Abstract
Objective:
The aim of our retrospective study was to assess the etiology of recurrent pregnancy loss (RPL) in Saudi couples attending a specialized RPL clinic at King Fahad Hospital of the University, Al-Khobar, Saudi Arabia.
Patients and Methods:
A total of 59 couples attending the RPL clinic between January 2010 and December 2013 and who had completed their workup and investigations for RPL were included in the study. Data were collected from patients’ charts and computer-based laboratory results.
Results:
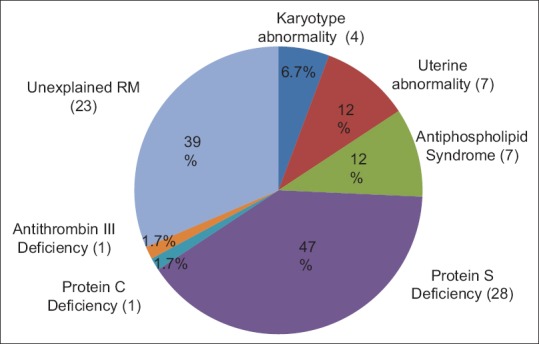
Protein S deficiency was found in 47% of patients, a chromosomal abnormality in 6.7%, uterine abnormality in 12%, antiphospholipid syndrome (APS) in 12%, and antithrombin III and Protein C deficiency in 1.7%. However, no patient had Factor V Leiden mutation. In 39% of the patients, there was no identifiable cause, and therefore, they had been diagnosed as unexplained RPL.
Conclusion:
The most common cause of RPL was Protein S deficiency followed by congenital uterine anomalies and APS. Almost 40% of couples with RPL remain with unknown etiology.
Keywords: Loss, pregnancy, recurrent, Saudi, thrombophilia, uterine anomaly
Abstract
ملخص البحث: هدفت هذه الدراسة لمعرفة أسباب حدوث الاجهاض المتكرر لدى الزوجات السعوديات واللاتي يراجعن العيادات المتخصصة في مستشفى الملك فهد الجامعي بالخبر. شملت الدراسة الزوجات السعوديات اللاتي استكملن الفحوصات اللازمة لمعرفة أسباب الاجهاضات المتكررة في الفترة ما بين يناير 2010 إلى ديسمبر 2013. بينت مراجعة التحاليل والفحوصات الشاملة أن أكثر الأسباب هو نقص بروتين (%47) S بينما لم تبين سبب واضح لدى (%39) من الحالات. يوصي الباحثون بالكشف عن بروتين S مبكراً لدى السيدات السعوديات ممن يعانين من الاجهاض المتكرر.
INTRODUCTION
Recurrent miscarriage (RM) or recurrent pregnancy loss (RPL) is defined as three or more consecutive pregnancy losses occurring before 20 weeks post menstruation.[1,2,3] The incidence is estimated to occur in 1% of fertile couples. But the incidence increases to 5% when two or more losses occur.[4] Clinicians have further classified pregnancy loss into three types, preclinical, embryonic and fetal loss; preclinical loss as demise <6 weeks; embryonic loss as demise at >6 weeks but <10 weeks gestation; fetal loss as demise at >10 weeks but <20 weeks gestation. Some clinicians use the presence or absence of fetal cardiac activity to determine the stage of pregnancy loss as either embryonic (loss before fetal cardiac activity has been identified) or fetal (loss after the fetal cardiac activity has been identified).[3,5] Other clinicians have further classified RPL as recurrent “early” pregnancy loss when this occurs before 12 weeks gestation and late loss is that which occurs after 12 weeks’ and before 20 weeks’ gestation.[6] The cause of RPL has been widely investigated, including parental and fetal chromosomal abnormalities, structural uterine abnormalities, antiphospholipid syndrome (APS), some thrombophilia, autoimmune disease, and endocrinological disorders such as polycystic ovarian syndrome and untreated diabetes.[4,7,8,9,10,11] Despite intensive and thorough investigations, no identifiable cause could be found in more than 50% of couples.[12] These couples with unexplained RPL experience even further stress and higher levels of anxiety because of the uncertainty as to whether they should stop trying to conceive or continue with the knowledge that further pregnancies may also result in a loss. Providing tender loving care, support and reassurance to these couples have been known to result in a 75% success rate in a future pregnancy.[13,14] The aim of our study is to assess the causes of RPL in Saudi couples attending a specialized RPL clinic at King Fahd Hospital of the University, Al-Khobar, Saudi Arabia.
PATIENTS AND METHODS
This is a retrospective study of all Saudi couples with RPL, who were referred to the RPL specialized clinic at King Fahd University Hospital in Al Khobar, Saudi Arabia between January 2010 and December 2013. All cases were ascertained to have had three or more spontaneous miscarriages, with pregnancies confirmed by βhCG. Demographic data, such as age, the number of live births, the number of miscarriages, and the number of early and late pregnancy losses were recorded. Peripheral karyotyping of the couple was performed. The detection of the lupus anticoagulant was based upon dilute Russell viper venom test time together with a platelet neutralization procedure. The anticardiolipin antibodies were detected by a standardized enzyme-linked immunosorbent assay, and the titer was considered elevated if medium or high titers of both IgG and IgM isotypes were present in blood. A diagnosis of APS is made if one of the following clinical criteria is present: (a) Three or more unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal abnormalities excluded, or (b) one or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation, with normal fetal morphology documented by ultrasound or by direct examination of the fetus, or (c) one or more premature births of a morphologically normal neonate at or before the 34th week of gestation because of severe preeclampsia or eclampsia or severe placental insufficiency. In addition, persistent abnormality of one of the following tests when measured at least twice, >6 weeks apart: (a) Lupus anticoagulant, (b) antiphospholipid antibodies.[15] Protein S, Protein C, antithrombin III, and Factor V (FV) global Protein C were also tested by functional immunosorbent assays. The uterine cavity was assessed by an office hysteroscopy or a hysterosalpingography, and uterine findings were reported as a normal uterine cavity, congenital (such as septate, bicornuate, unicornuate) or acquired abnormality (such as an endometrial polyp, submucous fibroid, or uterine synechiae). Couples who had not completed their investigations were excluded from the study.
RESULTS
A total of 59 Saudi couples with a history of repeated miscarriages were included. As shown in Table 1, the mean female age was 32.83 ± 6.64 standard deviation. The number of previous miscarriages varied from 3 to 23 miscarriages. The number of early losses ranged between 2 and 16 whereas the number of late losses ranged from none to seven losses. Nineteen females out of 59 (32%) were nulliparous with no live birth while the remaining 40 (68%) had one live birth. Forty females had only early losses, (all losses <12 weeks of gestation), one had only late losses (losses >12 weeks of gestation) and 18 had mixed losses some <12 weeks of gestation and others >12 weeks of gestation. Identifiable causes of RM are shown in Figure 1. Uterine cavity abnormalities were found in seven women (11.9%), specifically septate uterus in two patients, endometrial polyp in three patients, uterine synechia in one patient. Cervical incompetence in one patient. Abnormal karyotype was seen in four patients (6.8%) (two females and two males) mainly robertsonian translocation and inversion. The APS was diagnosed in 7 (11.9%) women whereas Protein S deficiency was diagnosed in 28 (47.5%) women. Protein C deficiency as well as antithrombin III was found in only one female (1.7%). FV Leiden mutation was not found in any of the women studied. Finally, 23 (39%) women were diagnosed as having unexplained RM. Table 2 shows the frequencies of the type of loss in relation to the identified cause.
Table 1.
Demographic characteristics of the women with three or more miscarriages
| Characteristic | Total 59 patients |
|---|---|
| Age mean±SD | 32.83±6.64 |
| Number of previous miscarriages (range) | 3-23 |
| Number of early losses (range) | 2-16 |
| Number of late losses (range) | 0-7 |
| Number of nulliparous (%) | 19 (32) |
| Number of patients with previous live birth (%) | 40 (68) |
SD – Standard deviation
Figure 1.
The frequency of identifiable causes of recurrent pregnancy loss (#)
Table 2.
Frequency of identifiable cause of recurrent pregnancy loss in relation to the type of loss
| Cause | Early loss (number of patients) | Mixed loss (early and late loss) (number of patients) |
|---|---|---|
| APS | 3 | 4 |
| Protein S deficiency | 21 | 6 |
| Uterine abnormality | 5 | 2 |
| Chromosomal abnormality | 3 | 1 |
| Antithrombin III deficiency | 1 | 0 |
| Protein C deficiency | 1 | 0 |
| Unexplained | 14 | 8 |
APS – Antiphospholipid syndrome
DISCUSSION
Our study demonstrated that the most common cause of RPL in Saudi females in the Eastern Province of Saudi Arabia was Protein S deficiency, which was found in almost half of the study population. This is followed by couples who had no identifiable cause for RPL, with a prevalence of 39%. APS and uterine cavity abnormalities were found in 12% of the patients. The most common uterine cavity abnormality was endometrial polyp followed by a uterine septum. Protein C deficiency and antithrombin III deficiency were found in 1.7% whereas FV Leiden mutation was not detected in this study population. Furthermore, our study showed a 6.7% rate of parental karyotype abnormality. All uterine cavity abnormalities were corrected at the time of diagnostic hysteroscopy either by the removal of the polyp or resection of the septum hysteroscopically. All patients who were diagnosed with thrombophilia were offered low dose aspirin and low molecular weight heparin throughout the pregnancy, and six weeks postpartum. The strength of our study is that all patients were attending a specialized RPL clinic at the same hospital and under the care of the same consultant and investigations were carried out in one laboratory. Patients who attended the clinic but did not complete their follow-up visits and investigations were not included in the study. The small sample size is due to the inclusion of only those couples who had undergone a thorough and complete investigation.
The prevalence of uterine anomalies in the RPL population has been reported to range between 1.8% and 37.6%, which is in accordance with our study. This wide range is due to the different criteria used in defining RPL (two vs. three or more consecutive pregnancy losses) as well as the different techniques and criteria used in the diagnosis of uterine anomalies.[16] It has been found that correction of a septate uterus resulted in a decline in miscarriage rate from 88% to 14%.[17] Chromosomal abnormalities of either parent has also been implicated as a cause of RPL. Furthermore, their pregnancies may result in a live birth with multiple congenital malformations and/or mental disability secondary to an unbalanced chromosomal arrangement. The composite prevalence of major chromosomal abnormalities in either parent was about 3.5%, 5 to 6 times higher than the general population, most commonly a balanced reciprocal or robertsonian translocation.[7] Our study showed a prevalence rate of 6.7% for parental karyotype abnormality, which is higher than that reported in the literature. Chromosomal abnormalities of the embryo were found to account for 30–57% of further miscarriages in couples with RPL.[5,18,19,20,21,22] However, it has been reported that as the number of miscarriages increases, the risk of euploid pregnancy loss increases.[5,20] Not surprisingly, it was found that advanced maternal age (older than 36) was associated with a significantly higher rate of aneuploidy. In other words, in women younger than 36, recurrent loss was due primarily to causes other than chromosomal abnormalities.[5] In our study, more than two-thirds of the population studied were younger than 36 years of age. Thrombophilias, both inherited as well as acquired, have been investigated and studied thoroughly as a cause of RPL and adverse pregnancy outcome. APS (an acquired thrombophilia) is the most treatable well-recognized cause of RPL. The prevalence of APS in our study was 12%, which is similar to several other studies, where it was reported to be approximately 15% in comparison to <2% in women with a low-risk obstetric history.[23] It has been reported that 10.9% of Saudi females with RPL had APS compared to 5.6% in normal pregnancies and 1.2% in healthy blood donors.[24] The live birth rate in women with RPL associated with APS with no pharmacological intervention has been reported to be as low as 10%.[25] It was concluded in a meta-analysis of randomized controlled trials that the only treatment or treatment combination that leads to a significant increase in the live birth rate among women with APS is aspirin plus unfractionated heparin. The reduction rate of miscarriage was 54% in patients receiving aspirin plus unfractionated heparin compared with aspirin alone: Relative risk 0.46, 95% confidence interval (0.29–0.71).[26] Recently, an increased incidence of early and recurrent fetal loss has been suggested in women with inherited thrombophilia, including FV Leiden mutation, activated Protein C resistance, prothrombin G20210A mutation, and Protein S deficiency. The incidence of FV Leiden mutation varies among different populations. Some sources revealed a frequency as high as 15% in Caucasians, with the highest incidence rate of 31.2% found in Jews.[27] The prevalence of FV Leiden mutation in the Saudi population ranged between 0.5 and 2%, which is quite low compared to that of Egyptians 16.5–18.5%, Turkish 24.6%, Greek 22.6%, Lebanese 14.65%, Tunisians 13.6%, Jewish 10%, and Italian populations 6%, but it is similar to that of Indians 1.3% and Americans 0.8–1.8%.[28,29,30,31,32,33,34,35,36,37,38] Another study found FV Leiden was present in 6.42% and 7.04% of Kuwaitis of Iranian and Iraqi origin, respectively, but it was absent (0%) among Kuwaitis of Saudi origin.[39] This might explain why no patients in our study were diagnosed with FV Leiden mutation. Protein S deficiency and Protein C deficiency are relatively less common, where the prevalence in the general population ranges between 0 and 0.5%.[40] Surprisingly, in the Saudi population, Protein S deficiency has a prevalence rate of 14.5% while prothrombin gene mutation has a prevalence of 1.1%.[28] The association of inherited thrombophilia and fetal loss varies from one study to another. A meta-analysis has reported a consistently stronger association of inherited thrombophilia with late fetal loss than with an early loss.[10] A meta-analysis of 16 case–control studies reported that carriers of FV Leiden or prothrombin gene mutation have double the risk of experiencing RPL compared with women without these thrombophilic mutations.[41] However, some studies have reported a similar risk of fetal loss in women with the FV Leiden mutation compared to those without.[42,43] Furthermore, several studies have found no correlation between the prothrombin gene mutation and RPL.[44,45,46,47] The association of RPL and antithrombin III deficiency and Protein C deficiency is less clear. In general, the association of inherited thrombophilia with fetal loss is not as clear as APS.[48]
CONCLUSION
Our study demonstrated the uniqueness of the Saudi population in terms of their thrombophilia abnormalities compared to neighboring Middle Eastern populations. Whilst a bigger epidemiological study may shed more light, our study shows that Protein S deficiency, APS and uterine abnormality should be the first three investigations to be carried out in Saudi women with RPL. Chromosomal study reserved to selected cases, bearing in mind that in up to 40% of couples, no cause for RPL can be identified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors appreciate and thank Professor Laila Bashawri, Consultant Hematologist for her guidance and interpreting the laboratory results and the hospital personnel for collection of the data.
REFERENCES
- 1.Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673–5. doi: 10.1016/0140-6736(90)92159-f. [DOI] [PubMed] [Google Scholar]
- 2.Berry CW, Brambati B, Eskes TK, Exalto N, Fox H, Geraedts JP, et al. The Euro-Team Early Pregnancy (ETEP) protocol for recurrent miscarriage. Hum Reprod. 1995;10:1516–20. doi: 10.1093/humrep/10.6.1516. [DOI] [PubMed] [Google Scholar]
- 3.Bricker L, Farquharson RG. Types of pregnancy loss in recurrent miscarriage: Implications for research and clinical practice. Hum Reprod. 2002;17:1345–50. doi: 10.1093/humrep/17.5.1345. [DOI] [PubMed] [Google Scholar]
- 4.Hogge WA, Byrnes AL, Lanasa MC, Surti U. The clinical use of karyotyping spontaneous abortions. Am J Obstet Gynecol. 2003;189:397–400. doi: 10.1067/s0002-9378(03)00700-2. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: A case-control study. Hum Reprod. 2002;17:446–51. doi: 10.1093/humrep/17.2.446. [DOI] [PubMed] [Google Scholar]
- 6.Farquharson RG, Jauniaux E, Exalto N ESHRE Special Interest Group for Early Pregnancy (SIGEP) Updated and revised nomenclature for description of early pregnancy events. Hum Reprod. 2005;20:3008–11. doi: 10.1093/humrep/dei167. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson MD, Sierra S. Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangement. Hum Reprod. 2006;21:1076–82. doi: 10.1093/humrep/dei417. [DOI] [PubMed] [Google Scholar]
- 8.Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: A critical appraisal. Hum Reprod Update. 2008;14:415–29. doi: 10.1093/humupd/dmn018. [DOI] [PubMed] [Google Scholar]
- 9.Greaves M, Cohen H, MacHin SJ, Mackie I. Guidelines on the investigation and management of the antiphospholipid syndrome. Br J Haematol. 2000;109:704–15. doi: 10.1046/j.1365-2141.2000.02069.x. [DOI] [PubMed] [Google Scholar]
- 10.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: A meta-analysis. Lancet. 2003;361:901–8. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 11.Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Semin Reprod Med. 2006;24:33–9. doi: 10.1055/s-2006-931799. [DOI] [PubMed] [Google Scholar]
- 12.Quenby SM, Farquharson RG. Predicting recurring miscarriage: What is important? Obstet Gynecol. 1993;82:132–8. [PubMed] [Google Scholar]
- 13.Clifford K, Rai R, Regan L. Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Hum Reprod. 1997;12:387–9. doi: 10.1093/humrep/12.2.387. [DOI] [PubMed] [Google Scholar]
- 14.Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod. 1999;14:2868–71. doi: 10.1093/humrep/14.11.2868. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: Report of an international workshop. Arthritis Rheum. 1999;42:1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update. 2001;7:161–74. doi: 10.1093/humupd/7.2.161. [DOI] [PubMed] [Google Scholar]
- 17.Homer HA, Li TC, Cooke ID. The septate uterus: A review of management and reproductive outcome. Fertil Steril. 2000;73:1–14. doi: 10.1016/s0015-0282(99)00480-x. [DOI] [PubMed] [Google Scholar]
- 18.Franssen MT, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: Index [corrected]-control study. BMJ. 2006;332:759–63. doi: 10.1136/bmj.38735.459144.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai G. Karyotype of the abortus in recurrent miscarriage. Fertil Steril. 2001;75:678–82. doi: 10.1016/s0015-0282(00)01801-x. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73:300–4. doi: 10.1016/s0015-0282(99)00495-1. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104:784–8. doi: 10.1097/01.AOG.0000137832.86727.e2. [DOI] [PubMed] [Google Scholar]
- 22.Carp H, Feldman B, Oelsner G, Schiff E. Parental karyotype and subsequent live births in recurrent miscarriage. Fertil Steril. 2004;81:1296–301. doi: 10.1016/j.fertnstert.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 23.Rai RS, Regan L, Clifford K, Pickering W, Dave M, Mackie I, et al. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: Results of a comprehensive screening approach. Hum Reprod. 1995;10:2001–5. doi: 10.1093/oxfordjournals.humrep.a136224. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mishari AA, Gader AG, Al-Jabbari AW, Al-Momen AK, El Rab MO, Babay ZH, et al. The prevalence of lupus anticoagulant in normal pregnancy and in women with recurrent fetal loss – Recommendations for laboratory testing for lupus anticoagulant. Ann Saudi Med. 2004;24:429–33. doi: 10.5144/0256-4947.2004.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rai RS, Clifford K, Cohen H, Regan L. High prospective fetal loss rate in untreated pregnancies of women with recurrent miscarriage and antiphospholipid antibodies. Hum Reprod. 1995;10:3301–4. doi: 10.1093/oxfordjournals.humrep.a135907. [DOI] [PubMed] [Google Scholar]
- 26.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;2:CD002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet. 1995;346:1133–4. doi: 10.1016/s0140-6736(95)91803-5. [DOI] [PubMed] [Google Scholar]
- 28.Al-Jaouni SK. Primary thrombophilia in Saudi Arabia. Saudi Med J. 2003;24:614–6. [PubMed] [Google Scholar]
- 29.Saour JN, Shoukri MM, Mammo LA. The Saudi thrombosis and familial thrombophilia registry.Design, rational, and preliminary results. Saudi Med J. 2009;30:1286–90. [PubMed] [Google Scholar]
- 30.Settin AA, Alghasham A, Ali A, Dowaidar M, Ismail H. Frequency of thrombophilic genetic polymorphisms among Saudi subjects compared with other populations. Hematology. 2012;17:176–82. doi: 10.1179/102453312X13376952196575. [DOI] [PubMed] [Google Scholar]
- 31.Ulu A, Elsobky E, Elsayed M, Yıldız Z, Tekin M, Akar N. Frequency of five thrombophilic polymorphisms in the Egyptian population. Turk J Hematol. 2006;23:100–3. [PubMed] [Google Scholar]
- 32.Bauduer F, Lacombe D. Factor V Leiden, prothrombin 20210A, methylenetetrahydrofolate reductase 677T, and population genetics. Mol Genet Metab. 2005;86:91–9. doi: 10.1016/j.ymgme.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Kalkanli S, Ayyildiz O, Tiftik N, Batun S, Isikdogan A, Ince H, et al. Factor V Leiden mutation in venous thrombosis in southeast Turkey. Angiology. 2006;57:193–6. doi: 10.1177/000331970605700209. [DOI] [PubMed] [Google Scholar]
- 34.Mougiou A, Androutsopoulos G, Karakantza M, Theodori E, Decavalas G, Zoumbos N. Inherited thrombophilia screening in Greek women with recurrent fetal loss. Clin Exp Obstet Gynecol. 2008;35:172–4. [PubMed] [Google Scholar]
- 35.Bouaziz-Borgi L, Almawi WY, Mtiraoui N, Nsiri B, Keleshian SH, Kreidy R, et al. Distinct association of factor V-Leiden and prothrombin G20210A mutations with deep venous thrombosis in Tunisia and Lebanon. Am J Hematol. 2006;81:641–3. doi: 10.1002/ajh.20582. [DOI] [PubMed] [Google Scholar]
- 36.Maalej L, Hadjkacem B, Ben Amor I, Smaoui M, Gargouri A, Gargouri J. Prevalence of factor V Leiden in South Tunisian blood donors. J Thromb Thrombolysis. 2011;32:116–9. doi: 10.1007/s11239-011-0582-9. [DOI] [PubMed] [Google Scholar]
- 37.Martinelli I, Taioli E, Cetin I, Marinoni A, Gerosa S, Villa MV, et al. Mutations in coagulation factors in women with unexplained late fetal loss. N Engl J Med. 2000;343:1015–8. doi: 10.1056/NEJM200010053431405. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann FH, Salazar-Sanchez L, Schröder W, Grimm R, Schuster G, Jimenez-Arce G, et al. Prevalence of molecular risk factors FV Leiden, FV HR2, FII 20210G>A and MTHFR 677C>T in different populations and ethnic groups of Germany, Costa Rica and India. IJHG. 2001;1:33–9. [Google Scholar]
- 39.Dashti AA, Jadaon MM. Race differences in the prevalence of the factor V Leiden mutation in Kuwaiti nationals. Mol Biol Rep. 2011;38:3623–8. doi: 10.1007/s11033-010-0474-7. [DOI] [PubMed] [Google Scholar]
- 40.Gris JC, Quéré I, Monpeyroux F, Mercier E, Ripart-Neveu S, Tailland ML, et al. Case-control study of the frequency of thrombophilic disorders in couples with late foetal loss and no thrombotic antecedent – The Nîmes Obstetricians and Haematologists Study5 (NOHA5) Thromb Haemost. 1999;81:891–9. [PubMed] [Google Scholar]
- 41.Kovalevsky G, Gracia CR, Berlin JA, Sammel MD, Barnhart KT. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss: A meta-analysis. Arch Intern Med. 2004;164:558–63. doi: 10.1001/archinte.164.5.558. [DOI] [PubMed] [Google Scholar]
- 42.Vossen CY, Preston FE, Conard J, Fontcuberta J, Makris M, van der Meer FJ, et al. Hereditary thrombophilia and fetal loss: A prospective follow-up study. J Thromb Haemost. 2004;2:592–6. doi: 10.1111/j.1538-7836.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 43.Roqué H, Paidas MJ, Funai EF, Kuczynski E, Lockwood CJ. Maternal thrombophilias are not associated with early pregnancy loss. Thromb Haemost. 2004;91:290–5. doi: 10.1160/TH03-09-0596. [DOI] [PubMed] [Google Scholar]
- 44.Altintas A, Pasa S, Akdeniz N, Cil T, Yurt M, Ayyildiz O, et al. Factor V Leiden and G20210A prothrombin mutations in patients with recurrent pregnancy loss: Data from the southeast of Turkey. Ann Hematol. 2007;86:727–31. doi: 10.1007/s00277-007-0327-1. [DOI] [PubMed] [Google Scholar]
- 45.Serrano F, Lima ML, Lopes C, Almeida JP, Branco J. Factor V Leiden and prothrombin G20210A in Portuguese women with recurrent miscarriage: Is it worthwhile to investigate? Arch Gynecol Obstet. 2011;284:1127–32. doi: 10.1007/s00404-010-1834-1. [DOI] [PubMed] [Google Scholar]
- 46.Silver RM, Zhao Y, Spong CY, Sibai B, Wendel G, Jr, Wenstrom K. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115:14–20. doi: 10.1097/AOG.0b013e3181c88918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodger MA, Betancourt MT, Clark P, Lindqvist PG, Dizon-Townson D, Said J, et al. The association of factor V Leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: A systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010;7:e1000292. doi: 10.1371/journal.pmed.1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawood, Feroza. Inherited and Acquired Thrombophilia in Pregnancy. INTECH Open Access Publisher. 2011 [Google Scholar]


