Abstract
Background:
Allergic disorders, particularly bronchial asthma, are one of the most common chronic childhood diseases. Bronchial asthma is more prevalent among children of the Eastern Province of Saudi Arabia compared with the other provinces. Environmental factors play major roles in the disease pathogenesis in genetically predisposed hosts. In this study, we characterize the pattern of allergenicity in asthmatic children in the Eastern Province of Saudi Arabia.
Materials and Methods:
This study is a retrospective, cross-sectional analysis of skin sensitization profiles of 100 Saudi asthmatic children living in Al-Khobar, Saudi Arabia. The group compromised 32 females and 68 males, aged 5–14 years with a mean age of 8.98 ± 2.93 years. Skin prick tests were performed in a regional tertiary center, in the period between January 2011 and December 2012, using a variety of indoor and outdoor allergens.
Results:
The most common indoor sensitizing allergens found were the house dust mite (54%), cat fur (53%) and the German cockroach (26%). Among outdoor allergens, Salsola kali was the most common (48%), followed by Timothy grass (23%) and Chenopodium album (23%). Among trees, Prosopis glandulosa was found to be the most prevalent sensitizer (19%). Among the molds, Alternaria and Aspergillus species were the most prevalent (21% each).
Conclusion:
A high rate of sensitization to aeroallergens was found in asthmatic children living in Al-Khobar city. The pattern of sensitization found in our study reflects the newly altered nature of an ancient humid desert that has been influenced by the recent artificial modernization of the region.
Keywords: Aeroallergens, allergy, bronchial asthma, Saudi Arabia, skin test
Abstract
ملخص البحث: تلعب العوامل البيئية دورا رئيسياً في حدوث مرض الحساسية مثل الربو وخاصة لمن يحمل العوامل الوراثية لذلك. في هذه الدراسة حدد الباحثون نمط الحساسية لدى الأطفال المصابين بالربو في المنطقة الشرقية بالمملكة العربية السعودية عن طريق دراسة مقطعية استرجاعية شملت مائة طفل سعودي بمدينة الخبر بالمنطقة الشرقية. وجد الباحثون أن المسبب الأكثر شيوعاً للحساسية داخل البيت هو عث الغبار ونبات يدعى ( Salsola kali ) خارج البيت. خلصت الدراسة إلى وجود معدل عالي من المواد المثيرة للحساسية في الهواء لدى الأطفال المصابين بالربو بمدينة الخبر. ويعكس هذا النمط من الحساسية التغير من الجو الصحراوي الرطب إلى التطور الحديث في هذه المنطقة.
INTRODUCTION
Allergic disorders are considered one of the most common chronic childhood disorders. Asthma affects up to 25% of school-aged children in Saudi Arabia, and its prevalence has increased dramatically over the last few decades.[1] The rising prevalence has been attributed, in part, to the influence of climatic and environmental factors on the allergic and genetically predisposed host.[2,3,4] The highest prevalence of asthma was found to affect children residing in the eastern region of Saudi Arabia, with an estimated prevalence of 33.7% compared with 17.7% and 14.1% in the central and western regions, respectively.[5]
The pattern and extent of allergen sensitization to various indoor and outdoor allergens are believed to contribute to the severity and progression of asthma symptoms.[6,7,8] A significant reduction in the risk of future asthma development was observed in high-risk children when specific environmental avoidance measures were practiced during their early years of life.[9] Once asthma has developed, avoidance of allergenic triggers significantly reduces airway hyperactivity and improves the overall quality of life in asthmatic children.[6,10]
Al-Khobar is a heavily populated city, located on the Arabian Gulf coast, in the eastern region of Saudi Arabia. For most of the year, the city experiences high humidity, which contributes to the survival of various species that possess different allergenicities.[2,11,12] Several studies have evaluated the pattern of allergen sensitization of asthmatic children in Saudi Arabia and other countries near the Arabian Gulf.[2,7,13,14,15] No recent reports are available on the allergen sensitization pattern of asthmatic children in Al-Khobar area.
MATERIALS AND METHODS
This is a retrospective cross-sectional study analyzing the results of allergy skin prick tests (SPTs) performed on 100 Saudi asthmatic children aged 5–14 years. These children were selected from patients living in the city of Al-Khobar and had been referred to a regional tertiary center. The referrals were for the purpose of evaluation of recurrent episodes of wheeze, confirmation of the diagnosis of asthma or performance of SPT. The tests were performed between January 2011 and December 2012.
Subjects who met the following criteria were included in the study (a) Saudi children aged 5–14 years with a clinical diagnosis of asthma made by the consulting allergist based on the Global Initiative for Asthma guidelines and the Saudi Initiative for Asthma guidelines;[16,17] (b) subjects tested with the same panel of allergens; and (c) subjects with a valid skin test, defined as a positive reaction (wheal diameter ≥3 mm) to histamine (positive control) and a negative reaction to saline (negative control). Subjects excluded from the study were those with (a) uncertain diagnosis; (b) nonreactive skin, as evidenced by a reaction to histamine equal to or less than the reaction to saline; (c) dermatographic or hyper-reactive skin, defined by a positive reaction to saline and (d) use of antihistamine within a week prior to the SPT or prolonged use of a systemic steroid. Asthmatic patients included in the study were divided according to their age into three groups, namely, Group A: 5–7 years; Group B: 8–10 years; and Group C: 11–14 years. These groups were used to calculate the rate of atopy and assess the monosensitization ratio among children of different ages. The study was approved by the Institutional Review Board committee.
The SPT, which is considered the most reliable method to test for specific IgE antibodies against specific environmental allergens, was performed by an allergist using standardized commercial glycerinated extracts in a standardized procedure according to previously published guidelines.[18,19,20] One drop of each extract was applied to the volar surface of the arm and then the skin was pricked using a disposable acrylic needle (Alyostal® Stallergenes, France) to introduce the specific allergen. Negative (saline) and positive (1 mg/ml histamine dihydrogen phosphate) controls were required to validate each test. The responses were evaluated 15 min later, with a positive reaction defined as a wheal ≥3 mm in diameter more than the negative control.[19] A patient was considered atopic if he/she had a positive reaction to at least one of the allergens. All extracts were purchased from the same vendor (Stallergenes, France), and all patients were tested with the same lot by the same allergist. The SPT included 20 indoor and outdoor allergens chosen based on their frequencies in the region.[1,11,12,21] The allergen panel included a combination of house dust mites (HDM) species; cat fur; German cockroaches; tree, weed and grass pollens as well as molds. Table 1 shows the composition of each extract.
Table 1.
Standard panel for aeroallergen extracts and their composition (Stallergenes, France)
| Allergen | Composition |
|---|---|
| House dust mite | Dermatophagoides pteronyssinus Dermatophagoides farinae |
| Cockroach | Blattella germanica (German cockroach) |
| Animal dander | Felinis domesticus (cat fur) |
| Molds | Alternaria alternata |
| Cladosporium mix (Cladosporium cladosporioides, Cladosporium herbarum) | |
| Penicillium mix (Penicillium digitatum, Penicillium expansum, Penicillium notatum) | |
| Aspergillus mix (Aspergillus fumigatus, Aspergillus nidulans, Aspergillus niger) | |
| Tree pollen | Prosopis glandulosa (mesquite tree) |
| Phoenix dactylifera (palm tree) | |
| Acacia (acacia species) | |
| Weed pollen | Ragweed mix |
| Chenopodium album (lamb’s quarter) | |
| Artemisia vulgaris (mugwort) | |
| Amaranthus spinosus (rough pigweed) | |
| Salsola kali (Russian thistle) | |
| Plantago lanceolata (plantain) | |
| Grass pollen | Phleum pratense (Timothy grass) |
| Lolium perenne (perennial ryegrass) | |
| Cynodon dactylon (Bermuda grass) |
Statistical analyses were performed, including cell counts and percentages which were reported for the categorical data. The mean and standard deviation or median (range) were reported for continuous data. Chi-square tests and Fisher's exact tests were used to analyze differences among the three age groups. All tests were two-sided with a P < 0.05 considered to be significant. Analyses were carried out using R software version 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 100 Saudi asthmatic children were included in the study. The patients in the cohort were aged between 5 and 14 years with a mean age of 8.98 ± 2.93 years, and included 32 females and 68 males (P = 0.0003). All subjects had resided in the Eastern Province for 5 years before the skin test. Of the 100 asthmatic patients enrolled, 86 patients had exhibited atopy, defined as sensitization to at least one allergenic extract. The rate of atopy was 75.68% (28/37) in Group A, 83.33% (25/30) in Group B and 93.93% (31/33) in Group C. Comparison of the data revealed that the overall difference in the rate of atopy among the three groups was trending toward significant (P = 0.11). We further looked at pairwise comparisons: Group C versus Group B (P = 0.243), Group A versus Group C (P = 0.049) (significant) and Group A versus Group B (P = 0.55). Hence, Group A had a significantly lower atopy rate than did Group C, but no significant difference in atopy between Group A and Group B or Group B and Group C.
Monosensitization, defined as sensitization to a single allergen, was observed in 13 patients, with the most common monosensitizing agent being the HDM. Monosensitization was more common in the youngest age group, as 21.62% (8/37) of Group A subjects were monosensitive compared with 10% (3/30) in Group B and 6.06% (2/33) in Group C, with an overall P = 0.662. The maximum number of positive reactions in a single patient was 12 in Group A, 14 in Group B and 20 in Group C.
The most common indoor sensitizing allergen was the HDM, which had sensitized 54% of the subjects. The sensitization rates for individual dust mite species were 48%for Dermatophagoides farina and 45% for Dermatophagoides pteronyssinus. Sensitization to both species occurred in 38% of the subjects. The other common indoor sensitizing allergens were cat fur (53% of subjects) and the German cockroach (26% of subjects) [Figure 1]. In addition, 32% of the cohort was sensitized to molds. The most common among the molds were the Aspergillus species and Alternaria alternata (21% each), followed by Cladosporium (13%) and Penicillium (6%). Among the outdoor allergens, the most common sensitizer was weed pollen, which sensitized 61% of the subjects. Among the weed family, Salsola kali (Russian thistle) was the most prevalent pollen sensitizer (48%), followed by Chenopodium album (lamb's quarter) (23%), Artemisia vulgaris (mugwort) (19%), Plantago lanceolate (plantain) (17%), Amaranthus spinosus (rough pigweed) (16%) and ragweed mix (8%). Furthermore, 32% of the children were sensitized to at least one type of grass. The most prevalent was Timothy grass (23%), followed by perennial rye (12%) and Bermuda grass (10%). Sensitization to tree pollens occurred in 33% of the subjects, with the most common tree pollen allergens being Prosopis glandulosa (mesquite tree) (19%), followed by acacia species (17%) and Phoenix dactylifera (date palm) (11%) [Figure 2].
Figure 1.
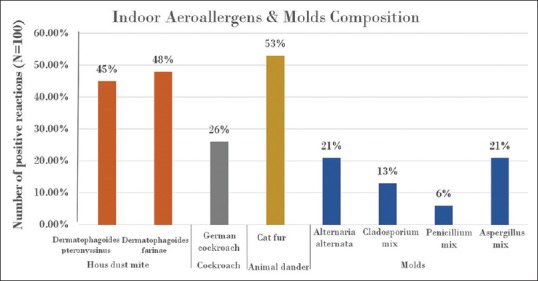
Incidence of skin prick test positive reactions for indoor allergens and molds
Figure 2.
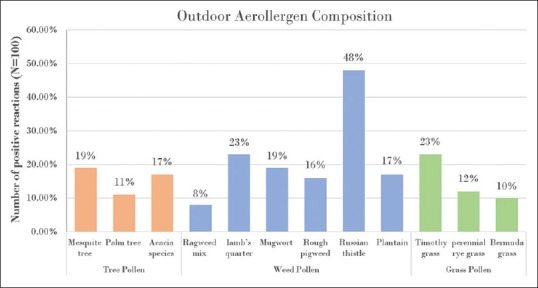
Incidence of skin prick tests positive reactions for pollens
DISCUSSION
In this study, the pattern of immediate-type hypersensitivity reactions to aeroallergens among atopic asthmatic children was determined. The study was performed in the city of Al-Khobar, in the Eastern Province of the Kingdom of Saudi Arabia. In our study, the majority of the asthmatic population was found to be atopic, as 86% of the children tested positive to at least one allergenic extract. Moreover, the difference in the rate of atopy among the three groups indicated that atopy does not occur equally at all ages, with the highest atopy rate being exhibited by the oldest age group. A recent study in Riyadh, in the central region of Saudi Arabia, found 39% of children with airway allergy (asthma or allergic rhinitis) to be atopic.[14] In another study that compared the sensitization rate between two major Saudi cities, atopy was present in 59.2% of asthmatic children residing in Riyadh, whereas 72.5% of asthmatic children in Makkah, a city in western Saudi Arabia, were found to be atopic.[2] These differences across the country's regions could be the result of genetic and geographical variations, in addition to unique host-environment interactions. Of note, the proportion of males in our study was significantly higher than females. Gender differences in the occurrence of asthma and atopy have been observed, with a predominance of the disease occurring in boys before puberty.[22]
The most common aeroallergen causing sensitization in our cohort of asthmatic children was the HDM, which is consistent with findings from other Saudi regions, neighboring Gulf countries and international reports.[7,13,14,21,23,24,25] In addition, the HDM was the most common monosensitizing agent found in our study, in agreement with other reports.[26] Monosensitization appeared to be more prevalent among the younger age groups, consistent with the natural history of atopy and the evolution of the disease, as allergic individuals become sensitized over time to multiple environmental agents.[27] However, the study was not powered enough to yield significant differences in monosensitization rates among the three groups.
Another important indoor allergen is the cockroach, which is known to cause significant morbidities in inner-city asthmatics, including increased rates of hospitalizations and the use of corticosteroids.[28] Our population exhibited a sensitization rate of 26% to an extract derived from the German cockroach (Blattella germanica) species. Other cockroach species are known to contribute to asthma symptoms, including Periplaneta americana (American cockroach) and Blattella orientalis (oriental cockroach).[29] Testing for these other species may have resulted in an even higher rate of sensitization to cockroaches in our population.
Amongst the outdoor aeroallergens, the most prevalent sensitizer was the Russian thistle, followed by Timothy grass and C. album from the Chenopodiaceae family of weed pollens. C. album was found previously to be the most common sensitizer among adults with respiratory allergies residing in the Eastern Province, exhibiting a sensitization rate of 53%.[21] Aerobiological surveys in the past have also identified Chenopodiaceae and grass pollens to be the most common botanical aeroallergens isolated in the region.[12] Comparably, our study demonstrated that weed pollens were the most prevalent sensitizers found in our region, as 61% of the entire cohort was sensitized to at least one type of weed pollen. This was followed by sensitization to tree pollen (33%), grass pollen and molds (32% each). Furthermore, among the tree pollens we tested, P. glandulosa was found to be the most prevalent sensitizer. Indeed, a large number of trees of various species of the Proposis genus are planted on the side roads in the city of Al-Khobar. In fact, an allergological study performed earlier on the Proposis family of trees in the Kingdom found that the sensitization rate of atopic individuals to Prosopis juliflora antigen in Al-Hofuf city, in the Eastern Province of Saudi Arabia, was 11.11%.[30] Our population was tested for P. glandulosa species and showed a sensitization rate of 17.6%. The variations in the obtained results may be attributed to the different species used for antigen testing or to the abundancy of other pollen antigens in Al-Hofuf city, which is a major agricultural district. In addition, allergen concentration in the environment plays an important role in determining the level of sensitization among the atopic individuals.[31] Therefore, the recent expansion of the Prosopis trees in the city had probably contributed to the higher sensitization rates to the related antigen. Currently, no allergological surveys have assessed the levels of Prosopis pollen antigens in Al-Khobar, despite the concerns of the Ministry of Health and Ministry of Municipal and Rural Affairs over the tree's known allergenic properties.[30]
In regards to sensitization to molds, in previous studies, Alternaria and Cladosporium species were found to be major contributors to the allergenic nature of the outdoor environment in Al-Khobar city.[32,33] Our study demonstrated that Alternaria and Aspergillus species were the most common sensitizing molds. In a previous study, the Alternaria species had been linked to the development of asthma in children and was found also to be related to the severity of the disease.[34] The unique high humidity that characterizes the region does contribute to the abundancy of molds and may explain the higher rates of bronchial asthma among the children residing in the Eastern Province of Saudi Arabia.[5] In contrast, the sensitization pattern of our population to trees and grass pollens is probably not related to the geographical distribution or climate but rather to the recent regional modernization. Interestingly, the findings reflect the region's newly altered nature within a preserved ancient humid dessert, as, in addition to the high concentration of weeds, there is an abundance of ornamental trees and grasses that are artificially maintained through irrigation systems. This combination has probably contributed to a unique sensitization pattern among our allergic individuals and may have resulted in the higher rate of sensitization and atopy noted in our study when compared to other regions of the Kingdom.[26,35]
Notably, the high rate of sensitization noted here may also reflect the highly selective nature of the population. Our subjects were selected from a specialized allergy clinic in a tertiary hospital; therefore, most of the subjects had moderate-to-severe asthma in comparison with the predominantly milder asthma present in the general population. This difference can be avoided in the future by designing a large community-based survey in parallel to pollen surveys. The information obtained from such surveys can help shed more light on the influence of regional climatic factors on disease activity. This is in order to gain more control on the disease processes and to plan for future preventive programs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author would like to thank Ms. Zahra Al-Matar, medical student at University of Dammam, for her assistance in the data collection. The author would also like to acknowledge Dr. Bashayer Al-Awam, pediatric demonstrator at King Fahd Hospital of the University, University of Dammam, Saudi Arabia, for her technical advice.
REFERENCES
- 1.Al Frayh AR, Shakoor Z, Gad El Rab MO, Hasnain SM. Increased prevalence of asthma in Saudi Arabia. Ann Allergy Asthma Immunol. 2001;86:292–6. doi: 10.1016/s1081-1206(10)63301-7. [DOI] [PubMed] [Google Scholar]
- 2.Al-Frayh A, Gad-El-Rab MO, Al-Najjar A, Hasnain SM. A comparative study of immediate skin test reactivity to inhalant allergens in asthmatic children of two different regions in Saudi Arabia. Ann Saudi Med. 1992;12:468–71. doi: 10.5144/0256-4947.1992.468. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Giner F, Kauffmann F, de Cid R, Kogevinas M. Gene-environment interactions in asthma. Occup Environ Med. 2006;63:776–86. doi: 10.1136/oem.2004.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks GB. Environmental factors and gene-environment interactions in the aetiology of asthma. Clin Exp Pharmacol Physiol. 2006;33:285–9. doi: 10.1111/j.1440-1681.2006.04360.x. [DOI] [PubMed] [Google Scholar]
- 5.Al Frayh AS. A 17 year trend for the prevalence of asthma and allergic diseases among children in Saudi Arabia. J Allergy Clin Immunol. 2005;115:S232. [Google Scholar]
- 6.Sheffer AL. Allergen avoidance to reduce asthma-related morbidity. N Engl J Med. 2004;351:1134–6. doi: 10.1056/NEJMe048177. [DOI] [PubMed] [Google Scholar]
- 7.Koshak EA. Skin test reactivity to indoor allergens correlates with asthma severity in jeddah, Saudi Arabia. Allergy Asthma Clin Immunol. 2006;2:11–9. doi: 10.1186/1710-1492-2-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 9.Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax. 2012;67:1046–51. doi: 10.1136/thoraxjnl-2012-202150. [DOI] [PubMed] [Google Scholar]
- 10.Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, et al. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: A community guide systematic review. Am J Prev Med. 2011;41(2 Suppl 1):S5–32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Hasnain SM, Al-Frayh AR, Subiza JL, Fernández-Caldas E, Casanovas M, Geith T, et al. Sensitization to indigenous pollen and molds and other outdoor and indoor allergens in allergic patients from Saudi Arabia, United Arab Emirates, and Sudan. World Allergy Organ J. 2012;5:59–65. doi: 10.1097/WOX.0b013e31825a73cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.al-Nahdi M, al-Frayh R, Hasnain SM. An aerobiological survey of allergens in al Khobar, Saudi Arabia. Allerg Immunol (Paris) 1989;21:278–82. [PubMed] [Google Scholar]
- 13.Al-Tamemi SH, Al-Shidhani AN, Al-Abri RK, Jothi B, Al-Rawas OA, Al-Riyami BM. The pattern of sensitisation to inhalant allergens in omani patients with asthma, allergic rhinitis and rhinoconjunctivitis. Sultan Qaboos Univ Med J. 2008;8:319–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Almogren A. Airway allergy and skin reactivity to aeroallergens in Riyadh. Saudi Med J. 2009;30:392–6. [PubMed] [Google Scholar]
- 15.Tabbara KS, Ibrahim A, Ajjawi R, Saleh F. Atopic profile of asthmatic children in Bahrain. East Mediterr Health J. 2012;16:1214–20. doi: 10.26719/2010.16.12.1214. [DOI] [PubMed] [Google Scholar]
- 16.Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: A roadmap to asthma control. Eur Respir J. 2015;46:622–39. doi: 10.1183/13993003.00853-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Moamary MS, Alhaider SA, Al-Hajjaj MS, Al-Ghobain MO, Idrees MM, Zeitouni MO, et al. The Saudi initiative for asthma – 2012 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2012;7:175–204. doi: 10.4103/1817-1737.102166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepys J. Skin testing. Br J Hosp Med. 1975;14:412–7. [Google Scholar]
- 19.Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 20.Dreborg S, Frew A. Position paper: Allergen standardization and skin tests. The European Academy of allergology and clinical immunology. Allergy. 1993;48(Suppl 14):48–82. [PubMed] [Google Scholar]
- 21.Suliaman FA, Holmes WF, Kwick S, Khouri F, Ratard R. Pattern of immediate type hypersensitivity reactions in the Eastern Province, Saudi Arabia. Ann Allergy Asthma Immunol. 1997;78:415–8. doi: 10.1016/S1081-1206(10)63205-X. [DOI] [PubMed] [Google Scholar]
- 22.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–90. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sattar HA, Mobayed H, al-Mohammed AA, Ibrahim AS, Jufairi AA, Balamurugan P, et al. The pattern of indoor and outdoor respiratory allergens in asthmatic adult patients in a humid and desert newly developed country. Eur Ann Allergy Clin Immunol. 2003;35:300–5. [PubMed] [Google Scholar]
- 24.Lin S, Jones R, Munsie JP, Nayak SG, Fitzgerald EF, Hwang SA. Childhood asthma and indoor allergen exposure and sensitization in Buffalo, New York. Int J Hyg Environ Health. 2012;215:297–305. doi: 10.1016/j.ijheh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Montealegre F, Meyer B, Chardon D, Vargas W, Zavala D, Hart B, et al. Comparative prevalence of sensitization to common animal, plant and mould allergens in subjects with asthma, or atopic dermatitis and/or allergic rhinitis living in a tropical environment. Clin Exp Allergy. 2004;34:51–8. doi: 10.1111/j.1365-2222.2004.01855.x. [DOI] [PubMed] [Google Scholar]
- 26.de Jong AB, Dikkeschei LD, Brand PL. Sensitization patterns to food and inhalant allergens in childhood: A comparison of non-sensitized, monosensitized, and polysensitized children. Pediatr Allergy Immunol. 2011;22:166–71. doi: 10.1111/j.1399-3038.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 27.Fasce L, Tosca MA, Olcese R, Milanese M, Erba D, Ciprandi G. The natural history of allergy: The development of new sensitizations in asthmatic children. Immunol Lett. 2004;93:45–50. doi: 10.1016/j.imlet.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Perzanowski MS, Platts-Mills TA. Further confirmation of the relevance of cockroach and dust mite sensitization to inner-city asthma morbidity. Clin Exp Allergy. 2009;39:1291–3. doi: 10.1111/j.1365-2222.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- 29.Helm RM, Squillace DL, Jones RT, Brenner RJ. Shared allergenic activity in Asian (Blattella asahinai), German (Blattella germanica), American (Periplaneta americana), and Oriental (Blatta orientalis) cockroach species. Int Arch Allergy Appl Immunol. 1990;92:154–61. doi: 10.1159/000235207. [DOI] [PubMed] [Google Scholar]
- 30.Al-Frayh A, Hasnain SM, Gad-El-Rab MO, Al-Turki T, Al-Mobeireek K, Al-Sedairy ST. Human sensitization to Prosopis juliflora antigen in Saudi Arabia. Ann Saudi Med. 1999;19:331–6. doi: 10.5144/0256-4947.1999.331. [DOI] [PubMed] [Google Scholar]
- 31.Carlsten C, Dimich-Ward H, Becker AB, Ferguson A, Chan HW, DyBuncio A, et al. Indoor allergen exposure, sensitization, and development of asthma in a high-risk birth cohort. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e740–6. doi: 10.1111/j.1399-3038.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasnain SM, Al-Frayh A, Gad-El-Rab MO, Al-Sedairy S. Airborne Alternaria spores: Potential allergic sensitizers in Saudi Arabia. Ann Saudi Med. 1998;18:497–501. doi: 10.5144/0256-4947.1998.497. [DOI] [PubMed] [Google Scholar]
- 33.Hasnain SM, Al-Frayh AS, Al-Suwaine A, Gad-El-Rab MO, Fatima K, Al-Sedairy S. Cladosporium and respiratory allergy: Diagnostic implications in Saudi Arabia. Mycopathologia. 2004;157:171–9. doi: 10.1023/b:myco.0000020592.72238.a6. [DOI] [PubMed] [Google Scholar]
- 34.Bush RK, Portnoy JM, Saxon A, Terr AI, Wood RA. The medical effects of mold exposure. J Allergy Clin Immunol. 2006;117:326–33. doi: 10.1016/j.jaci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Migueres M, Dávila I, Frati F, Azpeitia A, Jeanpetit Y, Lhéritier-Barrand M, et al. Types of sensitization to aeroallergens: Definitions, prevalences and impact on the diagnosis and treatment of allergic respiratory disease. Clin Transl Allergy. 2014;4:16. doi: 10.1186/2045-7022-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


