Summary
Objective
Weight management pharmacotherapies can improve metabolic diseases through weight‐dependent and weight‐independent effects. Lorcaserin is a selective 5‐hydroxytryptamine 2C receptor agonist. The objective of this analysis is to quantify the relative contribution of weight loss to the treatment effects of lorcaserin 10 mg twice a day on key metabolic parameters.
Methods
This retrospective analysis evaluated 6,897 patients with overweight or obesity (with or without diabetes mellitus) across three randomized, placebo‐controlled, double‐blind, 52‐week clinical trials that evaluated lorcaserin 10 mg twice daily (BID; NCT00395135, NCT00603902, and NCT00603291); 509 patients from only one of the studies had type 2 diabetes mellitus. A mediation analysis was applied to help rank the relative contribution of weight loss to metabolic study outcomes.
Results
According to this mediation analysis, lorcaserin 10 mg BID improved a spectrum of adiposopathic metabolic abnormalities with varying contributions attributable to weight loss. Improvements in waist circumference and blood pressure were almost exclusively attributable to weight loss. Less than 50% of the improvement in glucose parameters (fasting blood glucose and haemoglobin A1c) were attributable to weight loss.
Conclusions
Across Phase III clinical trials, lorcaserin 10 mg BID improved multiple cardiometabolic parameters through both weight‐loss dependent and independent mechanisms.
Keywords: Body weight, Metabolic disease, Obesity
Introduction
Obesity affects a multitude of individuals worldwide. Since 1980, its prevalence has doubled in more than 70 countries and continues to increase 1. Management of patients with overweight and obesity usually begins with implementing appropriate nutrition and physical activity, addressing potential secondary causes of fat weight gain, and engaging patients in behaviour modification. However, as with treatment of other metabolic diseases (e.g. high blood sugar, high blood pressure and abnormal lipid blood levels), these measures alone are often insufficient. In such cases, pharmacotherapy and perhaps even bariatric surgery may be beneficial in both reducing body weight and improving adiposopathic metabolic diseases. The overall objective in treating obesity is to not only improve the weight of patients but also improve the health of patients 2.
Some existing anti‐obesity pharmacotherapies not only reduce body fat but also improve metabolic diseases such as diabetes mellitus, hypertension and dyslipidemia, which are all major cardiovascular disease risk factors 3, 4. Lorcaserin is a selective 5‐hydroxytryptamine (5‐HT or serotonin) 2C receptor agonist that was approved by the US Food and Drug Administration for weight management pharmacotherapy in 2012. During the clinical trial development, lorcaserin not only reduced body weight, but also improved multiple metabolic parameters often caused or promoted by obesity 5. Clinical trials have suggested lorcaserin may be especially effective in improving glucose levels. Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus (BLOOM‐DM) was a 1‐year, randomized, placebo‐controlled study that evaluated 604 patients with type 2 diabetes mellitus (T2DM) treated with metformin, a sulfonylurea or both 6. Patients in the lorcaserin 10 mg twice daily (BID) group experienced significantly greater weight reduction (−4.5%) than the placebo group (−1.5%). The lorcaserin 10 mg BID group also experienced a significant reduction (27 mg dL−1) in fasting glucose compared with the reduction found with placebo (12 mg dL−1). Lorcaserin also promoted a significant reduction in haemoglobin A1c (HbA1c) of 0.9%, compared with a reduction of 0.4% with placebo (difference of 0.5%). A subsequent post hoc analysis of this Phase III BLOOM‐DM study supported the hypothesis that the glucose‐lowering effect of lorcaserin in patients with T2DM occurs with or without weight loss 7. This suggested lorcaserin may have beneficial metabolic effects (especially with regard to glucose parameters) independent of lorcaserin‐promoted weight loss.
Because anti‐obesity agents may have metabolic effects beyond reducing body fat alone, it may be useful to quantify the relative weight‐dependent and weight‐independent effects of anti‐obesity agents. Mediation analysis is a statistical application that quantifies multiple mechanisms that may help explain a cause‐and‐effect relationship 8. In a mediation analysis, key factors influencing the relationship between an independent variable (e.g. anti‐obesity agent) and a dependent variable (e.g. metabolic parameter) are examined via inclusion of a mediating variable (e.g. weight loss) 9. However, mediation analyses have limitations in that definitive proof of the findings of the mediation analysis requires clinical trials designed specifically to test the hypothesis. Nonetheless, the application of a mediation analysis can provide hypothesis‐generating insights into potential mechanisms of causality and rank the relative contributions of an independent variable mediator, on a dependent variable 9.
For example, a prior mediation analysis of liraglutide (an injectable, glucagon‐like peptide‐1 agonist) supported weight loss‐dependent and weight loss‐independent metabolic effects 9. Liraglutide‐induced weight loss was predominantly associated with improvements in waist circumference, triglycerides, diastolic blood pressure and high‐density lipoprotein (HDL) cholesterol levels, as well as Apnea‐Hypopnea Index and Impact of Weight on Quality of Life (IWQoL)‐Lite total and physical function scores (ranked 88–100%) 9. Liraglutide‐induced weight loss had an intermediate effect on systolic blood pressure, low‐density lipoprotein (LDL) cholesterol, total cholesterol and Short‐Form‐36 general health and physical function scores (ranked 59–67%) 9. Approximately 26–32% of the reductions in glucose parameters (e.g. fasting plasma glucose levels and HbA1c) were attributable to liraglutide‐promoted weight loss 9. This was expected, given that liraglutide is not only approved as a weight management pharmacotherapy but also as an anti‐DM agent. Thus, the findings of this prior mediation analysis were consistent with the known clinical effects of liraglutide (enhanced glucose‐dependent insulin secretion by the pancreatic beta cell, suppression of inappropriately elevated glucagon secretion and slowing of gastric emptying). It was also supportive of the potential for a weight‐management pharmacotherapy to have both weight loss‐dependent and weight loss‐independent effects.
The purpose of this report is to quantify the relative weight loss‐dependent and weight loss‐independent effects of lorcaserin. Lorcaserin clinical trial data were subjected to a mediator analysis to better quantify the relative contribution of lorcaserin to weight loss on metabolic parameters. In this case, the mediator analysis evaluated the association between an independent variable (i.e. treatment with lorcaserin 10 mg BID) and a dependent variable (outcome) through a third variable called a mediator (i.e. weight loss). The hypothesis prior to application of this mediator analysis was that lorcaserin would have both weight loss‐dependent and weight loss‐independent effects, especially with regard to glucose and HbA1c parameters.
Methods
The data for this mediation analysis were derived from patients with and without T2DM from three pivotal Phase III studies: BLOOM (NCT00395135, n = 3,182) 10, Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM; NCT00603902, n = 4,008) 11 and BLOOM‐DM (NCT00603291, n = 604) 6.
Non‐HDL cholesterol was calculated as total cholesterol less the cholesterol carried by HDL (total cholesterol – HDL cholesterol). Remnant cholesterol was calculated as total cholesterol less the cholesterol carried by LDL and HDL (total cholesterol – LDL cholesterol – HDL cholesterol). The American College of Cardiology/American Heart Association (ACC/AHA) Risk Score was performed on patients without T2DM and with/without metabolic syndrome (n = 5,658; ACC/AHA Risk Score non‐DM/non‐MetSyn) and patients with T2DM or metabolic syndrome (n = 2,097; ACC/AHA Risk Score DM/MetSyn).
Metabolic syndrome was defined as the presence of at least three of the following five parameters: waist circumference >35 in. in women and >40 in. in men; fasting plasma glucose levels >100 mg dL−1; triglyceride levels ≥150 mg dL−1; HDL cholesterol <50 mg dL−1 in women or <40 mg dL−1 in men; and/or systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg.
Mediation analysis investigates the association between an independent variable (exposure) and a dependent variable (outcome) through a third variable called a mediator 12. The independent variable was an exposure to treatment with lorcaserin 10 mg BID or placebo. The dependent variable (outcome) was assessed for changes from baseline at Week 52 last observation carried forward (LOCF). The mediator in this analysis was weight change from baseline to Week 52 LOCF 12.
In preliminary analyses among multiple baseline covariates in a stepwise linear regression model, age and diastolic blood pressure were significantly associated with weight change at Week 52 LOCF. Therefore, linear regression models applicable to age and diastolic blood pressure were applied to both outcomes (dependent variables) and weight loss (mediator). The mediation R‐package was used to conduct all analyses 13. The 95% confidence intervals were estimated using the quasi‐Bayesian Monte Carlo method, based on normal approximation 14.
Results
Baseline characteristics (mean) in the three Phase III trials were previously published and summarized as follows: BLOOM non‐T2DM 10: age (44.1 years), women (85.5%), body weight (100.1 kg), body mass index (BMI; 36.2 kg m−2); BLOSSOM non‐T2DM 11: age (43.8 years), women (79.8%), body weight (100.2 kg), BMI (35.9 kg m−2); BLOOM‐DM T2DM 6: age (52.7 years), women (54.2%), body weight (103.6 kg), BMI (36.0 kg m−2), HbA1c (8.06%). In these studies, lorcaserin 10 mg BID was superior to placebo in reducing body weight from baseline in each of the trials (BLOOM/BLOSSOM combined: −5.8 vs −2.5 kg modified intent‐to‐treat/LOCF, −7.9 vs 3.7 kg completers 15; BLOOM‐DM: −4.5% vs −1.5% modified intent‐to‐treat/LOCF, −5.5% vs −1.7% completers) 6. The proportion of participants at Week 52 achieving ≥5% weight loss were 47.1% and 22.6% for lorcaserin and placebo, respectively, in combined BLOOM/BLOSSOM trials 15 and 37.5% vs 16.1% in BLOOM‐DM 6. The proportion of participants at Week 52 achieving ≥10% weight loss were 22.4% and 8.7% for lorcaserin and placebo, respectively, in the combined BLOOM/BLOSSOM trials 15 and 16.3% vs 4.4% in BLOOM‐DM 6. Moreover, in BLOOM at Week 104, 67.9% (258/380) of patients who continued taking lorcaserin after Week 52 maintained ≥5% weight loss compared with 50.3% (88/175) of patients who were switched to placebo 10. Additionally, in the BLOOM‐DM study, HbA1c levels reduced by −0.9% vs −0.4% (baseline 8.1% for both) and fasting plasma glucose levels reduced by −27.4 vs −11.9 mg dL−1 (baseline approximately 160 mg dL−1 for both) with lorcaserin and placebo, respectively 6. The complete safety profiles were also previously published. Briefly, lorcaserin was very well tolerated in the lorcaserin development programmes (BLOOM, BLOSSOM, BLOOM‐DM), with headache (16.8% lorcaserin vs 10.1% placebo) and hypoglycaemia (29.3% lorcaserin vs 21% placebo) as most‐reported adverse events (AEs) for non‐T2DM and T2DM populations, respectively. Overall discontinuation rates due to AEs were 8.6% and 6.7% for lorcaserin and placebo, respectively. Headache, depression and dizziness were the three most common AEs (1.3% vs 0.8%, 0.9% vs 0.5%, 0.7% vs 0.2% for lorcaserin vs placebo, respectively) 6, 10, 11, 15.
The results of the mediation analysis are shown in Table 1 and Figure 1. A mediation analysis should be considered hypothesis generating, and thus, definitive statements of causality require confirmation by definitive clinical trials that test the hypothesis. In this mediation analysis, lorcaserin‐associated weight loss accounted for almost all the reductions in waist circumference and systolic and diastolic blood pressure. Other measured parameters that ranked ≥80%, as mediated by lorcaserin‐associated weight loss, included a reduction in the ACC/AHA Risk Score among patients without T2DM, reduction in triglyceride levels, improvement in the IWQoL survey, reduction in insulin blood levels, reduction in aspartate aminotransferase (AST), increase in HDL cholesterol and reduction in remnant cholesterol.
Table 1.
Proportion of outcome effects mediated by weight loss for lorcaserin 10 mg BIDa
| Outcome (%) | Estimate | Lower 95% CI | Upper 95% CI | P |
|---|---|---|---|---|
| Reduction in fasting plasma glucose | 33.69 | 16.59 | 69.38 | <0.0001 |
| Reduction in HbA1c | 46.57 | 34.43 | 65.71 | <0.0001 |
| Reduction in LDL‐cholesterol | 55.61 | 25.53 | 176.3 | 0.008 |
| Reduction in HOMA‐IR | 57.71 | 3.685 | 288.1 | 0.05 |
| Increase in AST/ALT ratio | 63.09 | 39.85 | 104.4 | <0.0001 |
| Reduction in ALT | 69.99 | 43.05 | 168.8 | <0.0001 |
| Reduction in total cholesterol | 71.03 | 43.44 | 167.3 | <0.0001 |
| Reduction in non‐HDL‐cholesterol | 72.57 | 51.56 | 120.4 | <0.0001 |
| Reduction in heart rate | 77.05 | 48.75 | 168.5 | <0.0001 |
| Reduction in ACC/AHA Risk Score (DM/MetSyn) | 78.36 | 37.33 | 306.2 | 0.016 |
| Reduction in remnant cholesterol | 86.64 | 65.23 | 132.5 | <0.0001 |
| Increase in HDL‐cholesterol | 88 | 54.86 | 158.5 | <0.0001 |
| Reduction in AST | 89.88 | 40.85 | 520.4 | 0.026 |
| Reduction in fasting insulin | 90.26 | −1452 | 1443 | 0.488 |
| Improvement in IWQoL | 94.25 | 68.19 | 135.2 | <0.0001 |
| Reduction in triglyceride levels | 95.9 | 68.83 | 168.1 | <0.0001 |
| Reduction in ACC/AHA Risk Score (non‐DM/non‐MetSyn) | 96.63 | 68.13 | 174.8 | <0.0001 |
| Reduction in waist circumference | 103 | 92.24 | 119.8 | <0.0001 |
| Reduction in diastolic blood pressure | 110 | 66.68 | 300.8 | 0.006 |
| Reduction in systolic blood pressure | 124.9 | 70.57 | 447.7 | 0.014 |
ACC/AHA, American College of Cardiology/American Heart Association; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BID, twice daily; CI, confidence interval; DM, diabetes mellitus; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment‐insulin resistance; IWQoL, impact of weight on quality of life; LDL, low‐density lipoprotein; MetSyn, metabolic syndrome.
Based upon data derived from 6,635 patients participating in three pivotal lorcaserin clinical trials: BLOOM (NCT00395135, n = 3,182), BLOSSOM (NCT00603902, n = 3,206) and BLOOM‐DM (NCT00603291, n = 509).
Figure 1.
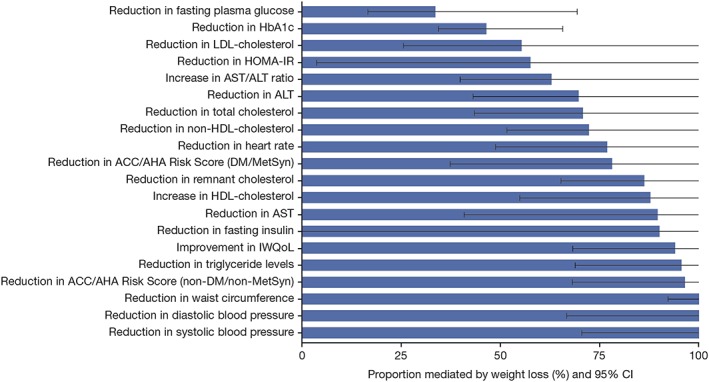
Relative contribution of weight loss (data truncated at 100%) on cardiometabolic parameters from Phase III clinical trials, ranked by effect size from smallest (least weight dependent) to largest (most weight dependent). ACC/AHA, American College of Cardiology/American Heart Association; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment‐insulin resistance; IWQoL, impact of weight on quality of life; LDL, low‐density lipoprotein; MetSyn, metabolic syndrome.
Measured parameters wherein lorcaserin‐associated weight loss had an intermediate effect (50–79%) included reductions in ACC/AHA Risk Score among patients with T2DM, reduction in heart rate, reduction in non‐HDL cholesterol, reduction in total cholesterol, reduction in alanine aminotransferase (ALT), reduction in AST/ALT ratio, reduction in homeostatic model assessment‐insulin resistance and reduction in LDL cholesterol. In addition, measured parameters wherein lorcaserin effects were mostly independent of weight loss (ranked <50%) included the glycaemic parameters of reduced fasting plasma glucose (33.7%) and reduced HbA1c (46.6%).
Discussion
Serotonin or 5‐HT is a neurotransmitter that not only regulates food intake and energy expenditure but also mediates glucose homeostasis 16, 17, 18. Lorcaserin is a selective 5‐HT2C receptor agonist. Human data support the hypothesis that lorcaserin‐mediated improvement in metabolic parameters may be mediated by both weight loss‐dependent and weight loss‐independent effects, especially regarding improvements in glycaemic parameters 7. This report further explores this hypothesis via application of a statistical model of mediation analysis, which helps quantify and rank the relative contribution of weight loss to various metabolic and clinical parameters. According to this mediation analysis, the reduction in waist circumference plus diastolic and systolic blood pressure were almost entirely due to lorcaserin‐associated weight loss. Conversely, <50% of the lorcaserin‐associated improvements in glucose parameters (fasting plasma glucose levels and HbA1c) were attributable to weight loss.
No therapeutic agent that specifically targets serotonergic pathways is yet approved for treatment of DM. However, due to the reported effects of serotonergic agents in improving glucose metabolism, the serotonin pathway has often been considered a therapeutic treatment target for novel anti‐DM agents 19. In human clinical trials, data support various serotonergic agents as favourably affecting glucose metabolism via mechanisms that appear to be independent of weight loss and not associated with increased insulin levels 20, 21, 22. Such effects would be consistent with the tissue expression and metabolic effects of serotonergic receptors, both in peripheral tissues and in the central nervous system 5, 23. However, many serotonergic agents previously reported to favourably affect glucose and other metabolic parameters were not specific in their binding to serotonergic receptors.
So how might serotonergic agents affect glucose parameters? Lorcaserin is highly selective for the 5‐HT2C central receptors 24, which are G‐coupled receptors found in the brain choroid plexus, cerebral cortex, basal ganglia and limbic system. Lorcaserin is thought to act upon the arcuate nucleus area of the hypothalamus via neural areas having energy balance functions (e.g. pro‐opiomelanocortin [POMC], agouti‐related peptide and cocaine‐regulated and amphetamine‐regulated transcript). Specifically, lorcaserin is believed to activate POMC neurons, resulting in second‐order signalling via melanocortin 4 (MC4) receptors 25, 26. Centrally, activation of MC4 receptors may reduce appetite and thus contribute to weight loss.
Increased MC4‐receptor activation may also increase sympathetic nerve activity 27, which may promote adipose tissue lipolysis 28. Adipocytes and adipose tissue are not inert; they are metabolically active. During positive caloric balance and onset of overweight and obesity, adipocyte hypertrophy and visceral adiposity become the anatomical findings of adiposopathy (i.e. fat cell and fat organ endocrine and immune dysfunction) 29. Reductions in adipocyte size and adipose tissue expansion with MC4 activation potentially improve adipocyte and adipose tissue functionality and thus improve metabolic parameters such as hyperglycaemia 30. Furthermore, during calorie restriction, sympathetic nervous system activity (as might be promoted by MC4 activation) may be required for energy liberation and reduction in adipose tissue, especially for visceral‐fat reduction during early weight loss 31. These mechanisms may help explain why weight loss via MC4 activation (as occurs with lorcaserin) may improve metabolic parameters such as markers of glucose homeostasis.
While fat weight loss alone may help improve multiple metabolic parameters 32, MC4 activity may affect glucose levels beyond its effects upon body weight. In rodent models of MC4 deficiency, young, lean MC4‐knockout mice have impaired insulin tolerance and increased insulin levels before onset of detectable hyperphagia or obesity 33. Potential weight‐independent mechanisms explaining how MC4 activation may improve glucose metabolism include increased insulin sensitivity, increased glucose transporter 4 expression and reduced hepatic glucose production 33, 34, 35. MC4 activation may also facilitate leptin‐promoted improvement in glucose metabolism 36. With specific regard to lorcaserin, murine models of T2DM suggest lorcaserin improves glycaemic control in the absence of reduction in food intake or body weight. These improvements in glycaemic parameters require sufficient POMC activity and functional MC4‐receptor signalling, both of which contribute to lorcaserin‐mediated increased insulin sensitivity, reduction in hepatic glucose production and increased glucose disposal 37, 38.
Given all the aforementioned information, it may not be surprising that, at least since 2007 (before the development and approval of lorcaserin), 5‐HT2C receptor agonists were proposed as having the potential to improve glucose tolerance and reduce plasma insulin levels in murine models of obesity and T2DM. In these earlier studies, improvement in glucose metabolism was found even at concentrations of 5‐HT2C receptor agonists that had no effect on ingestive behaviour, energy expenditure, locomotor activity, body weight or fat mass 38.
Regarding non‐glycaemic metabolic parameters in this mediation analysis, lorcaserin‐associated weight loss accounted for almost all reductions in waist circumference and systolic and diastolic blood pressure. Metabolic parameters that ranked ≥80%, as mediated by lorcaserin‐associated weight loss, included a reduction in the ACC/AHA Risk Score among patients without T2DM, reduction in triglyceride levels, improvement in the IWQoL survey, reduction in insulin blood levels, reduction in AST, increase in HDL cholesterol and reduction in remnant cholesterol. Lorcaserin‐associated weight loss had an intermediate effect (50–79%) on reduction in ACC/AHA Risk Score among patients with T2DM, a reduction in heart rate, a reduction in non‐HDL cholesterol, a reduction in total cholesterol, a reduction in ALT, a reduction in AST/ALT ratio, a reduction in homeostatic model assessment‐insulin resistance and a reduction in LDL cholesterol.
Conclusion
This report of a mediation analysis of lorcaserin is generally consistent with clinical trial data collected over the past several decades regarding the potential weight loss‐independent effects of serotonergic agents, especially with regard to improving glycaemic parameters. This mediation analysis of lorcaserin is also consistent with specific animal and human studies that have examined the effects of lorcaserin, with the greatest evidence of weight loss‐independent effects being those related to glycaemic control. Further information regarding the effects of lorcaserin on glucose metabolism, and/or patients with DM, will likely be found pending the results of the ongoing Cardiovascular And Metabolic Effects of Lorcaserin In Overweight And Obese Patients‐Thrombolysis in Myocardial Infarction 61 (CAMELLIA‐TIMI61) lorcaserin cardiovascular outcomes trial.
Funding
This study was funded by Eisai Inc.
Disclosures
In the past 12 months, Dr. Harold Bays' research site has received research grants from Eisai Inc.
Carlos Perdomo, Russell Knoth and Manoj Malhotra are employees of Eisai Inc.
Elena Nikonova is a former employee of Eisai Inc.
Author Contributions
HB proposed the study design and participated in the data analysis, data interpretation, literature search, generation of figures and writing of the manuscript.
All authors contributed to data interpretation, critically reviewed each draft of the manuscript, approved the final manuscript for submission and accept accountability for all aspects of the work.
Acknowledgements
Editorial support, under the direction of the authors, was provided by Stephanie Agbu, PhD, of CMC AFFINITY, a division of Complete Medical Communications Inc., Hackensack, USA, funded by Eisai Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Bays, H. , Perdomo, C. , Nikonova, E. , Knoth, R. , and Malhotra, M. (2018) Lorcaserin and metabolic disease: weight‐loss dependent and independent effects. Obesity Science & Practice, 4: 499–505. 10.1002/osp4.296.
Trial registration: NCT00395135, NCT00603902, and NCT00603291
References
- 1. Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 2018; 137: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bays HE, Seger J, Primack C, et al. Obesity Algorithm, presented by the Obesity Medicine Association. www.obesityalgorithm.org. Accessed June 29, 2018.
- 3. Bays H, Rodbard HW, Schorr AB, Gonzalez‐Campoy JM. Adiposopathy: treating pathogenic adipose tissue to reduce cardiovascular disease risk. Curr Treat Options Cardiovasc Med 2007; 9: 259–271. [DOI] [PubMed] [Google Scholar]
- 4. Bays H. Adiposopathy, metabolic syndrome, quantum physics, general relativity, chaos and the Theory of Everything. Expert Rev Cardiovasc Ther 2005; 3: 393–404. [DOI] [PubMed] [Google Scholar]
- 5. Bays HE. Lorcaserin: drug profile and illustrative model of the regulatory challenges of weight‐loss drug development. Expert Rev Cardiovasc Ther 2011; 9: 265–277. [DOI] [PubMed] [Google Scholar]
- 6. O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo‐controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM‐DM study. Obesity (Silver Spring) 2012; 20: 1426–1436. [DOI] [PubMed] [Google Scholar]
- 7. Magkos F, Nikonova E, Fain R, Zhou S, Ma T, Shanahan W. Effect of lorcaserin on glycemic parameters in patients with type 2 diabetes mellitus. Obesity (Silver Spring) 2017; 25: 842–849. [DOI] [PubMed] [Google Scholar]
- 8. Lange T, Hansen KW, Sorensen R, Galatius S. Applied mediation analyses: a review and tutorial. Epidemiol Health 2017; 39: e2017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bays H, Pi‐Sunyer X, Hemmingsson JU, Claudius B, Jensen CB, Van Gaal L. Liraglutide 3.0 mg for weight management: weight‐loss dependent and independent effects. Curr Med Res Opin 2017; 33: 225–229. [DOI] [PubMed] [Google Scholar]
- 10. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med 2010; 363: 245–256. [DOI] [PubMed] [Google Scholar]
- 11. Fidler MC, Sanchez M, Raether B, et al. A one‐year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 2011; 96: 3067–3077. [DOI] [PubMed] [Google Scholar]
- 12. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 13. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Software 2014; 59: 1–38. [Google Scholar]
- 14. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods 2010; 15: 309–334. [DOI] [PubMed] [Google Scholar]
- 15. Aronne L, Shanahan W, Fain R, et al. Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad Med 2014; 126: 7–18. [DOI] [PubMed] [Google Scholar]
- 16. Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH. Hyperactivity and reduced energy cost of physical activity in serotonin 5‐HT(2C) receptor mutant mice. Diabetes 2003; 52: 315–320. [DOI] [PubMed] [Google Scholar]
- 17. Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 2006; 51: 239–249. [DOI] [PubMed] [Google Scholar]
- 18. Berglund ED, Liu C, Sohn JW, et al. Serotonin 2C receptors in pro‐opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest 2013; 123: 5061–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh CM, Park S, Kim H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab J 2016; 40: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pestell RG, Crock PA, Ward GM, Alford FP, Best JD. Fenfluramine increases insulin action in patients with NIDDM. Diabetes Care 1989; 12: 252–258. [DOI] [PubMed] [Google Scholar]
- 21. Potter van Loon BJ, Radder JK, Frolich M, Krans HM, Zwinderman AH, Meinders AE. Fluoxetine increases insulin action in obese type II (non‐insulin dependent) diabetic patients. Int J Obes Relat Metab Disord 1992; 16: S55–S61. [PubMed] [Google Scholar]
- 22. Scheen AJ, Paolisso G, Salvatore T, Lefebvre PJ. Improvement of insulin‐induced glucose disposal in obese patients with NIDDM after 1‐wk treatment with d‐fenfluramine. Diabetes Care 1991; 14: 325–332. [DOI] [PubMed] [Google Scholar]
- 23. Wyler SC, Lord CC, Lee S, Elmquist JK, Liu C. Serotonergic control of metabolic homeostasis. Front Cell Neurosci 2017; 11: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5‐hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 2008; 325: 577–587. [DOI] [PubMed] [Google Scholar]
- 25. Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating “sick fat” through improving fat function with antidiabetes therapies. Am J Cardiol 2012; 110: 4B–12B. [DOI] [PubMed] [Google Scholar]
- 26. Burke LK, Heisler LK. 5‐hydroxytryptamine medications for the treatment of obesity. J Neuroendocrinol 2015; 27: 389–398. [DOI] [PubMed] [Google Scholar]
- 27. Sohn JW, Harris LE, Berglund ED, et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 2013; 152: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res 2015; 2015: 341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bays HE. Adiposopathy: is "sick fat" a cardiovascular disease? J Am Coll Cardiol 2011; 57: 2461–2473. [DOI] [PubMed] [Google Scholar]
- 30. Gaggini M, Carli F, Gastaldelli A. The color of fat and its central role in the development and progression of metabolic diseases. Horm Mol Biol Clin Investig 2017; 31. [DOI] [PubMed] [Google Scholar]
- 31. Sipe LM, Yang C, Ephrem J, Garren E, Hirsh J, Deppmann CD. Differential sympathetic outflow to adipose depots is required for visceral fat loss in response to calorie restriction. Nutr Diabetes 2017; 7: e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bays H. Adiposopathy, “sick fat,” Ockham's razor, and resolution of the obesity paradox. Curr Atheroscler Rep 2014; 16: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 2000; 141: 3072–3079. [DOI] [PubMed] [Google Scholar]
- 34. Morgan DA, McDaniel LN, Yin T, et al. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes 2015; 64: 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossi J, Balthasar N, Olson D, et al. Melanocortin‐4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 2011; 13: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system may be required for chronic CNS‐mediated antidiabetic and cardiovascular actions of leptin. Diabetes 2009; 58: 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burke LK, Ogunnowo‐Bada E, Georgescu T, et al. Lorcaserin improves glycemic control via a melanocortin neurocircuit. Mol Metab 2017; 6: 1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin‐4 receptor signaling pathways. Cell Metab 2007; 6: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]


