Summary
Background
Fractalkine (FKN) is an inflammatory cytokine that has been shown with increased serum levels in diabetic patients and is considered to contribute to the adipose tissue inflammation by supporting monocyte adhesion to adipocytes which has an important role in the pathogenesis of type 2 diabetes mellitus (T2DM). Our aim was to evaluate the effects of glucose ingestion on the serum fractal - kine levels in healthy subjects with normal glucose tolerance (NGT) and newly diagnosed T2DM patients.
Methods
A total of 67 patients were included in this study, and they were divided into NGT (n=34) and T2DM (n=33) groups according to their oral glucose tolerance test (OGTT) results. The serum FKN and C-reactive protein (CRP) levels were measured at 0 and 120 minutes during an OGTT following overnight fasting.
Results
The 0-minute (basal) and 120-minute OGTT FKN levels were found to be significantly higher in the T2DM group when compared to the NGT group (p=0.012 and p=0.001, respectively). However, no significant differences were observed in terms of the changes in the basal and 120-minute OGTT FKN levels in the T2DM and NGT groups (p=0.433 and p=0.06, respectively). A significant positive correlation was observed between the 120-minute OGTT FKN and glucose levels in the study group consisting of all of the patients (r=0.331, p=0.006).
Conclusions
In this study, basal and post-glycemic load FKN levels were found to be higher in newly diagnosed T2DM patients than those with NGT; however, there was no additional change in FKN levels by glycemic load.
Keywords: fractalkine, inflammation, oral glucose tolerance test, type 2 diabetes mellitus
Kratak sadržaj
Uvod
Fraktalkin (FKN) jeste inflamatorni citokin otkriven u povišenim serumskim nivoima kod obolelih od dijabetesa i za koji se smatra da doprinosi inflamaciji adipoznog tkiva tako što podstiče adheziju monocita za adipocite što ima važnu ulogu u patogenezi dijabetes melitusa tipa 2 (T2DM). Naš cilj bio je procena efekata unosa glukoze na nivo serumskog FKN-a kod zdravih ispitanika sa normalnom tolerancijom glukoze (NGT) i pacijenata sa T2DM.
Metode
Ukupno 67 pacijenata je obuhvaćeno u ovoj studiji i podeljeni su na NGT (n = 34) i T2DM (n = 33) grupe u skladu sa rezultatima oralnog testa tolerancije glukoze (OGTT). Nivoi serumskog FKN-a i C-reaktivnog proteina (CRP) mereni su na 0 i 120 minuta tokom OGTT-a nakon noćnog posta.
Rezultati
Utvrđeno je da su 0-minutni (bazalni) i 120-minutni nivoi OGTT FKN-a znatno viši u T2DM grupi u odnosu na NGT grupu (p = 0,012 i p = 0,001). Međutim, nisu zabeležene značajne razlike u pogledu promena u bazalnom i 120-minutnom nivou OGTT FKN-a u T2DM i NGT grupama (p = 0,433 i p = 0,06). Značajna pozitivna korelacija je utvrđena između 120-minutnog OGTT FKN-a i nivoa glukoze u studijskoj grupi koja se sastoji od svih pacijenata (r = 0,331, p = 0,006).
Zaključak
Prema našem saznanju, efekti unosa glukoze na nivo serumskog FKN-a su prvi put procenjeni u ovoj studiji. U ovoj studiji, bazalni nivoi FKN-a i nivoi posle opterećenja glukozom bili su viši kod pacijenata sa novodijagnostikovanim T2DM nego sa NGT, ali nije bilo dodatnih promena nivoa FKN-a u odnosu na opterećenje glukozom.
Ključne reči: fraktalkin, upala; oralni test tolerancije glukoze; dijabetes mellitus tip 2
Introduction
Several studies have demonstrated that chronic low-grade inflammation plays an important role in the pathogenesis of type 2 diabetes mellitus (T2DM) (1, 2, 3, 4, 5). The only described member of the CX3C chemokines family is fractalkine (FKN). The only known receptor of FKN (CX3CR1) is a G-protein coupled receptor and it is mainly expressed by monocytes, natural killer and T cells (6). FKN is known to have a promotive effect on both chemotaxis and adhesion during the inflammatory state and due to these characteristics, it is regarded as responsible for the migration of leukocytes to the tissue (7). T2DM is closely associated with adipose tissue inflammation. Monocyte accumulation in the adipose tissue first leads to local cytokine production and subsequent systemic inflammation and insulin resistance (8). The dual feature of FKN on leukocyte chemotaxis and adhesion mechanisms is considered to be a pathogenetic factor leading to T2DM and early-term adipocyte dysfunction (9). Recent research has focused on assessing the contribution of fractalkine (FKN), a newly described inflammatory cytokine, in the pathogenesis of T2DM. An increase in the serum FKN levels has been demonstrated in T2DM patients (10, 11).
Previous studies have shown that acute hyperglycemia or food intake can cause an increase in the inflammatory cytokines (12, 13). However, the effects of glucose ingestion on the serum FKN levels has not been evaluated before. Therefore, our aim was to evaluate the effects of glucose ingestion on the serum FKN levels in healthy subjects with normal glucose tolerance (NGT) and patients with T2DM.
Materials and Methods
Sixty-seven patients, who were asymptomatic but had a risk factor for T2DM development and therefore underwent an oral glucose tolerance test (OGTT), were included in the study. According to the OGTT results, the patients were divided into the newly diagnosed T2DM and NGT groups. Those patients with a history of diabetes mellitus, any malignant or inflammatory disease, and not in the age range of 18–65 years old were excluded. Each subject provided informed written consent, and the research protocols were approved by the ethical committee of our institution in accordance with the Helsinki Declaration. The age, gender, comorbid disease information, systolic and diastolic blood pressures, height, body weight, and body mass index (BMI) measurements of each of the participating patients were recorded.
Seventy-five grams of glucose were administered orally between 08:00 am and 09:00 am after overnight fasting. Blood samples were taken at 0 and 120 minutes during the OGTT for the glucose, FKN, and C-reactive protein (CRP) measurements. The blood samples taken at 0 minutes were used to test the triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels. The criteria proposed by the American Diabetes Association (ADA) were used for the diagnoses of NGT and T2DM (14).
The plasma glucose was measured via the hexokinase method (Architect; Abbott Diagnostics, Lake Forest, IL, USA). The TG, TC, HDL-C, and LDL-C concentrations were assayed using enzymatic methods, while the serum FKN levels were analyzed using an enzyme-linked immunosorbent assay (ELISA) with a Rayto-2600 Microplate Reader (India) and Quantikine ELISA kit (R&D Systems, USA) in accordance with the manufacturers’ instructions. The serum CRP level was assayed with an Architect c8000 (Abbott Diagnostics, Lake Forest, IL, USA) using the immunoturbidimetric method.
SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis of the data. The mean comparison of two groups was carried out using the Student's t-test for the parametric data, and the median comparison of two groups was carried out using the Mann-Whitney U test for the nonparametric data. The paired Student’s t test was used to compare the 0 and 120-minute OGTT FKN and CRP levels. The normally distributed data were expressed as the mean ± standard deviation, while the non-normally distributed data were expressed as the median (minimum-maximum). The correlation analysis of the FKN and CRP levels with the other parameters was carried out using a Spear man correlation test. A p value <0.005 was considered to be significant.
Results
The 67 patients included in this study were divided into newly diagnosed T2DM (n=33) and NGT (n=34) groups according to their OGTT results. BMI, systolic and diastolic blood pressure and TG levels were significantly higher in the T2DM group and HDL levels were significantly higher in the NGT group (Table I).
Table I.
Demographic features and laboratory results of groups.
| Parameter | T2DM | NGT | p |
|---|---|---|---|
| Number (male/female) | 33 (16/17) | 34 (16/18) | 0.900 |
| Age (yr) | 50.0 ± 10.2 | 48.4 ± 9.4 | 0.503 |
| BMI (kg/m2) | 27.99 ± 2.31 | 25.37 ± 3.05 | <0.001 |
| Systolic blood pressure (mmHg) | 122 ± 8 | 117 ± 8 | 0.010 |
| Diastolic blood pressure (mmHg) | 73 ± 6 | 68 ± 7 | 0.001 |
| Triglyceride (mmol/L) | 2.22 ± 1.13 | 1.35 ± 0.81 | 0.001 |
| Total cholesterol (mmol/L) | 5.50 ± 0.83 | 5.01 ± 1.10 | 0.047 |
| LDL cholesterol (mmol/L) | 3.34 ± 0.74 | 3.03 ± 0.94 | 0.145 |
| HDL cholesterol (mmol/L) | 1.13 ± 0.25 | 1.34 ± 0.35 | 0.008 |
| 0-minute glucose (mmol/L) | 6.05 ± 0.89 | 5.03 ± 0.34 | <0.001 |
| 120-minute glucose (mmol/L) | 14.6 ± 5.52 | 5.60 ± 1.12 | <0.001 |
| 0-minute fractalkine (ng/mL) | 0.37 ± 0.03 | 0.26 ± 0.25 | 0.012 |
| 120-minute fractalkine (ng/mL) | 0.37 ± 0.03 | 0.23 ± 0.22 | 0.001 |
| 0-minute CRP (ng/mL) | 3.79 ± 1.28 | 3.20 ± 1.80 | 0.125 |
| 120-minute CRP (ng/mL) | 4.29 ± 1.13 | 2.93±1.79 | <0.001 |
BMI: Body mass index, NGT: Normal glucose tolerance, T2DM: Type 2 diabetes mellitus, CRP: C-reactive protein
The 0-minute (basal) OGTT FKN level was significantly higher in the T2DM group when compared to the NGT group (p=0.012). In addition, the 120-minute OGTT FKN level was also significantly higher in the T2DM group when compared to the NGT group (p=0.001). Although the baseline CRP levels were high in the T2DM group, no significant difference was observed in the NGT group (p=0.125). The 120-minute OGTT CRP level was significantly higher in the T2DM group than in the NGT group (p=0.001) (Table II).
Table II.
Fractalkine, glucose and CRP results of groups.
| Parameter | T2DM | NGT | p |
|---|---|---|---|
| 0-minute glucose (mmol/L) | 6.05 ± 0.89 | 5.03 ± 0.34 | <0.001 |
| 120-minute glucose (mmol/L) | 14.6 ± 5.52 | 5.60 ± 1.12 | <0.001 |
| 0-minute fractalkine (ng/mL) | 0.374 ± 0.33 | 0.26 ± 0.25 | 0.012 |
| 120-minute fractalkine (ng/mL) | 0.367 ± 0.34 | 0.23 ± 0.22 | 0.001 |
| 0-minute CRP (ng/mL) | 3.79 ± 1.28 | 3.20 ± 1.80 | 0.125 |
| 120-minute CRP (ng/mL) | 4.29 ± 1.13 | 2.93±1.79 | <0.001 |
NGT: Normal glucose tolerance, T2DM: Type 2 diabetes mellitus, CRP: C-reactive protein
No significant differences were observed in terms of the changes in the basal and 120-minute OGTT FKN levels in the T2DM and NGT groups (p=0.433 and p=0.06, respectively). In addition, there were no significant differences in terms of the changes in the basal and 120-minute OGTT CRP levels in the T2DM and NGT groups (p=0.205 and p=0.06, respectively) (Table II).
A significant positive correlation was observed between the 120-minute OGTT FKN and glucose levels in the study group consisting of all of the patients (r=0.331, p=0.006) (Figure I).
Figure 1.
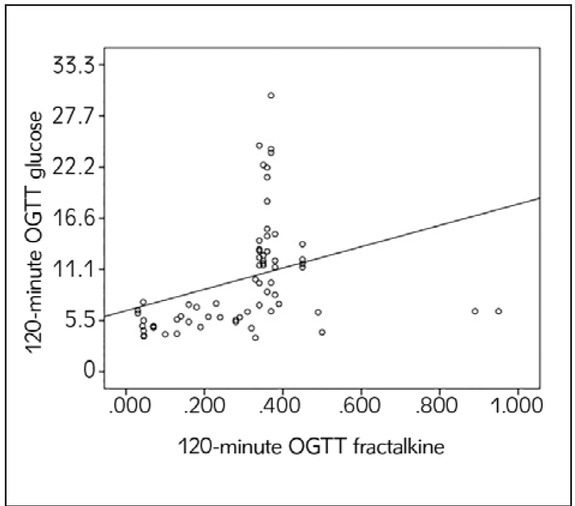
Correlation between 120-minute OGTT FKN and glucose levels.
Discussion
Progressive b cell dysfunction is a condition occurring during the natural course of T2DM, but the detailed mechanism of this condition has not yet been elucidated. However, increased evidence points toward the chronic low-grade inflammation caused by proinflammatory cytokines and chemokines (1, 2, 3, 4, 5). As a chemoattractant and adhesion factor, FKN directs the recruitment of inflammatory cells in the inflammation area, such as expanding white adipose tissue in obesity and T2DM. FKN has been reported to be associated with monocyte adhesion to adipocytes, while the FKN expression in adipocytes and stromal vascular cells has been associated with insulin resistance and T2DM (11). In T2DM patients, the serum FKN levels were demonstrated to increase (11, 12). Moreover, a relationship has been shown between an FKN receptor (CX3CR1) polymorphism and obesity and T2DM (11, 15); however, the data obtained from animal studies are contradictory. The CX3CR1 gene knockout in obese mice fed a high-fat diet was shown not to improve adipose tissue inflammation or peripheral insulin resistance (16). Recently, in another study conducted by Lee et al. (17), the b cells of mice with another strain of CX3CR1 gene knockout were demonstrated to have defective insulin secretion, and they developed hyperglycemia with a high-fat diet. In the same study, an increase in the insulin release and an improvement in glucose tolerance were reported with the in vivo administration of FKN (17). The results of these studies indicate that there are still unexplained points regarding the relationship between glucose metabolism and FKN.
Previous in vitro studies have reported that the IL-6 and TNF-α expression of blood cells increased in media including high glucose, and that the intake of glucose or elevated blood glucose were associated with an acute inflammatory response, oxidative stress, and inflammation at the cellular and molecular levels (12, 18, 19, 20, 21). In addition, glucose was shown to increase the production of reactive oxygen species from the leucocytes as well as NF-kB binding to transcription targets, resulting in an increase in the levels of the systemic cytokines (such as ICAM-1, IL-6, and TNF-α) (13, 22, 23, 24). In a study conducted by Hofso et al. (25), independent of obesity and the glucose tolerance status, there was suppression of the osteoprotegerin level with the OGTT, and a temporary increase was observed in the visfatin level. In another study conducted recently by Choi et al. (26), in which the levels of 16 different cytokines were assessed, independent of the glucose tolerance status, a significant reduction was reported in the leptin, retinol binding protein-4, CRP, osteopontin, angiogenin, macro phage-derived chemokine, and macrophage colony stimulating factor levels with the OGTT, where as a significant increase was noted in the IL-6, IL-8, and monocyte chemoattractant protein-3 levels. The results of these studies suggest that a glucose intake causes an inflammatory response, and that the relationship between a glucose intake and the inflammatory cytokine levels is governed by complex mechanisms.
The fasting FKN levels were significantly higher in the T2DM patients when compared to the NGT group in our study, which was similar to previous studies. To our knowledge, the effect of glucose ingestion on FKN levels was investigated for the first time in this study. In our results, no significant change was observed in the post-OGTT FKN levels in either the T2DM or NGT groups. However, while examining the group including all of the patients, a significantly positive correlation was detected in the 120-minute OGTT glucose and FKN levels. These results suggest that there may be a relationship between the glucose and FKN levels, but the results of this cross-sectional study are not adequate for elucidating the complex relationship between glucose metabolism and FKN, and there is a need for studies with broad participation.
Different results have been reported regarding the effects of a glucose intake on the CRP levels. Some studies have reported an increase in the CRP levels with a glucose intake (12), while others have reported that glucose does not have any effects on the CRP levels (25, 27). Interestingly, the CRP levels were shown to decrease in response to hyperinsulinemia in the healthy subjects in the study presented recently by Ruotsalainen et al. (28). The same study reported no decrease in the CRP levels in response to hyperinsulinemia in patients with insulin resistance and a parental T2DM history (28). This condition may be attributed to the anti-inflammatory effect of hyperinsulinemia. In our study, although not statistically significant, the increase in the CRP levels of the T2DM patients with a glucose intake might have occurred due to the decreased insulin response, while the decrease in the NGT patients might have occurred due to an adequate insulin response. As a result of this, the CRP levels of the T2DM group after a glucose intake might have been found to be significantly higher.
This study has a number of limitations. First of all, conducting this research with small patient groups weakens our statistical findings. Secondly, establishing a cause–effect relationship was not possible due to the cross-sectional design of our study. Thirdly, we presented the short-term effects of a glucose intake on the FKN levels, which may not reflect the situation in patients with T2DM who are exposed to elevated glucose levels for long periods of time.
In conclusion, in this study, basal and postglycemic load FKN levels were found to be higher in newly diagnosed T2DM patients than those with NGT however, there was no additional change at FKN levels by glycemic load. Overall, the results of this study may be important in terms of providing basic knowledge for further studies.
Acknowledgments
This work was presented in part in abstract form at the 19th European Congress of Endocrinology, 2017, in Lisbon, Portugal. This study has been partially supported by Selcuk University under grant no: BAP 16102008.
List of abbreviations
- FKN
fractalkine
- NGT
normal glucose tolerance
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- 1.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. NatMed. 2012;18:363. doi: 10.1038/nm.2627. –. [DOI] [PubMed] [Google Scholar]
- 2.Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA. Involvement of oxidative stress and the JNK pathway in glucose toxicity. Rev Diabet Stud. 2004;1:165. doi: 10.1900/RDS.2004.1.165. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maedler K, Sergeev P, Ehses JA. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA. 2004;101:8138. doi: 10.1073/pnas.0305683101. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114:525. doi: 10.1002/jcb.24402. –. [DOI] [PubMed] [Google Scholar]
- 5.Klisic A, Kavaric N, Soldatovic I, Bjelakovic B, Kotur- Stevuljevic J. Relationship between cardiovascular risk score and traditional and nontraditional cardiometabolic parameters in obese adolescent girls. J Med Biochem. 2016;35:282. doi: 10.1515/jomb-2016-0005. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM. Control of microglial neurotoxicity by the fractalkine receptor. Nature Neuroscience. 2006;9:917. doi: 10.1038/nn1715. et al. –. [DOI] [PubMed] [Google Scholar]
- 7.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv. 2010;10:263. doi: 10.1124/mi.10.5.3. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann K, Sypniewska G. Secreted frizzled-related protein 4 (SFRP4) and fractalkine (CX3CL1) - Potential new biomarkers for b-cell dysfunction and diabetes. Clin Biochem. 2014;47:529. doi: 10.1016/j.clinbiochem.2014.03.007. –. [DOI] [PubMed] [Google Scholar]
- 9.Cefalu WT. Fractalkine: a cellular link between adipose tissue inflammation and vascular pathologies. Diabetes. 2011;60:1380. doi: 10.2337/db11-0239. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao K, Lu H, Huang R, Zhang S, Hong X, Shi H. Changes of dendritic cells and fractalkine in type 2 diabetic patients with unstable angina pectoris: a preliminary report. Cardiovasc Diabetol. 2011;10:50. doi: 10.1186/1475-2840-10-50. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60:1512. doi: 10.2337/db10-0956. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111:2518. doi: 10.1161/01.CIR.0000165070.46111.9F. et al. –. [DOI] [PubMed] [Google Scholar]
- 13.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067. doi: 10.1161/01.cir.0000034509.14906.ae. et al. –. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81. doi: 10.2337/dc14-S081. –. [DOI] [PubMed] [Google Scholar]
- 15.Sirois-Gagnon D, Chamberland A, Perron S, Brisson D, Gaudet D, Laprise C. Association of common polymorphisms in the fractalkine receptor (CX3CR1) with obesity. Obesity. 2011;19:222. doi: 10.1038/oby.2010.125. –. [DOI] [PubMed] [Google Scholar]
- 16.Morris DL, Oatmen KE, Wang T, Del Proposto JL, Lumeng CN. CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with dietinduced obesity. Obesity. 2012;20:1189. doi: 10.1038/oby.2012.7. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Kesh - wani M. The fractalkine/CX3CR1 system regulates beta cell function and insulin secretion. Cell. 2013;153:413. doi: 10.1016/j.cell.2013.03.001. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowalska I, Straczkowski M, Szelachowska M, Kinalska I, Prokop J, Bachórzewska-Gajewska H. Circulating Eselectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in men with coronary artery disease assessed by angiography and disturbances of carbohydrate metabolism. Metabolism. 2002;51:733. doi: 10.1053/meta.2002.32802. et al. –. [DOI] [PubMed] [Google Scholar]
- 19.Morohoshi M, Fujisawa K, Uchimura I, Numano F. The effect of glucose and advanced glycosylation end products on IL-6 production by human monocytes. Ann N Y Acad Sci. 1995;748:562. doi: 10.1111/j.1749-6632.1994.tb17362.x. –. [DOI] [PubMed] [Google Scholar]
- 20.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970. doi: 10.1210/jcem.85.8.6854. –. [DOI] [PubMed] [Google Scholar]
- 21.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism. 2006;55:1177. doi: 10.1016/j.metabol.2006.04.016. et al. –. [DOI] [PubMed] [Google Scholar]
- 22.Dhindsa S, Tripathy D, Mohanty P, Ghanim H, Syed T, Aljada A. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism. 2004;53:330. doi: 10.1016/j.metabol.2003.10.013. et al. –. [DOI] [PubMed] [Google Scholar]
- 23.Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701. doi: 10.2337/diabetes.53.3.701. et al. –. [DOI] [PubMed] [Google Scholar]
- 24.Puhalo Sladoje D, Kisi} B, Miri} D. The monitoring of protein markers of inflammation and serum lipid concentration in obese subjects with metabolic syndrome. J Med Biochem. 2017;36:366. doi: 10.1515/jomb-2017-0009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofso D, Ueland T, Hager H, Jenssen T, Bollerslev J, Godang K. Inflammatory mediators in morbidly obese subjects: associations with glucose abnormalities and changes after oral glucose. Eur J Endocrinol. 2009;161:451. doi: 10.1530/EJE-09-0421. et al. –. [DOI] [PubMed] [Google Scholar]
- 26.Choi HJ, Jeon SY, Hong WK, Jung SE, Kang HJ, Kim JW. Effect of glucose ingestion in plasma markers of inflammation and oxidative stress: analysis of 16 plasma markers from oral glucose tolerance test samples of normal and diabetic patients. Diabetes Res Clin Pract. 2013;99:27. doi: 10.1016/j.diabres.2012.01.005. et al. –. [DOI] [PubMed] [Google Scholar]
- 27.Metzig AM, Schwarzenberg SJ, Fox CK, Deering MM, Nathan BM, Kelly AS. Postprandial endothelial function, inflammation, and oxidative stress in obese children and adolescents. Obesity. 2011;19:1279. doi: 10.1038/oby.2010.318. –. [DOI] [PubMed] [Google Scholar]
- 28.Ruotsalainen E, Stancáková A, Vauhkonen I, Salmen - niemi U, Pihlajamäki J, Punnonen K. Changes in cytokine levels during acute hyperinsulinemia in offspring of type 2 diabetic subjects. Atherosclerosis. 2010;210:536. doi: 10.1016/j.atherosclerosis.2009.11.036. et al. –. [DOI] [PubMed] [Google Scholar]


