Abstract
Background:
Inorganic arsenic exposure is ubiquitous and both exposure and inter-individual differences in its metabolism have been associated with cardiometabolic risk. A more efficient arsenic metabolism profile (lower MMA%, higher DMA%) has been associated with reduced risk for arsenic-related health outcomes. This profile, however, has also been associated with increased risk for diabetes-related outcomes.
Objectives:
The mechanism behind these conflicting associations is unclear; we hypothesized the one-carbon metabolism (OCM) pathway may play a role.
Methods:
We evaluated the influence of OCM on the relationship between arsenic metabolism and diabetes-related outcomes (HOMA2-IR, waist circumference, fasting plasma glucose) using metabolomic data from an OCM-specific and P180 metabolite panel measured in plasma, arsenic metabolism measured in urine, and HOMA2-IR and FPG measured in fasting plasma. Samples were drawn from baseline visits (2001–2003) in 59 participants from the Strong Heart Family Study, a family-based cohort study of American Indians aged ≥14 years from Arizona, Oklahoma, and North/South Dakota.
Results:
In unadjusted analyses, a 5% increase in DMA% was associated with higher HOMA2-IR (geometric mean ratio (GMR)= 1.13 (95% CI: 1.03, 1.25)) and waist circumference (mean difference=3.66 (0.95, 6.38). MMA% was significantly associated with lower HOMA2-IR and waist circumference. After adjustment for OCM-related metabolites (SAM, SAH, cysteine, glutamate, lysophosphatidylcholine 18.2, and three phosphatidlycholines), associations were attenuated and no longer significant.
Conclusions:
These preliminary results indicate that the association of lower MMA% and higher DMA% with diabetes-related outcomes may be influenced by OCM status, either through confounding, reverse causality, or mediation.
Keywords: Metabolomics, diabetes, arsenic metabolism, one carbon metabolism, American Indians
1. INTRODUCTION
Inorganic arsenic is a known human carcinogen and chronic exposure has been associated with increased risk for numerous health outcomes including metabolic effects, such as type 2 diabetes and the metabolic syndrome.1–6 After ingestion, inorganic arsenic is metabolized through multiple oxidative methylation and reduction reactions ultimately converting inorganic arsenic (AsIII and AsV) (iAs) to the methylated metabolites monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA), which are excreted in the urine together with inorganic arsenic.7–9 Typically, arsenic metabolism is evaluated in epidemiological studies by computing relative percentages of inorganic arsenic, MMA, and DMA over their sum (iAs%, MMA%, DMA%).10–13
Inter-individual differences in methylation capacity have been associated with risk for subsequent health outcomes. Several studies have reported higher percentages of MMA (MMA%) and lower percentages of DMA (DMA%) in the urine to be associated with greater risk for many arsenic-induced health effects including skin lesions14–16, cancers of the skin, bladder, lung17–22 and cardiovascular disease,23–25 even after controlling for arsenic exposure levels. However, for metabolic-related health outcomes, including diabetes and metabolic syndrome, higher MMA% and lower DMA% is associated with a lower risk.3, 26–28 The reasons for the contrasting association between arsenic metabolism and metabolic outcomes versus other arsenic-associated health outcomes are not clear. Some studies have suggested that one-carbon metabolism (OCM) may play a role given its strong association with both metabolic outcomes29–35 and arsenic metabolism.8, 9, 36–42 OCM, a biochemical pathway that is dependent on folate, facilitates the generation of S-adenosylmethionine (SAM), a central metabolite, which serves as the methyl donor for numerous methylation reactions including the methylation of arsenic.43
In this study, we explored potential mechanistic pathways that may explain the associations between arsenic metabolism and metabolic outcomes through the targeted evaluation of specific metabolites. The association between arsenic metabolism and metabolic profiles has been relatively unexplored. However, metabolic profiling has already identified metabolites, including those related to OCM, that can predict risk for diabetes beyond traditional diabetes risk factors.44–48 Our analyses expanded on these findings by evaluating associations between OCM- and diabetes-associated metabolites, arsenic metabolism biomarkers, and diabetes-related outcomes including waist circumference, fasting plasma glucose and HOMA2-IR. Our selection of these outcomes was informed by previous work conducted in our study population that have reported associations between arsenic metabolism and diabetes3, a homeostasis model assessment index (HOMA2-IR),49 body mass index (BMI)50, waist circumference51 and metabolic syndrome51; all of these outcomes are linked to insulin resistance. Previous evaluation of the association between arsenic metabolism and other metabolic outcomes in this study population, including triglycerides and high density lipoprotein cholesterol, yielded null results and were therefore not evaluated in this analysis.
Using data from the Strong Heart Family Study (SHFS), a family-based cohort study comprised of American Indian tribal members aged 14 years and older from Arizona, Oklahoma, and North and South Dakota, we conducted a pilot study including 59 participants. We analyzed nine OCM-specific metabolites in addition to the Biocrates P180 metabolite panel (188 endogenous metabolites including amino acids, lipids, and carbohydrates, many of them related to OCM) in baseline plasma samples collected in 2001–2003. For our analyses, of the 188 metabolites, we a priori selected only metabolites that are involved in OCM or have been prospectively associated with incident diabetes in previous studies (n=33 metabolites) (Supplemental Table 1).44–48, 52–57 Given the small sample size, our analyses are considered exploratory and aim to describe the relationship of OCM-specific metabolites and previously identified incident diabetes-associated metabolites with arsenic metabolism and metabolic outcomes including fasting glucose levels, insulin resistance and waist circumference.
2. METHODS
2.1. Study population
The SHFS recruited a total of 3,838 participants for baseline visits in 1998–1999 and 2001–2003 from three centers: Arizona, Oklahoma and North/South Dakota. The age range of the participants in the SHFS was 14 to 93 (mean 42) years. At each visit, in-person interviews, physical examinations and biological specimens were obtained. Methods have been described in detail previously.58 For this pilot study, we randomly selected 20 participants per study region among those with a baseline visit in 2001–2003 and with complete data on the following variables: urinary arsenic and creatinine, education, smoking status, body mass index (BMI), estimated glomerular filtration rate (eGFR), alcohol intake, dietary intake information and telomeres. One participant was excluded because of a missing sample, leaving 59 participants in the pilot evaluation of plasma metabolites. All participants provided informed consent before participation and study protocols were approved by multiple institutional review boards, participating communities and The Indian Health Service.
2.2. Data Collection
2.2.1. Urinary Arsenic
Morning spot urine samples were collected in polypropylene tubes, frozen within 1 to 2 hours of collection, shipped buried in dry ice and stored at −70°C in the Penn Medical Laboratory, MedStar Research Institute, Washington, DC for up to 18 years. The freezers have been operating under a strict quality control system to guarantee secure sample storage. For arsenic analyses, urine samples were thawed and up to 1.0 mL from each urine sample was transferred to a small vial, transported on dry ice to the Trace Element Laboratory at Graz University, Austria and stored at −80°C until analyses. The urine concentrations of arsenite, arsenate, MMA and DMA were measured using high performance liquid chromatography/inductively coupled plasma mass spectrometry (HPLC/ICPMS). The limits of detection were 0.1 μg/L for arsenite, arsenate, MMA and DMA. The inter-assay coefficients of variation for arsenite, arsenate, MMA, DMA and total arsenic were 14.7%, 6.9%, 6.4%, 6.0% and 4.7% respectively. For participants with concentrations below the limit of detection (less than 5% for all species) for total arsenic or for the arsenic species we imputed the corresponding limit of detection divided by the square root of two.
2.2.2. Outcome Measurements
Participants were asked to fast for 12 hours before blood sample collection. Biological specimen aliquots were stored at −70°C at the Texas Biomedical Research Institute (Texas Biomed) in San Antonio, TX. Most baseline laboratory determinations have been performed at the MedStar Health Research Institute, Washington DC under strict quality control procedures including enzymatic methods for fasting plasma creatinine and glucose and radioimmunoassay for fasting insulin. HOMA2-IR was used as a surrogate measure of insulin resistance and was calculated with the computed solved model for HOMA2-IR59 using fasting glucose and insulin values.60, 61 Waist circumference was measured by centrally trained nurses following a standardized protocol at the same visit of spot urine and fasting blood sample collection.58
2.2.3. OCM Metabolomics and P180 Panel
Plasma samples were used to measure OCM nutrients and nutrient intermediates. Some OCM intermediates can be affected by freeze-thaw cycles and were measured in samples that had never been thawed before. Targeted OCM metabolomic analysis of plasma was by liquid chromatography tandem mass spectrometery (LC-MS/MS) at the Center of Metabolomics, Baylor Research Institute under the direction of Dr. Teodoro Bottiglieri. Targeted metabolomic analysis of SAM, S-adenosylhomocysteine (SAH), methionine, choline, betaine, cysteine, cystathionine, homocysteine (Hcys), 5-methyltetrahydrofolate (5-MTHF), in plasma samples were performed using validated LC-MS/MS methods.62–64 This metabolic panel provides a quantitative comprehensive assessment of OCM. Each plasma sample (<300 μL) was processed by addition of labeled-isotope internal standards followed by deproteinization. Processed extracts of samples were transferred to a 96 well microtiter plate for analysis. The compounds were detected by multiple reaction monitoring using positive-electrospray ionization as previously described.62–64 Sample injection and separation was performed by a Shimadzu Nexera UPLC System interfaced with a 5500QTRAP® (Sciex). All data were collected and analyzed using Analyst software version 1.5.2. Stable isotopes were used for each metabolite to account for matrix effects related ion-suppression, and the quantitative values obtained were expressed as μmol/L. Quality control samples (2 levels) were positioned at the start, in the middle and at the end of each batch of samples analyzed. The inter assay coefficients of variability for all metabolites were <20%, with most <15%.
The P180 Panel (Biocrates Life Sceinces, Innsbruck, Austria) covers carefully selected metabolic pathways which includes 188 metabolites from 6 compound classes including hexoses, amino acids, biogenic amines, acylcarnitines, glycerophospholipids and sphingolipids. For a more targeted analysis, we selected metabolites that have been previously associated with incident diabetes.
2.2.4. Other Variables
A standardized questionnaire was conducted during the in-person interviews and included sociodemographic data (age, sex), smoking history, alcohol use, and medical history.65 Physical exam measures (waist circumference, height and weight) were performed by centrally trained nurses following a standardized protocol; this visit included collection of spot urine and fasting blood samples.58 Estimated daily averages of dietary intake of OCM related micronutrients, including vitamins B2, B6 and folate, as well as supplement data, were measured during the baseline visit through an interviewer-administered Block 119-item food frequency questionnaire (FFQ). The Block questionnaire is one of the most widely used questionnaires with demonstrated reliability and validity.66 To enhance accuracy of the questionnaire in this cohort, additional questions relating to foods commonly consumed by American Indians were included.66
2.3. Statistical Analysis
Arsenic exposure was estimated as the sum of inorganic (arsenite, arsenate) and methylated (MMA and DMA) arsenic species adjusted by urine creatinine to correct for urine dilution (∑As). ∑As was log-transformed to better approximate a normal distribution. Arsenic metabolism was estimated as percentages of inorganic arsenic, MMA and DMA, (iAs%, MMA%, DMA%) by dividing the corresponding concentration for each species by the sum of the inorganic and methylated species.67, 68 Arsenic species percentages were modeled in their original scale.
Arsenic, metabolites and outcome related variables were coded into related groups in the analyses: 1) Arsenic exposure and metabolism biomarkers (∑As, iAs%, MMA% and DMA%); 2) OCM-specific metabolites (Hcys, cysteine, methionine, betaine, choline, SAM, SAH, SAM/SAH, cystathionine and 5-MTHF); 3) Amino-acids (glutamine, glutamate, glycine, histidine, isoleucine, leucine, phenylalanine, serine, tryptophan, tyrosine, valine); 4) Acylcarnitines (propionylcarnitine); 5) Glycerophospholipids (lysophosphatidylcholine (LPC) a C16:0, LPC 18.2, phosphatidylcholine (PC) aa 32:1, PC aa 36:1, PC aa 38:3, PC aa 40:5, PC ae 34:3, PC ae 40:6, PC ae 44:4 and PC ae 44:5); 6) Sphingolipids (sphingomyelin (SM) C16:1); 7) Diabetes related outcomes (fasting plasma glucose (FPG), HOMA2-IR and waist circumference).
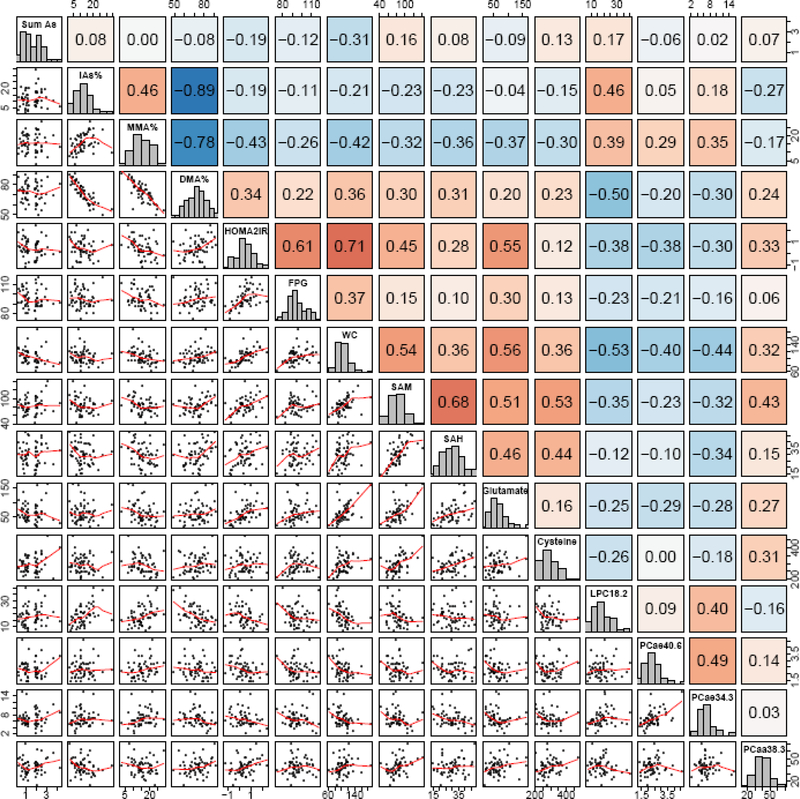
First, we computed Spearman correlations for metabolites, biomarkers and outcomes within and between the seven group types listed in the previous paragraph and displayed those correlations graphically in correlation globes (Figure 1). Correlations greater than |0.15| were represented by colored lines between the two metabolites and were displayed as solid colored lines for correlations with p-values < 0.05 and as transparent colored lines for p-values ≥ 0.05. Metabolites with correlation p-values < 0.05 between both an arsenic metabolism biomarker (iAs%, MMA%, DMA%) and a metabolic outcome (HOMA2-IR, FPG, waist circumference) were considered “metabolites of interest” and evaluated further. P-values were not corrected for multiple comparisons due to the limited sample size. Correlations between metabolites of interest and both arsenic metabolism biomarkers and diabetes-related outcomes were also graphically and numerically displayed through a correlation and scatterplot matrix (Figure 2).
Figure 1. Correlation matrices between and within metabolite and biomarker groups.
Transparent lines reflect correlations ≥ |15|. Solid lines represent correlations with p-values ≤ 0.05. Abbreviations: ∑As (sum of inorganic arsenic and its methylated metabolites); iAs (inorganic arsenic); DMA (dimethylarsinic acid); FPG (fasting plasma glucose); LPC (lysophosphatidylcholine); MMA (monomethylarsonic acid); OCM (one carbon metabolism); PC (phosphatidylcholine); SAH (S-Adenosylhomocysteine); SAM (S-Adenosylmethionine); WC (waist circumference)
Figure 2. Spearman Correlations between arsenic metabolism biomarkers, diabetes-related outcomes, and metabolites of interest.
∑As and HOMA2-IR are log-transformed. P-value <0.05 for correlations ≥0.26; P-value <0.01 for correlations ≥0.33; P-value <0.001 for correlations ≥0.42. Blue shading reflects negative correlations, red shading reflects positive correlations. Darker shades of both colors reflect stronger correlations. Abbreviations: Sum As (sum of inorganic arsenic and its methylated metabolites); DMA (dimethylarsinic acid); FPG (fasting plasma glucose); HOMA (homeostasis model assessment); iAs (inorganic arsenic); LPC (lysophosphatidylcholine); MMA (monomethylarsonic acid); PC (phosphatidylcholine); SAH (S-Adenosylhomocysteine); SAM (S-Adenosylmethionine); WC (waist circumference)
Linear regression analyses were conducted to evaluate the associations of diabetes-related outcomes, including HOMA2-IR, FPG and waist circumference, separately, with each metabolite of interest as well as with the arsenic metabolism biomarkers iAs%, MMA% and DMA%. HOMA2-IR was log-transformed in analyses to better approximate a normal distribution. FPG and waist circumference were normally distributed and therefore included in analyses in their original scale. Metabolites of interest were also normally distributed, however, they were standardized (N,0) to reduce variability. Results are presented as the geometric mean ratios (GMR) for HOMA2-IR, and mean differences for waist circumference and FPG, comparing the 75th to 25th percentile (IQR increase) of each metabolite of interest or 5% increase in arsenic metabolism biomarker. Crude models report unadjusted associations and adjusted models report associations fully adjusted for all other metabolites. Variance inflation factors (VIF) were used to evaluate multicollinearity in adjusted models which included all OCM metabolites. Finally, we constructed directed acyclic graphs (DAGs) of possible pathways between arsenic metabolism, one carbon metabolism and diabetes-related outcomes based on our findings (Figure 3).
Figure 3. Directed acyclic graphs of proposed pathways between arsenic metabolism, one carbon metabolism and diabetes-related outcomes.
Abbreviations: OCM (one carbon metabolism)
3. RESULTS
The median age of the study population was 35 years with slightly more females (53%) than males (Table 1). Most participants were overweight or obese (76%) and met or exceeded the recommended daily allowance (RDA) for intake of vitamins B269 and B670 despite most participants not taking supplements of either vitamin (78%). The majority of participants were below the RDA for of folate intake70 (63%) and did not take folate supplements (75%), although RDAs used to categorize inadequate folate intake are based on dietary folate equivalents (DFEs), which incorporate different forms of folate (e.g., folic acid) and could not be measured in this dataset. Therefore, the true percentage of participants with inadequate folate intake is likely lower. Evaluation of plasma folate (5-MTHF) confirmed no participants were folate deficient (5-MTHF ≤9 nmo/L) (data not shown). Further, only seven participants had hyperhomocysteinemia (homocysteine >11.4 μmol/L and 10.4 μmol/L for men and women, respectively) suggesting vitamin B12 status was also likely sufficient in this population as well (data not shown). Median (IQR) plasma folate and homocysteine levels were 51.3 (40.2, 63.9) and 7.53 (5.85, 8.98), respectively. Forty-two percent of participants were never smokers, 22% were former smokers and 36% were current smokers. A total of 14% of participants reported never drinking alcohol, 25% reported former drinking and 61% reported current drinking. Median (IQR) for ∑As, iAs%, MMA% and DMA% were 3.66 (2.64, 8.27) μg/g creatinine, 10.7 (7.29, 16.4)%, 15.3 (11.3, 19.9)% and 72.9 (65.9, 78.8)%, respectively. Median (IQR) for diabetes-related outcomes were 1.44 (1.06, 2.62) for HOMA2-IR, 93.0 (89.0, 101.5) mg/dL for FPG and 105.0 (91.5, 117.5) cm for waist circumference. Participant characteristics were similar in the pilot sample compared to the full cohort.
Table 1.
Participant Characteristics, Pilot versus Full Strong Heart Family Study Cohort
| Pilot (N=59) | Full Cohort (N=1577)a | |
|---|---|---|
| Age (years), median (IQR) | 35.3 (26.4, 46.3) | 35.3 (23.6, 46.3) |
| Sex | ||
| Male, n (%) | 28 (47.5) | 654 (41.6) |
| Female, n (%) | 31 (52.5) | 923 (58.4) |
| Smoking | ||
| Never, n (%) | 25 (42.4) | 647 (41.1) |
| Ever, n (%) | 13 (22.0) | 325 (20.6) |
| Current, n (%) | 21 (35.6) | 604 (38.3) |
| Alcohol Intake | ||
| Never, n (%) | 8 (13.6) | 173 (11.0) |
| Ever, n (%) | 15 (25.4) | 406 (25.8) |
| Current, n (%) | 36 (61.0) | 997 (63.3) |
| BMI (kg/m2) | 31.6 (25.9, 38.0) | 29.8 (25.5, 35.0) |
| Vitamin B6 intake (mg) | 1.60 (1.05, 2.65) | 1.70 (1.10, 2.60) |
| <RDAb, n (%) | 21 (35.6) | 545 (34.6) |
| ≥RDAb, n (%) | 38 (64.4) | 1032 (65.4) |
| Vitamin B2 intake (mg) | 1.60 (1.00, 2.30) | 1.70 (1.10, 2.70) |
| <RDAc, n (%) | 19 (32.2) | 358 (22.7) |
| ≥RDAc, n (%) | 40 (67.8) | 1219 (77.3) |
| Folate intake (μg) | 348 (226, 518) | 360 (234, 559) |
| <RDAd, n (%) | 37 (62.7) | 890 (56.4) |
| ≥RDAd, n (%) | 22 (37.3) | 687 (43.6) |
| Plasma Folate (nmol/L) | 51.3 (40.2, 63.9) | --- |
| Plasma Homocysteine (μmol/L) | 7.53 (5.85, 8.98) | --- |
| ΣAs (μg/g) | 3.66 (2.64, 8.27) | 4.59 (3.02, 7.49) |
| iAs% | 10.7 (7.29, 16.4) | 10.0 (6.94, 14.1) |
| MMA% | 15.3 (11.3, 19.9) | 14.5 (11.1, 18.2) |
| DMA% | 72.9 (65.9, 78.8) | 74.6 (67.8, 80.8) |
| HOMA2-IR | 1.44 (1.06, 2.62) | 1.44 (0.97, 1.44) |
| Fasting Plasma Glucose (mg/dL) | 93.0 (89.0, 101.5) | 93.0 (87.0, 100.0) |
| Waist Circumference (cm) | 105.0 (91.5, 117.5) | 99.0 (88.0, 111.0) |
Abbreviations: RDA (recommended dietary allowance); ΣAs (sum of inorganic arsenic and its methylated metabolites); iAs (inorganic arsenic); MMA (monomethylarsonic acid);DMA (dimethylarsinic acid)
Excluding participants with missing data on variables in table
RDA for B6: Males (≤50 years=1.3 mg; >50=1.7 mg); Females (14–18 years=1.2 mg; 19–50 years=1.3 mg; >50 years=1.5 mg)
RDA for B2: Males (≥14 years =1.3 mg); Females (14–18 years=1.0 mg; >18 years=1.1 mg)
RDA for folate: Males and Females ≥14 years=400 μg
Eight metabolites were identified as metabolites of interest due to correlations with both an arsenic metabolism variable (iAs%, MMA% or DMA%) and a metabolic outcome (HOMA2-IR, FPG, waist circumference) that reached statistical significance p<0.05. These metabolites included SAM, SAH, cysteine, glutamate, LPC 18.2, PC ae 34:3, PC ae 40:6 and PC aa 38:3. Spearman correlations between arsenic metabolism variables, diabetes-related outcomes and metabolites of interest are displayed in Figure 2. SAM, SAH, cysteine, glutamate and PC aa 38:3 were positively correlated with adverse diabetes-related outcomes and DMA%, and negatively correlated with MMA% and iAs%. LPC 18:2 and acyl-alkyl PCs (PC ae 34:3 and PC ae 40:6) were negatively correlated with adverse diabetes-related outcomes and DMA% and positively correlated with MMA% and iAs%. DMA% had a significant positive correlation with HOMA2-IR (0.34) and waist circumference (0.36). MMA% was significantly negatively correlated with HOMA2-IR, FPG and waist circumference.
In unadjusted regression analyses evaluating HOMA2-IR as the outcome, a 5% increase of MMA% was associated with a GMR of 0.75 (95% CI: 0.63, 0.89) (Table 2). After adjustment for all eight metabolites (SAM, SAH, cysteine, glutamate, LPC 18.2, PC ae 34:3, PC ae 40:6 and PC aa 38:3), higher MMA% remained associated with reduced HOMA2-IR although the association was markedly attenuated and no longer significant (0.90 (0.74, 1.09)). For DMA%, the positive association with HOMA2-IR (GMR 1.13, 95% CI: 1.03, 1.25) became entirely null (GMR 0.99, 95%CI 0.89, 1.11) after adjustment for the other metabolites. Six of the eight metabolites were also significantly associated with HOMA2-IR in unadjusted models (increased risk for SAM, SAH, glutamate and PC aa 38:3; decreased risk for LPC 18:2 and PC ae 40:6). After adjustment for other metabolites, associations were attenuated and no longer significant except for PC aa 38:3 which remained borderline significantly associated with increased HOMA2-IR. iAs% was not significantly associated with HOMA2-IR or any other diabetes-associated outcome (data not shown).
Table 2.
Associations between MMA%, DMA% and OCM Metabolites with Diabetes-Related Outcomes
| Model 1a: Unadjusted | Model 2b: Metabolite Adjusted | Model 3c: Metabolite Adjusted +MMA% | Model 4d: Metabolite Adjusted +DMA% | |
|---|---|---|---|---|
| Geometric Mean Ratio (95%CI) of HOMA2-IRe | ||||
| MMA% | 0.75 (0.63, 0.89) | 0.90 (0.74, 1.09) | --------------------- | --------------------- |
| DMA% | 1.13 (1.03, 1.25) | 0.99 (0.89, 1.11) | --------------------- | --------------------- |
| SAM | 1.46 (1.22, 1.76) | 1.19 (0.87, 1.63) | 1.23 (0.90, 1.70) | 1.19 (0.86, 1.64) |
| SAH | 1.41 (1.08, 1.85) | 1.16 (0.81, 1.67) | 1.10 (0.75, 1.60) | 1.17 (0.79, 1.73) |
| Cysteine | 1.10 (0.82, 1.48) | 0.76 (0.56, 1.03) | 0.74 (0.55, 1.01) | 0.76 (0.56, 1.04) |
| Glutamate | 1.45 (1.19, 1.77) | 1.13 (0.90, 1.42) | 1.11 (0.88, 1.40) | 1.13 (0.90, 1.43) |
| LPC 18:2 | 0.74 (0.59, 0.91) | 0.80 (0.65, 0.99) | 0.84 (0.67, 1.06) | 0.79 (0.61, 1.03) |
| PC ae 40:6 | 0.74 (0.59, 0.93) | 0.80 (0.62, 1.03) | 0.83 (0.64, 1.09) | 0.80 (0.61, 1.04) |
| PC ae 34:3 | 0.81 (0.66, 1.00) | 1.04 (0.82, 1.31) | 1.02 (0.81, 1.29) | 1.04 (0.82, 1.31) |
| PC aa 38:3 | 1.35 (1.09, 1.67) | 1.23 (1.00, 1.52) | 1.22 (0.99, 1.50) | 1.23 (0.99, 1.53) |
| Mean Difference (95%CI) of Waist Circumferencee | ||||
| MMA% | −7.83 (−12.8, −2.90) | 0.93 (−4.04, 5.90) | --------------------- | --------------------- |
| DMA% | 3.66 (0.95, 6.38) | −1.16 (−3.99, 1.67) | --------------------- | --------------------- |
| SAM | 10.3 (4.99, 15.6) | −3.70 (−11.8, 4.39) | −4.00 (−12.3, 4.32) | −4.36 (−12.6, 3.92) |
| SAH | 11.3 (3.73, 18.8) | 5.84 (−3.49, 15.2) | 6.35 (−3.45, 16.1) | 7.28 (−2.72, 17.3) |
| Cysteine | 9.22 (1.12, 17.3) | 3.46 (−4.43, 11.4) | 3.67 (−4.37, 11.7) | 3.52 (−4.4, 11.4) |
| Glutamate | 12.8 (7.55, 18) | 8.43 (2.52, 14.3) | 8.62 (2.57, 14.7) | 8.46 (2.53, 14.4) |
| LPC 18:2 | −11.5 (−17.3, −5.81) | −8.52 (−14, −3.05) | −8.97 (−15.0, −2.94) | −10.0 (−16.7, −3.41) |
| PC ae 40:6 | −10.2 (−16.6, −3.9) | −7.28 (−13.9, −0.69) | −7.63 (−14.5, −0.72) | −7.98 (−14.8, −1.15) |
| PC ae 34:3 | −8.9 (−14.4, −3.43) | −1.17 (−7.08, 4.75) | −1.04 (−7.05, 4.96) | −0.76 (−6.78, 5.26) |
| PC aa 38:3 | 5.49 (−0.797, 11.8) | 2.48 (−2.92, 7.87) | 2.56 (−2.90, 8.02) | 2.96 (−2.58, 8.50) |
| Mean Difference (95%CI) of FPGe | ||||
| MMA% | −2.08 (−4.35, 0.18) | −1.28 (−4.24, 1.68) | --------------------- | --------------------- |
| DMA% | 0.75 (−0.49, 1.99) | 0.14 (−1.56, 1.85) | --------------------- | --------------------- |
| SAM | 2.28 (−0.23, 4.80) | 1.75 (−3.1, 6.60) | 2.15 (−2.80, 7.11) | 1.83 (−3.17, 6.82) |
| SAH | 2.07 (−1.43, 5.57) | 1.17 (−4.42, 6.76) | 0.47 (−5.37, 6.31) | 1.00 (−5.04, 7.03) |
| Cysteine | 0.75 (−2.94, 4.43) | −1.27 (−6.00, 3.46) | −1.56 (−6.35, 3.23) | −1.28 (−6.06, 3.50) |
| Glutamate | 2.08 (−0.57, 4.73) | 0.59 (−2.95, 4.13) | 0.32 (−3.29, 3.93) | 0.59 (−2.99, 4.16) |
| LPC 18:2 | −1.77 (−4.56, 1.02) | −1.61 (−4.89, 1.67) | −0.98 (−4.58, 2.61) | −1.42 (−5.42, 2.57) |
| PC ae 40:6 | −1.84 (−4.81, 1.13) | −1.77 (−5.71, 2.18) | −1.28 (−5.39, 2.84) | −1.68 (−5.8, 2.44) |
| PC ae 34:3 | −0.59 (−3.18, 1.99) | 1.46 (−2.08, 5.01) | 1.29 (−2.28, 4.87) | 1.41 (−2.22, 5.04) |
| PC aa 38:3 | 0.32 (−2.49, 3.14) | −0.64 (−3.87, 2.60) | −0.75 (−4.01, 2.50) | −0.70 (−4.04, 2.64) |
Abbreviations: DMA (dimethylarsinic acid); FPG (fasting plasma glucose); LPC (lysophosphatidylcholine); MMA (monomethylarsonic acid); PC (phosphatidylcholine); SAH (S-Adenosylhomocysteine); SAM (S- Adenosylmethionine)
Model 1: unadjusted
Model 2: adjusted for the seven OCM-related metabolites (SAM, SAH, cysteine, glutamate, LPC 18:2, PC ae 40:6, PC ae 34:3, PC aa 38:3)
Model 3: Model 2 adjustments plus additional adjustment for MMA%
Model 4: Model 2 adjustments plus additional adjustment for DMA%
Per 5% increases for arsenic metabolites and per IQR increases for OCM metabolites
Similarly, in unadjusted analyses for waist circumference, an inverse association was observed with a 5% increase in MMA% (−7.83, 95% CI: −12.8, −2.90, cm) and a positive association was observed with a 5% increase in DMA% (3.66, 95% CI: 0.95, 6.38, cm) (Table 2). After adjustment for the other metabolites, the association with MMA% was attenuated to the null (0.93, 95% CI: −4.04, 5.90, cm) and the association with DMA% even reversed direction (−1.16, 95% CI: −3.99, 1.67, cm). Several other metabolites were also significantly associated with waist circumference in unadjusted models in the same direction as the associations seen between these metabolites and HOMA2-IR. After adjustment for the other metabolites, glutamate remained significantly associated with increased waist circumference and both LPC 18:2 and PC ae 40:6 remained significantly associated with reduced waist circumference. The direction of associations between MMA%, DMA% and OCM metabolites with FPG was the same as for the other diabetes-related outcomes, however none of the associations were significant. Variance inflation factor (VIF) values were below 4 for all variables in fully OCM-adjusted models, and therefore, multicollinearity was not considered a significant concern.
4. DISCUSSION
In this pilot study of 59 men and women from the SHFS, we observed significant correlations between eight metabolites (SAM, SAH, cysteine, glutamate, LPC 18:2, PC ae 34:3, PC ae 40:6 and PC aa 38:3) and both a metabolic outcome (HOMA2-IR, FPG, waist circumference) and at least one arsenic metabolism biomarker (iAs%, MMA% or DMA%). Before adjustment, higher MMA% was associated with lower HOMA2-IR and waist circumference, and higher DMA% was associated with higher HOMA2-IR and waist circumference. After adjustment for these eight “metabolites of interest”, associations between arsenic metabolism and diabetes-related outcomes were substantially attenuated and no longer significant. For the metabolites of interest, glutamate, LPC 18:2 and PC ae 40:6 remained statistically significantly associated with waist circumference even after adjustment for other metabolites and for arsenic metabolism biomarkers. Together, our results suggest that these metabolites play a key role in the relationship between arsenic metabolism and diabetes-related outcomes and may explain the difference in association seen between arsenic metabolism and diabetes-related outcomes compared to the associations reported for arsenic metabolism and other health outcomes.
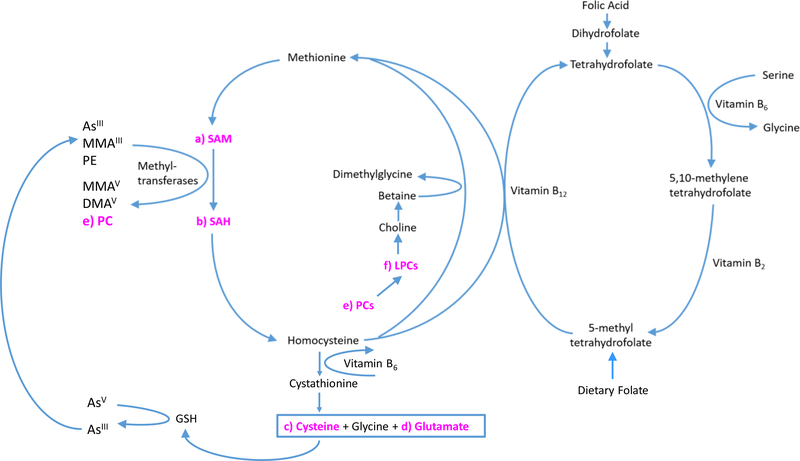
Inorganic arsenic exposure has been associated with numerous health outcomes including cancer, cardiovascular disease, skin lesions and birth outcomes.1, 2, 15, 71–74 Further, an arsenic metabolism profile reflecting higher percentages of MMA% and lower percentages of DMA%, has been associated with greater risk for these outcomes, even after controlling for exposure levels.14, 75–77 These findings, as well as the shorter half-life and rapid excretion in the urine of DMA compared to iAs, has led to the characterization of higher DMA% and lower iAs% and MMA% in urine as a more efficient arsenic metabolism profile.78 Inorganic arsenic exposure has also been associated with diabetes at high (≥100 μg/L)79–83 and moderate (<100 μg/L)27, 28, 67, 84–88 levels of exposure, with mixed findings at low levels.3, 89–91 However, in contrast to other arsenic-associated health outcomes, a more efficient arsenic metabolism profile (i.e., higher DMA% but lower iAs% and MMA%) has been associated with increased risk for diabetes3, 77 and other diabetes-related outcomes including higher BMI,50 insulin resistance92, elevated waist circumference51 and metabolic syndrome.93 The mechanism behind the reported contrasting associations is unclear. Some studies have suggested the association between higher DMA% and increased risk for metabolic-related outcomes may be due to DMAIII, as trivalent methylated arsenicals are considered the most toxic metabolites; this intermediate is difficult to measure analytically as it rapidly oxidizes to DMAV.3, 27, 28 Other studies have considered confounding by diet, particularly greater intake of OCM nutrients.3, 26 All eight of the metabolites of interest in our study that were related both to arsenic metabolism and to a metabolic outcome are connected to the OCM pathway, as outlined below (study metabolites underlined) and in Figure 4, and provide a potentially relevant mechanism behind the association between arsenic metabolism and diabetes-related outcomes.
Figure 4. Study Metabolites in the One Carbon Metabolism Pathway.
One carbon metabolism metabolites of interest (i.e., metabolites significantly correlated with both arsenic metabolism and a metabolic outcome) are highlighted in pink (a-f). OCM facilitates the generation of SAM (a), the methyl donor for numerous substrates, which are essential to many biological processes including arsenic metabolism. SAH (b) is a product of SAM-dependent methylation reactions, and is hydrolyzed to Hcys, which can be remethylated to form methionine and activated to regenerate SAM. SAH is a potent inhibitor of most transmethylation reactions, including arsenic, through its binding with methyltransferases. SAH is only removed from methyltransferases if Hcys is pulled forward for the remethylation of Hcys to form methionine or diverted to the transsulfuration pathway for glutathione synthesis. Glutathione is a tripeptide consisting of cysteine (c), glutamate (d), and glycine and is critical to the body’s antioxidant response (in its reduced form as GSH), including detoxification of heavy metals. Choline can provide methyl groups for the remethylation of Hcys through conversion to betaine. Choline is also used in the synthesis of phosphatidylcholine (PC) (e), an abundant phospholipid involved in maintenance of hepatic lipid metabolism. PCs may also be synthesized through the methylation of phosphatidylethanolamine (PE) using SAM as the methyl donor. aaPCs consist of glycerol linked to phosphocholine and two fatty acid residues, and removal of one fatty acid produces LPCs (f). aePC’s comprise an ether linkage to one alkyl chain and one polyunsaturated fatty acid. Abbreviations: As (inorganic arsenic); DMA (dimethylarsinic acid); GSH (glutathione); LPCs (lysophosphatidilycholines); MMA (monomethylarsonic acid); PCs (phosphatidylcholines); SAH (s-adenosylhomocysteine); SAM (S-adenosylmethionine)
OCM facilitates the generation of SAM, the methyl donor for numerous substrates, which are essential to many biological processes including cellular signaling, DNA methylation, the synthesis of proteins, lipids, hormones and carbohydrates, and arsenic metabolism.43 SAH is a product of SAM-dependent methylation reactions, and is hydrolyzed to Hcys, which can be remethylated to form methionine and activated to regenerate SAM.43 SAH is a potent inhibitor of most transmethylation reactions, including arsenic, through its binding with methyltransferases.41 SAH is removed from methyltransferases when Hcys is pulled forward for the remethylation of Hcys to form methionine or diverted to the transsulfuration pathway for glutathione synthesis. Glutathione is a tripeptide consisting of cysteine, glutamate, and glycine and is critical to the body’s antioxidant response (in its reduced form as GSH), including detoxification of heavy metals.94 OCM is dependent on essential nutrients - including folate, vitamin B12, vitamin B6, vitamin B2 and choline – for the recruitment and transfer of methyl groups.9 Indeed, in our study we observed greater SAM in participants with sufficient folate intake versus those with low intake. Choline can provide methyl groups for the remethylation of Hcys through conversion to betaine. Choline is also used in the synthesis of phosphatidylcholine (PC), an abundant phospholipid involved in maintenance of hepatic lipid metabolism.95 Four of the eight OCM-related metabolites included in this analysis were PCs, which may also be synthesized through the methylation of phosphatidylethanolamine using SAM as the methyl donor.96 aaPCs consist of glycerol linked to phosphocholine and two fatty acid residues, and removal of one fatty acid produces LPCs. aePC’s comprise an ether linkage to one alkyl chain and one polyunsaturated fatty acid.48
The positive associations that we observed in our study between glutamate and both HOMA2-IR and waist circumference, before and after adjustment for other OCM metabolites and arsenic metabolism, are consistent with several previous prospective and cross-sectional studies, reporting a positive relationship between glutamate and diabetes,97–101 including prospective metabolomic analyses.54, 56, 102 Further, elevated cerebral glutamate measured via proton magnetic resonance spectroscopy in occipitoparietal grey matter has been reported in adults with metabolic syndrome.103 In vitro studies in human islet cells have shown chronic exposure to glutamate through treated cell media causes dose-dependent increases in insulin secretion as well as β-cell apoptosis.104 The mechanism behind these associations is likely due to glutamate’s role in a regulatory system in which dietary protein ingestion stimulates ß-cells to stimulate insulin secretion: The increase in branched-chain amino acids following a protein-containing meal activates glutamate dehydrogenase in ß-cells which oxidizes glutamate to α-ketoglutarate and there is a consequent increase in the ATP/ADP ratio.105 Insulin secretion is very sensitive to this because it closes the ATP-gated K+ channel which depolarizes the cell and activates a voltage-gated Ca2+ channel.105 The increase in Ca2+ then triggers insulin release.105 Further, excess extracellular levels of glutamate have been shown to inhibit the glutamate/cysteine antiporter system, which depletes the cells of cysteine, in turn limiting the production of glutathione, thus decreasing the antioxidant defense.104, 106
SAM, SAH and cysteine were also significantly associated with increases in HOMA2-IR and waist circumference before adjustment for the other OCM metabolites and arsenic metabolism. Research on these metabolites and diabetes outcomes is limited, with some evidence suggesting the association may be due to reverse causality. An in vitro study found that when hepatic cells were exposed to elevated insulin and glucose, SAM concentrations increased.107 In addition, animal models have shown insulin resistance and diabetes can alter one carbon metabolism-related metabolites and enzymes, including increases in hepatic SAM, and that administration of insulin can prevent these perturbations.108, 109
SAM, SAH, glutamate and cysteine all correlated positively with a more efficient arsenic metabolism profile characterized by higher DMA%. This is consistent with observational studies from Bangladesh which reported greater SAM to be associated with lower iAs%110 and greater cysteine to be associated with lower MMA% and higher DMA%,111 as well as indirect evidence provided by experimental studies reporting a protective effect of SAM in regard to arsenic toxicity.112, 113 We found one study that has evaluated the association between glutamate and arsenic metabolism.114 In contrast to our findings, this study reported glutamate having an inverse correlation with DMA%, however, the study was conducted in pregnant women which may have influenced results. Further, both glutamate and cysteine are critical for glutathione synthesis, and increases in glutathione when glutathione levels are low have been shown to accelerate arsenic metabolism, supporting our findings.115
The findings in our study between PCs and diabetes-related outcomes are consistent with previous studies which have reported inverse associations between ae PCs and LPCs with diabetes risk and BMI, but positive associations between aa PCs and diabetes risk.116–118 The mechanisms behind the contrasting associations between ae PCs and LPCs versus aa PCs are not clear and warrant further investigation. Further, some evidence suggests the association between PCs and diabetes is due to reverse causality. Animal studies have shown significant alterations in both hepatic and blood PC levels in diabetic versus control rats.109 Regardless, the strong associations between the PCs and LPCs with multiple diabetes-related outcomes in our study are not surprising given the established link between PCs (the most abundant phospholipid), dyslipidemia and obesity-associated insulin resistance.119
Research on the association between arsenic metabolism and PCs is limited. An experimental study found a strong effect of arsenic (+3 oxidation state) methyltransferase (As3mt), the key enzyme for inorganic arsenic methylation, knockout on both plasma and urine PCs in mice.120 This is the first study to evaluate the association between arsenic metabolism and PCs in humans. Our particularly strong (meeting Bonferroni correction criteria (p=0.0015)) correlations between LPC and all three arsenic metabolite percentages is intriguing. LPC is formed by the hydrolysis of aaPCs, during which a fatty acid is freed.52 In turn, LPCs are hydrolyzed to choline.121 As choline assists in the remethylation of Hcys to methionine in the OCM pathway through conversion to betaine, we might expect LPCs to be associated with enhanced arsenic metabolism. However, our results suggest the opposite and may reflect the fact that the PC/LPC pathway for choline synthesis, one of two choline synthesis pathways, consumes three molecules of SAM per molecule of choline.96, 122, 123 This is supported by the significant inverse correlation (−0.35) between SAM and LPC 18:2 in this study.
Together, our results provide evidence that OCM metabolites may have an instrumental role in the previously observed associations between arsenic metabolism and diabetes-related outcomes. In our regression analyses, we show strong attenuations and loss of significance for associations between both DMA% and MMA% with HOMA2-IR and waist circumference after adjustment for OCM metabolites. We propose 3 potential pathways for how these OCM metabolites may influence the observed relationships between arsenic metabolism and diabetes-related outcomes. First, as represented in DAG 1 (Figure 3), OCM metabolites may be acting as confounders. All of the OCM metabolites were positively associated with HOMA2-IR and waist circumference were also positively associated with enhanced arsenic metabolism capacity. Likewise, the metabolites negatively correlated with those two diabetes-related outcomes were also associated with reduced arsenic metabolism capacity. Further, the previous paragraphs have summarized the consistency of these findings with other studies. In this way, OCM metabolites could cause a spurious association between enhanced arsenic metabolism and higher HOMA2-IR and waist circumference.
Second, as represented in DAG 2 (Figure 3), the associations seen between both OCM metabolites and arsenic metabolism with diabetes-related outcomes may be a result of reverse causality. Despite multiple metabolomic studies showing an association between most of our metabolites of interest with incident diabetes, it is still possible diabetes converters were in early stages of disease progression, causing early metabolite alterations, which in turn affected arsenic metabolism.
Finally, as represented in DAG 3 (Figure 3), OCM may still directly affect diabetes-related outcomes and arsenic metabolism (per DAG 1), however, in this scenario, part of the effect of OCM metabolites on HOMA2-IR and waist circumference is through its influence on arsenic metabolism. The results of this study provide evidence that arsenic metabolism alone is not responsible for the associations reported between arsenic metabolism and diabetes-related outcomes. However, it is still possible arsenic metabolism has some effect on diabetes-related outcomes and is not simply confounded by OCM metabolites (DAG 1) or an outcome of insulin resistance (DAG 2); instead it is acting as a partial mediator in the association between OCM and diabetes outcomes. Robust mechanistic evidence is lacking for this pathway, however, the trivalent forms of DMA (DMAIII) and MMA (MMAIII) have been shown to be potent inhibitors of insulin signal transduction in experimental models,124, 125 providing some explanation for greater diabetes risk with higher DMA%, although we are not able to distinguish between DMAIII and DMAV. Still it is unclear how this would lead to lower MMA% being inversely associated with diabetes outcomes, unless it was just an indirect association due to an increased risk with DMA%. Another theory postulates that increased methylation reactions of methyl-consuming xenobiotics, such as arsenic, may lead to a depletion of the endogenous methyl pool and generation of reactive oxygen species, and in turn tissue injury.126 We feel this to be unlikely, as the methylation of As consumes a very small proportion of SAM (< 4%).127
This study was limited by a small sample size and therefore conclusions should be interpreted with caution. Further, due the small sample size, final models did not include other confounders for arsenic metabolism diabetes-related outcomes, such as age and sex. Final models also did not account for family relatedness. However, in sensitivity analyses adjusting for those factors resulted in similar findings. Finally, availability of just a single urine and blood sample prevented prospective analyses, which are needed to confirm direction of associations as well as mediation analyses.
5. CONCLUSIONS
We found that several OCM-related metabolites were significantly and relatively strongly associated with both arsenic metabolism and diabetes-related outcomes. LPC 18:2 had the strongest association with arsenic metabolism, a novel finding that warrants additional research. In addition, glutamate, LPC 18:2 and PC ae 40:6 remained significantly associated with waist circumference after adjustment for both arsenic metabolism and all other metabolites, highlighting a potential role of these three metabolites in the development or physiological consequences of central adiposity. Adjusting arsenic metabolism biomarkers for all OCM-related metabolites in models evaluating the association between arsenic metabolism and both HOMA2-IR and waist circumference resulted in significant attenuations and losses in statistical significance. Our findings provide evidence that the OCM pathway may be - either directly or indirectly - influencing the previously reported relationship between arsenic metabolism and diabetes-related outcomes. Further, this pathway may, in part, explain the contrasting associations seen between arsenic metabolism and diabetes-related outcomes versus arsenic metabolism and other arsenic-related health outcomes.
Supplementary Material
HIGHLIGHTS.
One carbon metabolism, arsenic and diabetes appear to be interconnected
Arsenic metabolism was associated with diabetes outcomes
Adjustment for one carbon metabolism metabolites removed this association
Glutamate and phosphatidylcholines may be of particular importance
Findings need confirmation in larger prospective cohorts
Acknowledgments
FUNDING SOURCES
This work was supported by grants 1F31ES027796-01, 5T32ES007141-33, R01ES025216, 5P30ES009089 and P42ES010349 from NIEHS and grants R01-HL090863, R01-HL109315, R01HL109301, R01HL109284, R01HL109282, R01HL109319, U01-HL41642, U01-HL41652, U01-HL41654, U01-HL65520, U01-HL65521 from NHLBI.
Abbreviations:
- 5-MTHF
5-methyltetrahydrofolate
- ∑As
Arsenic exposure measured as urine arsenic levels
- DMA
Dimethylarsinic acid
- DAGs
Directed acyclic graphs
- eGFR
Estimated glomerular filtration rate
- FPG
Fasting plasma glucose
- FFQ
Food frequency questionnaire
- HOMA2-IR
Homeostasis model assessment for insulin resistance
- Hcys
Homocysteine
- iAs
Inorganic arsenic
- LPC
Lysophosphatidylcholine
- MMA
Monomethylarsonic acid
- OCM
One carbon metabolism
- PC
Phosphatidylcholine
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- SHFS
Strong Heart Family Study
Footnotes
Disclaimer: The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
HUMAN SUBJECTS APPROVAL
All participants provided informed consent before participation in this study and study protocols were approved by multiple institutional review boards, participating communities and The Indian Health Service
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Moon K, Guallar E, and Navas-Acien A, Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep, 2012. 14(6): p. 542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naujokas MF, et al. , The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect, 2013. 121(3): p. 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo CC, et al. , Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care, 2015. 38(4): p. 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyler CR and Allan AM, The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr Environ Health Rep, 2014. 1: p. 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Molinaro P, and Chen Y, Arsenic Exposure and Subclinical Endpoints of Cardiovascular Diseases. Curr Environ Health Rep, 2014. 1(2): p. 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Council NR, Arsenic in drinking water, 2001 update, Council NR, Editor. 2001: Washington DC. [Google Scholar]
- 7.Chen JW, et al. , The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere, 2011. 84(1): p. 17–24. [DOI] [PubMed] [Google Scholar]
- 8.Hall MN, et al. , Influence of cobalamin on arsenic metabolism in Bangladesh. Environ Health Perspect, 2009. 117(11): p. 1724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall MN and Gamble MV, Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol, 2012. 2012: p. 595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahter M, Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett, 2000. 112–113: p. 209–17. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez A and Marcos R, Genetic variations associated with interindividual sensitivity in the response to arsenic exposure. Pharmacogenomics, 2008. 9(8): p. 1113–32. [DOI] [PubMed] [Google Scholar]
- 12.Steinmaus CM, Yuan Y, and Smith AH, The temporal stability of arsenic concentrations in well water in western Nevada. Environ Res, 2005. 99(2): p. 164–8. [DOI] [PubMed] [Google Scholar]
- 13.Huang YK, et al. , Changes in urinary arsenic methylation profiles in a 15-year interval after cessation of arsenic ingestion in southwest Taiwan. Environ Health Perspect, 2009. 117(12): p. 1860–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahsan H, et al. , Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev, 2007. 16(6): p. 1270–8. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, et al. , Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol, 2009. 239(2): p. 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenzuela OL, et al. , Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol Appl Pharmacol, 2009. 239(2): p. 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinmaus C, et al. , Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. J Occup Environ Med, 2006. 48(5): p. 478–88. [DOI] [PubMed] [Google Scholar]
- 18.Agusa T, et al. , Exposure, metabolism, and health effects of arsenic in residents from arsenic-contaminated groundwater areas of Vietnam and Cambodia: a review. Rev Environ Health, 2010. 25(3): p. 193–220. [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, et al. , Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med, 2003. 45(3): p. 241–8. [DOI] [PubMed] [Google Scholar]
- 20.Yu RC, et al. , Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev, 2000. 9(11): p. 1259–62. [PubMed] [Google Scholar]
- 21.Huang YK, et al. , Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control, 2008. 19(8): p. 829–39. [DOI] [PubMed] [Google Scholar]
- 22.Steinmaus C, et al. , Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol, 2010. 247(2): p. 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YK, et al. , Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol, 2007. 218(2): p. 135–42. [DOI] [PubMed] [Google Scholar]
- 24.Huang YL, et al. , Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ, 2009. 407(8): p. 2608–14. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, et al. , A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect, 2013. 121(7): p. 832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nizam S, et al. , Differences in urinary arsenic metabolites between diabetic and non-diabetic subjects in Bangladesh. Int J Environ Res Public Health, 2013. 10(3): p. 1006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez MA, et al. , Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environ Health Perspect, 2016. 124(1): p. 104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Razo LM, et al. , Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health, 2011. 10: p. 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahabir S, et al. , Measures of adiposity and body fat distribution in relation to serum folate levels in postmenopausal women in a feeding study. Eur J Clin Nutr, 2008. 62(5): p. 644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun KV, et al. , Dietary Intakes of Folic Acid and Methionine in Early Childhood Are Associated with Body Composition at School Age. J Nutr, 2015. 145(9): p. 2123–9. [DOI] [PubMed] [Google Scholar]
- 31.Bird JK, et al. , Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J Nutr, 2015. 145(1): p. 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian S, et al. , Dietary nutrient intake and metabolic syndrome risk in Chinese adults: a case-control study. Nutr J, 2013. 12: p. 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazidi M, Pennathur S, and Afshinnia F, Link of dietary patterns with metabolic syndrome: analysis of the National Health and Nutrition Examination Survey. Nutr Diabetes, 2017. 7(3): p. e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe AJ, et al. , Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am J Clin Nutr, 2017. 105(6): p. 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunanti IR, et al. , Low serum vitamin B-12 and folate concentrations and low thiamin and riboflavin intakes are inversely associated with greater adiposity in Mexican American children. J Nutr, 2014. 144(12): p. 2027–33. [DOI] [PubMed] [Google Scholar]
- 36.Spratlen MJ, et al. , Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: Evidence from the Strong Heart Study. Food Chem Toxicol, 2017. 105: p. 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heck JE, et al. , Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr, 2007. 85(5): p. 1367–74. [DOI] [PubMed] [Google Scholar]
- 38.Hall M, et al. , Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect, 2007. 115(10): p. 1503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamble MV, et al. , Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr, 2006. 84(5): p. 1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamble MV, et al. , Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. Am J Clin Nutr, 2005. 81(6): p. 1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamble MV, et al. , Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect, 2005. 113(12): p. 1683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters BA, et al. , Folic Acid and Creatine as Therapeutic Approaches to Lower Blood Arsenic: A Randomized Controlled Trial. Environ Health Perspect, 2015. 123(12): p. 1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ralph Carmel DWJ, ed. Homocysteine in Health and Disease. 2001, Cambridge University Press. [Google Scholar]
- 44.Zhao J, et al. , Novel metabolic markers for the risk of diabetes development in American Indians. Diabetes Care, 2015. 38(2): p. 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhee EP, et al. , Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest, 2011. 121(4): p. 1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang TJ, et al. , Metabolite profiles and the risk of developing diabetes. Nat Med, 2011. 17(4): p. 448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drogan D, et al. , Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin Chem, 2015. 61(3): p. 487–97. [DOI] [PubMed] [Google Scholar]
- 48.Floegel A, et al. , Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes, 2013. 62(2): p. 639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grau-Perez M, K.C., Gribble MO, Balakrishnan P, Spratlen MS, Vaidya D, Francesconi KA, Goessler W, Guallar E, Silbergeld EK, Umans JG, Best LG, Lee ET, Howard BV, Cole SA, Navas-Acien A, Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. Environmental Health Perspectives, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gribble MO, et al. , Body composition and arsenic metabolism: a cross-sectional analysis in the Strong Heart Study. Environ Health, 2013. 12: p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spratlen MJ, G.-P.M., Best LG, Yracheta J, Lazo M, Vaidya D, Balakrishnan P, Gamble MV, Francesconi KA, Goessler W, Cole SA, Umans JG, Howard BV, Navas-Acien A, The Association of Arsenic Exposure and Arsenic Metabolism with the Metabolic Syndrome and its Individual Components: Prospective Evidence from the Strong Heart Family Study. American Journal of Epidemiology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang-Sattler R, et al. , Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol, 2012. 8: p. 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang TJ, et al. , 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest, 2013. 123(10): p. 4309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walford GA, et al. , Metabolite Profiles of Diabetes Incidence and Intervention Response in the Diabetes Prevention Program. Diabetes, 2016. 65(5): p. 1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menni C, et al. , Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes, 2013. 62(12): p. 4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peddinti G, et al. , Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrannini E, et al. , Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes, 2013. 62(5): p. 1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.North KE, et al. , Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol, 2003. 157(4): p. 303–14. [DOI] [PubMed] [Google Scholar]
- 59.Levy JC, Matthews DR, and Hermans MP, Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care, 1998. 21(12): p. 2191–2. [DOI] [PubMed] [Google Scholar]
- 60.Wallace TM, Levy JC, and Matthews DR, Use and abuse of HOMA modeling. Diabetes Care, 2004. 27(6): p. 1487–95. [DOI] [PubMed] [Google Scholar]
- 61.Matthews DR, et al. , Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985. 28(7): p. 412–9. [DOI] [PubMed] [Google Scholar]
- 62.Arning E and Bottiglieri T, Quantitation of S-Adenosylmethionine and S-Adenosylhomocysteine in Plasma Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Methods Mol Biol, 2016. 1378: p. 255–62. [DOI] [PubMed] [Google Scholar]
- 63.Arning E and Bottiglieri T, Quantitation of 5-Methyltetrahydrofolate in Cerebrospinal Fluid Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Methods Mol Biol, 2016. 1378: p. 175–82. [DOI] [PubMed] [Google Scholar]
- 64.Butler LM, et al. , Prediagnostic levels of serum one-carbon metabolites and risk of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev, 2013. 22(10): p. 1884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yurgalevitch SM, et al. , Physical activity and lipids and lipoproteins in American Indians ages 45–74. Med Sci Sports Exerc, 1998. 30(4): p. 543–9. [DOI] [PubMed] [Google Scholar]
- 66.Fretts AM, et al. , Associations of processed meat and unprocessed red meat intake with incident diabetes: the Strong Heart Family Study. Am J Clin Nutr, 2012. 95(3): p. 752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gribble MO, et al. , Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol, 2012. 176(10): p. 865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopenhayn-Rich C, et al. , Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect, 1996. 104(6): p. 620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NIH, Riboflavin Fact Sheet for Health Professionals, N.I.o.H.O.o.D. Supplements, Editor. 2016.
- 70.NIH, Folate Dietary Supplement Fact Sheet, N.I.o.H.O.o.D. Supplements, Editor. 2016.
- 71.Garcia-Esquinas E, et al. , Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol Biomarkers Prev, 2013. 22(11): p. 1944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moon KA, et al. , Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med, 2013. 159(10): p. 649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bloom MS, et al. , Maternal arsenic exposure and birth outcomes: a comprehensive review of the epidemiologic literature focused on drinking water. Int J Hyg Environ Health, 2014. 217(7): p. 709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quansah R, et al. , Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect, 2015. 123(5): p. 412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei B, et al. , Arsenic methylation and skin lesions in migrant and native adult women with chronic exposure to arsenic from drinking groundwater. Environ Geochem Health, 2017. 39(1): p. 89–98. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, et al. , Associations of arsenic metabolites, methylation capacity, and skin lesions caused by chronic exposure to high arsenic in tube well water. Environ Toxicol, 2017. 32(1): p. 28–36. [DOI] [PubMed] [Google Scholar]
- 77.Kuo CC, et al. , The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ Health Perspect, 2017. 125(8): p. 087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Challenger F, Biological methylation. Adv Enzymol Relat Subj Biochem, 1951. 12: p. 429–91. [DOI] [PubMed] [Google Scholar]
- 79.Maull EA, et al. , Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect, 2012. 120(12): p. 1658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tseng CH, et al. , Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect, 2000. 108(9): p. 847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahman M, et al. , Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol, 1998. 148(2): p. 198–203. [DOI] [PubMed] [Google Scholar]
- 82.Lai MS, et al. , Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol, 1994. 139(5): p. 484–92. [DOI] [PubMed] [Google Scholar]
- 83.Wang SL, et al. , Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect, 2003. 111(2): p. 155–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim NH, et al. , Arsenic exposure and incidence of type 2 diabetes in Southwestern American Indians. Am J Epidemiol, 2013. 177(9): p. 962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.James KA, et al. , A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res, 2013. 123: p. 33–8. [DOI] [PubMed] [Google Scholar]
- 86.Navas-Acien A, et al. , Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA, 2008. 300(7): p. 814–822. [DOI] [PubMed] [Google Scholar]
- 87.Feseke SK, et al. , Arsenic exposure and type 2 diabetes: results from the 2007–2009 Canadian Health Measures Survey. Health Promot Chronic Dis Prev Can, 2015. 35(4): p. 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim Y and Lee BK, Association between urinary arsenic and diabetes mellitus in the Korean general population according to KNHANES 2008. Sci Total Environ, 2011. 409(19): p. 4054–62. [DOI] [PubMed] [Google Scholar]
- 89.Steinmaus C, et al. , Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology, 2009. 20(6): p. 807–15. [DOI] [PubMed] [Google Scholar]
- 90.Makris KC, et al. , A preliminary assessment of low level arsenic exposure and diabetes mellitus in Cyprus. BMC Public Health, 2012. 12: p. 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, et al. , No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environ Health Perspect, 2010. 118(9): p. 1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grau-Perez Maria, C.-C.K., Gribble Matthew O., Balakrishnan Poojitha, Vaidya Dhananjay, Francesconi Kevin A., Goessler Walter, Guallar Eliseo, Silbergeld Ellen, Umans Jason G., Best Lyle G., Lee Elisa T., Howard Barbara V., Cole Shelley A., Navas-Acien Ana, The association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. 2017. [DOI] [PMC free article] [PubMed]
- 93.Chen JW, et al. , Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere, 2012. 88(4): p. 432–8. [DOI] [PubMed] [Google Scholar]
- 94.Reed MC, et al. , A mathematical model of glutathione metabolism. Theor Biol Med Model, 2008. 5: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.da Silva RP, et al. , Novel insights on interactions between folate and lipid metabolism. Biofactors, 2014. 40(3): p. 277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Z and Vance DE, Phosphatidylcholine and choline homeostasis. J Lipid Res, 2008. 49(6): p. 1187–94. [DOI] [PubMed] [Google Scholar]
- 97.Hack V, et al. , Elevated venous glutamate levels in (pre)catabolic conditions result at least partly from a decreased glutamate transport activity. J Mol Med (Berl), 1996. 74(6): p. 337–43. [DOI] [PubMed] [Google Scholar]
- 98.Davalli AM, Perego C, and Folli FB, The potential role of glutamate in the current diabetes epidemic. Acta Diabetol, 2012. 49(3): p. 167–83. [DOI] [PubMed] [Google Scholar]
- 99.Cheng S, et al. , Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation, 2012. 125(18): p. 2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newgard CB, et al. , A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab, 2009. 9(4): p. 311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao Y, et al. , Metabonomic variations in the drug-treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res, 2009. 8(4): p. 1623–30. [DOI] [PubMed] [Google Scholar]
- 102.Palmer ND, et al. , Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab, 2015. 100(3): p. E463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haley AP, et al. , Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metab Brain Dis, 2010. 25(4): p. 397–405. [DOI] [PubMed] [Google Scholar]
- 104.Di Cairano ES, et al. , The glial glutamate transporter 1 (GLT1) is expressed by pancreatic beta-cells and prevents glutamate-induced beta-cell death. J Biol Chem, 2011. 286(16): p. 14007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brosnan JT and Brosnan ME, Glutamate: a truly functional amino acid. Amino Acids, 2013. 45(3): p. 413–8. [DOI] [PubMed] [Google Scholar]
- 106.Albrecht P, et al. , Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets, 2010. 9(3): p. 373–82. [DOI] [PubMed] [Google Scholar]
- 107.Chiang EP, et al. , Effects of insulin and glucose on cellular metabolic fluxes in homocysteine transsulfuration, remethylation, S-adenosylmethionine synthesis, and global deoxyribonucleic acid methylation. J Clin Endocrinol Metab, 2009. 94(3): p. 1017–25. [DOI] [PubMed] [Google Scholar]
- 108.Martinez JA, et al. , Epigenetics in adipose tissue, obesity, weight loss, and diabetes. Adv Nutr, 2014. 5(1): p. 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wijekoon EP, et al. , Homocysteine metabolism in ZDF (type 2) diabetic rats. Diabetes, 2005. 54(11): p. 3245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Howe CG, et al. , Folate and cobalamin modify associations between S-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed Bangladeshi adults. J Nutr, 2014. 144(5): p. 690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hall MN, et al. , Folate, Cobalamin, Cysteine, Homocysteine, and Arsenic Metabolism among Children in Bangladesh. Environ Health Perspect, 2009. 117(5): p. 825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramirez T, et al. , S-adenosyl-L-methionine is able to reverse micronucleus formation induced by sodium arsenite and other cytoskeleton disrupting agents in cultured human cells. Mutat Res, 2003. 528(1–2): p. 61–74. [DOI] [PubMed] [Google Scholar]
- 113.Ramirez T, et al. , S-adenosyl-L-methionine counteracts mitotic disturbances and cytostatic effects induced by sodium arsenite in HeLa cells. Mutat Res, 2008. 637(1–2): p. 152–60. [DOI] [PubMed] [Google Scholar]
- 114.Laine JE, et al. , Neonatal Metabolomic Profiles Related to Prenatal Arsenic Exposure. Environ Sci Technol, 2017. 51(1): p. 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lawley SD, et al. , Mathematical modeling of the effects of glutathione on arsenic methylation. Theor Biol Med Model, 2014. 11: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pallares-Mendez R, et al. , Metabolomics in diabetes, a review. Ann Med, 2016. 48(1–2): p. 89–102. [DOI] [PubMed] [Google Scholar]
- 117.Weir JM, et al. , Plasma lipid profiling in a large population-based cohort. J Lipid Res, 2013. 54(10): p. 2898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lacruz ME, et al. , Cardiovascular Risk Factors Associated With Blood Metabolite Concentrations and Their Alterations During a 4-Year Period in a Population-Based Cohort. Circ Cardiovasc Genet, 2016. 9(6): p. 487–494. [DOI] [PubMed] [Google Scholar]
- 119.Meikle PJ and Summers SA, Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol, 2017. 13(2): p. 79–91. [DOI] [PubMed] [Google Scholar]
- 120.Huang MC, Douillet CC, and Styblo M, Knockout of arsenic (+3 oxidation state) methyltransferase results in sex-dependent changes in phosphatidylcholine metabolism in mice. Arch Toxicol, 2016. 90(12): p. 3125–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garcia-Sevillano MA, et al. , Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal Bioanal Chem, 2014. 406(5): p. 1455–69. [DOI] [PubMed] [Google Scholar]
- 122.Hall MN, et al. , Supplementation with Folic Acid, but Not Creatine, Increases Plasma Betaine, Decreases Plasma Dimethylglycine, and Prevents a Decrease in Plasma Choline in Arsenic-Exposed Bangladeshi Adults. J Nutr, 2016. 146(5): p. 1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeisel SH and da Costa KA, Choline: an essential nutrient for public health. Nutr Rev, 2009. 67(11): p. 615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walton FS, et al. , Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol, 2004. 198(3): p. 424–33. [DOI] [PubMed] [Google Scholar]
- 125.Douillet C, et al. , Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol, 2013. 267(1): p. 11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou SS, et al. , Dietary methyl-consuming compounds and metabolic syndrome. Hypertens Res, 2011. 34(12): p. 1239–45. [DOI] [PubMed] [Google Scholar]
- 127.Gamble MV and Hall MN, Relationship of creatinine and nutrition with arsenic metabolism. Environ Health Perspect, 2012. 120(4): p. A145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.