Abstract
Background
The optimal chemotherapy regimen for non-small-cell lung cancer (NSCLC) patients with interstitial lung disease (ILD) remains unknown. Therefore, in this study, we investigated the real-world efficacy and safety of carboplatin (CBDCA) plus nab-paclitaxel (nab-PTX) as a first-line regimen for NSCLC patients with ILD.
Patients and methods
We retrospectively reviewed advanced NSCLC patients with ILD who had received CBDCA plus nab-PTX as a first-line chemotherapy regimen between April 2013 and March 2018. Patients were diagnosed with ILD based on the findings of a pretreatment high-resolution computed tomography of the chest.
Results
The 34 patients enrolled in this study were included in the efficacy and safety analysis. Collagen vascular disease or a history of exposure to dust or asbestos was not reported for any patients. The median age of patients was 71 years (range, 59–83 years), and 32 patients had a performance status of 0 or 1. The overall response rate was 38.2%. The median progression-free survival and overall survival were 5.8 months and 12.7 months, respectively. Chemotherapy-related acute exacerbation of ILD was observed in two patients (5.7%). Other toxicities were feasible, and no treatment-related deaths occurred.
Conclusion
CBDCA plus nab-PTX, as a first-line chemotherapy regimen for NSCLC, showed favorable efficacy and safety in patients with preexisting ILD.
Keywords: interstitial lung disease, non-small-cell lung cancer, carboplatin, nab-paclitaxel
Introduction
Lung cancer is the leading cause of cancer mortality.1 Preexisting interstitial lung disease (ILD) is a risk factor associated with drug-related ILD.2 The rate of complications of idiopathic pulmonary fibrosis (IPF) in lung cancer patients is 2%–8%.3 Chemotherapy-induced acute exacerbation of ILD (AE-ILD) can, at times, be fatal, and occurs in 22.7% of non-small-cell lung cancer (NSCLC) patients with ILD.4 As ILD patients have been excluded from most clinical trials, the standard chemotherapy used is unknown. Previous studies have evaluated the safety and efficacy of platinum doublet chemotherapy in NSCLC patients with ILD,5–13 indicating that median survival time (MST) of NSCLC patients with ILD ranges from 7 to 16 months. A prospective study has also demonstrated the performance of carboplatin (CBDCA) plus soluble-paclitaxel (sol-PTX) in these patients.14 In Japan, CBDCA plus sol-PTX has therefore been considered an option for the treatment of NSCLC patients with ILD in clinical practice.
Nab-paclitaxel (nab-PTX), with a mean particle size of 130 nm, was developed to improve the therapeutic index of paclitaxel. Compared to sol-PTX, nab-PTX has demonstrated a 10-fold higher mean concentration (Cmax) of free PTX in serum.15 In a Phase III study (CA031 study), nab-PTX plus CBDCA vs sol-PTX plus CBDCA administered weekly resulted in a significantly higher overall response rate (ORR; primary end point; 33% vs 25%, P=0.005) and showed an improved safety profile in chemo-naïve patients with advanced NSCLC.16
Based on the information presented above, this retrospective real-world study was performed to determine whether the combination of nab-PTX and CBDCA is an effective and feasible treatment option for patients with advanced NSCLC and ILD.
Patients and methods
Patient selection
To meet the eligibility criteria for this retrospective cohort study, patients must have had NSCLC (proven via histological or cytological examination), stage IV or IIIB disease (as defined by the Union for International Cancer Control TNM classification, seventh edition), a clinical diagnosis of ILD, and an Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 3, and should have received combination chemotherapy with nab-PTX plus CBDCA as first-line treatment at Kitasato University Hospital between April 2013 and March 2018. In addition, target lesions measurable via chest radiography, high-resolution computed tomography (CT) of the chest and abdomen, or by other procedures such as magnetic resonance imaging (MRI) of the head, positron emission tomography (PET), or combined PET/CT imaging were also required for inclusivity. Patients with insufficient organ function could not receive the combination of nab-PTX and CBDCA in the present study. The classification of ILD was reviewed by two experienced observers (AT and NN). The subtype of ILD was estimated following the guidelines for management of incidental pulmonary nodules (from the Fleischner Society, 2017) detected in the CT images.17 This study was approved by the institutional ethics review board of the Kitasato University Hospital. Informed consent was waived because of the retrospective nature of the study. The patient data were maintained with confidentiality, in compliance with the Declaration of Helsinki.
Treatment
Patients were administered 100 mg/m2 nab-PTX on days 1, 8, and 15, and CBDCA was administered every 4 weeks on day 1 (area under the curve [AUC]). The treatment protocol stated that 50 µg/m2/day or 2 µg/kg/day recombinant human granulocyte colony-stimulating factor (G-CSF) should be used in accordance with the national health insurance coverage of Japan. Indications of G-CSF administration were as follows: 1) body temperature over 37.5°C with a neutrophil count of ≤1,000/mm3, 2) a neutrophil count of 500/mm3, and 3) a neutrophil count of 500/mm3 before completing the same chemotherapy that resulted in a neutrophil count of ≤1,000/mm3. Treatment was discontinued owing to disease progression, unacceptable toxicity such as AE-ILD, patient refusal of further treatment, or a decision by the head doctor to terminate treatment. The maximum number of cycles of platinum doublet chemotherapy was limited to six.
Response evaluation
Lesions were evaluated using plain chest radiography, CT of the chest and abdomen, PET or bone scintigraphy, and CT or MRI of the cranium. To evaluate the tumors, CT imaging of the chest and abdomen was performed every two cycles, at the very least. PET or bone scintigraphy, as well as CT or MRI of the cranium, was performed at 6-month intervals or earlier if patients had significant tumor-associated symptoms. Tumor control was assessed based on the Response Evaluation Criteria in Solid Tumors guideline (version 1.1). The best overall response and maximum tumor control were recorded as the tumor response.
Toxicity assessment and dose modification
Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. At our institution, a dose reduction was performed when there was a grade 4 neutropenia lasting ≥4 days, febrile neutropenia, grade 4 thrombocytopenia, grade ≥3 peripheral neuropathy, and grade 4 non-hematologic adverse events (AEs). If any of the above events occurred, the doses of CBDCA and nab-PTX were reduced to a target AUC of 5 mg/min/mL and 75 mg/m2, respectively, in subsequent cycles. Patients received supportive care as required. Prior to starting the treatment regimen and on day 1, patients were required to have an absolute neutrophil count of ≥1,500/mm3, a platelet count of ≥100,000/mm3, and a grade ≤2 peripheral neuropathy. On days 8 and 15, patients were required to have an absolute neutrophil count of ≥1,000/mm3, a platelet count of ≥50,000/mm3, and a grade ≤2 peripheral neuropathy. AE-ILD was confirmed if the following criteria were met: 1) exacerbation of dyspnea within 1 month, 2) newly developed diffuse pulmonary opacity, and 3) absence of heart failure and infectious lung disease, as previously described.18,19
Statistical analysis
Progression-free survival (PFS) was defined as the interval between the date of starting first-line chemotherapy and the day of disease progression or patient death. Overall survival (OS) was defined as the interval between the date of starting first-line chemotherapy and the day of patient death or patient’s last follow-up. Survival curves were plotted using the Kaplan–Meier method. Statistical analysis was performed using the SPSS software program, version 23.0 (IBM Corp., Armonk, NY, USA), for Windows.
Results
Patient characteristics
The 34 patients who satisfied the criteria for this retrospective cohort study were included in the efficacy and safety analysis. The patients’ demographic data are shown in Table 1. The median age of the patients was reported as 71 years (range, 59–83 years). Thirty-three patients (97%) were current smokers, and 32 (94%) had an ECOG performance status of 0 or 1. The histological subtypes of cancer were squamous cell carcinoma (n=16), adenocarcinoma (n=12), and unspecified carcinoma (n=6). The subtype of ILD was typical usual interstitial pneumonia (UIP) pattern in 16 patients (45%), probable UIP in 11 patients (34%), alternative UIP in six patients (18%), and indeterminate UIP in one patient (3%). No patient had collagen vascular disease or a history of exposure to dust or asbestos. ILD was therefore diagnosed in all patients as idiopathic interstitial pneumonia. The median number of the treatment cycles was 4 (range, 1–4 cycles).
Table 1.
Patient characteristics
| Total N=34 (%) | |
|---|---|
|
| |
| Age (years), median (range) | 71 (59–83) |
|
| |
| Gender | |
|
| |
| Male | 29 (85) |
| Female | 5 (15) |
|
| |
| Performance status | |
|
| |
| 0–1 | 32 (94) |
| 2–3 | 2 (6) |
|
| |
| Smoking status | |
|
| |
| Current smoker | 33 (97) |
| Never or former light smoker | 1 (3) |
|
| |
| Histology | |
|
| |
| Squamous carcinoma | 16 (47) |
| Adenocarcinoma | 12 (35) |
| Not otherwise specified | 6 (18) |
|
| |
| Stage | |
|
| |
| IIIA | 2 (6) |
| IIIB | 2 (6) |
| IV or recurrence | 30 (88) |
|
| |
| Type of IP, n (%) | |
|
| |
| UIP | 16 (47) |
| Probable UIP | 11 (32) |
| Alternative UIP | 6 (18) |
| Indeterminate UIP | 1 (3) |
|
| |
| Number of cycles, median (range) | 1 (1–4) |
Abbreviations: IP, interstitial pneumonia; UIP, usual interstitial pneumonia.
Response and survival data
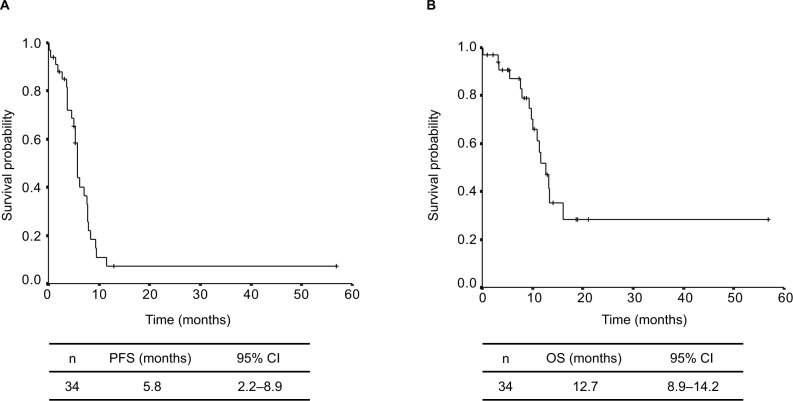
A partial response was observed in 13 patients, stable disease in 12 patients, and progressive disease in eight patients, and the response was not evaluable in one patient. The ORR was 38.2% (95% CI: 22%–55%, Table 2). The median follow-up time was 10.4 months, and the median PFS and OS for all patients were 5.8 (95% CI: 2.2–8.9) months and 12.7 (95% CI: 8.9–14.2) months, respectively (Figure 1). There was no significant difference in PFS between squamous lung cancer group and non-squamous lung cancer group (5.8 months vs 5.7 months, P=0.81). There was also no significant difference in OS between them (12.7 months vs 10.0 months, P=0.78). There was no significant difference in PFS between UIP/probable UIP group and alternative UIP/indeterminate UIP group (5.8 months vs 3.7 months, P=0.49). There was also no significant difference in OS between them (13.2 months vs 11.6 months, P=0.75). Of the 34 patients who had disease progression after the administration of nab-PTX plus CBDCA, 17 patients received second-line chemotherapy with 8 patients, 4 patients, 2 patients, 2 patients and one patient receiving S-1, docetaxel, pemetrexed, pembrolizumab, and nivolumab, respectively. OS of those patients from the start of first-line chemotherapy was 15.6 (95% CI: 5.6–18.7) months.
Table 2.
Tumor response to chemotherapy with CBDCA plus weekly nab-PTX
| Total N=34 | |
|---|---|
|
| |
| Complete response | 0 |
| Partial response | 13 |
| Stable disease | 12 |
| Progressive disease | 8 |
| Response not evaluable | 1 |
| Response rate, % (95% CI) | 38.2 (21.9–54.5) |
| Disease control rate, % (95% CI) | 73.5 (58.7–88.3) |
Abbreviations: CBDCA, carboplatin; nab-PTX, nab-paclitaxel.
Figure 1.
Kaplan–Meier plots of survival: progression-free survival (A) and overall survival (B).
Abbreviations: OS, overall survival; PFS, progression-free survival.
Toxicity
The chemotherapy-related AEs are summarized in Table 3; the most common AEs were hematological toxicities such as neutropenia and leukopenia. A grade ≥3 neutropenia and leukopenia occurred in 43.3% and 36.7% of patients, respectively. Febrile neutropenia occurred in six patients (20%), while peripheral neuropathy occurred in 6.7% of patients. A first-line chemotherapy-related AE-ILD of grade 2 was observed in two patients. The subtype of the existing ILD before chemotherapy was reported as typical UIP pattern in both patients, and no subsequent chemotherapy was administered to these patients. Treatment-related death was not observed. Among the 34 patients, seven received five of AUC values of the CBDCA and 75 mg/m2 of nab-PTX doses (among the seven patients, AUC was reduced once more to 4.5 in five patients). In the first cycle of chemotherapy, nab-PTX was discontinued on days 15 and 8 in 10 and two patients, respectively. Among the 17 patients who received second-line chemotherapy, AE-ILD was observed in two (11.7%): one on pemetrexed and the other on docetaxel. The subtype of the existing ILD was typical UIP pattern in both patients, and no subsequent chemotherapy was administered to them.
Table 3.
Treatment-related adverse events
| Grade | ||||
|---|---|---|---|---|
| ≤2 | 3 | 4 | ≥3 (%) | |
| Leukopenia | 8 | 9 | 2 | 36.7 |
| Neutropenia | 11 | 6 | 7 | 43.3 |
| Thrombocytopenia | 12 | 0 | 0 | 0 |
| Anemia | 10 | 2 | 0 | 6.7 |
| Febrile neutropenia | – | 4 | 2 | 20 |
| Nausea | 3 | 0 | – | 0 |
| Anorexia | 3 | 1 | 0 | 3.3 |
| Constipation | 6 | 0 | 0 | 0 |
| Fatigue | 4 | 3 | – | 10 |
| Peripheral neuropathy | 7 | 1 | 1 | 6.7 |
| Mucositis | 2 | 1 | 0 | 3.3 |
| AST/ALT | 5 | 0 | 0 | 0 |
| Creatinine | 3 | 0 | 0 | 0 |
| Hyperglycemia | 5 | 0 | 0 | 0 |
| Uric acid | 3 | 0 | 0 | 0 |
| Hyponatremia | 4 | 1 | 0 | 3.3 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
The optimal chemotherapy regimen for NSCLC patients with preexisting ILD remains unclear owing to the lack of randomized trials. A Japanese prospective Phase II study that evaluated the safety and efficacy of CBDCA plus weekly paclitaxel for advanced NSCLC with ILD (n=18) showed that the MST of patients was 10.6 months.4 The results of a Phase III trial (CA013) revealed the significantly high response rate of CBDCA plus nab-PTX compared to CBDCA plus sol-PTX in advanced NSCLC patients without ILD.16 In Japan, combination chemotherapy with CBDCA plus weekly nab-PTX has been conventionally used for advanced NSCLC patients with preexisting ILD in clinical practice. Based on the results of our study, we confirmed that CBDCA plus nab-PTX can be an effective option for the first-line treatment of NSCLC patients with preexisting ILD. It is important to emphasize that the number of objective patients in our study was the largest compared to any study that has evaluated CBDCA plus weekly nab-PTX as a treatment option for NSCLC patients with preexisting ILD.20–22
Although immune checkpoint inhibitors such as nivolumab and pembrolizumab (programmed death 1 [PD-1] inhibitors) have proven to be beneficial in patients with NSCLC in two Phase III trials, the incidence of drug-induced ILD is higher in patients treated with PD-1 inhibitors than in those treated with cytotoxic drugs (5% in the nivolumab group vs 0% in the docetaxel group, 5.8% in the pembrolizumab group vs 0.7% in the platinum-based chemotherapy group).23,24 In contrast, chemotherapy-induced ILD was not observed when CBDCA was administered with a weekly nab-PTX in the CA031 study.16 A previous study reported that the incidence of severe nivolumab-related pneumonitis was 19% and 5% in patients with ILD and without ILD, respectively.25 This suggests that the incidence of pneumonitis associated with nivolumab may be higher than that with CBDCA plus weekly nab-PTX; it is also observed to be more frequent in patients with preexisting ILD. These findings indicate that chemotherapy regimens including nab-PTX may be reasonable for NSCLC patients with preexisting ILD compared to the use of immune checkpoint inhibitors. Several studies based on CBDCA plus weekly nab-PTX or CBDCA plus sol-PTX have evaluated its safety and efficacy for NSCLC patients with preexisting ILD (Table 4). These studies indicated that the incidence of drug-related AE-ILD was 0%–12%, and response rate, PFS, and OS were 27%–72%, 5.1–7.2 months, and 8.1–16.1 months, respectively. Therefore, it is reasonable to imply that the results of our study are comparable to those of the previously mentioned studies in terms of incidence of AE-ILD and clinical efficacy. While febrile neutropenia occurred in six patients (20%) indicating the higher incidence than that of CA0321 trial,16 the incidence of peripheral neuropathy and grade ≥3 neutropenia, anemia, and thrombocytopenia was consistent with the outcomes of the CA031 trial.16 While the association between preexisting ILD and the onset of febrile neutropenia under the administration of CBDCA plus weekly nab-PTX was not clear, appropriate prophylaxis of neutropenia by the administration of G-CSF can prevent febrile neutropenia in this patient cohort.
Table 4.
Summary of the previous reports on platinum doublet regimens for NSCLC patients with ILD, including the present study
| Regimen | N | Study design | AE-ILD | Response rate | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|
|
| ||||||
| CBDCA + weekly PTX4 | 18 | Prospective | 1 (5.6%) | 61% | 5.3 | 10.6 |
| CBDCA + weekly PTX5 | 15 | Retrospective | 4 (27%) | 31% | 2.5 | 7.0 |
| CBDCA + PTX | 11 | Retrospective | 1 (9%) | 27% | 4.3 | 9.7 |
| CBDCA + PTX + BEV6 | 10 | 0 (0%) | 40% | 5.3 | 16.1 | |
| CBDCA + PTX + BEV7 | 25 | Retrospective | 3 (12%) | 72% | 7.2 | 8.5 |
| CBDCA + nab-PTX20 | 12 | Retrospective | 1 (8.3%) | 66.7% | 5.1 | 14.9 |
| CBDCA + nab-PTX21 | 8 | Retrospective | 0 (0%) | 57% | 5.6 | 8.1 |
| CBDCA + nab-PTX (present study) | 34 | Retrospective | 2 (6.3%) | 38.2% | 5.8 | 12.7 |
Abbreviations: AE-ILD, acute exacerbation of interstitial lung disease; BEV, bevacizumab; CBDCA, carboplatin; ILD, interstitial lung disease; nab-PTX, nab-paclitaxel; NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival; PTX, paclitaxel.
Kenmotsu et al reported that the incidence of AE-ILD was significantly higher in lung cancer patients with the UIP pattern than in those without the UIP pattern.26 The subtype of ILD in two patients having AE-ILD in our study had the typical UIP pattern, thus corresponding with the previously mentioned study. Another finding in our study was that among the 17 patients who received second-line chemotherapy, AE-ILD was observed in 11.7% of these patients. Kenmotsu et al reported that AE-ILD occurred in 30% of NSCLC patients with preexisting ILD who received subsequent chemotherapy after platinum-based chemotherapy.11 The results of our study suggest that AE-ILD should be scrutinized during the second-line chemotherapy of NSCLC patients with preexisting ILD. Meanwhile, Imai et al reported that post-progression survival after first-line chemotherapy has a greater effect on OS in patients with lung cancer.27,28 This suggests that a successful subsequent chemotherapy after first-line therapy may affect the OS of NSCLC patients with ILD.
There were several limitations in our retrospective study. The results obtained cannot be considered definitive owing to the study’s retrospective, single-centered design and the relatively small sample size used. However, it is important to note that the number of patients included in this study was the largest of all studies that have evaluated CBDCA plus nab-PTX in this setting. The diagnosis of ILD was based on CT findings instead of a histological diagnosis, which also serves as a limitation of our study. Likewise, the diagnosis of the exacerbation of ILD was based on CT findings and we could not confirm the exacerbation of ILD via histological analysis. A consensus statement of the American Thoracic Society/European Respiratory Society indicates the criteria for the clinical diagnosis of IPF according to CT findings.29 Furthermore, studies using high-resolution CT scanning to diagnose ILD have reported sensitivities of 43%–78% and specificities of 90%–97% for confirmed radiological diagnosis.30–34 Therefore, we consider that it is appropriate to diagnose the subtype and exacerbation of ILD via clinical and radiological findings in clinical practice.
In conclusion, CBDCA plus nab-PTX showed favorable efficacy and safety as a first-line chemotherapy for NSCLC patients with preexisting ILD. Further assessment in a large prospective study is warranted to confirm these findings.
Acknowledgments
The authors gratefully thank the staff members of the Department of Respiratory Medicine, Kitasato University School of Medicine, for their suggestions and assistance. This study was supported by Research Grant of Kitasato University School of Medicine for Young Researchers (2018).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 2.Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004;91(Suppl 2):S18–S23. doi: 10.1038/sj.bjc.6602063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91(Suppl 2):S3–S10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minegishi Y, Sudoh J, Kuribayasi H, et al. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer. 2011;71(1):70–74. doi: 10.1016/j.lungcan.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Shukuya T, Ishiwata T, Hara M, et al. Carboplatin plus weekly paclitaxel treatment in non-small cell lung cancer patients with interstitial lung disease. Anticancer Res. 2010;30(10):4357–4361. [PubMed] [Google Scholar]
- 6.Shimizu R, Fujimoto D, Kato R, et al. The safety and efficacy of paclitaxel and carboplatin with or without bevacizumab for treating patients with advanced nonsquamous non-small cell lung cancer with interstitial lung disease. Cancer Chemother Pharmacol. 2014;74(6):1159–1166. doi: 10.1007/s00280-014-2590-x. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto Y, Kenmotsu H, Watanabe N, et al. Efficacy and safety of combined carboplatin, paclitaxel, and bevacizumab for patients with advanced non-squamous non-small cell lung cancer with pre-existing interstitial lung disease: a retrospective multi-institutional study. Anticancer Res. 2015;35(7):4259–4263. [PubMed] [Google Scholar]
- 8.Sekine A, Satoh H, Baba T, et al. Safety and efficacy of S-1 in combination with carboplatin in non-small cell lung cancer patients with interstitial lung disease: a pilot study. Cancer Chemother Pharmacol. 2016;77(6):1245–1252. doi: 10.1007/s00280-016-3040-8. [DOI] [PubMed] [Google Scholar]
- 9.Okuda K, Hirose T, Oki Y, et al. Evaluation of the safety and efficacy of combination chemotherapy with vinorelbine and platinum agents for patients with non-small cell lung cancer with interstitial lung disease. Anticancer Res. 2012;32(12):5475–5480. [PubMed] [Google Scholar]
- 10.Kinoshita T, Azuma K, Sasada T, et al. Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett. 2012;4(3):477–482. doi: 10.3892/ol.2012.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenmotsu H, Naito T, Mori K, et al. Effect of platinum-based chemotherapy for non-small cell lung cancer patients with interstitial lung disease. Cancer Chemother Pharmacol. 2015;75(3):521–526. doi: 10.1007/s00280-014-2670-y. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe N, Niho S, Kirita K, et al. Vinorelbine and cisplatin in patients with advanced non-small cell lung cancer with interstitial pneumonia. Anticancer Res. 2015;35(3):1697–1701. [PubMed] [Google Scholar]
- 13.Choi MK, Hong JY, Chang W, et al. Safety and efficacy of gemcitabine or pemetrexed in combination with a platinum in patients with non-small-cell lung cancer and prior interstitial lung disease. Cancer Chemother Pharmacol. 2014;73(6):1217–1225. doi: 10.1007/s00280-014-2458-0. [DOI] [PubMed] [Google Scholar]
- 14.Minegishi Y, Takenaka K, Mizutani H, et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern Med. 2009;48(9):665–672. doi: 10.2169/internalmedicine.48.1650. [DOI] [PubMed] [Google Scholar]
- 15.Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 17.Macmahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 18.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103(6):1808–1812. doi: 10.1378/chest.103.6.1808. [DOI] [PubMed] [Google Scholar]
- 19.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda Y, Hattori Y, Tohnai R, et al. The safety and efficacy of carboplatin plus nanoparticle albumin-bound paclitaxel in the treatment of non-small cell lung cancer patients with interstitial lung disease. Jpn J Clin Oncol. 2018;48(1):89–93. doi: 10.1093/jjco/hyx142. [DOI] [PubMed] [Google Scholar]
- 21.Fujita T, Hiroishi T, Shikano K, et al. The Safety and Efficacy of Treatment with Nab-paclitaxel and Carboplatin for Patients with Advanced Squamous Non-small Cell Lung Cancer Concurrent with Idiopathic Interstitial Pneumonias. Intern Med. 2018;57(13):1827–1832. doi: 10.2169/internalmedicine.0404-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa H, Nakahara Y, Yokoba M, Mitsufuji H, Sasaki J, Masuda N. Safety and efficacy of carboplatin plus nab-paclitaxel for treating advanced non-small-cell lung cancer with interstitial lung disease. Mol Clin Oncol. 2017;7(4):604–608. doi: 10.3892/mco.2017.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 25.Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9(7):847–855. doi: 10.1111/1759-7714.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6(7):1242–1246. doi: 10.1097/JTO.0b013e318216ee6b. [DOI] [PubMed] [Google Scholar]
- 27.Imai H, Kaira K, Minato K. Clinical significance of post-progression survival in lung cancer. Thorac Cancer. 2017;8(5):379–386. doi: 10.1111/1759-7714.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai H, Mori K, Watase N, et al. Clinical impact of post-progression survival on overall survival in elderly patients with extensive disease small-cell lung cancer. Thorac Cancer. 2016;7(6):655–662. doi: 10.1111/1759-7714.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Thoracic Society, European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty KR, King TE, Raghu G. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170(8):904–910. doi: 10.1164/rccm.200402-147OC. [DOI] [PubMed] [Google Scholar]
- 31.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 32.Johkoh T, Müller NL, Cartier Y, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999;211(2):555–560. doi: 10.1148/radiology.211.2.r99ma01555. [DOI] [PubMed] [Google Scholar]
- 33.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: A prospective study. Chest. 1999;116(5):1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]
- 34.Swensen SJ, Aughenbaugh GL, Myers JL. Diffuse lung disease: diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiology. 1997;205(1):229–234. doi: 10.1148/radiology.205.1.9314990. [DOI] [PubMed] [Google Scholar]