Abstract
During embryogenesis, tissues and organs are progressively shaped into their functional morphologies. While the information about tissue and organ shape is encoded genetically, the sculpting of embryonic structures in the 3D space is ultimately a physical process. The control of physical quantities involved in tissue morphogenesis originates at cellular and subcellular scales, but it is their emergent behavior at supracellular scales that guides morphogenetic events. In this review, we highlight the physical quantities that can be spatiotemporally tuned at supracellular scales to sculpt tissues and organs during embryonic development of animal species, and connect them to their cellular and molecular origins.
Introduction
From the branching geometry of lung or kidneys to the structure of limbs and digits, tissue and organ morphology is intimately related to proper organ function and, therefore, to the survival of the organism. A myriad of works over the past several decades have revealed a critical role of signaling molecules in orchestrating cellular events during tissue and organ morphogenesis[1]. However, despite their key role in developmental processes, even a detailed knowledge of the signaling molecules and their connections to cellular events cannot, per se, provide a complete understanding of morphogenetic events during development. Tissues and organs are also physical objects (materials) that are sculpted in the 3D space during embryonic development, as highlighted by D’Arcy Thompson a century ago[2] and generally acknowledged today. While the current knowledge of the molecular control of morphogenesis dwarfs our understanding of how embryonic structures are physically built, the development of new techniques to quantify mechanics within living embryos[3] and the advent of interdisciplinary approaches[4], have sparked new and rapidly increasing interest in the physical aspects of embryonic development.
From a physical perspective, embryonic tissues are complex, active materials, with the ability to self-shape, remodel and, in some cases, even self-heal or regenerate. Regardless of their complexity, any material is subject to fundamental physical laws that constrain how mechanical forces propagate in the structure and how addition of new material (e.g., via cell proliferation) is spatiotemporally redistributed. However, there are several physical quantities that remain largely unconstrained by physical law and can be genetically controlled and spatiotemporally modulated by cells to sculpt tissues into virtually any desired shape. Similar to sculpting inert materials (e.g., clay molding or even 3D printing), shaping embryonic tissues and organs requires fine spatiotemporal control of several physical quantities to attain the desired functional morphology.
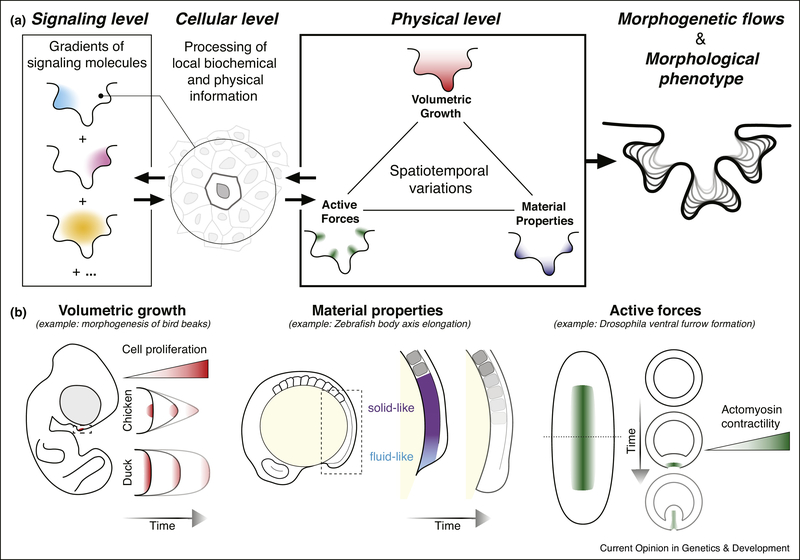
Inspection of the fundamental physics governing material (tissue) morphogenesis highlights three physical quantities that can be modulated and, therefore, can be used to shape tissues and organs: volumetric growth, tissue material properties and active forces (Fig. 1A; Box 1). Both inhomogeneities and anisotropies (Box 1) in any of these quantities, or combinations of them, can be employed to sculpt the desired shape. For instance, the spatiotemporal control of a growth zone (proliferation zone) in the frontonasal mass of developing bird beaks has been shown to directly affect the adult beak shape in several avian species (Fig. 1B). While a narrow proliferation zone gives rise to the slender chicken beak, widening the proliferation zone widens the beak into a bill, as observed in ducks[5]. More strikingly, offsetting the proliferation zone posteriorly and dorsally bends the tissue, making the curved cockatoos beak[6]. Tight spatiotemporal control of apoptotic tissue regions (negative growth or loss of tissue) via programmed cell death can also be used to shape tissues and it is an essential mechanism of digit formation in vertebrates[7].
Figure 1 – Sculpting tissues via spatiotemporal variations of key physical quantities.
A. The functional, morphological phenotype is ultimately achieved by controlling in space and time three physical quantities (physical level): volumetric growth, tissue material properties and active forces. Such control is, at least partially, due to gradients in signaling molecules in the tissue (signaling level). Cells within the tissue both sense and respond to local values of biochemical and physical cues (cellular level). B. Examples of morphogenetic processes for which spatiotemporal control of specific physical quantities have been identified. A localized cell proliferation zone (controlled region of volumetric growth) is essential to shaping bird beaks. Changes in the shape of the proliferation zone change the shape of the resulting beak, as illustrated for chicken and duck (dorsal views of developing beak) [5,6] (left). A fluid-to-solid transition between tissue physical states along the anteroposterior axis guides posterior body elongation in zebrafish[10]. In this case, the spatiotemporal control of fluid-like and solid-like tissue regions shapes the tissue (middle). In Drosophila, high levels of actomyosin contractility are restricted to a ventral region to enable invagination of the ventral furrow[9] (right). Dotted line indicates location of transverse cross sections shown on right.
Box 1
Three physical quantities can be spatiotemporally tuned to guide tissue morphogenesis:
Volumetric growth –
the change in volume of a tissue (including negative growth, or shrinkage), generally involving exchange of matter with the environment. Such changes can be due to cell proliferation or programmed cell death, but also due to cell volume changes or extracellular matrix deposition.
Active forces –
forces generated by molecular and cellular mechanisms that consume energy (ATP or GTP-consuming processes), such as actomyosin contraction or polymerization of actin filament networks or microtubules. These forces are to be differentiated from passive forces that are not directly generated by cellular processes that employ energy consumption. These include elastic and dissipative forces (shear, crowding pressure, etc.). Mechanical stresses, defined as force per unit surface, can be active or passive following the definition detailed above for forces.
Tissue material properties –
The material (or mechanical) properties of a material, including living tissues, dictate how the material deforms or flows in response to mechanical forces. At supracellular, tissue scales, the mechanical properties not only depend on cellular structures (cortical tension or adhesion levels) but also on the local tissue architecture and extracellular structures (such as matrix). Tissue mechanical parameters include its stiffness (resistance to deformation), viscosity (resistance to flow), or viscoelasticity (time-dependent elastic/viscous behavior), but also its plastic behavior, characterized by a yield stress, which quantifies the minimal mechanical stress needed to make the tissue flow like a fluid: below the yield stress, the tissue behaves like a solid and above like a fluid.
Any of these physical quantities can be isotropic or anisotropic and homogeneous or inhomogeneous, which are defined as:
Isotropic, anisotropic, homogenous and inhomogeneous physical quantities –
A physical quantity is isotropic if its properties are equal along all spatial orientations. Anisotropic physical quantities display differences along different spatial orientations. A physical quantity is homogenous if it is spatially uniform, i.e. if its magnitude does not change with spatial position. In contrast, inhomogeneous physical quantities vary from point to point in space, displaying differential variations in their magnitude. For instance, cell proliferation could be anisotropic (cells dividing along a specific spatial orientation, e.g., along an embryonic axis) and homogeneous (the division rate being the same at every point of the tissue). In contrast, cell proliferation could be isotropic (the cell division axis being randomly oriented) and inhomogeneous (the proliferation rate changing with location in the tissue).
In contrast to the abovementioned examples, tissues can be shaped in the absence of growth. For instance, during ventral furrow formation in Drosophila, the regional control of active forces in the tissue via spatially-graded actomyosin activity, drives the tissue invagination necessary for gastrulation[8] (reviewed in[9]) (Fig. 1B). Beyond active forces, direct in vivo and in situ measurements of tissue mechanics during posterior axis elongation in zebrafish have recently revealed the existence of a fluid-to-solid transition in the tissue state that guides body elongation[10] (Fig. 1B). While the posterior-most elongating tissue is maintained in a fluid-like state, enabling tissue remodeling and changes in shape, the tissue progressively transits into a solid-like state as it moves anteriorly, establishing tissue architecture and mechanically supporting body elongation[10].
Below we discuss the cellular and molecular processes that affect each of these physical quantities during tissue morphogenesis, as well as the interplay (or coupling) between them.
Volumetric growth
While uniform, isotropic volumetric growth causes the size of a tissue or organ to change, inhomogeneous growth or anisotropic growth can both lead to shape changes (Box 1). A number of cellular processes can lead to tissue volumetric growth, including cell proliferation or apoptosis (negative growth), changes in cell size, and deposition/degradation of extracellular matrix (ECM).
Cell proliferation/apoptosis is the most studied type of volumetric growth. In many embryonic tissues, cell proliferation is inhomogeneous, with some highly proliferative domains and regions with little proliferation which, as described above, can drive important morphogenetic changes (Fig. 1B). Several signaling molecules, such as BMP4 in beak development[5] or FGF, RA and BMP signaling in digit formation[11], have been shown to control the spatial localization of cell proliferation/apoptosis (Fig. 2; Table 1). Beyond the regional control of proliferation and/or programmed cell death, oriented cell divisions along specific spatial directions (anisotropic growth) are also known to contribute to tissue shape changes[12], as observed during germ band extension and wing imaginal disc morphogenesis in Drosophila[13,14], or during zebrafish gastrulation[15].
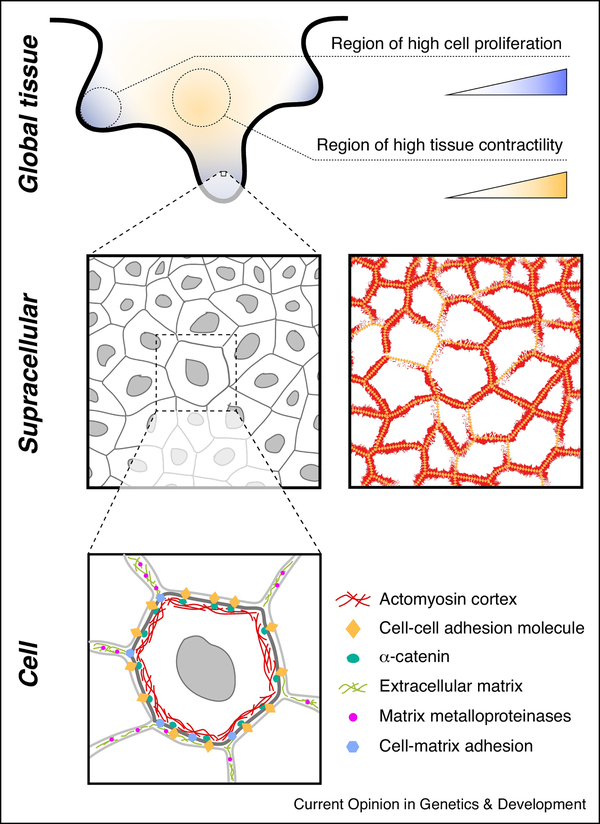
Figure 2 – Control of physical quantities across scales.
The control of physical quantities at different scales (global tissue scale; supracellular scale; cell/subcellular scales) involves many molecular players and structures (Table 1). Multiple physical quantities are controlled simultaneously for coordinated morphogenesis. Global tissue scale: A tissue may contain gradients of growth (illustrated here by cell proliferation), active forces (illustrated here by tissue contractility), and material properties. These global gradients of physical parameters are defined by gradients of signaling molecules and signaling pathway activity. Supracellular scale: Emergent collective behavior arising from the interactions of cells in the tissue defines physical quantities at the supracellular scale. Therefore, physical quantities at these scales depend on the local tissue architecture, as well as the molecules involved in maintaining physical interactions between cells (e.g., connection of cytoskeletal structures across cells: adhesion complexes (orange diamonds), α-catenin and β-catenin, cortical actomyosin (red), etc.). Cell scale: Several cellular structures and molecules control cell mechanics. Active forces can be generated at the cell cortex via actomyosin contractility, via actin polymerization at the cell surface, via osmotic pressure changes, etc. Changes in physical quantities at one scale typically affect other scales in the tissue, leading to coordinated development and morphogenesis.
Table 1:
Biological control of physical quantities across scales
| Physical Quantity | |||
|---|---|---|---|
| Scale | Volumetric Growth | Active Forces | Material Properties |
| Global tissue | • Spatial inhomogeneities in cell proliferation controlled by gradients in signaling molecules (BMP4, RA, FGF, etc.; see e.g.[5]) • Global tissue mechanical forces affect both orientation (anisotropy) and rate of cell proliferation (see e.g.[58,62]). |
• Spatial inhomogeneities in active forces controlled by transcription factors spatial localization (see e.g., {Heer:2017hm, Leptin:1990ub}) • Force anisotropy controlled by planar cell polarity (see e.g., {Zallen:2004wg, Rauzi:2008gz, Wallingford:2000c}) |
• Unknown signaling control. |
| Supracellular | • Cell proliferation/apopt osis. Cell shape and local forces affect both orientation and rate of cell division (see e.g., [60,61]). • Extracellular matrix deposition (see e.g.{Kalson:2015ez,Rozario:2010fz}). |
• Supracellular actomyosin networks(see e.g. [21,40,41]) • Supracellular forces depend on cell adhesion and the connection of cell cortex and adhesion complexes (e.g. α-catenin) (see e.g. {Mongera:2018wv,Vasquez:2016dy,Lecuit:2015hd,Lecuit:2011ec }) • Collective cell migration[74] |
• Supracellular mechanical properties depend on cortical tensions (seee.g.{Heisenberg:2013tla, Lecuit:2007cw, Zhou:2009hz}), cell-cell or cell-matrix adhesion{Mongera:2018 wv, Serwane:2017ht}, extracellular matrix properties and remodeling (via matrix metalloproteinases{Bon nans:2014kn}), cell-cell rearrangements and extracellular spaces{Mongera:2018wv}, etc.{Khalilgharibi:2016c z, Campas:2016gd}. |
| Cell/subcellular | •Molecularregulators of cell growth, shrinkage, proliferation and apoptosis control cell and local tissue growth (see e.g., [43,75,76]). | • Acto-myosincontractility (e.g. cortical tension[40,41]) • Osmotic pressure changes (through ion channels[43]) • Actin polymerization, especially during collective migration (e.g. formation of filopodia and lamellipodia[74]). |
• Intracellular cytoskeletal structures{Khalilgharibi :2016cz}, especially the cell cortex. Adhesion molecules. Connection of actin cortex to adhesion molecules {Vasquez:2016dy, Lecuit:2015hd}. • Force generating molecules, especially non-muscle myosin II force generation at the cell cortex[40,41]. • Secretion of extracellular matrix components and matrix metalloproteinases[17,77]. |
Secretion of extracellular matrix (ECM) by cells can considerably contribute to volumetric growth in specialized tissues, such as in cartilage, tendon or bone[16,17]. In contrast, in the less specialized tissues, such as those found at earlier developmental stages, ECM deposition does not strongly contribute to the volumetric growth of the tissue, but rather affects the tissue material properties (see below) and provides biochemical and biophysical cues for cells[17,18].
Although growth contributes to shaping tissues, in most cases it is not possible to explain morphogenetic events solely from volumetric growth, as has been shown, for instance, in limb morphogenesis[19,20]. Typically, spatiotemporal modulations in tissue material properties or active forces must occur in conjunction with growth to shape functional structures.
Active forces
Perhaps the most studied physical quantity in relation to morphogenesis is mechanical force and, more specifically, active cellular forces. Several cellular processes and structures can generate active forces and affect tissue form, including actomyosin contractility and cell volume changes.
The control of regions in the tissue with high actomyosin activity has been shown to be key for gastrulation movements in the fly embryo. During ventral furrow formation, a region of the tissue defined by the expression of Twist and Snail displays increased and pulsed actomyosin constriction that initiates the invagination of the tissue and gastrulation movements[8,21,22] (Fig. 1B). The large-scale spatial distribution of myosin and its anisotropy in the tissue are thought to drive global tissue morphogenetic flows during gastrulation[23,24]. Also in Drosophila, germband elongation has been shown to involve planar-polarized (anisotropic) distributions of actomyosin contractility[25]. Myosin is more strongly localized at junctions oriented along the dorso-ventral direction, and less strongly localized at anterior-posterior junctions[26–28]; this anisotropy in myosin localization and in myosin flows within the cell[29] result in local anisotropic forces[28,30] that cause polarized cell intercalation, generating convergent extension movements and axis elongation[28,31,32]. Anisotropic force generation is also essential to morphogenetic events in vertebrates, including gastrulation movements[33] and early body elongation[34,35], where planar cell polarity controls force anisotropy during convergent extension[36]. Recent quantitative measurements of supracellular forces during zebrafish posterior axis elongation revealed a posterior-to-anterior increase in actively-generated mediolateral (anisotropic) forces associated to the thinning of the body axis, but not to its elongation[10]. Finally, recent experiments showed that tissue morphology in medaka hir mutants is strongly affected by gravity[37]. These defects appeared to be a result of reduced actomyosin contractility leading to a failure to correctly assemble fibronectin fibrils, presumably leading to reduced tissue tension and the flattening of the body.
Several of the abovementioned processes require the transmission and coordination of active forces across multiple cells. This coordination relies on the formation of supracellular actin cables that physically connect the cytoskeleton (and especially the cortices) of multiple cells through cell-cell adhesion proteins, such as E-cadherin, and connectivity between adhesions and the actin cytoskeleton via α-catenin and β-catenin[38–42] (Fig. 2; Table 1).
Changes in cell volume driven by osmotic changes can also generate constrictive forces in a tissue that can drive global tissue shape changes. An example of this is the caspase-mediated cell volume decrease seen in the Drosophila amnioserosa during dorsal closure. The collective cell shrinkage produces a contractile force, that works with the supracellular actin cable at the leading edge of the dorsal epithelium to close the epithelium over the dorsal surface of the embryo[43].
Tissue material properties
While active forces power cell movements, the material properties of the tissue define the morphogenetic movements that result from both active and passive forces in the tissue. There are several cellular processes and structures that impact the tissue material properties, including cortical actomyosin contractility, cell-cell or cell-matrix adhesion or ECM physicochemical state.
In tissues with little to no ECM between cells (except at tissue boundaries), tissue material properties depend strongly on the supracellular tissue architecture (Fig. 2), which is largely controlled both by the mechanics of cell-cell contacts, as well as on cellular processes like cell rearrangements and divisions[12,40]. Measurements in tissue explants from amphibian embryos have shown that different tissues are characterized by different elastic and viscous properties[44,45] and that axial tissues during body elongation display an actomyosin-dependent temporal stiffening[45], which is thought to help increase the tissue mechanical integrity and maintain tissue architecture as development proceeds[45]. In addition, recent direct in vivo measurements of tissue material properties during zebrafish body axis elongation show the existence of an anteroposterior, N-cadherin-dependent gradient in tissue viscoelasticity[46]. Since the material properties of the cellular microenvironment are known to strongly affect cell behavior, it is possible that spatial variations in tissue material properties act as differential biophysical cues. Indeed, recent experiments in amphibian embryos have revealed that head mesoderm stiffening triggers the collective migration of neural crest cells and coordinates key morphogenetic events, namely gastrulation movements and neural crest migration[47]. Other in vivo measurements of mechanical properties have focused on the mechanics at the cellular and subcellular scales[30,48] and revealed the material properties of cell-cell contacts directly. Recent experiments indicate that the viscoelastic dissipation at cell-cell contacts may stabilize the cell shape changes necessary for tissue morphogenesis during germ band extension in Drosophila[49].
More recently, comprehensive measurements of both forces and tissue material properties in vivo have revealed a fluid-to-solid tissue transition that guides posterior body axis elongation in zebrafish[10]. Posterior tissues were shown to display a less constrained cellular microenvironment (more extracellular spaces) and higher cell-cell contact active fluctuations, driving cellular rearrangements and effectively ‘melting’ the tissue into a fluid-like state (plastic behavior; Box 1). After remodeling at the posterior end of the body, tissues progressively move anteriorly and turn solid-like through a jamming transition caused by increasing cellular confinement and smaller cell-cell contact active fluctuations. The solid-like tissue state helps establish tissue architecture and mechanically supports the posterior extension of fluid-like tissues[10]. Cellular movements observed in chicken embryos during axis elongation[50], which are under the control of FGF signaling, are consistent with the physical mechanical of axis elongation reported in zebrafish embryos. In line with these observations, recent experiments suggest that cellular jamming also occurs during Drosophila gastrulation in epithelial tissues[51], affecting cell shapes and potentially restricting morphogenetic movements.
Spatiotemporal variations in matrix deposition or remodeling have been shown to affect morphogenetic events. For instance, branching morphogenesis is strongly dependent on proper fibronectin deposition for cleft formation[52] and also on the controlled spatiotemporal remodeling of the basement membrane[53]. While direct in vivo and in situ measurements of ECM mechanical properties within developing 3D tissues have never been achieved, it is thought that spatial variations in ECM assembly/remodeling can lead to spatiotemporal variations in its mechanical properties, thereby affecting morphogenesis[54]. Recent quantitative experiments have shown that the biased deposition of basement membrane during Drosophila oogenesis leads to anisotropic and inhomogeneous matrix mechanical properties, which constrict growth of the egg chamber medially and bias growth along the antero-posterior axis[55,56].
Interplay between physical quantities
Since the same molecular and cellular structures can affect multiple physical quantities, changes in a particular molecule or structure can lead to simultaneous changes in different physical quantities (Fig. 2). Indeed, changes in myosin II activity affecting cortical contractility can affect both active force generation and the tissue material properties[45]. More generally, the three physical quantities described above can be coupled, meaning that changes in one quantity may lead to concomitant changes of another one. The interplay (or coupling) between physical quantities is essential to morphogenetic events, as highlighted in several key developmental processes.
Interplay between mechanical forces and cell proliferation.
When cells proliferate in a confined environment, the buildup of isotropic stresses can affect cell proliferation and anisotropic stresses can affect the orientation of cell division[12,57]. For instance, during the development of the Drosophila wing imaginal disc, cells in the center of the wing pouch are thought to be compressed and barely proliferate, whereas cells in the periphery experience circumferential tension and proliferate more, dividing along the directions of maximal tension1[57,58]. At pupal stages, a large scale tissue contraction subjects epithelial cells in the wing blade to anisotropic tension, which orients cells divisions (and coordinates other cell behaviors) to elongate the wing proximo-distally to properly shape it[59]. In the Drosophila dorsal thorax, the inhomogeneous distributions of anisotropic stresses have also been shown correlate with the spatial distribution of cell division orientation[60], with local cell shapes and strains affecting the cell division axis[61]. In zebrafish, cells of the enveloping cell layer divide along the direction of maximal tension during epiboly, thereby reducing the anisotropic stress in the tissue and aiding tissue movement toward the vegetal pole[62]. In mouse embryos, tension anisotropy in the limb ectoderm results in oriented cell divisions that facilitate cellular rearrangements and limb bud outgrowth[63].
Cell proliferation can also affect mechanics and morphogenesis. Recent experiments showed that spatially uniform cell proliferation can drive a mechanical instability causing the simultaneous formation of branches in the developing murine airway epithelium[64,65]. Moreover, differential proliferation in adjacent, physically connected tissues, has also been shown to drive a mechanical instability causing the looping pattern in the avian gut[66].
Interplay between mechanical forces and tissue material properties.
Active cell-scale forces have been recently shown to be necessary to fluidize embryonic tissues that would otherwise be in a solid-like state, directly relating cell-generated forces to tissue material properties[10]. Mechanical forces can both promote the secretion of new ECM or align previously established ECM, thereby affecting the tissue material properties and their anisotropy. During the formation of the heart in zebrafish, shear stress generated by blood flow promotes the synthesis of fibronectin1b via activation of the transcription factor klf2, resulting in the reorganization of cells in the heart tube and the proper development of the atrioventricular valve[67]. In tooth development, odontogenic differentiation is triggered by physical compaction of the mesenchyme (mesenchymal condensation), a process which results in the deposition of collagen VI which stabilizes the forming odontogenic stem cell niche[68,69]. Beyond the mechanical stimulation of matrix deposition, forces generated by cells in the ECM can orient the matrix fibers and lead to anisotropic mechanical properties. Recent in vitro experiments show that active cellular pulling on the ECM reorganizes the matrix, leading to the alignment of matrix fibers[70,71] that generates anisotropic mechanical properties which help define the direction of collective cell migration[72]. Finally, it has recently been shown that the interplay between tissue stiffness and active force generation in adjacent tissue layers specifies the follicle pattern in the developing avian skin[73].
The couplings between physical quantities themselves and with signaling events contain essential information to gain a holistic understanding of tissue morphogenesis. With the development of new techniques to directly probe physical quantities in vivo[3], it is becoming possible to quantitatively study the molecular control of physical quantities and how these physical cues affect genetic programs during embryonic development.
Acknowledgements
GS-V acknowledges financial support from an Otis Williams postdoctoral fellowship. OC is supported by grants from the National Institute of General Medical Sciences, the National Institute of Dental and Craniofacial Research and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (award numbers R01GM113241, R01DE027620 and R01HD095797 respectively), the National Science Foundation (NSF CAREER award, CMMI-1562910) and the Human Frontier Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilbert S: Developmental Biology. Sinauer Associates; 2013. [Google Scholar]
- 2.Thompson DW: On Growth and Form. Cambridge University Press; 1917. [Google Scholar]
- 3.Campas O: A toolbox to explore the mechanics of living embryonic tissues. Semin Cell Dev Biol 2016, 55:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oates AC, Gorfinkiel N, González-Gaitán M, Heisenberg C-P: Quantitative approaches in developmental biology. Nat Rev Genet 2009, 10:517–530. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, Jiang T-X, Suksaweang S, Widelitz RB, Chuong C-M: Molecular shaping of the beak. Science 2004, 305:1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Jiang T, Shen J, Widelitz R, Chuong C: Morphoregulation of avian beaks: Comparative mapping of growth zone activities and morphological evolution. Dev Dynam 2006, 235:1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Garijo A, Steller H: Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development 2015, 142:3253–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. •.Heer NC, Miller PW, Chanet S, Stoop N, Dunkel J, Martin AC: Actomyosin-based tissue folding requires a multicellular myosin gradient. Development 2017, 144:1876–1886. The authors identify a gradient of actomyosin contractility radiating out from the Drosophila ventral midline, in a manner dependent on upstream signaling factors fog and T48. They use modelling to demonstrate that the contractility gradient, and not contractility itself, is what promotes folding of the ventral furrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heer NC, Martin AC: Tension, contraction and tissue morphogenesis. Development 2017, 144:4249–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ••.Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J, Campàs O: A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 2018, doi: 10.1038/s41586-018-0479-2. The authors quantitatively measure both forces and material properties in vivo and in situ during posterior body axis elongation in zebrafish. They reveal distinct roles for cell-scale and supracellular forces, a spatial gradient in mediolateral forces and the existence of a fluid-to-solid jamming transition that guides body axis elongation. The work presents a novel mechanism of tissue morphogenesis based on the regional control of fluid-like and solid-like tissue states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltcheva MM, Anderson MJ, Harfe BD, Lewandoski M: BMPs are direct triggers of interdigital programmed cell death. Dev Biol 2016, 411:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecuit T, Le Goff L: Orchestrating size and shape during morphogenesis. Nature 2007, 450:189–192. [DOI] [PubMed] [Google Scholar]
- 13.da Silva SM, Vincent J-P: Oriented cell divisions in the extending germband of Drosophila. Development 2007, 134:3049–3054. [DOI] [PubMed] [Google Scholar]
- 14.Baena-López LA, Baonza A, García-Bellido A: The Orientation of Cell Divisions Determines the Shape of Drosophila Organs. Current Biology 2005, 15:1640–1644. [DOI] [PubMed] [Google Scholar]
- 15.Gong Y, Mo C, Nature SF: Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 2004, 430:689–693. [DOI] [PubMed] [Google Scholar]
- 16.Kalson NS, Lu Y, Taylor SH, Starborg T, Holmes D, Kadler KE: A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. eLife 2015, 4: e05958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozario T, DeSimone DW: The extracellular matrix in development and morphogenesis: A dynamic view. Dev Biol 2010, 341:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frantz C, Stewart KM, Weaver VM: The extracellular matrix at a glance. J Cell Sci 2010, 123:4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, Swoger J, Sharpe J: The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol 2010, 8:e1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morishita Y, Kuroiwa A, Suzuki T: Quantitative analysis of tissue deformation dynamics reveals three characteristic growth modes and globally aligned anisotropic tissue deformation during chick limb development. Development 2015, 142:1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin AC, Kaschube M, Wieschaus EF: Pulsed contractions of an actin. Nature 2009, 457:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leptin M, Grunewald B: Cell shape changes during gastrulation in Drosophila. Development 1990, 110:73–84. [DOI] [PubMed] [Google Scholar]
- 23.Streichan SJ, Lefebvre MF, Noll N, Wieschaus E, Shraiman B: Global morphogenetic flow is accurately predicted by the spatial distribution of myosin motors. eLife 2018, 7:e27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He B, Doubrovinski K, Polyakov O, Wieschaus E: Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 2014, 508:392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zallen JA, Wieschaus E: Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell 2004, 6:343–355. [DOI] [PubMed] [Google Scholar]
- 26. •.Munjal A, Philippe J-M, Munro E, Lecuit T: A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 2015, 524:351–355. The authors study Drosophila germband extension, which is dependent on myosin II contractility, they find that myosin II pulsatility is required for morphogenesis. They demonstrate this pulsing depends on self-organized positive and negative biomechanical feedback between myosin II advection and dissociation. [DOI] [PubMed] [Google Scholar]
- 27.Kasza KE, Farrell DL, Zallen JA: Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc Natl Acad Sci U S A 2014, 111:11732–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauzi M, Verant P, Lecuit T, Lenne P-F: Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol 2008, 10:1401–U57. [DOI] [PubMed] [Google Scholar]
- 29.Rauzi M, Lenne P-F, Lecuit T: Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 2010, 468:1110–1114. [DOI] [PubMed] [Google Scholar]
- 30. •.Bambardekar K, Clément R, Blanc O, Chardès C, Lenne P-F: Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc Natl Acad Sci U S A 2015, doi: 10.1073/pnas.1418732112. The authors used optical tweezers to quantitatively measure junctional tension and mechanical properties in epithelial tissues within developing embryos. They provide quantitative evidence for anisotropic junctional tension and quantitatively measure the viscoelastic material properties of cellular junctions in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collinet C, Rauzi M, Lenne P-F, Lecuit T: Local and tissue-scale forces drive oriented junction growth during tissue extension. Nature cell biology 2015, 17:1247–1258. [DOI] [PubMed] [Google Scholar]
- 32.Bertet C, Sulak L, Lecuit T: Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 2004, 429:667–671. [DOI] [PubMed] [Google Scholar]
- 33.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW: Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 2000, 405:76–81. [DOI] [PubMed] [Google Scholar]
- 34.Williams ML, Solnica-Krezel L: Regulation of gastrulation movements by emergent cell and tissue interactions. Curr Opin Cell Biol 2017, 48:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller R: Shaping the vertebrate body plan by polarized embryonic cell movements. Science 2002, 298:1950–1954. [DOI] [PubMed] [Google Scholar]
- 36.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM: Dishevelled controls cell polarity during Xenopus gastrulation. Nature 2000, 405:81–85. [DOI] [PubMed] [Google Scholar]
- 37. ••.Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, Hata S, Sasaki T, Krens SFG, Osada Y, et al. : YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 2015, doi: 10.1038/nature14215. The authors describe a medaka fish YAP mutant, hir, that is unable to withstand deformation due to gravity during development. They show that actomyosin mediated tissue tension is reduced in hir mutant embryo, which alongside evidence from cultured 3D spheroids suggest that the YAP Rho GTPase pathway is important for controlling the material properties of tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasquez CG, Martin AC: Force transmission in epithelial tissues. Dev Dyn 2016, 245:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecuit T, Yap AS: E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol 2015, 17:533–539. [DOI] [PubMed] [Google Scholar]
- 40.Heisenberg C-P, Bellaiche Y: Forces in Tissue Morphogenesis and Patterning. Cell 2013, 153:948–962. [DOI] [PubMed] [Google Scholar]
- 41.Lecuit T, Lenne P-F, Munro E: Force Generation, Transmission, and Integration during Cell and Tissue Morphogenesis. Annu Rev Cell Dev Biol 2011, 27:157–184. [DOI] [PubMed] [Google Scholar]
- 42.Lecuit T, Lenne P-F: Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 2007, 8:633–644. [DOI] [PubMed] [Google Scholar]
- 43. •.Saias L, Swoger J, D’Angelo A, Hayes P, Colombelli J, Sharpe J, Salbreux G, Solon J: Decrease in Cell Volume Generates Contractile Forces Driving Dorsal Closure. Developmental Cell 2015, 33:611–621. This study shows that tissues can generate morphogenetic force and movement by changing cell volume. By studying Drosophila dorsal closure, the authors show that dorsal closure requires cell shrinkage in the amniosera, which is triggered by caspase activation at the onset of the apoptotic program in this tissue. Cell shrinkage in the amniosera is shown to work in tandem with actin cable contraction in the dorsal tissue to ensure dorsal closure. [DOI] [PubMed] [Google Scholar]
- 44.Luu O, David R, Ninomiya H, Winklbauer R: Large-scale mechanical properties of Xenopus embryonic epithelium. Proc Natl Acad Sci U S A 2011, 108:4000–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Kim HY, Davidson LA: Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 2009, 136:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. •.Serwane F, Mongera A, Rowghanian P, Kealhofer DA, Lucio AA, Hockenbery ZM, Campas O: In vivo quantification of spatially varying mechanical properties in developing tissues. Nat Methods 2017, 14:181–186. The authors develop the first technique to measure mechanical properties in 3D tissues within developing embryos. Using magnetically-responsive microdroplets, they find that during body axis elongation the tissue material properties display spatial differences along the anteroposterior axis, with decreasing stiffness and viscosity towards the elongating posterior region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. ••.Barriga EH, Franze K, Charras G, Mayor R: Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 2018, 554:523–527. The authors show that neural crest migration in triggered in vivo by the stiffening of the underlying mesoderm. This shows that mechanical cues coordinate cell migration in vivo and that two distinct morphogenetic events, namely collective neural crest migration and gastrulation movements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doubrovinski K, Swan M, Polyakov O, Wieschaus EF: Measurement of cortical elasticity in Drosophila melanogasterembryos using ferrofluids. Proc Natl Acad Sci U S A 2017, doi: 10.1073/pnas.1616659114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clément R, Dehapiot B, Collinet C, Lecuit T, Lenne P-F: Viscoelastic Dissipation Stabilizes Cell Shape Changes during Tissue Morphogenesis. Current biology 2017, 27:3132–3142.e4. [DOI] [PubMed] [Google Scholar]
- 50.Bénazéraf B, Francois P, Baker RE, Denans N, Little CD, Pourquié O: A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 2010, 466:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. •.Atia L, Bi D, Sharma Y, Mitchel JA, Gweon B, Koehler SA, DeCamp SJ, Lan B, Kim JH, Hirsch R, et al. : Geometric constraints during epithelial jamming. Nat Phys 2018, doi: 10.1038/s41567-018-0089-9. The authors study the relation between average cell shape anisotropy and its variation in epithelial monolayers, and show that similar patterns are observed in vitro and in vivo, during Drosophila gastrulation. The work suggests that the observed, seemingly universal, relation is related to cellular jamming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakai T, Larsen M, Yamada KM: Fibronectin requirement in branching morphogenesis. Nature 2003, 423:876–881. [DOI] [PubMed] [Google Scholar]
- 53.Ingber DE: Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol 2006, 50:255–266. [DOI] [PubMed] [Google Scholar]
- 54.Mammoto T, Ingber DE: Mechanical control of tissue and organ development. Development 2010, 137:1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chlasta J, Milani P, Runel G, Duteyrat J-L, Arias L, Lamiré L-A, Boudaoud A, Grammont M: Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development 2017, 144:4350–4362. [DOI] [PubMed] [Google Scholar]
- 56. ••.Crest J, Diz-Muñoz A, Chen D-Y, Fletcher DA, Bilder D: Organ sculpting by patterned extracellular matrix stiffness. eLife 2017, 6:e24958 The authors measure stiffness of the basement membrane of the Drosophila egg chamber and find an anterior-posterior stiffness gradient that is not present in mutants where the egg chamber fails to elongate. By manipulating the basement membrane, the authors find that its mechanical properties are important for egg chamber morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwank G, Basler K: Regulation of Organ Growth by Morphogen Gradients. Cold Spring Harb Perspect Biol 2010, 2:a001669–a001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeGoff L, Rouault H, Lecuit T: A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development 2013, 140:4051–4059. [DOI] [PubMed] [Google Scholar]
- 59.Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper J-C, Jülicher F, Eaton S: Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 2010, 142:773–786. [DOI] [PubMed] [Google Scholar]
- 60. •.Guirao B, Rigaud SU, Bosveld F, Bailles A, Lopez-Gay J, Ishihara S, Sugimura K, Graner F, Bellaiche Y: Unified quantitative characterization of epithelial tissue development. eLife 2015, 4:773 The authors provide a full quantification of multiple cell behaviors, namely cell divisions, apoptoses, cell size, cell rearrangements in the tissue, and relate them to tissue deformations and growth both in the case of the Drosophila dorsal thorax and the wing pupal epithelia. Using force inference methods, they relate the observed junctional tensions to different cell behaviors in the dorsal thorax. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosveld F, Markova O, Guirao B, Martin C, Wang Z, Pierre A, Balakireva M, Gaugue I, Ainslie A, Christophorou N, et al. : Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature 2016, doi: 10.1038/nature16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campinho P, Behrndt M, Ranft J, Risler T, Minc N, Heisenberg C-P: Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nature cell biology 2013, 15:1405–1414. [DOI] [PubMed] [Google Scholar]
- 63.Lau K, Tao H, Liu H, Wen J, Sturgeon K, Sorfazlian N, Lazic S, Burrows JTA, Wong MD, Li D, et al. : Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nat Cell Biol 2015, 17:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson CM, Gleghorn JP, Pang M-F, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC, Stone HA: Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 2017, 144:4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. •.Varner VD, Gleghorn JP, Miller E, Radisky DC, Nelson CM: Mechanically patterning the embryonic airway epithelium. Proc Natl Acad Sci U S A 2015, 112:9230–9235. The authors use 3D embryonic lung culture combined with modeling to show that growth induced physical instability can potentially initiate the formation of branches during morphogenesis. This suggests that branching morphogenesis could arise from a purely physical instability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, Tabin CJ: On the growth and form of the gut. Nature 2012, 476:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steed E, Faggianelli N, Roth SEP, Ramspacher C, Concordet J-P, Vermot J: klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. Nature Communications 2016, 7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mammoto T, Mammoto A, Jiang A, Jiang E, Hashmi B, Ingber DE: Mesenchymal condensation-dependent accumulation of collagen VI stabilizes organ-specific cell fates during embryonic tooth formation. Dev Dyn 2015, 244:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mammoto T, Mammoto A, Torisawa Y-S, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D, et al. : Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell 2011, 21:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piotrowski-Daspit AS, Nerger BA, Wolf AE, Sundaresan S, Nelson CM: Dynamics of Tissue-Induced Alignment of Fibrous Extracellular Matrix. Biophys J 2017, 113:702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Feng J, Jones CAR, Mao X, Sander LM, Levine H, Sun B: Stress-induced plasticity of dynamic collagen networks. Nature Communications 2017, 8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gjorevski N, Piotrowski AS, Varner VD, Nelson CM: Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Scientific Reports 2015, doi: 10.1038/srep11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. ••.Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM: Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 2017, 357:811–815. The authors study formation of feather follicles in chicken embryo to demonstrate that contractility-driven cellular pulling in the dermis leads to cellular self-organisation, patterning the formation of feather follicles in the overlying dermis. This study demonstrates that mechanical patterning can occur upstream of molecular patterning in organ formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedl P, Hegerfeldt Y, Tusch M: Collective cell migration in morphogenesis and cancer. Int J Dev Biol 2004, 48:441–449. [DOI] [PubMed] [Google Scholar]
- 75.Fuchs Y, Steller H: Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 2015, 16:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edgar BA, Lehner CF: Developmental control of cell cycle regulators: a fly’s perspective. Science 1996, 274:1646–1652. [DOI] [PubMed] [Google Scholar]
- 77.Bonnans C, Chou J, Werb Z: Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014, 15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]