Abstract
Patient: Female, 70
Final Diagnosis: Infective endocarditis
Symptoms: Dyspnea
Medication: —
Clinical Procedure: —
Specialty: Cradiology
Objective:
Challenging differential diagnosis
Background:
Infective endocarditis is prevalent worldwide and the modified Duke criteria have been used universally to diagnose this condition. However, making the correct diagnosis is rather difficult because the clinical presentation and findings of blood tests are non-specific.
Case Report:
A 70-year-old female complaining of dyspnea for 5 days with acute mitral regurgitation was transferred to our hospital. She had acute heart and respiratory failure and disseminated intravascular coagulation. Although infective endocarditis was suspected, repeated blood cultures and transesophageal echocardiography could not reveal any findings of infective endocarditis. Because the etiology of her condition was not determined by various examinations, mitral annuloplasty was required to treat her mitral regurgitation, and was performed for definitive diagnosis and treatment revealing the presence of vegetation on the mitral valve. Enterococcus faecalis was detected by cultures of the mitral valve and blood after the surgery.
Conclusions:
It can be very difficult to diagnose infective endocarditis correctly, especially when a case fails to fulfill the modified Duke criteria. In such a case, only cardiac surgery might enable us to make an accurate diagnosis and save a patient’s life.
MeSH Keywords: Disseminated Intravascular Coagulation , Endocarditis , Enterococcus Faecalis, Mitral Valve Annuloplasty , Mitral Valve Insufficiency
Background
Although infective endocarditis (IE) is prevalent worldwide, the yearly incidence of the disease in Japan is as low as 32.4 per 1 000 000 people [1]. Making the correct diagnosis of IE is rather difficult because the clinical presentations and findings of blood tests are non-specific [2]. Although the modified Duke criteria are usually helpful to make a diagnosis of IE, they could be invalid in atypical cases [3]. Certainly, the presence of acute mitral regurgitation (MR) could be a clue to make a diagnosis of IE. However, the incidence of acute MR complicated with IE itself is low [4]. Furthermore, the case of IE in the native valve complicated with disseminated intravascular coagulation (DIC) and acute MR occurring in patients who do not have any underlying systemic disease is rare. To our knowledge, there is only one previous case report, which was caused by methicillin-sensitive Staphylococcus aureus in a 24-year-old female patient [5].
We herein report a diagnostically challenging case of IE developing in the native mitral valve in a patient complicated with acute MR and DIC without underlying systemic disease. Since the patient failed to fulfill definitively the modified Duke criteria, only mitral annuloplasty made it possible to make the correct diagnosis.
Case Report
A 70-year-old Japanese female patient without smoking history or remarkable past history, had had shortness of breath while going up or down stairs for 5 days. Her condition gradually worsened to the point of feeling dyspnea even while standing still, and she subsequently visited her family doctor. Since chest x-ray showed pulmonary congestion, and transthoracic echocardiography revealed MR and mitral valve cusp prolapse, she was assumed to have acute MR and transferred to our hospital on the same day. On admission, her body temperature was 36.8°C, heart rate 94 beats/min, blood pressure 150/88 mmHg, respiratory rate 26/min and oxygen saturation 99% with 2 L/min of oxygen delivered via a nasal cannula. Physical findings showed jugular venous distention, apical systolic murmur, and coarse crackles in both lung fields. However, leg edema, conjunctival hemorrhage, or other cutaneous manifestations including purpura of the tip of digit were not revealed. Laboratory findings on admission are shown in Table 1. The patient had a white blood cell count of 9 200/µL, C-reactive protein of 1.95 mg/dL, high-sensitivity troponin T of 0.020 ng/mL, and N-terminal pro-brain natriuretic peptide of 1 464 pg/mL. In addition, she also had DIC, which was diagnosed based on the DIC scoring system of the Japanese Association for Acute Medicine (score=5) [6]. Arterial blood gas analysis showed type I respiratory failure. Urinalysis suggested the presence of glomerulonephritis without urinary tract infection. Electrocardiogram did not show ST-T changes or left ventricular high voltage. Echocardiograph revealed an ejection fraction of 70%, MR, prolapse of posterior mitral leaflet P3, expansion of the left atrium with left atrial volume index of 66 mL/m2 and left ventricular wall thickening with posterior wall thickness of 14 mm. However, no vegetation or wall motion abnormality was detected (Figure 1). Chest x-ray and contrast-enhanced thoracic computed tomography showed increased shadows in central parts of both lung fields, bilateral pleural effusion. Based on the aforementioned findings, her condition was diagnosed as congestive heart failure due to acute MR. Because acute myocardial infarction (AMI) was deniable due to the absence of chest pain and typical findings of AMI on electrocardiogram and echocardiograph, IE was the most likely cause of her acute MR and DIC. However, 2 sets of blood cultures on admission were negative and transesophageal echocardiography (TEE) failed to reveal vegetation or thickness of valves (Figure 2). Even with blood cultures performed 4 times during her admission and the second TEE, her condition did not definitively fulfill the modified Duke criteria [3]. Subsequently performed examinations to detect the cause of DIC, such as infectious diseases, malignancies, or collagen diseases, were all negative. Meanwhile, brain magnetic resonance imaging revealed 2 acute or sub-acute cerebral infarctions (Figure 3), suggesting the etiology of cardiogenic embolisms from mitral valves because of the involvements of both sides of the brain. Although surgical intervention on the mitral valve was required, control of heart failure and DIC was considered to be the priority. Her heart failure was temporally stabilized by the 39th hospital day only by conservative treatments. DIC had been improved by the 94th hospital day after transfusing human leukocyte antigen (HLA)-matched platelets because of the presence of HLA class I antibody. On the 170th hospital day, mitral annuloplasty was performed in another hospital following the patient’s wishes. Vegetation on a large portion of the mitral valve was found intraoperatively (Figure 4), and Enterococcus faecalis was detected by culture of the mitral valve. Because blood culture after surgery was also positive for E. faecalis, which denied the possibility of contamination, we made the definitive diagnosis of IE. A course of ampicillin was administered for 6 weeks after blood cultures turned negative. She had been followed at our hospital on an outpatient basis for 6 months without showing any symptoms and signs of recurrence of IE.
Table 1.
Laboratory data on admission.
| Complete blood count | Biochemistry | Blood gas analysis (O2 2 L/min) | ||||||
| White blood cell | 9 200 | /µL | Total protein | 5.9 | g/dL | pH | 7.449 | |
| Neutrophil | 77.6% | Albumin | 3.0 | g/dL | PaCO | 32.0 | mmHg | |
| Lymphocyte | 13.3% | BUN | 24.1 | mg/dL | PaO | 56.2 | mmHg | |
| Red blood cell | 372 | ×104/µL | Creatinine | 0.74 | mg/dL | Bicarbonate | 21.8 | mmol/L |
| Hemoglobin | 11.9 | g/dL | Total bilirubin | 1.2 | mg/dL | Lactate | 0.8 | mmol/L |
| Hematocrit | 35.6% | Glucose | 115 | g/dL | ||||
| Urinalysis | ||||||||
| MCV | 95.7 | fL | AST | 19 | U/L | Qualitative | ||
| MCHC | 33.4% | ALT | 16 | U/L | pH | 5.5 | ||
| Reticulocyte | 3.8% | LDH | 317 | U/L | Specific gravity | 1.018 | ||
| Platelet | 3.1 | ×104/µL | ALP | 240 | U/L | Protein | 3+ | |
| Reticulated platelets | 21.8% | Amylase | 40 | U/L | Occult blood | 2+ | ||
| Congealing system | CK | 60 | U/L | Nitrite | (–) | |||
| PT-INR | 1.21 | CK-MB | 3 | U/L | White blood cell | (–) | ||
| APTT | 30.7 | second | Sodium | 140 | mEq/L | Sediment | ||
| Fibrinogen | 437.9 | mg/dL | Potassium | 3.9 | mEq/L | Red blood cell | 20–29 | /HPF |
| FDP | 13.9 | μg/mL | Chlorine | 107 | mEq/L | White blood cell | 1–4 | /HPF |
| D-dimer | 7.82 | μg/mL | NT-pro BNP | 1 464 | pg/mL | Epithelial cast | 1–2 | /10 LPF |
| PIC | 3.56 | μg/mL | Troponin T | 0.020 | ng/mL | Granular cast | 1–9 | /LPF |
| TAT | 6.6 | ng/mL | CRP | 1.95 | mg/dL | Erythrocyte morphology | Mixed | |
MCV – mean cell volume; MCHC – mean corpuscular hemoglobin concentration; PT-INR – prothrombin time-international normalized ratio; APTT – activated partial thromboplastin time; FDP – fibrin/fibrinogen degradation products; PIC – plasmin-α2 plasmin inhibitor complex; TAT – thrombin-antithrombin III complex; BUN – blood urea nitrogen; AST – aspartate aminotransferase; ALT – alanine aminotransferase; LDH – lactate dehydrogenase; ALP – alkaline phosphatase; CK – creatine kinase; CK-MB – creatine kinase-MB; NT-pro BNP – N-terminal pro-brain natriuretic peptide; CRP – C-reactive protein.
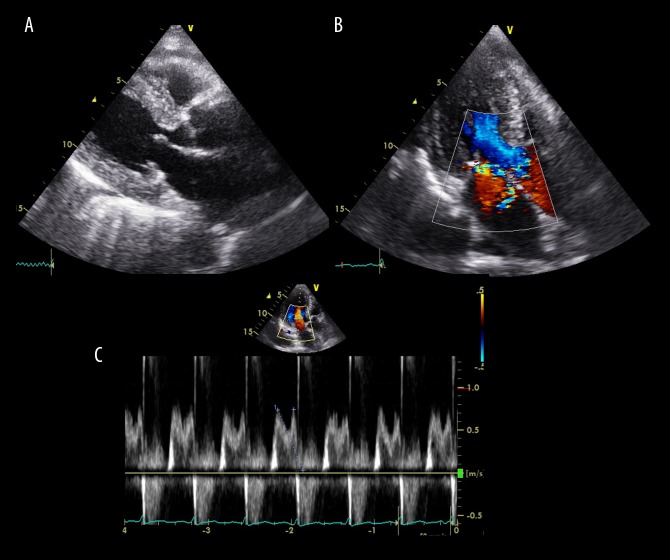
Figure 1.
Transthoracic echocardiography performed on admission. (A) Brightness mode image, (B) color Doppler image, and (C) pulse Doppler image. Ejection fraction of 70%, left atrial volume index of 66 mL/m2 and posterior wall thickening of 14 mm are shown. Mitral regurgitation (grades III to IV) and prolapse of posterior mitral leaflet P3 are revealed (B, C) without findings of vegetation and wall motion abnormalities (A).
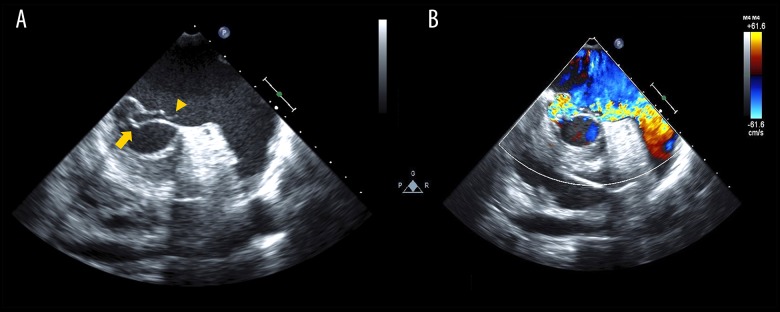
Figure 2.
First transesophageal echocardiography performed following transthoracic echocardiography. (A) Brightness mode image and (B) color Doppler image. Prolapse of posterior mitral leaflet P3 (arrow) and ruptured chordae tendineae (arrowhead) are shown without the finding of vegetation and hyperplasia of valves (A). The mitral regurgitation jet runs from the site of prolapse to left atrial appendage along the anterior leaflet (B).
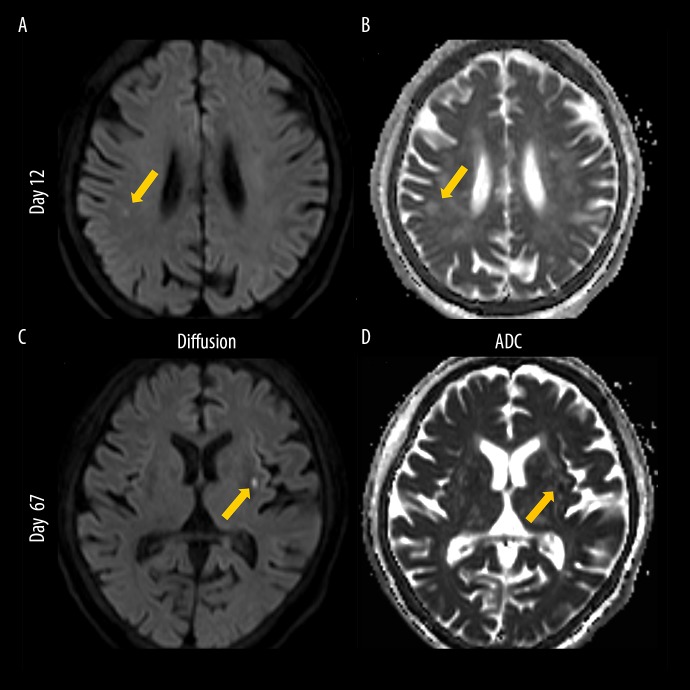
Figure 3.
Brain magnetic resonance imaging. (A) Diffusion-weighted image taken on day 12, (B) apparent diffusion coefficient (ADC) image taken on day 12, (C) diffusion-weighted image taken on day 67, and (D) ADC image taken on day 67. On day 12, the diffusion-weighted image shows high signal intensity in the right temporoparietal lobe (arrow, A) and ADC value of the lesion is low to equal (arrow, B). On day 67, the diffusion-weighted image shows high signal intensity in the left putamen (arrow, C) and ADC value of the lesion is low to equal (arrow, D). Images of these lesions are compatible with sub-acute to acute phase cerebral infarction, which is considered to be caused by cardiogenic emboli because of the involvements of both sides of the brain.
Figure 4.
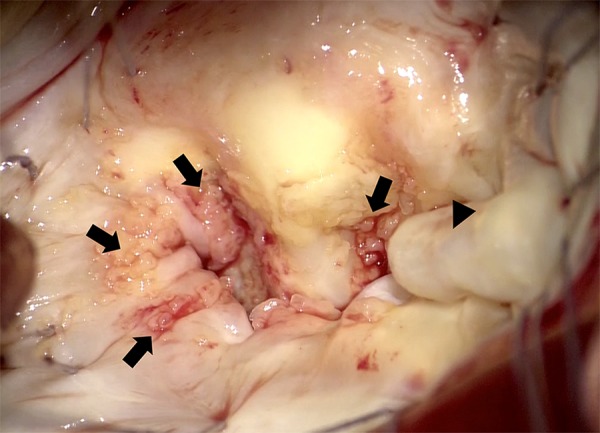
Intraoperative findings of the mitral valve. Widespread attachment of vegetation on the mitral valve (arrows) and prolapse of posterior mitral leaflet P3 (arrowhead) are shown.
The objective for this case report is to inform others that it could be very difficult to diagnose IE correctly if it fails to fulfill the modified Duke criteria. In such a case, only cardiac surgery might enable us to make an accurate diagnosis and save a patient’s life.
Discussion
Our patient had IE complicated with acute MR and DIC, which did not definitively fulfill the modified Duke criteria despite twice repeated TEE and 8 sets of blood cultures, and her condition was eventually diagnosed only by mitral valve surgery. Because coexistence of acute MR and DIC is an atypical and rare complication of IE, we had extreme difficulty in making an accurate diagnosis, despite multidisciplinary collaboration among the departments of general medicine, cardiology, cardiovascular surgery, and hematology in our hospital. Since we could not be convinced that the cause of her DIC was MR and IE, and her heart failure was temporally stabilized by medical treatment, we tried to find the cause of DIC first temporally pending mitral annuloplasty.
The unusual presentations of this patient made the diagnosis extremely difficult. The modified Duke criteria have been used universally to diagnose IE [3]. Positive blood cultures and confirmation of the presence of vegetation by echocardiography are the decision-making points of these criteria [3]. On one hand, 3 consecutive sets of blood cultures are useful bacteriological tools [2]. However, 10–30% of IE patients could have negative blood cultures [7]. The 2 major causes are antibiotic treatments prior to examinations and the involvement of fastidious organisms, which are difficult to culture [7]. On the other hand, among echocardiography examinations, TEE is the most useful imaging modality for detecting vegetation with sensitivity of 86%, specificity 97%, positive predictive value (PPV) of 89%, and negative predictive value of 96% [8]. Although repetition of TEE could improve sensitivity and specificity, the third TEE is reported to detect only 20% of IEs. Additionally, performing TEE more than 4 times is futile and would not provide additional useful findings [9]. Among cases posing a diagnostic challenge, 0.9% of patients who were diagnosed as having non-infectious endocarditis and underwent heart valve operations were reported to be diagnosed with IE only after cardiac operations [10]. In such cases, histological findings and bacterial detection on the valve using PCR were extremely useful [11,12]. In particular, histological examination could fulfill the strict version of the modified Duke criteria, which is evaluated by the presence of vegetation and bacteria on the valve, with both specificity and PPV of 100% [13]. Despite the lack of prior antibiotic therapy, our case did not definitively fulfill the modified Duke criteria on the basis of negative results of 4 blood cultures (8 sets) and 2 TEEs. Finally, we could make the correct diagnosis of IE only by performing a surgical operation on the mitral valve. This made it possible to detect vegetation and perform bacterial culture of the valve, which grew E. faecalis.
Another important feature of this case was the complication of DIC. DIC usually develops in the presence of underlying major disorders including sepsis, malignancies, trauma, major surgery, organ damages, obstetric complications, vascular abnormalities, severe hepatic failure, or immunological diseases [14]. Of all disorders, sepsis, trauma, and major surgeries account for about 75% of underlying disorders of DIC [6]. Our patient was diagnosed as having acute DIC on admission. Refractoriness to platelet transfusion and uncontrollable bleeding tendency, which were apparent by the presence of general subcutaneous bleeding, appeared in the clinical course. Her DIC was supposedly started by IE in the early disease phase, which deteriorated by a vicious circle of excessive consumption of platelets due to the production of HLA class I antibody, the presence of which was confirmed by laboratory examination. In short, uncontrolled bleeding tendency, which was improperly treated by HLA-unmatched platelet transfusion, worsened her DIC. Our hypotheses might be supported by the fact that the patient did not require additional platelet transfusions after administering 2 sets of HLA-matched platelet transfusions, which markedly improved bleeding tendency and DIC, promoting the disappearance of generalized subcutaneous bleeding.
Conclusions
IE on native mitral valve complicated with acute MR and DIC without underlying systemic disorders, which fails to fulfill definitively the modified Duke criteria, is rare and difficult to be diagnosed correctly. Only cardiac surgery might enable us to make an accurate diagnosis and save a patients’ life in such cases. Our case suggests that following guidelines or criteria should never replace clinical reasoning.
Acknowledgments
The authors thank the Department of Cardiovascular Surgery at Nagasaki University Hospital, especially Kiyoyuki Eishi, MD, PhD, for successfully performing cardiac operation on our patient and offering perioperative information including intra-operative pictures. We also thank Christina Croney, PhD, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Footnotes
Conflict of interest
None.
References:
- 1.Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin infect Dis. 2012;54(9):1230–39. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 2.Thimas JC, Bernard DP. Infective endocarditis. Lancet. 2016;387:882–93. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 3.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–38. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 4.Lorusso R, Gelsomino S, De Cicco G, et al. Mitral valve surgery in emergency for severe acute regurgitation: Analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg. 2008;33(4):573–82. doi: 10.1016/j.ejcts.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto W, Hamawaki M, Hashizume K, Eishi K. A patient in whom survival was achieved by acute-stage surgery for infective endocarditis complicated by a cerebral hemorrhage. Ann Thorac Cardiovasc Surg. 2009;15(4):257–60. [PubMed] [Google Scholar]
- 6.Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: Results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–50. doi: 10.1097/01.CCM.0000295317.97245.2D. [DOI] [PubMed] [Google Scholar]
- 7.Naber CK, Erbel R. Infective endocarditis with negative blood cultures. Int J Antimicrob Agents. 2007;30(Suppl.1):S32–36. doi: 10.1016/j.ijantimicag.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Sekar P, Johnson JR, Thurn JR, et al. Comparative sensitivity of transthoracic and transesophageal echocardiography in diagnosis of infective endocarditis among veterans with Staphylococcus aureus bacteremia. Open Forum Infect Dis. 2017;4(2):1–8. doi: 10.1093/ofid/ofx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieira MLC, Grinberg M, Pomerantzeff PMA, et al. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart. 2004;90(9):102–4. doi: 10.1136/hrt.2003.025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: Epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg. 2004;78(5):1623–29. doi: 10.1016/j.athoracsur.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 11.Morris AJ, Drinkovic D, Pottumarthy S, et al. Gram stain, culture, and histopathological examination findings for heart valves removed because of infective endocarditis. Clin infect Dis. 2003;36(6):697–704. doi: 10.1086/367842. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz P, Bouza E, Marín M, et al. Heart valves should not be routinely cultured. J Clin Microbiol. 2008;46(9):2897–901. doi: 10.1128/JCM.02173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greub G, Lepidi H, Rovery C, et al. Diagnosis of infectious endocarditis in patients undergoing valve surgery. Am J Med. 2005;118(3):230–38. doi: 10.1016/j.amjmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]