Abstract
Background:
How family environment and parental factors affect health status and symptoms in childhood cancer survivors is understudied. We examined the influence of family cohesion, parent distress, and overprotection on child symptom burden and health-related quality of life (HRQOL) and family strain in survivors of childhood acute lymphoblastic leukemia (ALL).
Methods:
Parents of 213 children treated with chemotherapy-only completed a survey when survivors were at least five-years post-diagnosis. Family Environment Scale, Brief Symptom Inventory-18, Parent Protection Scale, PedsQL, and Impact on Family were used to assess family cohesion, parental distress, overprotection, child symptom burden and HRQOL, and family strain, respectively. Path analysis was conducted to quantify effects of family cohesion on family strain through parental distress, overprotection, child symptoms, and HRQOL.
Results:
Lower family cohesion (β=0.06, 95% CI=0.01 to 0.13), higher parental distress (β=0.35, 95% CI=0.20 to 0.45), and overprotection (β=0.17, 95% CI=0.01 to 0.32) were associated with more child symptom burden. More symptom burden were associated with poorer child HRQOL (β=0.66, 95% CI=0.57 to 0.75), which in turn was associated with more family strain (β=0.11, 95% CI=0.01 to 0.22). Lower maternal education was associated with overprotection (β=−0.23, 95% CI=−0.33 to −0.12), more child symptoms (β=−0.30, 95% CI=−0.41 to −0.16), poorer child HRQOL (β=−0.36, 95% CI=−0.46 to −0.21), and more family strain (β=−0.15, 95% CI=−0.23 to −0.08).
Conclusions:
Family and parental factors contributed to health outcomes of childhood ALL survivors. Interventions to enhance family cohesion, decrease parental distress and overprotection, and ameliorate child symptoms may improve family functioning.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer among children younger than 15 years of age.1 Modern treatments have increased the overall 5-year survival rates to over 85%;2 most survivors, parents, and families adapt well to this traumatic event, indicating psychological competent state and resilience.3,4 However, some survivors are still vulnerable to significant late-effects, which can disrupt family functioning5 as family members must adjust their daily routines to cope with the child’s acute and late effects. Sparse studies have examined how child, parental, and family factors influence health outcomes of childhood cancer survivors in a systematic manner.
Investigating health outcomes and family adjustment among pediatric cancer survivors can be conceptualized by the social-ecology model,6 which articulates that a child’s well-being is dependent on the surrounding environment in addition to personal factors (e.g., age, treatment exposure, etc.). This theory specifically places a child in the center of the model, with concentric circles representing the influence of parental (e.g., parenting style and distress), familial (e.g., cohesion), and community/societal (e.g., health care system) factors drawn outward from the most immediate to more distal levels of influence. Health impacts of ecological factors have been well-articulated in general pediatrics but not in cancer survivorship. For more distal factors, evidence suggests that children with cancer living in families of low expressiveness (i.e., poor communication), low cohesion (i.e., poor emotional bonding), or high conflict are likely to develop somatization (e.g., pain, headache, fatigue, etc.) as part of internalizing/externalizing behavioral problems.5,7 Maintaining a cohesive family environment may prevent children with cancer as well as parents/caregivers from developing emotional disorders.8
Parental factors, including parenting style and psychological states, are key elements of ecological systems influencing a child’s socialization, psychological development, and health status. A recent review study reported that 10-44% of parents and 0-13% of pediatric patients had severe posttraumatic stress symptoms during/after cancer treatments;9 high parental and pediatric posttraumatic stress placed children at risk of symptom burden, typically emotional distress and somatization.10,11 When caring for children with cancer, parents may adopt a protective or indulgent strategy in response to perceived health vulnerability.12 In fact, after a diagnosis of leukemia, 30-40% of parents used preventive-focused parenting strategy with pessimistic communication and restraints on the child’s activities.13 However, parental overprotection has been linked to anxiety,14 depression,15 posttraumatic stress syndrome,16 impulsive behaviors,12 and poor HRQOL13 in children.
Previous childhood cancer research has demonstrated pairwise associations between treatment, family environment, parenting style, parental emotional distress, child health status, and family strain. For example, it is evident that parental stress is a predictor of functional impairment in childhood cancer survivors.17 Low cohesion and higher control within the family are associated with elevated internalizing problems in children with leukemia.18 In addition, family environment mediates associations between parental distress and children’s adjustment to cancer.19 Nevertheless, the complex interplay among clinical factors and parental behaviors within the family system remains unknown. This is a considerable limitation because without applying an ecological framework and advanced analytic methods (e.g., path analysis) to elucidate these complex relationships, clinicians may not identify opportunities to improve health and family outcomes. This study aimed to investigate the influence of family and parental factors on children’s health outcomes and family strain in survivors of childhood ALL, with attention given to identifying potential mechanisms (Figure 1). We hypothesized that low family cohesion would be associated with parent emotional distress and overprotective behavior. This distress and overprotection would place a child at risk of lower HRQOL, through higher symptom burden, especially somatic symptoms. Further, adverse family/parental factors and lower HRQOL would be associated with more family stress.
Figure 1:
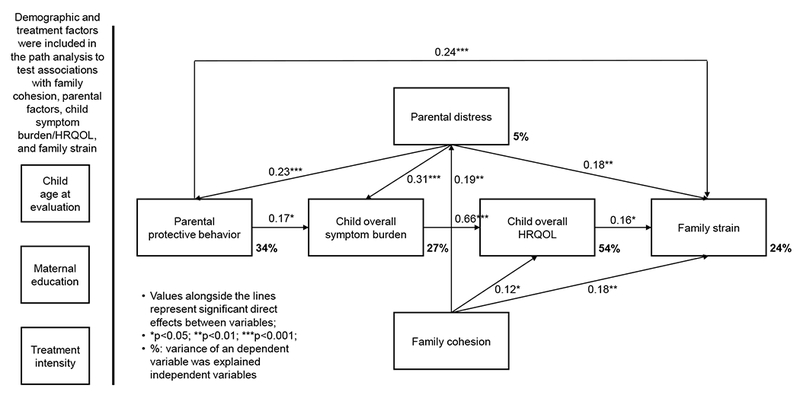
Associations of family and parental factors with child symptom burden, HRQOL, and family strain
METHODS
Participants
Potential participants were parents of 408 ALL childhood survivors treated on the chemotherapy-only protocol “Total Therapy XV” at St. Jude Children’s Research Hospital (SJCRH) from 2000 to 2010.2 This is a cross-sectional study through which we actively recruited all eligible patients for the study participation. For those survivors who were attending annual visits, we coordinated the research appointments with their visit. However, for those not attending annual visits, we scheduled appointments for specific campus visits per their preference. To be eligible, parents must have been a primary guardian of a cancer survivor who was at least five years from diagnosis, and eight years of age at the time of assessment. Parents were excluded (N=88) if their child died (N=35), was treated for a secondary cancer or relapse (N=30), had a pre-existing non-cancer-related neurodevelopmental disorder (N=7), had a genetic disorder associated with cognitive impairment (N=11), had a subsequent brain injury unrelated to cancer (N=4), or if parents were not proficient in English (N=1). Recruitment was not attempted for individuals who were permanently discharged from pediatric follow-up care (N=18; i.e. became independent adults). Of the 302 eligible participants, a survey was completed by parents of 213 cancer survivors (response rate=71%) during follow-up care between February 2010 and December 2015. The protocol was approved by SJCRH Institutional Review Board (reference number: FWA00004775), and all parents provided written informed consent.
Measures
The Family Environment Scale, a 90-item questionnaire, was included to assess environmental characteristics of the family.20 A nine-item family cohesion subscale was used to evaluate the degree of commitment, help, and support that family members provided to each other. Items were rated using a true/false response scale. Mean item scores of the subscale were calculated, with higher scores indicating lower levels of family cohesion.
The Brief Symptom Inventory-18 was used to measure parental emotional distress.21 This scale contains 18 items assessing three aspects of distress (anxiety, depression, and somatization) with six items on each subscale. Items were rated using a five-point Likert scale ranging from “1=not at all” to “5=extremely.” Mean item scores of individual subscales were calculated and transformed to sex-specific T-scores, with higher scores indicating greater distress. Global distress scores calculated from the mean subscale scores were used.
Parental protective behavior was measured using the Parent Protection Scale.22 This scale contains 25 items assessing four overprotection domains (supervision, separation problems, dependence, and control). Items were rated using a four-point Likert scale ranging from “0=never” to “3=always.” Mean item scores were calculated, with higher scores indicating higher levels of parental protective behavior.
The proxy-version of the PedsQL-Cancer Module version-323 was used to measure symptom burden (i.e., perceived abnormal physical, emotional, and cognitive symptoms). PedsQL-Cancer contains 27 items assessing eight domains: pain/hurt, nausea, procedural anxiety, treatment anxiety, worry, perceived cognitive problems, perceived physical appearance, and communication. A classical HRQOL framework,24 and our childhood survivorship publication25 suggest that symptom burden is a key determinant of HRQOL deficits. Items were rated using a five-point Likert scale ranging from “0=never” to “4=almost always.” Per scoring instruction, the overall symptom scores were calculated based on the mean subscale scores. Higher scores indicate higher symptom burden.
The proxy-version of the PedsQL-Generic Core Module version-426 was used to measure child HRQOL (i.e., whether disease/treatment influences one’s daily activities). PedsQL-Generic contains 23 items assessing four domains: physical, emotional, social, and school functioning. Items were rated using a five-point Likert scale ranging from “0=never” to “4=almost always.” Per scoring instruction, the overall HRQOL scores were calculated based on the mean subscale scores. Higher scores indicate higher HRQOL.
The Impact on Family scale was used to measure family strain.27 This scale contains 33 items assessing four family impact domains: financial burden, familial/social impact, personal strain, and mastery. Items were rated using a four-point Likert scale ranging from “1=strongly agree” to “4=strongly disagree.” Per scoring instruction, mean scores of 24 selected items with valid measurement properties were calculated, with higher scores indicating higher family strain.
Statistical Analysis
Pearson’s correlation coefficients were examined for associations among variables of interest. Path analytic methodology was used to test associations among multiple variables (Figure 1). In contrast to the use of traditional regression models to analyze effects of an independent variable on a dependent variable, path analysis possesses the advantage for understanding the mechanisms among multiple variables. This approach typically introduces a set of linear regressions simultaneously in the same framework, where a dependent variable occurs through independent variables in a single regression, and a dependent variable used in one regression model serves as an independent variable in other regressions.28
Specifically, path analysis quantified associations of independent variables (e.g., family cohesion) with dependent variables (e.g., family strain) through the influence of mediators (e.g., parental emotional distress, protective behavior, child symptoms, and child HRQOL). The effect of independent variables on dependent variables can be differentiated by the direct effects and indirect effects through the influence of mediating variables. Important covariates (age at evaluation, treatment intensity, maternal education, etc.) were included in the analysis if each of these variables were significantly (p<0.1) associated with family cohesion, parental emotional distress, protective behavior, child symptoms, HRQOL, or family strain in bivariate analyses. Overall symptom scores instead of eight respective symptom domain scores were used in path modeling for the primary results. Our factor analysis revealed an essential unidimensional solution for all symptom domains (an eigenvalue>8.5). Chi-square statistic, root-mean-square error of approximation (RMSEA), and comparative fit index (CFI) were estimated to evaluate the model fit of path analysis, with non-significant chi-square, RMSEA ≤0.06 and CFI ≥0.95 for good model fit. We also tested two plausible alternative models by flipping the order of variables in path analysis: alternative model 1 assumes that parental distress influences family cohesion, which in turn influences parental protective behaviors and so on; alternative model 2 assumes that child symptoms influence parental distress, which in turn influences child HRQOL and so on. All analyses were performed using Mplus 7.4 (Muthen & Muthen, Los Angeles, CA), and 2-sided tests (significance level 0.05) were implemented.
RESULTS
Characteristics of Survivors and Parents
The majority of survivors were male (51.2%) and non-Hispanic white (73.7%; Table 1). The mean ages at diagnosis and evaluation were 6.6 (SD 4.5) and 14.3 (SD 4.8) years, respectively. More than 42% of survivors received standard/high risk treatment. The mean years of maternal education was 13.7 (SD 2.6) years.
Table 1:
Characteristics of children and parents and outcomes of interest (N=213)
| Demographics | Mean (SD)/Range | Median (IQR) |
|---|---|---|
| Child age at diagnosis (years) | 6.6 (4.5)/1-18 | 5.0 (5.5) |
| Child age at evaluation (years) | 14.3 (4.8)/8-23 | 13.2 (7.1) |
| Maternal education (years) | 13.7 (2.6)/4-20 | 13.0 (4.0) |
| Child’s sex | N (percentage) | |
| Male | 109 (51.2%) | |
| Female | 104 (48.8%) | |
| Parent’s sex | ||
| Male | 40 (18.8%) | |
| Female | 173 (81.2%) | |
| Child’s race/ethnicity | N (percentage) | |
| White, non-Hispanic | 157 (73.7%) | |
| Black, non-Hispanic | 27 (12.7%) | |
| Hispanic | 17 (8.0%) | |
| Other | 12 (5.6%) | |
| Treatment Factors | Mean (SD) | Median (IQR) |
| Time since diagnosis (years) | 7.7 (1.7) | 7.5 (2.7) |
| Treatment intensity | N (percentage) | |
| Standard/High risk | 91 (42.7%) | |
| Low risk | 122 (57.3%) | |
| Survey Outcomes | Mean (SD) | Median (IQR) |
| Family cohesiona | 2.4 (1.5) | 2.0 (2.0) |
| Parental distressa, b | 42.6 (9.6) | 39.0 (11.0) |
| Parental protective behaviora, b | 23.9 (7.9) | 24.0 (9.5) |
| Child overall symptom burden c | 84.5 (14.8) | 83.5 (21.0) |
| Child HRQOLc | 80.6 (15.9) | 84.5 (21.0) |
| Family straina | 44.6 (13.8) | 43.0 (22.0) |
IQR: Interquartile range
Higher scores indicate worse outcomes
Based on clinically meaningful cut-points, 16% of parents of childhood cancer survivors had global distress, and 22% of parents engaged in overprotective behaviors
Inter-Correlations among Variables
Lower family cohesion, higher parental emotional distress, and excessive parental protective behavior were significantly associated with more child symptom burden, poorer child HRQOL, and higher family strain (Table 2). Collinearity among variables can limit interpretation of path analyses; though given only moderate correlations among variables of interest, collinearity is less of a concern in the present analysis.
Table 2:
| Family cohesion | Parental distress | Parental protective behavior | Child overall symptom burden | Child HRQOL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r (95% CI) | p-value | r (95% CI) | p-value | r (95% CI) | p-value | r (95% CI) | p-value | r (95% CI) | p-value | |
| Parental distress | 0.16 (0.02, 0.29) | 0.03 | ||||||||
| Parental protective behavior | −0.01 (−0.15. 0.13) | 0.89 | 0.29 (0.16, 0.42) | <0.001 | ||||||
| Child overall symptom burden | 0.18 (0.04, 0.31) | 0.01 | 0.38 (0.25, 0.49) | <0.001 | 0.29 (0.16, 0.42) | <0.001 | ||||
| Child HRQOL | 0.28 (0.14, 0.40) | <0.001 | 0.28 (0.15, 0.41) | <0.001 | 0.25 (0.11, 0.38) | <0.001 | 0.70 (0.62, 0.77) | <0.001 | ||
| Family strain | 0.28 (0.14, 0.40) | <0.001 | 0.34 (0.21, 0.46) | <0.001 | 0.33 (0.20, 0.45) | <0.001 | 0.38 (0.25, 0.50) | <0.001 | 0.33 (0.20, 0.45) | <0.001 |
r represents the Person’s correlation coefficient
Higher scores indicate worse outcomes for all variables (for the purpose of interpretation among variables)
Associations of Cohesion and Parental Factors on Symptoms, HRQOL, and Family Strain
Model fit statistics indicate satisfactory results for associations among variables of interest: chi-square statistic=23.7 (p=0.10), RMSEA=0.05, and CFI=0.98. Standardized coefficients for the direct and indirect effects are reported in Figure 1 and Table 3. Results suggest that parental emotional distress was the most significant factor directly and indirectly related to family strain (total effect β=0.28, 95% CI=0.14 to 0.41; p<0.001; Table 3). Specifically, parental distress was associated with family strain through the mechanisms of higher protective behavior, more child symptom burden, and poorer child HRQOL (indirect effect: 32.1% of the total effect; Table 3). Excessive parental protective behavior was the second most significant factor directly and indirectly associated with family strain (total effect β=0.26, 95% CI=0.10 to 0.40; p=0.001; direct effect: 85.7% of total effect; Table 3). Child symptom burden was the most significant factor directly alone associated with poor HRQOL of survivors (total effect β=0.66, 95% CI=0.57 to 0.75; p<0.001; direct effect: 100% of total effect; Table 3). Parental distress was the second most significant factor indirectly alone associated with poor HRQOL of survivors through the mechanism of child symptoms (total effect β=0.23, 95% CI=0.14 to 0.32; p<0.001; indirect effect: 100% of total effect; Table 3). Additionally, parental distress was the most significant factor directly and indirectly associated with child symptoms (total effect β=0.35, 95% CI=0.20 to 0.45; p<0.001; direct effect: 88.6% of total effect; Table 3), and directly associated with parental protective behavior (total effect β=0.23, 95% CI=0.11 to 0.35; p<0.001; Figure 1). Low family cohesion was directly alone associated with parental distress (total effect β=0.19, 95% CI=0.03 to 0.34; p=0.02; Figure 1). Compared to the primary model, fit indices of two alternative models (particularly alternative model 2) were reasonable; however, associations between parental distress and family cohesion, and between symptom burden and overprotection became not-significant (Supplement Table S1a/S1b).
Table 3:
Impact of demographic, treatment, family, and parental factors on child symptom burden and HRQOL, and family straina,b,c
| Total effect | Indirect effect | Variance explained (%)d | |||
|---|---|---|---|---|---|
| β (95% CI)a,b | p-value | β (95% CI)a,c | p-value | ||
| Child overall symptom burdene | 26.6% | ||||
| Treatment intensityf | 0.24 (0.11, 0.36) | <0.001 | 0 | n.a.g | |
| Age at evaluation | −0.13 (−0.21, −0.05) | 0.002 | −0.13 (−0.21, −0.05) | 0.002 | |
| Maternal education | −0.30 (−0.41, −0.16) | <0.001 | −0.05 (−0.10, −0.01) | 0.029 | |
| Family cohesione | 0.06 (0.01, 0.13) | 0.039 | 0.06 (0.01, 0.13) | 0.039 | |
| Parental distresse | 0.35 (0.20, 0.45) | <0.001 | 0.04 (0.01, 0.10) | 0.065 | |
| Parental protective behaviore | 0.17 (0.01, 0.32) | 0.035 | 0 | n.a. | |
| Child HRQOLe | 54.3% | ||||
| Treatment intensity | 0.16 (0.07, 0.25) | <0.001 | 0.16 (0.07, 0.25) | <0.001 | |
| Age at evaluation | −0.08 (−0.14, −0.03) | 0.003 | −0.08 (−0.14, −0.03) | 0.003 | |
| Maternal education | −0.36 (−0.46, −0.21) | <0.001 | −0.22 (−0.31, −0.12) | <0.001 | |
| Family cohesion | 0.17 (0.06, 0.28) | 0.002 | 0.04 (0.01, 0.10) | 0.040 | |
| Parental distress | 0.23 (0.14, 0.32) | <0.001 | 0.23 (0.14, 0.32) | <0.001 | |
| Parental protective behavior | 0.11 (0.01, 0.23) | 0.046 | 0.11 (0.01, 0.23) | 0.046 | |
| Child overall symptom burden | 0.66 (0.57, 0.75) | <0.001 | 0 | n.a. | |
| Family straine | 23.8% | ||||
| Treatment intensity | 0.03 (0.00, 0.07) | 0.095 | 0.03 (0.00, 0.07) | 0.095 | |
| Age at evaluation | −0.16 (−0.24, −0.09) | <0.001 | −0.16 (−0.24, −0.09) | <0.001 | |
| Maternal education | −0.15 (−0.23, −0.08) | <0.001 | −0.15 (−0.23, −0.08) | <0.001 | |
| Family cohesion | 0.25 (0.11, 0.36) | <0.001 | 0.07 (0.02, 0.15) | 0.021 | |
| Parental distress | 0.28 (0.14, 0.41) | <0.001 | 0.09 (0.05, 0.15) | 0.001 | |
| Parental protective behavior | 0.26 (0.10, 0.40) | 0.001 | 0.02 (0.00, 0.06) | 0.189 | |
| Child overall symptom burden | 0.11 (0.01, 0.22) | 0.047 | 0.11 (0.01, 0.22) | 0.047 | |
| Child HRQOL | 0.17 (0.01, 0.33) | 0.039 | 0 | n.a. | |
Overall model fit indices: X2-statistic=23.7 (p=0.10); RMSEA=0.05; CFI=0.98.
β represents standardized coefficients: the magnitude of the effects in 1SD unit
Total effect represents direct effects plus indirect effects
Direct effect was reported in Figure 1
% of total variance in specific dependent/outcome variable explained by independent variables
Higher scores indicate worse outcomes for all variables (for the purpose of interpretation among variables)
Standard/high vs. low risk of treatment
p-value is not applicable
Associations of Demographic and Treatment Factors on Cohesion, Parental Factors, Symptoms, HRQOL, and Family Strain
Lower maternal education was related to lower family cohesion (total effect β=−0.17, 95% CI=−0.31 to −0.01; p=0.03), excessive protective behavior (total effect β=−0.23, 95% CI=−0.33 to −0.12; p<0.001), more child symptom burden (total effect β=−0.30, 95% CI=−0.41 to −0.16; p<0.001; Table 3), poorer HRQOL of survivors (total effect β=−0.36, 95% CI=−0.46 to −0.21; p<0.001; Table 3), and family strain (total effect β=−0.15, 95% CI=−0.23 to −0.08; p<0.001; Table 3). Younger age at evaluation was related to excessive protective behavior (total effect β=−0.47, 95% CI=−0.59 to −0.31; p<0.001). Receiving more intensive chemotherapy in the standard/high risk arm versus the low risk arm was related to more child symptom burden (total effect β=0.24, 95% CI=0.11 to 0.36; p<0.001; Table 3).
Accounting for demographic and treatment variables, family cohesion, parental emotional distress and protective behavior, symptom burden, and HRQOL together explained 24% of variance in family strain (Figure 1). Family cohesion, parental distress, protective behavior, and symptom burden explained 54% of variance in HRQOL. Family cohesion, parental distress, and protective behavior explained 27% of variance in symptom burden. Family cohesion and parental distress explained 34% of variance in parental protective behavior. However, family cohesion explained only 5% of variance in parental distress.
DISCUSSION
This study demonstrates that higher treatment intensity, lower family cohesion, and more parental emotional distress and overprotective behavior function as a constellation of factors that was simultaneously related to more child symptom burden. More symptom burden significantly limited child HRQOL. Low child HRQOL, parental distress, and low family cohesion were significantly associated with higher family strain. Understanding this pathway to poor child health status and family strain leads to multiple opportunities for a design of social-ecological-based clinical interventions (e.g., managing early child symptoms, treating parental distress, coaching parenting behavior, and supporting family to improve communication) that is beyond the traditional rehabilitation or functional recovery approach.29
This study demonstrated that family and parental factors play a role in health outcomes of childhood cancer survivors. Specifically, we found that parental distress instead of low family cohesion was likely to directly contribute to overprotective behavior, and low family cohesion may exacerbate child overall symptom burden (specifically somatic symptoms such as pain) and compromise HRQOL through parental distress and overprotective behavior. In general pediatrics, prevalence of somatizations (headache, musculoskeletal, abdominal pain, fatigue, etc.) is 10–50%, depending upon the research methodologies.30 However, evidence suggests that parental overprotection may reinforce pediatric symptom burden in the form of somatization,31 which has been linked to functional impairment (e.g., missing school days, dropping out of sport activities or social connection, etc.). Future studies are encouraged to investigate this issue in childhood cancer survivors. From a broader social-ecological viewpoint, available social supports in the community and effective health care systems may also have an influence on health outcomes of cancer survivors, which in turn affects family functioning.32
HRQOL and family cohesion of our samples were comparable to that of healthy samples,20,33 suggesting psychological resilience. However, family strain of our samples was higher than healthy ones,34 and 16% of our parents had clinically meaningful global distress.21 Parental distress can be elevated if survivors are treated in facilities that require the family to relocate, due to difficulty of transition, a reduction in social support, job stress, etc.35,36 Per scoring instructions,22 22% of our parents engaged in overprotective behavior, which is equivalent type-I diabetic children who require regular blood sugar monitoring.37 Nevertheless, we found that parental overprotective behavior was indeed associated with higher child symptoms and poorer HRQOL. Overprotection may be counterproductive following therapy completion because it can prevent children from achieving independence and autonomy. Future research is warranted to explore the effects of specific developmentally-appropriate parenting strategies on outcomes of childhood cancer survivors.
Both parental emotional distress and overprotection had a stronger association with more child health status. Specifically, more parental distress and/or overprotective style were associated with more nausea, procedure anxiety, treatment procedures, perceived cognitive problems, physical appearance problems, and communication problems (Supplement Tables S2a-S2h). Compared with on-therapy patients,23 symptom issues of our survivors were less severe, but the magnitude did not differ by the meaningful difference threshold (i.e., 0.5SD) across the majority of domains, except for nausea, treatment anxiety, and procedure anxiety. The significant associations of overall symptom burden and somatization (e.g., pain) with treatment intensity further suggest that high symptom burden is associated with cancer treatment. Our finding suggests that as a result of medical traumatic stress, parental distress could directly and indirectly (via overprotective behavior) worsen the manifestation of overall symptom burden and specific somatic symptoms. Not surprisingly, children with more symptom burden had poorer HRQOL. Although this study was based on a cross-sectional design, our findings do provide support for conducting longitudinal studies to elucidate causality and directionality among family and parental factors and child health outcomes.
We found that lower maternal education was significantly related to low family cohesion, overprotective behavior, more child symptoms, and poor HRQOL. However, impact of low maternal education on the child’s symptom burden and HRQOL was mediated or explained by parental distress and overprotective behavior. Parents with lower education may be more likely to be unaware of risk for late-effects of cancer therapy and/or management of such risk, leading to more distress and overprotective behavior.
Study Limitations
This study has several limitations. First, study samples were recruited from a single pediatric cancer center, which may limit generalizability. Second, parental emotional distress, child symptoms, and HRQOL were reported by parents. When survivors undertook an on-campus medical evaluation (6-8 hours per day), we invited parents to participate in a survey. We did not ask children to complete the survey to avoid the burden of research participation. Therefore, associations of parental distress with child symptoms and HRQOL may be overestimated. Third, parental distress and protective behavior were collected from one parent, and the majority of the participating parents were mothers. There is evidence that fathers with better mental health may buffer the influence of mothers’ poorer mental health on children’s emotional and behavior problems.38 In addition, we did not collect detailed socio-demographic data (e.g., marital status, family income, etc.) from parents, which may confound associations among variables of interest. Finally, as mentioned previously, the use of cross-sectional design precludes the conclusion of temporal or causal relations among the family, parental, and child variables. It is possible that pre-morbid factors such as child and parental psychiatric history, overprotective parenting behaviors, family dynamics, and socioeconomic status may affect psychosocial adjustment and health outcomes of childhood cancer survivors.39,40 Although a collection of aforementioned pre-morbid data may be challenging during the acute therapeutic stage, the use of social worker notes or a brief screening tool to collect these risk factors may be a practical solution. Future studies utilizing a longitudinal framework to collect pre- and post-morbid data, and performing interventional designs are warranted to affirm temporal or causal relations.
Clinical Implications
This study lays the ground work for clinical practice. Given the influence of family cohesion and parental factors (e.g., protective behavior, emotional distress, and education), initial assessment for family cohesion, parental distress, and parenting overprotection at the acute care stage, as well as continuous monitoring through survivorship to identify problems for early interventions on maintaining family cohesion and adjusting overprotective behavior, may be an avenue for improving child and family outcomes.
In summary, family dynamics and parental factors are associated with symptom burden, suboptimal HRQOL, and poor family functioning of childhood ALL survivors. Interventions to address family cohesion, parental distress, and overprotection may improve child symptom, HRQOL, and family functioning.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
National Institutes of Health (MH085849 [to KRK], CA195547 [to MMH and LLR] and CA21765 [CORE]) and the American Lebanese Syrian Associated Charities
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. New Engl J Med 2009;360(26):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phipps S, Long A, Willard VW, et al. Parents of children with cancer: at-risk or resilient? J Pediatr Psychol 2015;40(9):914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillery R, Howard Sharp KM, Okado Y, Long A, Phipps S. Profiles of resilience and growth in youth with cancer and healthy comparisons. J Pediatr Psychol 2016;41(3):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderfer MA, Navsaria N, Kazak AE. Family functioning and posttraumatic stress disorder in adolescent survivors of childhood cancer. J Fam Psychol 2009;23(5):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronfenbrenner U Ecology of the family as a context for human development: research perspectives. Dev Psychol 1986;22(6): 723–742. [Google Scholar]
- 7.Pelcovitz D, Libov BG, Mandel F, Kaplan S, Weinblatt M, Septimus A. Posttraumatic stress disorder and family functioning in adolescent cancer. J Trauma Stress. 1998;11(2):205–221. [DOI] [PubMed] [Google Scholar]
- 8.Phipps S, Dunavant M, Lensing S, Rai SN. Psychosocial predictors of distress in parents of children undergoing stem cell or bone marrow transplantation. J Pediatr Psychol 2005;30(2):139–153. [DOI] [PubMed] [Google Scholar]
- 9.Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. Lancet Psychiatry. 2017;4(4):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steele RG, Dreyer ML, Phipps S. Patterns of maternal distress among children with cancer and their association with child emotional and somatic distress. J Pediatr Psychol 2004;29(7):507–517. [DOI] [PubMed] [Google Scholar]
- 11.Holley AL, Wilson AC, Noel M, Palermo TM. Post-traumatic stress symptoms in children and adolescents with chronic pain: a topical review of the literature and a proposed framework for future research. Eur J Pain. 2016;20(9):1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmbeck GN, Johnson SZ, Wills KE, et al. Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: the mediational role of behavioral autonomy. J Consult Clin Psychol 2002;70(1):96–110. [DOI] [PubMed] [Google Scholar]
- 13.Eiser C, Richard Eiser J, Greco V. Parenting a child with cancer: promotion and prevention-focused parenting. Pediatr Rehabil 2002;5(4):215–221. [DOI] [PubMed] [Google Scholar]
- 14.Spada MM, Caselli G, Manfredi C, et al. Parental overprotection and metacognitions as predictors of worry and anxiety. Behav Cogn Psychother 2012;40(3):287–296. [DOI] [PubMed] [Google Scholar]
- 15.Stein D, Williamson DE, Birmaher B, et al. Parent-child bonding and family functioning in depressed children and children at high risk and low risk for future depression. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1387–1395. [DOI] [PubMed] [Google Scholar]
- 16.Bokszczanin A Parental support, family conflict, and overprotectiveness: predicting PTSD symptom levels of adolescents 28 months after a natural disaster. Anxiety Stress Coping. 2008;21(4):325–335. [DOI] [PubMed] [Google Scholar]
- 17.Hile S, Erickson SJ, Agee B, Annett RD. Parental stress predicts functional outcome in pediatric cancer survivors. Psychooncology. 2014;23(10):1157–1164. [DOI] [PubMed] [Google Scholar]
- 18.Morris JAB, Blount RL, Cohen LC, Frank NC, Madan-Swain A, Brown RT. Family functioning and behavioral adjustment in children with leukemia and their healthy peers. Child Health Care 1997;26(2):61–75. [Google Scholar]
- 19.Robinson KE, Gerhardt CA, Vannatta K, Noll RB. Parent and family factors associated with child adjustment to pediatric cancer. J Pediatr Psychol 2007;32(4):400–410. [DOI] [PubMed] [Google Scholar]
- 20.Moos R, Moos B. Family Environment Scale Manual: Development, Applications, Research. 4th Ed. Palo Alto, CA: Consulting Psychologist Press; 1997. [Google Scholar]
- 21.Derogatis L Brief Symptom Inventory 18: Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 22.Thomasgard M, Metz WP, Edelbrock C, Shonkoff JP. Parent-child relationship disorders. Part I. Parental overprotection and the development of the Parent Protection Scale. J Dev Behav Pediatr 1995;16(4):244–250. [PubMed] [Google Scholar]
- 23.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 25.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort study. J Clin Oncol 2013;31(33):4242–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 27.Stein RE, Riessman CK. The development of an impact-on-family scale: preliminary findings. Med Care 1980;18(4):465–472. [DOI] [PubMed] [Google Scholar]
- 28.Kline RB. Principles and Practice of Structural Equation Modeling. 4th Ed. New York, NY: The Guilford Press; 2015. [Google Scholar]
- 29.Kazak AE. Evidence-based interventions for survivors of childhood cancer and their families. J Pediatr Psychol 2005;30(1):29–39. [DOI] [PubMed] [Google Scholar]
- 30.Campo JV. Annual research review: functional somatic symptoms and associated anxiety and depression--developmental psychopathology in pediatric practice. J Child Psychol Psychiatry. 2012;53(5):575–592. [DOI] [PubMed] [Google Scholar]
- 31.Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. Pain. 2006;122(1-2):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore AR, Buchanan ND, Fairley TL, Lee Smith J. Public health action model for cancer survivorship. Am J Prev Med 2015;49(6 Suppl 5):S470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cronin CM, Shapiro CR, Casiro OG, Cheang MS . The impact of very low-birth-weight infants on the family is long lasting. A matched control study. Arch Pediatr Adolesc Med 1995;149(2):151–158. [DOI] [PubMed] [Google Scholar]
- 35.Norberg AL, Boman KK. Parent distress in childhood cancer: a comparative evaluation of posttraumatic stress symptoms, depression and anxiety. Acta Oncol 2008;47(2):267–274. [DOI] [PubMed] [Google Scholar]
- 36.Sultan S, Leclair T, Rondeau E, Burns W, Abate C. A systematic review on factors and consequences of parental distress as related to childhood cancer. Eur J Cancer Care (Engl) 2016;25(4):616–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hullmann SE, Wolfe-Christensen C, Ryan JL, et al. Parental overprotection, perceived child vulnerability, and parenting stress: a cross-illness comparison. J Clin Psychol Med Settings. 2010;17(4):357–365. [DOI] [PubMed] [Google Scholar]
- 38.Kahn RS, Brandt D, Whitaker RC. Combined effect of mothers’ and fathers’ mental health symptoms on children’s behavioral and emotional well-being. Arch Pediatr Adolesc Med 2004;158(8):721–729. [DOI] [PubMed] [Google Scholar]
- 39.Kazak AE. Families of chronically ill children: a systems and social-ecological model of adaptation and challenge. J Consult Clin Psychol 1989;57(1):25–30. [DOI] [PubMed] [Google Scholar]
- 40.Manne S, Miller D, Meyers P, Wollner N, Steinherz P, Redd WH. Depressive symptoms among parents of newly diagnosed children with cancer: a 6-month follow-up study. Child Health Care 1996;25(3): 191–209. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


