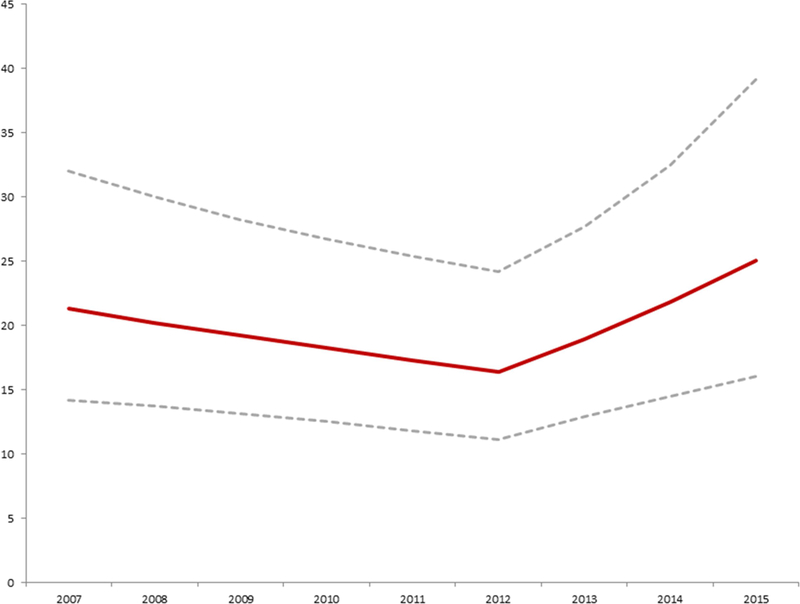
Probability of operative ASD closure versus transcatheter ASD closure
Conditional standardization was used to calculate an adjusted probability of operative ASD closure vs. transcatheter device closure, for a hypothetical white male six-year-old boy with no co-morbid conditions (maroon line with 95% CI represented by the dashed grey lines). This was based on the mixed effects multivariate generalized linear model summarized in Table 2. The probability of operative ASD closure decreased significantly from 2007 until 2012 (OR: 0.95 per year, p=0.02). In 2013, there was a significant shift in probability favoring ASD (OR: 1.21 per year, p=0.006).
(From O’Byrne et al 2017 Increasing Propensity to Pursue Operative Closure of Atrial Septal Defects Following Changes in the Instructions for Use of the Amplatzer Septal Occluder Device
An Observational Study Using Data from the Pediatric Health Information Systems Database. Am Heart J, 192: 85–97; with permission.)
Another trend seen in this data is a trend towards closing ASD in progressively younger patients. From the outset of TC-ASD, smaller children have had ASD closed. In the ASO device trial, there was no restriction on age or size for the TC-ASD arm, and the median age of subjects was 9.8 years with a range from 0.6 to 82 years[4]. Similarly, the first multicenter study reporting real-world use of the ASO device, reporting the results of 478 cases from 13 centers performed between 2004 and 2007 demonstrated an equally broad range (infancy to the ninth decade of life)[5]. In that series, 33% of reported cases were performed in patients <16 kg. Since that report, multiple case series demonstrate that TC-ASD can be performed even in patients <10 kg[32,41–45]. At the same time, the majority of cases continue to be performed in school-age patients. Contemporary data from the IMPACT® registry that the median age of TC-ASD is 5–7 years)[9,37] with a similar series from the C3PO registry reporting that 85% of subjects were older than 3 years[10].
Though natural history studies of large ASD demonstrated a decrease in life expectancy, childhood symptoms due to congestive heart failure were rare as was the development of pulmonary vascular disease [46–48]. Other series have demonstrated that the majority of small defects found in infants close spontaneously[49–51]. The natural history of larger defects has not been well defined. In cross-sectional analyses, an association has been demonstrated between older patient age at diagnosis and larger defect size[50,51]. This may be due to spontaneous closure of smaller defects, but some have hypothesized that some ASD increase in size over time [52]. This has been used as a justification for early intervention. No studies have followed ASD longitudinally to confirm if some ASD do grow over time. Even if some ASD did grow, it is unclear whether how often that growth would complicate TC-ASD.
Analysis of data from the PHIS registry demonstrated that the age of patients undergoing ASD closure at primary pediatric hospitals in the US decreased progressively between 2007 and 2015[9], independent of measurable patient-level confounders. It is impossible to determine in this study design the reasons behind this trend. Sensitivity analyses demonstrated that this was not driven by the aforementioned trend to increasing use of O-ASD and was present regardless of closure method. There is also no evidence that the trend is the result of increasing prevalence of pulmonary disease or prematurity that might aggravate the physiological effects of an atrial level shunt. This trend has the potential to have real ramifications on patient safety. The effect of small size on the risk of adverse events or technical failure has been equivocal in multiple studies [8–10]. However, McElhinney and colleagues demonstrated that larger defect size to patient size was a risk factor for device erosion[28]. Therefore, for TC-ASD, the optimal age for closure is not clear, and it remains the shared responsibility of referring physicians and interventional cardiologists to balance these risks and benefits.
These studies have demonstrated that there is more uncertainty regarding which patients should be referred for TC-ASD than what one might have expected. This is underscored, by variation in the relative utilization of TC-ASD and O-ASD, even at relatively large primary pediatric hospitals. Though TC-ASD accounted for >80% of ASD closure procedures at US pediatric hospitals[9], some hospitals utilized TC-ASD for as few as 30% of their ASD closure cases, while others were as high as 100%[9]. Even after adjusting for differences in case-mix, there remained significant inter-hospital variability in the choice between O-ASD and TC-ASD[9]. The systematic differences between hospitals underscores the lack of consensus about how ASD should be treated. Similar differences between hospitals have been seen in the both the distribution of indications for TC-ASD and how different hospitals define right ventricular volume overload [53]. Taken together, these observations demonstrate the lack of consensus in practice for TC-ASD and the potential benefit of standardization of practice.
Minimally invasive cardiac surgery:
In considering the potential risk of device erosion, an important question is whether O-ASD can be made safer and less morbid for patients. Minimally invasive cardiac surgery (MICS), through “mini-sternotomy” or video assisted thoracoscopic surgery (VATS) (with or without robotic assistance), has the potential to reduce procedural morbidity relative to open heart surgery with reductions in length of stay along with improved cosmesis compared to conventional surgical correction of ASD[54]. However, to date the largest case series of MICS report lengths of stay (median between 5–7 days) that are similar to conventional studier and much longer than that following TC-ASD (1 day or less) [55–58]. Enthusiasm and expertise in MICS remains limited to a few centers and is not yet a compelling alternative to conventional O-ASD and TC-ASD.
PFO closure as secondary prophylaxis for stroke:
The incidence of cryptogenic stroke in young adults is between 15 and 35%[59]. A possible mechanism for these strokes is transient right to left shunt through a patent foramen ovale allowing embolization of thrombus from the systemic venous circulation into the systemic arterial circulation (and eventually the brain). As a result, there has been significant interest in evaluating the relative benefit of device closure of PFO as secondary prophylaxis after a stroke. Three randomized clinical trials (RCT) from 2012–2013 using the Amplatzer PFO occluder (St. Jude Medical, St. Paul MN) [60,61] and the STARFlex closure system (NMT Medical, Boston MA)[62]failed to demonstrate benefit over medical therapy. Therefore 2016 American Academy of Neurology recommendations stated that there was insufficient evidence to endorse device closure of PFO after cryptogenic stroke[63]. However, two recent RCT have demonstrated a significant benefit of TC-PFO over medical therapy: the REDUCE[64]and CLOSE [65] trials. The two more recent studies differed from prior studies in that they restricted enrollment to patients in whom a right to left shunt could elicited on bubble-study echocardiogram. A number of meta-analyses have been applied to these data, pooling data from previous trials. The most recent of these[66] demonstrated that the benefit of device closure was greater in patients with larger right to left shunt (i.e. a larger number of microbubbles) on agitated saline contrast echocardiogram and in the subgroup of patients <45 years. The pooled benefit over medical therapy was still relatively modest (number needed to treat between 28 and 64 to prevent one stroke over 4 years depending on which estimate of stroke recurrence risk was used), but supported the benefit of TC-PFO over medical therapy[66]. Concerns over the increase in risk of atrial fibrillation with TC-PFO appear to be largely driven by events in cases in which the STARFlex system was used and there was no significant difference in major adverse events between TC-PFO and medical therapy groups in this meta-analysis[66]. Additionally, reanalysis of the RESPECT trial data after longer follow up time (median: 5.9 years, compared to previously published data after a median of 2.1 years) demonstrated significant benefit to TC-PFO over medical therapy consistent with REDUCE and CLOSE, and demonstrating increased benefit in larger shunts on agitated saline injection [67].
At the present time, both the Amplatzer PFO occluder and the Gore Cardioform device are approved by the United States Food and Drug Administration for the closure of PFO as secondary prophylaxis for stroke. As these results disseminate, it is likely that pediatric/congenital cardiologists will be asked to be involved in PFO closure in both adults and in children. At the present time, several questions are important to consider when translating the data from clinical trials and meta-analyses to clinical practice. First, the definition of cryptogenic stroke differs between studies and needs to be clarified both to provide an accurate measurement of recurrence risk and to determine patients in whom TC-PFO closure is less likely to be effective. Second, in light of relatively modest benefits in absolute risk reduction, it is important to determine what factors (in addition to relatively young age and larger shunt) identify populations that would benefit more from TC-PFO. Third, as with any new application of a technique there is the potential for new or unexpected adverse events along with the anticipated benefits. As an example, several studies have shown that a small but significant minority of patients with ASD and PFO devices develop aortic insufficiency after device closure[68–71]. Addressing all of these issues will require continued vigilance and ongoing clinical effectiveness research as PFO closure becomes more widespread.
Another important issue for pediatric/congenital cardiologists is the applicability of these findings to the pediatric population. The incidence of arterial stroke is lower in children than in older patients, but the proportion of cryptogenic strokes is similar to that in adults, between 15 and 27%[72–74]. It is tempting to extrapolate data from clinical trials in older populations since the benefit TC-PFO are greater robust in younger (i.e. <45 years) adults[66,75], and because children and adolescents will presumably spend more time at risk than even young adults. Documented issues with maintaining patients on chronic antiplatelet or anticoagulant regimens are likely to be even more challenging in the pediatric population. However, at this time there is still of dearth of data in children and it is not a given that recurrence risk for cryptogenic stroke will be similar between pediatric and adult populations. In addition, to the cryptogenic stroke population an open question is whether patients long-term central venous lines or transvenous pacing leads should be screened for PFO and which of these patients should have their PFO treated.
Also, transient ischemic attacks (TIA) are a potentially problematic indication for TC-PFO. TIA are not only much more frequent than cerebrovascular accidents, but also are more problematic from a diagnostic perspective, since they are not always accompanied by findings on neuro-imaging studies and have overlap in symptoms with migraines and other conditions. Diagnosis of TIA in a pediatric population is also more challenging due to the cognitive development of the population. Further research is necessary to clarify these issues and until that time, there is uncertainty in regards to what the appropriate care of these children should be.
Non-implant defect closure:
While most closure techniques for atrial septal defects and PFO’s rely on implantation of a permanent device within the defect such as an Amplatzer device or Cardioform device, the ideal closure device would not utilize a foreign body. This “ide al” ASD closure device would obviate the need for concern over thrombus formation, embolization, erosion, arrhythmias, and the ability to access the left atrium later in life. Clearly, with the increase in interventions for atrial fibrillation, mitral valve dysfunction and left atrial appendage occlusion, transcatheter access to the left atrium is valuable and permanent implants in the atrial septum can make this much more difficult. Although the initial “non-device” closure techniques have been used predomina ntly for closure of PFOs (patent foramen ovale), it is important to consider these creative techniques as similar technology could someday be utilized for ASD closures.
The initial concept for non-device closure of ASDs utilized radiofrequency (RF) energy to essential “weld” together the tissue flaps or tunne l which created a PFO. By applying RF energy to heat tissue flaps while pushing them together or utilizing a vacuum to keep the septum primum and secundum opposed to one another, the injury and heat created from the RF energy allows the tissue to become adherent and then to heal together. The pathology of radiofrequency closure of PFOs in animals has been described and reported in a porcine model[76] and the first human implant was performed in 2005[77]. This is marketed as the RFx closure system (Cierra Inc, Redwood City, CA). In a study of 144 patients lead by Dr. Horst Sievert, the RFx device was found to be safe as there were no significant adverse events in any of the patients except one patient who received a blood transfusion for blood loss during the procedure. However, at 6 month follow up 45% of the PFOs continued to have a significant shunt. The device was much more effective (72% closure at 6 months) in closing smaller PFOs with stretch diameters less than 8 mm[78].
Another new device that is being used in Europe and now also in North America is the NobleStitch Device device made by HeartStitch (Fountain Valley, CA, USA). This device works by passing two sutures through different aspects of the PFO and creating a knot to suture the PFO into a closed position (Figure 5). This device and its accessories are CE marked for cardiovascular suturing and PFO closure in Europe. Although this device is not approved in the United States for PFO closure, it is now being used off label for this procedure. The first reported series of cases with this device were reported in a prospective study from 12 centers in Italy[79]. This study enrolled 192 patients who were considered acceptable candidates for suture-mediated PFO closure. The NobleStitch EL system was technically successfully in 96% of the patients with no procedural or long-term complications reported. At follow up time of about 7 months, contrast echocardiography with Valsalva showed no shunting in 75% but a “significant” shunt was seen in 11% of th e patients. Although use of radiofrequency energy may be difficult to translate to and significant ASD, it may be possible in the future to utilize stitch-based device to close small ASD and even larger ASD with transcatheter patch placement in the future. It may also become possible to employ these sort of techniques along with device closure to minimize the risks or either erosion or embolization for high risk ASDs.
Figure 5: NobleStitch El Device for PFO closure.
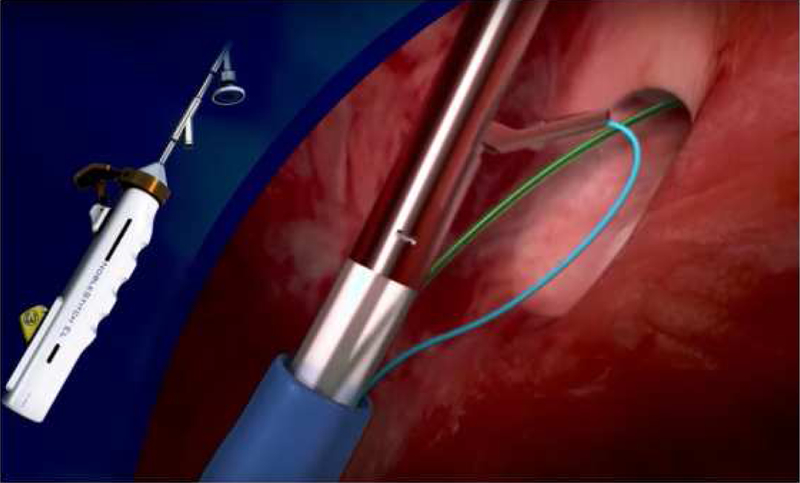
Left) The NobleStitch El device, Right) Drawing of mechanism of action of the device demonstrating mechanism of action of stitch placement. (Courtesy of HeartStitch (Fountain Valley, CA; with permission.)
CONCLUSION
Transcatheter device closure of defects in the inter-atrial setpum have been performed for over forty years and have demonstrated excellent safety and efficacy. The controversy surrounding the risk of device erosion has brought to light the lack of consensus between different centers how to approach this lesion. Continued innovation in development of devices and epidemiologic surveillance of practice are necessary to provide the best care for patients with ASD.
KEY POINTS:
Transcatheter device closure of ostium secundum atrial defects (ASD) has a lower risk of mortality and morbidity, shorter length of stay, and lower cost than operative closure of the same defect.
Erosion of devices after transcatheter closure of ASD remain an important consideration in transcatheter closure of ASD. The best data to date indicates that patients with erosion were more likely than controls to have smaller superior rims, larger defects relative to the septum and patient size, and were more likely to have an oversized device. Though these findings suggest steps that might improve outcomes, further research is necessary to determine whether a specific subset of patients can be identified whose risk of device erosion exceeds the risks of open-heart surgery.
Transcatheter ASD closure remains the predominant method of ASD closure in children and young adults, but in recent years (coincident with concern for device erosion) there is a significant trend towards increasing referral for operative ASD closure. Simultaneously, the patient population referred for ASD closure is progressively younger with time. The effect of these trends on outcomes is not clear at this time, but deserves attention.
Recent data has demonstrated the benefit of device closure of patent foramen ovale as secondary prophylaxis for strokes in older adults. There is a dearth of data regarding the relative risks and benefits of this practice in younger patients with cryptogenic stroke and PFO. Given the high prevalence of PFO and increasing incidence of stroke in medically complicated pediatric patients, closure of PFO for this indication is likely to be an increasingly important issue for pediatric/congenital cardiologists.
A potential innovation in closure of ASD and PFO are approaches that avoid implantation of a permanent device. An example are catheter delivered sutures to close atrial defects such as the NobleStitch device.
Acknowledgements:
Funding Sources: Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). The funding agencies had no role in the drafting of the manuscript or influencing its content. This manuscript represents the opinion of the authors alone. There are no other relevant financial disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No other relevant disclosures.
Conflicts of Interest: Dr. O’Byrne has no significant conflicts to disclose. Dr Levi has…
REFERENCE:
- 1.Hoffman JIE, Kaplan S: The Incidence of Congenital Heart Disease. J Am Coll Cardiol 2002, 39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2.King TD, Thompson SL, Steiner C, Mills NL: Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976, 235:2506–2509. [PubMed] [Google Scholar]
- 3.King TD, Mills NL: Historical Perspective on ASD Device Closure. In Transcatheter Closure of ASDs and PFOs Edited by Hijazib ZM, Feldman T, Abdullah Al-Qbandi MH, Sievert H. Cardiotext; 2010:37–64. [Google Scholar]
- 4.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K, Amplatzer Investigators: Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002, 39:1836–1844. [DOI] [PubMed] [Google Scholar]
- 5.Everett AD, Jennings J, Sibinga E, Owada C, Lim DS, Cheatham J, Holzer R, Ringewald J, Bandisode R, Ringel R: Community Use of the Amplatzer Atrial Septal Defect Occluder: Results of the Multicenter MAGIC Atrial Septal Defect Study. Pediatr Cardiol 2008, 30:240–247. [DOI] [PubMed] [Google Scholar]
- 6.Wang J-K, Tsai S-K, Wu M-H, Lin M-T, Lue H-C: Short- and intermediate-term results of transcatheter closure of atrial septal defect with the Amplatzer Septal Occluder. Am Heart J 2004, 148:511–517. [DOI] [PubMed] [Google Scholar]
- 7.Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM: Long-Term Follow Up of Secundum Atrial Septal Defect Closure with the Amplatzer Septal Occluder. Cong Heart Dis 2010, 5:32–37. [DOI] [PubMed] [Google Scholar]
- 8.O’Byrne ML, Glatz AC, Sunderji S, Mathew AE, Goldberg DJ, Dori Y, Rome JJ, Gillespie MJ: Prevalence of Deficient Retro-Aortic Rim and Its Effects on Outcomes in Device Closure of Atrial Septal Defects [Internet]. Pediatr Cardiol 2014, 35:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Byrne ML, Gillespie MJ, Kennedy KF, Dori Y, Rome JJ, Glatz AC: The influence of deficient retro-aortic rim on technical success and early adverse events following device closure of secundum atrial septal defects: An Analysis of the IMPACT Registry(®). [Internet]. Catheter Cardiovasc Interv 2017, 89:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Said H, Hegde S, Foerster S, Hellenbrand W, Kreutzer J, Trucco SM, Holzer R, Burch G, Mirani A, Nicolas R, et al. : Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv 2015, 85:227–233. [DOI] [PubMed] [Google Scholar]
- 11.Vincent RN, Raviele AA, Diehl HJ: Single-center experience with the HELEX septal occluder for closure of atrial septal defects in children. J Interv Cardiol 2003, 16:79–82. [DOI] [PubMed] [Google Scholar]
- 12.Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, Vincent R, Kanter K: Results of the U.S. Multicenter Pivotal Study of the HELEX Septal Occluder for Percutaneous Closure of Secundum Atrial Septal Defects. J Am Coll Cardiol 2007, 49:2215–2221. [DOI] [PubMed] [Google Scholar]
- 13.Latson LA, Jones TK, Jacobson J, Zahn E, Rhodes JF: Analysis of factors related to successful transcatheter closure of secundum atrial septal defects using the HELEX septal occluder. Am Heart J 2006, 151:1129.e7–1129.e11. [DOI] [PubMed] [Google Scholar]
- 14.Correa R, Zahn E, Khan D: Mid-term Outcomes of the Helex Septal Occluder for Percutaneous Closure of Secundum Atrial Septal Defects. Cong Heart Dis 2013, 8:428–433. [DOI] [PubMed] [Google Scholar]
- 15.de Hemptinne Q, Horlick EM, Osten MD, Millán X,Tadros V-X, Pighi M, Gonzalez Barlatey F, Alnasser SM, Miró J, Asgar AW, et al. : Initial clinical experience with the GORE(®) CARDIOFORM ASD occluder for transcatheter a trial septal defect closure. Catheter Cardiovasc Interv 2017, doi: 10.1002/ccd.26907. [DOI] [PubMed]
- 16.Preventza O, Sampath-Kumar S, Wasnick J, Gold JP: Late cardiac perforation following transcatheter atrial septal defect closure. Ann Thoracic Surg 2004, 77:1435–1437. [DOI] [PubMed] [Google Scholar]
- 17.Chun DS, Turrentine MW, Moustapha A, Hoyer MH: Development of aorta-to-right atrial fistula following closure of secundum atrial septal defect using the Amplatzer septal occluder. Cathet Cardiovasc Intervent 2003, 58:246–251. [DOI] [PubMed] [Google Scholar]
- 18.Trepels T, Zeplin H, Sievert H, Billinger K, Krumsdorf U, Zadan E, Horvath K: Cardiac perforation following transcatheter PFO closure. Cathet Cardiovasc Intervent 2003,58:111–113. [DOI] [PubMed] [Google Scholar]
- 19.Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS: Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: Review of registry of complications and recommendations to minimize future risk. Cathet Cardiovasc Intervent 2004, 63:496–502. [DOI] [PubMed] [Google Scholar]
- 20.Amplatzer Septal Occluder and Delivery System: Instructions for Use. professional.sjm.com 2012, [no volume].
- 21.Rare Serious Erosion Events Associated with St. Jude Amplatzer Atrial Septal Occluder (ASO) [Internet] United States Food and Drug Administration; 2013. [Google Scholar]
- 22.Mallula K, Amin Z: Recent Changes in Instructions for Use for the Amplatzer Atrial Septal Defect Occluder: How to Incorporate These Changes While Using Transesophageal Echocardiography or Intracardiac Echocardiography? Pediatr Cardiol 2012, 33:995–1000. [DOI] [PubMed] [Google Scholar]
- 23.DiBardino DJ, McElhinney DB, Kaza AK, Mayer JE Jr: Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg 2009, 137:1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaney JW, Li JS, Rhodes JF: Major Complications Associated with Transcatheter Atrial Septal Occluder Implantation: A Review of the Medical Literature and the Manufacturer and User Facility Device Experience (MAUDE) Database. Cong Heart Dis 2007, 2:1–9. [DOI] [PubMed] [Google Scholar]
- 25.DiBardino DJ, Mayer JE Jr: Continued controversy regarding adverse events after Amplatzer septal device closure: Mass hysteria or tip of the iceberg? J Thorac Cardiovasc Surg 2011, 142:222–223. [DOI] [PubMed] [Google Scholar]
- 26.Diab K, Kenny D, Hijazi ZM: Erosions, erosions, and erosions! Device closure of atrial septal defects: How safe is safe? Cathet Cardiovasc Intervent 2012, 80:168–174. [DOI] [PubMed] [Google Scholar]
- 27.Moore J, Hegde S, El-Said H, Beekman R, Benson L, Bergersen L, Holzer R, Jenkins K, Ringel R, Rome J, et al. : Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv 2013, 6:433–442. [DOI] [PubMed] [Google Scholar]
- 28.McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z: Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation 2016, 133:1738–1746. [DOI] [PubMed] [Google Scholar]
- 29.Taggart NW, Dearani JA, Hagler DJ: Late erosion of an Amplatzer septal occluder device 6 years after placement. J Thorac Cardiovasc Surg 2011, 142:221–222. [DOI] [PubMed] [Google Scholar]
- 30.Roberts WT, Parmar J, Rajathurai T: Very late erosion of Amplatzer septal occluder device presenting as pericardial pain and effusion 8 years after placement. Catheter Cardiovasc Interv 2013, 82:E592–4. [DOI] [PubMed] [Google Scholar]
- 31.Amin Z: Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement. [Internet]. Catheter Cardiovasc Interv 2014, 83:84–92. [DOI] [PubMed] [Google Scholar]
- 32.Petit CJ, Justino H, Pignatelli RH, Crystal MA, Payne WA, Ing FF: Percutaneous Atrial Septal Defect Closure in Infants and Toddlers: Predictors of Success. Pediatr Cardiol 2012, 34:220–225. [DOI] [PubMed] [Google Scholar]
- 33.O’Byrne ML, Glatz AC, Goldberg DJ, Shinohara R, Dori Y, Rome JJ, Gillespie MJ: Accuracy of Transthoracic Echocardiography in Assessing Retro-aortic Rim prior to Device Closure of Atrial Septal Defects. Cong Heart Dis 2014, doi: 10.1111/chd.12226. [DOI] [PMC free article] [PubMed]
- 34.Butera G, Romagnoli E, Carminati M, Chessa M, Piazza L, Negura D, Giamberti A, Abella R, Pomè G, Condoluci C, et al. : Treatment of isolated secundum atrial septal defects: Impact of age and defect morphology in 1,013 consecutive patients. Am Heart J 2008, 156:706–712. [DOI] [PubMed] [Google Scholar]
- 35.Kutty S, Abu Hazeem A, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA: Long-Term (5- to 20-Year) Outcomes After Transcatheter or Surgical Treatment of Hemodynamically Significant Isolated Secundum Atrial Septal Defect. Am J Cardiol 2012, 109:1348–1352. [DOI] [PubMed] [Google Scholar]
- 36.Kotowycz MA, Therrien J, Ionescu-Ittu R, Owens CG, Pilote L, Martucci G, Tchervenkov C, Marelli AJ: Long-Term Outcomes After Surgical Versus Transcatheter Closure of Atrial Septal Defects in Adults. JACC Cardiovasc Interv 2013, 6:497–503. [DOI] [PubMed] [Google Scholar]
- 37.Moore JW, Vincent RN, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Li Y, Ringel R, et al. : Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the National Cardiovascular Data Registry. [Internet]. J Am Coll Cardiol 2014, 64:2439–2451. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, et al. : An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009, 138:1139–1153. [DOI] [PubMed] [Google Scholar]
- 39.Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, Kogon B, Oster ME: Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. [Internet]. JACC Cardiovasc Interv 2016, 9:79–86. [DOI] [PubMed] [Google Scholar]
- 40.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC: Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. [Internet]. Am Heart J 2015, 169:727–735.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyss Y, Quandt D, Weber R, Stiasny B, Weber B, Knirsch W, Kretschmar O: Interventional Closure of Secundum Type Atrial Septal Defects in Infants Less Than 10 Kilograms: Indications and Procedural Outcome. J Interv Cardiol 2016, 29:646–653. [DOI] [PubMed] [Google Scholar]
- 42.Abu-Tair T, Wiethoff CM, Kehr J, Kuroczynski W, Kampmann C: Transcatheter Closure of Atrial Septal Defects using the GORE® Septal Occ luder in Children Less Than 10 kg of Body Weight [Internet] 2016, 37:778–783. [DOI] [PubMed] [Google Scholar]
- 43.Tanghöj G, Odermarsky M, Naumburg E, Liuba P: Early Complications After Percutaneous Closure of Atrial Septal Defect in Infants with Procedural Weight Less than 15 kg. Pediatr Cardiol 2016, 38:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer G, Smevik B, Kramer HH, Bjørnstad PG: Catheter-based closure of atrial septal defects in the oval fossa with the Amplatzer® devic e in patients in their first or second year of life. Cathet Cardiovasc Intervent 2009, 73:949–955. [DOI] [PubMed] [Google Scholar]
- 45.Fraisse A, Losay J, Bourlon F, Agnoletti G, Lusson JR, Godart F, De Geeter B, Petit J, Piechaud JF: Efficiency of transcatheter closure of atrial septal defects in small and symptomatic children. Cardiol Young 2008, 18:343–347. [DOI] [PubMed] [Google Scholar]
- 46.Campbel M: Natural history of atrial septal defect. Br Heart J 1970, 32:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig RJ, Selzer A: Natural History and Prognosis of Atrial Septal Defect. Circulation 1968, 37:805–815. [DOI] [PubMed] [Google Scholar]
- 48.Andersen M, Lyngborg K, Moller I, Wennevold A: The natural history of small atrial septal defects: Long-term follow-up with serial heart catheterizations. Am Heart J 1976, 92:302–307. [DOI] [PubMed] [Google Scholar]
- 49.Radzik D, Davignon A, van Doesburg N, Fournier A, Marchand T, Ducharme G: Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol 1993, 22:851–853. [DOI] [PubMed] [Google Scholar]
- 50.Hanslik A, Pospisil U, Salzer-Muhar U, Greber-Platzer S, Male C: Predictors of Spontaneous Closure of Isolated Secundum Atrial Septal Defect in Children: A Longitudinal Study. Pediatr 2006, 118:1560–1565. [DOI] [PubMed] [Google Scholar]
- 51.Helgason H, Jonsdottir G: Spontaneous closure of atrial septal defects. Pediatr Cardiol 1999, 20:195–1999. [DOI] [PubMed] [Google Scholar]
- 52.McMahon CJ, Feltes TF, Fraley JK, Bricker JT, Grifka RG, Tortoriello TA, Blake R, Bezold LI: Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart 2002, 87:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Byrne ML, Kennedy KF, Rome JJ, Glatz AC: Variation in practice patterns in device closure of atrial septal defects and patent ductus arteriosus: An analysis of data from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2018, 196:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacha E, Kalfa D: Minimally invasive paediatric cardiac surgery. Nat Rev Cardiol 2014, 11:24–34. [DOI] [PubMed] [Google Scholar]
- 55.Lee H, Yang J-H, Jun T-G, Kang I-S, Huh J, Park SW, Song J, Kim CS: The Mid-term Results of Thoracoscopic Closure of Atrial Septal Defects. Korean Circ J 2017, 47:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burkhart HM, Suri RM: Minimally invasive video assisted surgical closure of secundum atrial septal defect. Ann. Cardiothorac. Surg 2017, 6:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kodaira M, Kawamura A, Okamoto K, Kanazawa H, Minakata Y, Murata M, Shimizu H, Fukuda K: Comparison of Clinical Outcomes After Transcatheter vs. Minimally Invasive Cardiac Surgery Closure for Atrial Septal Defect. Circ. J 2017, 81:543–551. [DOI] [PubMed] [Google Scholar]
- 58.Schneeberger Y, Schaefer A, Conradi L, Brickwedel J, Reichenspurner H, Kozlik-Feldmann R, Detter C: Minimally invasive endoscopic surgery versus catheter-based device occlusion for atrial septal defects in adults: reconsideration of the standard of care. Interactive CardioVascular and Thoracic Surgery 2016, doi: 10.1093/icvts/ivw366. [DOI] [PubMed]
- 59.Ferro JM, Massaro AR, Mas J-L: Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol 2010, 9:1085–1096. [DOI] [PubMed] [Google Scholar]
- 60.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL: Closure of Patent Foramen Ovale versus Medical Therapy after Cryptogenic Stroke. N. Engl. J. Med 2013, 368:1092–1100. [DOI] [PubMed] [Google Scholar]
- 61.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, et al. : Percutaneous closure of patent foramen ovale in cryptogenic embolism. N. Engl. J. Med 2013, 368:1083–1091. [DOI] [PubMed] [Google Scholar]
- 62.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg MJ, et al. : Closure or medical therapy for cryptogenic stroke with patent foramen ovale. … Journal of Medicine 2012, 366:991–999. [DOI] [PubMed] [Google Scholar]
- 63.Messé SR, Gronseth G, Kent DM, Kizer JR, Homma S, Rosterman L, Kasner SE : Practice advisory: Recurrent stroke with patent foramen ovale (update of practice parameter). Neurol 2016, 87:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sondergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, et al. : Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N. Engl. J. Med 2017, 377:1033–1042. [DOI] [PubMed] [Google Scholar]
- 65.Mas J-L, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, et al. :Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N. Engl. J. Med 2017, 377:1011–1021. [DOI] [PubMed] [Google Scholar]
- 66.Akobeng AK, Abdelgadir I, Boudjemline Y, Hijazi ZM: Patent foramen ovale (PFO) closure versus medical therapy for prevention of recurrent stroke in patients with prior cryptogenic stroke: A systematic review and meta-analysis of randomized controlled trials. Cathet Cardiovasc Intervent 2018, 92:165–173. [DOI] [PubMed] [Google Scholar]
- 67.Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL: Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N. Engl. J. Med 2017, 377:1022–1032. [DOI] [PubMed] [Google Scholar]
- 68.Schoen SP, Boscheri A, Lange SA, Braun MU, Fuhrmann J, Kappert U, Strasser RH: Incidence of aortic valve regurgitation and outcome after percutaneous closure of atrial septal defects and patent foramen ovale. Heart 2008, 94:844–847. [DOI] [PubMed] [Google Scholar]
- 69.Wöhrle J, Kochs M, Spiess J, Nusser T, Hombach V, Merkle N: Impact of percutaneous device implantation for closure of patent foramen ovale on valve insufficiencies. Circulation 2009, 119:3002–3008. [DOI] [PubMed] [Google Scholar]
- 70.Loar RW, Johnson JN, Cabalka AK, Cetta F, Hagler DJ, Eidem BW, Taggart NW: Effect of percutaneous atrial septal defect and patent foramen ovale device closure on degree of aortic regurgitation. Catheter Cardiovasc Interv 2013, 81:1234–1237. [DOI] [PubMed] [Google Scholar]
- 71.O’Byrne ML, Glatz AC, Sunderji S, Mathew AE, Goldberg DJ, Dori Y, Rome JJ, Gillespie MJ: Prevalence of Deficient Retro-Aortic Rim and Its Effects on Outcomes in Device Closure of Atrial Septal Defects [Internet]. Pediatric Cardiology 2014, 35:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, deVeber GA, Ganesan V, on behalf of the International Pediatric Stroke Study Group: Arterial ischemic stroke risk factors: The international pediatric stroke study. Ann Neurol 2011, 69:130–140. [DOI] [PubMed] [Google Scholar]
- 73.Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, Parker AP, Wassmer E, Wraige E, Amin S, et al. : Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol 2014, 13:35–43. [DOI] [PubMed] [Google Scholar]
- 74.Gerstl L, Weinberger R, Kries von R, Heinen F, Schroeder AS, Bonfert MV, Borggraefe I, Tacke M, Vill K, Landgraf MN, et al. : Risk factors in childhood arterial ischaemic stroke: Findings from a population-based study in Germany. European Journal of Paediatric Neurology 2018, 22:380–386. [DOI] [PubMed] [Google Scholar]
- 75.Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL: Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N. Engl. J. Med 2017, 377:1022–1032. [DOI] [PubMed] [Google Scholar]
- 76.Hara H, Jones TK, Ladich ER, Virmani R, Auth DC, Eichinger JE, Sommer RJ, Van Tassel RA, Schwartz RS: Patent foramen ovale closure by radiofrequency thermal coaptation: first experience in the porcine model and healing mechanisms over time. Circulation 2007, 116:648–653. [DOI] [PubMed] [Google Scholar]
- 77.Sievert H, Fischer E, Heinisch C, Majunke N, Roemer A, Wunderlich N: Transcatheter closure of patent foramen ovale without an implant: initial clinical experience. Circulation 2007, 116:1701–1706. [DOI] [PubMed] [Google Scholar]
- 78.Sievert H, Ruygrok P, Salkeld M, Baumgartner H, Meier B, Windecker S, Juliard J-M, Aubry P, Tiefenbacher C, Krumsdorf U, et al. : Transcatheter closure of patent foramen ovale with radiofrequency: Acute and intermediate term results in 144 patients. Cathet Cardiovasc Intervent 2009, 59:NA–NA. [DOI] [PubMed] [Google Scholar]
- 79.Gaspardone A, De Marco F, Sgueglia GA, De Santis A, Iamele M, D’Ascoli E, Tusa M, Corciu A, Mullen M, Nobles A, et al. : Novel percutaneous suture-mediated patent foramen ovale closure technique: early results of the NobleStitch EL Italian Registry. EuroIntervention 2018, 14:e272–e279. [DOI] [PubMed] [Google Scholar]


