Abstract
Background:
Kato–Katz is the preferred method for the detection of Schistosoma mansoni eggs in stool. However, the sensitivity of this method is low and affected by day-to-day variation in egg excretion. Cathodic antigen urine tests have been proven to be sensitive for the diagnosis of S. mansoni infection in limited studies.
Aim:
To evaluate the accuracy and sensitivity of cathodic antigen urine tests for the diagnosis of S. mansoni infection.
Setting and Design:
This study was conducted in the Gezira Irrigation Scheme in the Gezira State, Sudan. Both S. mansoni and Schistosoma haematobium are endemic in the Gezira State. Kab-Algidad Village situated in Al Kamleen locality was selected for the study. This is a school-based, cross-sectional, comparative study.
Subjects and Methods:
Female school children, aged between 11 and 14 years who consented to participate, were enrolled in the study. Stool samples were examined using Kato–Katz technique and sodium dodecyl sulfate (SDS) digestion method. Urine samples were tested using the circulating cathodic antigen assays for the diagnosis of S. mansoni, and by centrifugation for S. haematobium.
Statistical Analysis Used:
Data were analyzed using the Scientific Package for Social Sciences version 15.
Results:
Cathodic antigen urine tests showed similar sensitivity to SDS and higher sensitivity compared to six Kato–Katz (reference diagnostic test).
Conclusion:
Cathodic antigen urine tests is a useful tool for mapping S. mansoni and may be used to evaluate the interruption of transmission.
Key words: Cathode antigen, diagnosis, Kato–Katz, Schistosoma haematobium, Schistosoma mansoni, sodium dodecyl sulfate, urine test
Abstract
ملخص البحث: تعنى هذه الدراسة المستقبلية لتقييم دقة وحساسية فحص البول لتشخيص عدوى البلهارسيا المانسونية، والتي أجريت في إحدى القرى السودانية المعروفة بتوطن هذه العدوى فيها. أجريت هذه الدراسة على طالبات المدارس اللاتي تتراوح أعمارهن بين 11 – 14 سنة وتم تحليل البراز والبول لهن. وخلصت الدراسة إلا أن كلا الفحصين اظهرا حساسية متشابهة، بينما كانت نتيجة فحص البول بواسطة (Cathodic Antigen) أكثر حساسية مقارنة بالــ (Kato – katz) وعليه فان الفحص عن طريق تحليل البول بواسطــــــــــــة (Cathodic Antigen) يعتبر كاختبار جيد لتشخيص البلهارسيا المانسونية.
INTRODUCTION
Schistosomiasis is a tropical disease that particularly affects poor people and is rife in settings where poverty is widespread, resources are scarce and access to basic services and livelihood opportunities are limited.[1,2,3] Currently, approximately 200 million people are infected with schistosomiasis and 779 million are at risk of infection in more than 76 countries.[4,5] Two main species, Schistosoma mansoni, causing intestinal schistosomiasis and Schistosoma haematobium, causing urogenital schistosomiasis, are endemic in Africa and account for about 85% of the global socio-economic burden of the disease.[1,2] In recent years, several countries have started implementing large campaigns to interrupt the transmission of schistosomiasis by Mass Drug Administration using Praziquantel as the drug of choice. To evaluate the efficiency of this drug in the interruption of the transmission of S. mansoni in low-transmission areas, a sensitive, simple and low-cost stool examination method is required. Kato–Katz is the preferred method to detect eggs in stool.[6,7] However, the sensitivity of this method is low, especially in low-transmission areas, and it is also affected by day-to-day variation in egg excretion.[8,9,10,11] Several immunological tests based on the detection of antibodies have been developed. Antibody detection is less sensitive in low-intensity infections and affected by previous treatment, cross reactivity with other parasites and particularly by antibody persistence after infection.[12] Circulating anodic and Cathodic antigens are schistosome genus-specific antigens and can be detected in the serum and urine of infected individuals with very high specificity (98%) and satisfactory sensitivity. The main disadvantages of antigen detection are related to the availability and cost of reagents and to relatively time-consuming and expensive assay.[13] The recently developed circulating cathodic antigen (CCA) urine-tests proved to be sensitive for the diagnosis of S. mansoni infection in limited studies and it is simple and easy to perform.[14,15,16] This study attempts to contribute to addressing a gap in the literature on CCA tests in low-transmission areas which tend to be scarce.
SUBJECTS AND METHODS
Ethical approval was obtained from Ahfad University for Women and the Federal Ministry of Health. Signed informed consent forms were obtained from parents and teachers. Male and female school children 11–14 years of age who completed the assent forms were enrolled in the study. The protocol was approved by WHO/EMRO. This study was conducted in the Gezira Irrigation Scheme which lies between the Blue Nile and the White Nile in the Gezira State, approximately 250 km south of the city of Khartoum. It occupies about five million feddans (1 feddan is equal to 1.38 acres); only 3 million feddans are suitable for irrigation. Both S. mansoni and S. haematobium are endemic in the Gezira State. Surveys conducted in Kamleen locality, Gezira State in 2011 showed a prevalence rate of 32% among school children.[17] Kab-Algidad village situated in Al Kamleen locality, approximately 120 km from Khartoum was selected for the study. It is situated at the tail end of the Gezira Irrigation Scheme.
This is a school-based cross-sectional, comparative study.
The sample size was calculated according to the Training in Tropical Diseases (TDR) diagnostics evaluation expert panel, available online under the title “Evaluation of the Diagnostic Tests for Infectious Diseases: General Principles.” At sensitivity of 85% and prevalence of 10%, the sample size would be 490 as follows:
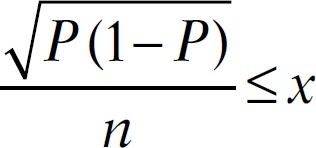
Which translates to:
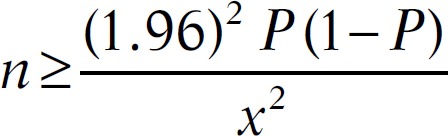
thus, P = 0.85 and x = 0.10
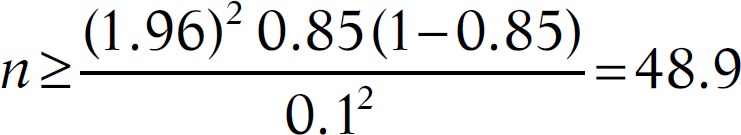
Therefore, to measure the sensitivity within ±10%, we required at least 49 samples that tested positive by the gold standard test. Therefore, to obtain 49 positive samples within 10% prevalence of infection in the study population, a total of 490 samples were required.
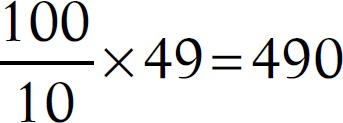 samples
samples
Three schools were selected, one mixed male and female school (setting A) and one school for boys and one for girls (setting B). Two stool and 2 urine samples were collected from each student on consecutive days after 10:00 A.M. One preparation, 2 preparations and 6 preparations were examined by Kato–Katz technique.[18] One preparation from each stool sample was examined by the digestion method.[19] In this method, 1 gm of feces was thoroughly mixed in 10 ml of 10% formal saline solution, centrifuged for 3 min at 3500 rpm, then the supernatant was discarded, and 10 ml of 0.05% of sodium dodecyl sulfate (SDS) solution was then added to the deposit and left to stand for at least 45 min to digest. After this period, 0.25 ml from the emulsified stool was transferred to microscope slide and examined under the microscope for the presence of Schistosomal eggs. Urine samples were tested using the CCA assays for the diagnosis of S. haematobium obtained from rapid diagnostics (Pretoria, South Africa) were performed at ambient temperature, following the manufacturer's instructions. Briefly, one drop of urine was added to the well of the test cassette and allowed to be absorbed entirely into the specimen pad within the well. Then, one drop of buffer (provided with the kit) was added. Results were read 20 min after adding the buffer. Results were determined by two persons and confirmed by the principal investigator as negative, trace (weak band) or positive (strong band). Each urine sample was examined by centrifugation at 3500 rpm for S. haematobium eggs.[20] Each infected child was weighed using a calibrated weighing scale, and a single dose of 40 mg/kg Praziquantel was administered.
Sensitivity was calculated as the number of true-positives (TP)/(TP + false-negative [FN]), specificity as true-negative (TN)/(TN + false-positive [FP]), positive predictive value (PPV) as TP/(TP + FP) and negative predictive value (NPV) as TN/(TN + FN).[21] Data were analyzed using the Scientific Package for Social Sciences version 15.
SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc http://www-01.ibm.com/support/docview.wss?uid = swg21476197
RESULTS
Table 1 shows the prevalence rates of S. mansoni and S. haematobium according to each diagnostic test in setting A (males and females). Prevalence rates for S. mansoni by six Kato–Katz “reference,” two Kato–Katz and one Kato–Katz was 28.3%, 23.6% and 9.4%, respectively. Two CCA revealed a prevalence of 34%. One SDS digestion method revealed a prevalence of 35%. Using both CCA and SDS methods resulted in the identification of a greater number of S. mansoni infected patients. These differences were statistically significant (P < 0.05).
Table 1.
Prevalence of Schistosoma mansoni and Schistosoma haematobium in setting A
| Diagnostic test | Number of children tested | Number of children positive | Percentage |
|---|---|---|---|
| Schistosoma mansoni diagnosis | |||
| Six Kato-Katz smear | 106 | 30 | 28.3 |
| Two Kato-Katz smear | 106 | 25 | 23.6 |
| One Kato-Katz smear | 106 | 10 | 9.4 |
| Two CCA | 106 | 36 | 34 |
| One SDS concentration | 106 | 37 | 35 |
| Schistosoma haematobium diagnosis | |||
| Two urine centrifugation | 106 | 0 | 0 |
CCA – Circulating cathodic antigen; SDS – Sodium dodecyl sulfate
Table 2 shows the prevalence of S. mansoni according to each diagnostic test in setting B. the prevalence of S. mansoni by six Kato–Katz, two Kato–Katz and one Kato–Katz was 20.6%, 14.7% and 8.4% respectively. Two CCA revealed a prevalence of 29.2%. One SDS method revealed a prevalence of 29.2%. The prevalence rates reported in setting B are lower than those reported in setting A. This may be due to the availability of adequate water supply and sanitation, which were not available in setting A.[22,23] The two urine samples were negative.
Table 2.
Prevalence of Schistosoma mansoni and Schistosoma haematobium in setting B
| Diagnostic test | Number of children tested | Number of children positive | Percentage |
|---|---|---|---|
| Schistosoma mansoni diagnosis | |||
| Six Kato-Katz smear | 394 | 81 | 20.6 |
| Two Kato-Katz smear | 394 | 58 | 14.7 |
| One Kato-Katz smear | 394 | 33 | 8.4 |
| Two CCA | 394 | 115 | 29.2 |
| One SDS concentration | 394 | 115 | 29.2 |
| Schistosoma haematobium diagnosis | |||
| Two urine centrifugation | 394 | 0 | 0 |
CCA – Circulating cathodic antigen; SDS – Sodium dodecyl sulfate
Table 3 shows the overall prevalence of S. mansoni and S. haematobium in both settings A and B. Prevalence rates for S. mansoni by six Kato–Katz two Kato–Katz and one Kato–Katz were 22.2%, 16.6%, and 8.6% respectively. Two CCA revealed a prevalence of 30.2%. One SDS revealed a prevalence of 30.4%. The two urine samples were negative. The overall assessment indicates low prevalence rates as a result of several rounds of mass chemotherapy.[24]
Table 3.
Prevalence of Schistosoma mansoni and Schistosoma haematobium in settings A and B
| Diagnostic test | Number of children tested | Number of children positive | Percentage |
|---|---|---|---|
| Schistosoma mansoni diagnosis | |||
| Six Kato-Katz smear | 500 | 111 | 22.2 |
| Two Kato-Katz smear | 500 | 83 | 16.6 |
| One Kato-Katz smear | 500 | 43 | 8.6 |
| Two CCA | 500 | 151 | 30.2 |
| One SDS concentration | 500 | 152 | 30.4 |
| Schistosoma haematobium diagnosis | |||
| Two urine centrifugation | 500 | 0 | 0 |
CCA – Circulating cathodic antigen; SDS – Sodium dodecyl sulfate
Of the 500 examined, 170 females were positives (34%) compared to 330 males (66%). Males were observed to have more water contact activities such as swimming and fishing in canals than females. S. haematobium was not found among the sample of 500.
Table 4 reports on the sensitivity, specificity and PPV NPV of different tests for the diagnosis of S. mansoni. CCA showed nearly similar sensitivity to six Kato–Katz (reference diagnostic test) and higher sensitivity as compared to two Kato–Katz. CCA also showed nearly similar sensitivity to SDS.
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive value of different tests for the diagnosis of Schistosoma mansoni
| Diagnostic test | Test result | Six Kato-Katz smears as reference diagnostic test | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Sensitivity | Specificity | PPV | NPV | ||
| One Kato-Katz | Positive | 43 (TP) | 0 (FP) | 43 | 38.7 | 100 | 100 | 85.1 |
| Negative | 68 (FN) | 389 (TN) | 457 | |||||
| Total | 111 | 389 | 500 | |||||
| Two Kato-Katz | Positive | 83 (TP) | 0 (FP) | 83 | 74.8 | 100 | 100 | 93.3 |
| Negative | 28 (FN) | 389 (TN) | 417 | |||||
| Total | 111 | 389 | 500 | |||||
| Two CCA | Positive | 110 (TP) | 41 (FP) | 151 | 99.1 | 89.5 | 72.8 | 99.7 |
| Negative | 1 (FN) | 348 (TN) | 349 | |||||
| Total | 111 | 389 | 500 | |||||
| One SDS | Positive | 108 (TP) | 44 (FP) | 152 | 97.3 | 88.7 | 71.1 | 99.1 |
| Negative | 3 (FN) | 345 (TN) | 348 | |||||
| Total | 111 | 389 | 500 | |||||
| Six Kato-Katz smear and SDS as reference diagnostic tests | ||||||||
| One Kato-Katz | Positive | 43 (TP) | 0 (FP) | 43 | 28.3 | 100 | 100 | 76.1 |
| Negative | 109 (FN) | 348 (TN) | 457 | |||||
| Total | 152 | 348 | 500 | |||||
| Two Kato-Katz | Positive | 83 (TP) | 0 (FP) | 83 | 54.6 | 100 | 100 | 83.5 |
| Negative | 69 (FN) | 348 (TN) | 417 | |||||
| Total | 152 | 348 | 500 | |||||
| Two CCA | Positive | 151 (TP) | 0 (FP) | 151 | 99.3 | 100 | 100 | 99.7 |
| Negative | 1 (FN) | 348 (TN) | 349 | |||||
| Total | 152 | 348 | 500 | |||||
PPV – Positive predictive value; NPV – Negative predictive value; CI – Confidence interval; SDS – Sodium dodecyl sulfate; CCA – Circulating cathodic antigen; TP – True-positive; FP – False-positive; TN – True-negative; FN – False-negative
DISCUSSION
In Kenya, the prevalence for 6 Kato–Katz was 38.8% compared to 62.4% by CCA.[15] In Côte d'Ivoire, CCA revealed a prevalence of 52.2% compared to a prevalence of 36.2% for Kato–Katz. The prevalence rates reported in Kenya and Côte d'Ivoire by Kato–Katz were higher than the prevalence rates reported in this study. This may be due to high endemicity of schistosomiasis in the two countries. Further studies were recommended for the evaluation of CCA in low-transmission areas in Côte d'Ivoire.[16] The new SDS method is reliable, easy to perform and sensitive for the detection of S. mansoni eggs in low-transmission areas. In this test, the eggs maintained their shape and the miracidia were clear. The disadvantage of the SDS method is that the digestion of the stool particles takes 45 min, but it was useful for confirmation of the CCA test and may also be useful in routine diagnosis in health settings.
Prevalence rates reported in setting B are lower than those reported in setting A. This may be due to the availability of adequate water supply and sanitation which were not available in setting A.[22,23]
The CCA method is not a quantitative method, which is required for the evaluation of the WHO strategic plan 2012–2020: Progression towards elimination of schistosomiasis prevalence of heavy intensity infection <1% in all sentinel sites.[24] WHO classifies morbidity levels by egg counts per gram, (epg), low category 1–99 epg, moderate 100–399 epg and high ≥400 epg.[25] In the present study, 111 samples were found to be positive by CCA [Table 3]. Of these, 59 showed light color intensity (+) and within the low epg category (1–99), 49 samples showed medium color intensity (++) within the range of (100–399) and 3 samples showed high color intensity, but they fall within the moderate egg count category. The high egg load is ≥400 epg.
CONCLUSION
It was concluded that CCA is a useful tool for mapping S. mansoni and evaluation of interruption of transmission. The cost of CCA is <$2.00 (<10,000= $1.76; 10,000–50,000= $1.59; >50,000= $1.46). The First Large-Scale Protocol to Formally Include Rapid Diagnostic Tests for Mapping of Schistosomiasis and Soil-Transmitted Helminthes was launched in Namibia.[26]
Financial support and sponsorship
Financial support was received from WHO/TDR EMRO and Ahfad University for Women.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We wish to thank WHO/TDR EMRO for their financial support and Ahfad University for Women for logistic support. We would also like to thank the communities and teachers of Kap Agidad Village for their cooperation.
REFERENCES
- 1.Savioli L, Renganathan E, Montresor A, Davis A, Behbehani K. Control of schistosomiasis – A global picture. Parasitol Today. 1997;13:444–8. doi: 10.1016/s0169-4758(97)01141-1. [DOI] [PubMed] [Google Scholar]
- 2.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 5.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 6.Montresor A, Crompton DW, Bundy DA, Savioli L. Geneva: 1998. [ResearchGate retrieved 19/03/2016]. Guidelines for the Evaluation of `Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level, WHO/CDS/SIP; pp. 1–48. https://www.researchgate.net/./237377179_WHO_1998_Gui . [Google Scholar]
- 7.Kongs A, Marks G, Verlé P, Van der Stuyft P. The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop Med Int Health. 2001;6:163–9. doi: 10.1046/j.1365-3156.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO/CDS/CPE/SIP. Geneva: WHO; 2001. Report of the Informal Consultation on Schistosomiasis in Low Transmission Areas: Control Strategies and Criteria for Elimination. [Google Scholar]
- 9.Teesdale CH, Fahringer K, Chitsulo L. Egg count variability and sensitivity of a thin smear technique for the diagnosis of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1985;79:369–73. doi: 10.1016/0035-9203(85)90384-0. [DOI] [PubMed] [Google Scholar]
- 10.van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000;77:69–80. doi: 10.1016/s0001-706x(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 11.Engels D, Sinzinkayo E, Gryseels B. Day-to-day egg count fluctuation in Schistosoma mansoni infection and its operational implications. Am J Trop Med Hyg. 1996;54:319–24. doi: 10.4269/ajtmh.1996.54.319. [DOI] [PubMed] [Google Scholar]
- 12.Elimination of Schistosomiasis from Low Transmission Areas. Report of a WHO Informal Consultation, Salvador, Bahia, Brazil. World Health Organization. 2009 [Google Scholar]
- 13.World Health Organization. Geneva: WHO; 2001. Report of the WHO Informal Consultation on Schistosomiasis on Schistosomiasis Control. WHO/CDS/CPE/SIP/1999. [Google Scholar]
- 14.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PN, et al. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Negl Trop Dis. 2011;5:e951. doi: 10.1371/journal.pntd.0000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchuem Tchuenté LA, Kueté Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, et al. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis. 2012;6:e1758. doi: 10.1371/journal.pntd.0001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulibaly JT, Knopp S, N'Guessan NA, Silué KD, Fürst T, Lohourignon LK, et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS Negl Trop Dis. 2011;5:e1384. doi: 10.1371/journal.pntd.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin MA, Swar M, Kardaman M, Elhussein D, Gibril N, Mahmoud A, et al. Treatment of pre-school children under 6 years of age for schistosomiasis: Safety, efficacy and acceptability of praziquantel. Sudan J Med Sci. 2012;7:67–76. [Google Scholar]
- 18.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 19.Hussein DM. PhD Thesis, University of Khartoum. 2005:44. [Google Scholar]
- 20.Ageel AR, Amin MA. Integration of schistosomiasis-control activities into the primary-health-care system in the Gizan region, Saudi Arabia. Ann Trop Med Parasitol. 1997;91:907–15. doi: 10.1080/00034989760293. [DOI] [PubMed] [Google Scholar]
- 21.Harper R, Reeves B. Reporting of precision of estimates for diagnostic accuracy: A review. BMJ. 1999;318:1322–3. doi: 10.1136/bmj.318.7194.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudra A. Evaluation of a health education programme in Tayba Qurashi Village, Central Sudan during 1983. J Trop Med Hyg. 1985;88:111–3. [PubMed] [Google Scholar]
- 23.Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ. 1991;69:609–21. [PMC free article] [PubMed] [Google Scholar]
- 24.WHO/EMRO Report. [Last retrieved on 2015 Jun 08]. Available from: http://www.who.int/neglected_disease/sudan intensifiesfightagainstScistosomiasis2013/en .
- 25.WHO. Schistosomiasis Progress Report 2001-2011 and Strategic plan 2011-12. ???: WHO. [retrieved 19/3/2016]. www.who.int/iris/./9789241503174_eng.pd .
- 26.Sousa-Figueiredo JC, Stanton MC, Katokele S, Arinaitwe M, Adriko M, Balfour L, et al. Mapping of schistosomiasis and soil-transmitted helminths in Namibia: The first large-scale protocol to formally include rapid diagnostic tests. PLoS Negl Trop Dis. 2015;9:e0003831. doi: 10.1371/journal.pntd.0003831. [DOI] [PMC free article] [PubMed] [Google Scholar]


