Summary
Background
The usual history of chronic heart failure (HF) is characterized by frequent episodes of acute decompensation (ADHF), needing urgent management in the emergency department (ED). Since the diagnostic accuracy of routine laboratory tests remains quite limited for predicting short-term mortality in ADHF, this retrospective study investigated the potential significance of combining red blood cell distribution width (RDW) with other conventional tests for prognosticating ADHF upon ED admission.
Methods
We conducted a retrospective study including visits for episodes of ADHF recorded in the ED of the Uni versity Hospital of Verona throughout a 4-year period. Demo - graphic and clinical features were recorded upon patient presentation. All patients were subjected to standard Chest X-ray, electrocardiogram (ECG) and laboratory testing in - cluding creatinine, blood urea nitrogen, B-type natriuretic peptide (BNP), complete blood cell count (CBC), sodium, chloride, potassium and RDW. The 30-day overall mortality after ED presentation was defined as primary endpoint.
Results
The values of sodium, creatinine, BNP and RDW were higher in patients who died than in those who survived, whilst hypochloremia was more frequent in patients who died than in those who survived. The multivariate model, incorporating these parameters, displayed a modest efficiency for predicting 30-day mortality after ED admission (AUC, 0.701; 95% CI, 0.662-0.738; p=0.001). Notably, the inclusion of RDW in the model significantly enhanced prediction efficiency, with an AUC of 0.723 (95% CI, 0.693-0.763; p<0.001). These results were confirmed with net reclassification improvement (NRI) analysis, showing that combination of RDW with conventional laboratory tests resulted in a much better prediction performance (net reclassification index, 0.222; p=0.001).
Conclusions
The results of our study show that prognostic assessment of ADHF patients in the ED can be significantly improved by combining RDW with other conventional laboratory tests.
Keywords: acute decompensated heart failure, red blood cell distribution width, net reclassification improvement, laboratory parameters
Kratak sadržaj
Uvod
Istoriju hronične srčane insuficijencije (SI) obično karakterišu česte epizode akutne dekompenzacije (ASD), koja zahteva urgentno lečenje u odeljenjima hitne službe (HS). Kako dijagnostička tačnost rutinskih laboratorijskih testova i dalje ima znatna ograničenja kad se radi o predviđanju kratkoročnog smrtnog ishoda u ASD, ova retrospektivna studija istraživala je potencijalni značaj kombinovanja širine distribucije eritrocita (ŠDE) i drugih uobičajenih testova za prognoziranje ASD posle prijema u HS.
Metode
Sproveli smo retrospektivnu studiju koja je uključila posete zbog epizoda ASD zabeležene u HS Univerzitetske bolnice u Veroni tokom perioda od 4 godine. Demografske i kliničke odlike su zabeležene posle prijema pacijenata. Svi pacijenti su podvrgnuti standardnom rendgenu grudnog koša, elektrokardiogramu (EKG) i laboratorijskom testiranju koje je obuhvatilo kreatinin, ureu u krvi, natriuretski peptid B-tipa (BNP), kompletnu krvnu sliku (CBC), natrijum, hlorid, kalijum i širinu distribucije eritrocita. Ukupan broj smrtnih ishoda u roku od 30 dana posle prijema u HS je definisan kao primarna krajnja tačka.
Rezultati
Vrednosti natrijuma, kreatinina, BNP i ŠDE bile su više kod pacijenata koji su umrli nego kod onih koji su preživeli, dok je hipohloremija bila češća kod pacijenata koji su umrli nego kod onih koji su preživeli. Multivarijantni model koji je uključio ove parametre pokazao je umerenu efikasnost za predviđanje smrtnog ishoda u roku od 30 dana po prijemu u HS (AUC, 0,701; 95% CI, 0,662–0,738; p=0,001). Primećeno je da je uključivanje ŠDE u ovaj model značajno poboljšalo efikasnost predikcije, sa AUC od 0,723 (95% CI, 0,693–0,763; p<0,001). Ovi rezultati su potvrđeni analizom neto pobošljanja reklasifikacije (net reclassification improvement, NRI), koja je pokazala da kombinovanje ŠDE sa uobičajenim laboratorijskim testovima ima za rezultat mnogo bolju predikciju (indeks neto reklasifikacije, 0,222; p=0,001).
Zaključak
Rezultati ove studije pokazuju da prognostička procena pacijenata sa ASD u HS može biti značajno pobošljana ukoliko se ŠDE kombinuje sa drugim uobičajenim laboratorijskim testovima.
Ključne reči: akutna srčana dekompenzacija, širina distribucije eritrocita, pobošljanje neto reklasifikacije, laboratorijski parametri
Introduction
Heart failure (HF) is a leading cause of hospitalization and death in Western countries. It has been recently estimated that more than 500,000 patients are hospitalized each year with a first diagnosis of HF in the United States (1). Due to the progressive aging of the population and improved management of other chronic conditions such as diabetes, hypertension and cardiac valvular disorders, the prevalence of HF is also constantly increasing, so that the epidemiologic burden of this condition now resembles a real epidemics, associated with considerable risk of mortality, morbidity and healthcare expenditure (2, 3).
The usual history of chronic HF is characterized by frequent episodes of acute decompensation (ADHF) needing urgent management in the emergency department (ED), which is then frequently followed by hospitalization (4, 5). Despite some progresses observed in the past decades, the mortality for ADHF remains considerably high, approaching 12% at 30 days after ED assessment (6), with a 30 days re-hospitalization rate as high as 20% (6, 7). This last aspect is mainly due to the fact that the frequent recurrence episodes contribute to worsen cardiac function, thus ultimately compromising the prognosis (7).
Laboratory diagnostics plays a crucial role during the initial evaluation of ADHF patients (8). Both traditional laboratory tests (hemoglobin, chloride, sodium, blood urea nitrogen (BUN), creatinine, natriuretic peptides) routinely performed upon ED admission (9, 10) and the vast array of innovative cardiac biomarkers (i.e., galectin-3, interleukin 1 and 6, soluble ST2) (11, 12) were found to be variably associated with, and predictive of, the clinical history of disease. Whilst the use of innovative biomarkers is an appealing perspective, still confined to highly specialized or research laboratories, several lines of evidence attest that the diagnostic accuracy of routine laboratory tests remains quite limited for predicting short-term mortality in ADHF (11, 12).
The red blood cell (RBC) distribution width (RDW) has been largely investigated for establishing the prognosis of patients admitted to the ED with many acute disorders (13). Notably, increased values of RDW measured upon ED admission were found to be associated with increased short-term mortality also in ADHF patients (14, 15). It is hence conceivable that the combination of RDW with other routine laboratory tests may enhance the efficiency of prognostic information provided by laboratory diagnostics in patients with ADHF.
Therefore, this study was aimed at assessing whether RDW determination during initial laboratory assessment of ADHF patients may allow to better predict 30-day mortality risk, by using an approach based on net reclassification improvement (NRI).
Materials and Methods
Patient population
We conducted a retrospective study based on reassessment of all urgent visits for an episode of ADHF recorded in the ED of the University Hospital in Verona between January 1, 2013 and December 31, 2016. HF has always been diagnosed according to the recent guidelines of the European Society of Cardiology (ESC), and thus based on the presence of suggestive diagnostic signs and symptoms including respiratory distress, dyspnea, paroxysmal nocturnal dyspnea, peripheral edema, jugular turgor, hepatojugular reflux, tachypnea and pulmonary stasis (16). The main demographic and clinical features were recorded upon patient presentation to the ED. The patients were all subjected to standard Chest X-ray, electrocardiogram (ECG) and laboratory testing. According to the local protocol, the following laboratory tests were performed: creatinine, BUN, B-type natriuretic peptide (BNP), complete blood cell count (CBC), sodium, chloride and potassium.
The patients records were then reevaluated by two emergency physicians to confirm the correctness of the diagnosis and to delete wrong records (i.e., all diagnoses not complying with the ESC diagnostic criteria). Additional exclusion criteria included: 1) impossibility to immediately perform standard Chest X-ray examination in the ED; 2) diagnosis of acute myocardial infarction (AMI) according to the ESC guidelines (17); 3) patients missing follow-up; 4) incomplete data of laboratory testing at ED presentation. The 30-day overall mortality after ED presentation was defined as the primary endpoint. Mortality data was obtained by consultation of the registry office. This retrospective study was carried out in agreement with the Helsinki Declaration, according to the terms of relevant local legislation, and was cleared by the local institutional review board.
Statistical analysis
Categorical variables were reported both as percentages and as number of events, and differences were analyzed with Fisher Exact Test or Chi-square Test. Continuous variables were reported as median and interquartile range (IQR), and differences were analyzed with Mann-Whitney U Test.
The results of laboratory tests were logarithmically transformed and then standardized (mean of 0 and SD of 1), as suggested by Schnabel et al. (18). The parameters that were found to be significantly different in ADHF patients who died or survived within 30 days after ED admission were then entered into a multivariable logistic regression analysis with the backwards elimination method (likelihood-ratio test). The odds ratio (OR) and the relative 95% confidence interval (95% CI) were finally estimated for variables which independently predicted the risk of death 30 days after ED admission. The diagnostic performance of the final model, obtained from multivariate analysis and including the significant predictors of 30-day mortality (i.e., p<0.05), was assessed by calculating the area under the curve (AUC) in an operating receiver characteristic (ROC) analysis.
The NRI approach (19) was used to verify whether the RDW value may ameliorate the overall prognostication by reclassification of patients whose risk had been previously estimated using conventional laboratory analyses. The NRI hence was applied to all patients who died or survived 30 days after ED admission, using the following risk categories: <5%, 5–10% and >10% (20, 21). These different cut-offs were then analyzed with survival analysis (Log-Rank test) to compare the 30-day cumulative mortality among the three risk groups. The statistical analysis was performed using STATA statistical software (Stata Corp LP, College Station, TX, USA). The statistical significance was set at p<0.05.
Results
A total number of 2278 visits for an episode of ADHF were recorded in the ED of the University Hospital of Verona throughout the 4-year study period. Of these, 554 ought to be excluded due to discordant diagnosis with ESC criteria (n, 122), diagnosis of AMI (n, 73), missing follow-up (n, 35) or lack of complete laboratory testing immediately at ED admission (n, 344). Therefore, the final study population consisted of 1704 ED visits (mean age 83 years, 51.6% women). Table I shows the demographic, clinic and laboratory data of the study population divided into patients who died (217/1704; 12.7%) and those who survived at 30 days. The values of sodium, creatinine, BNP and RDW were found to be higher in patients who died than in those who survived, whilst hypochloremia was more frequent in patients who died than in those who survived. In backward stepwise selection multivariable logistic regression, the risk of 30-day mortality was 67% higher per SD increase of BNP value, 39% higher per SD increase of creatinine value, 38% higher per SD increase of sodium value, and 66% higher per SD decrease of chloride value (Table II). The multivariate model, incorporating these parameters, displayed a modest efficiency for predicting 30-day mortality after ED admission (AUC, 0.701; 95% CI, 0.662–0.738; p=0.001). Notably, the inclusion of RDW in the model significantly enhanced the prediction efficiency, displaying a significant increase of AUC (0.723; 95% CI, 0.693–0.763; p<0.001) (Figure 1). The NRI resulting from the combination of RDW with the previous model based on BNP, creatinine, sodium and chloride is shown in Table III. In the category of ADHF patients who died within 30 days after ED admission, the incorporation of RDW in the predictive model allowed to reclassify 27 patients in higher risk categories (i.e., more accurate classification, bold font), whilst 20 patients were inaccurately reclassified as having a lower risk (i.e., less accurate classification, italic font). As regards the category of patients who survived, the incorporation of RDW in the predictive model allowed to reclassify 437 patients in a lower risk category (i.e., more accurate classification, bold font), whilst 155 patients were inaccurately reclassified as having a higher risk (i.e., less accurate classification, italic font). Overall, the NRI net reclassification index,
Table I.
Demographic, clinical and laboratory data of the study population.
| Variable | Patients survived | Patients died | p |
|---|---|---|---|
| ADHF episodes, n (%) | 1487 (87.3) | 217 (12.7) | |
| Sex, n (%) | 0.310 | ||
| Men | 712 (47.9) | 112 (51.6) | |
| Women | 775 (52.1) | 105 (48.4) | |
| Age (years) | 83 (76–88) | 87 (80–92) | 0.001 |
| Clinical history | |||
| Ischemic heart disease | 410 (27.6) | 53 (24.3) | 0.353 |
| Hypertensive cardiomyopathy | 1167 (78.5) | 154 (70.9) | 0.019 |
| Atrial fibrillation | 666 (44.8) | 101 (46.8) | 0.598 |
| Valvular heart disease | 300 (20.2) | 39 (18.2) | 0.574 |
| Chronic renal failure | 280 (18.8) | 47 (21.7) | 0.340 |
| Pacemaker | 232 (15.6) | 41 (18.7) | 0.260 |
| Drugs | |||
| Loop diuretics | 868 (58.4) | 131 (60.3) | 0.641 |
| Potassium-sparing diuretics | 247 (16.6) | 37 (17.0) | 0.918 |
| Beta-Blockers | 671 (45.1) | 79 (36.6) | 0.031 |
| ACE inhibitors/sartans | 578 (38.9) | 64 (29.4) | 0.011 |
| Laboratory data | |||
| Hb (g/L) | 124 (110–138) | 119 (105–134) | 0.002 |
| Creatinine (mmol/L) | 101 (81–134) | 120 (89–172) | 0.000 |
| Sodium (mol/L) | 136 (135–138) | 138 (134–142) | 0.035 |
| Chloride (mol/L) | 99 (96–102) | 97 (93–101) | 0.000 |
| Potassium (mol/L) | 4.3 (4.1–5.2) | 4.4 (3.9–5.1) | 0.122 |
| Leukocytes (x109/L) | 9.1 (7.1–11.6) | 9.2 (6.7–13.2) | 0.516 |
| BNP (pg/mL) | 7517 (3031–13504) | 11743 (6347–18376) | 0.000 |
| RDW (%) | 14.3 (13.4–15.6) | 15.2 (14.2–16.6) | 0.000 |
ACE, angiotensin converting enzyme; BNP, B-type natriuretic peptide; RDW, red blood cell distribution width.
Table II.
Multivariable logistic regression analysis for prediction of 30-day mortality in patients with acute decompensated heart failure (BNP, creatinine, serum natrium and chloride are considered as continuous variables).
| Coefficient | Standard error | Odds ratio | 95% CI | p | |
|---|---|---|---|---|---|
| Final model | |||||
| BNP | 0.511 | 0.088 | 1.666 | 1.403–1.979 | <0.001 |
| Creatinine | 0.331 | 0.071 | 1.392 | 1.212–1.599 | <0.001 |
| Hypernatremia | 0.323 | 0.110 | 1.381 | 1.112–1.715 | 0.003 |
| Hypochloremia | 0.509 | 0.111 | 1.665 | 1.340–2.068 | <0.001 |
| Final model + RDW | |||||
| BNP | 0.460 | 0.088 | 1.584 | 1.333–1.882 | <0.001 |
| Creatinine | 0.278 | 0.072 | 1.321 | 1.148–1.520 | <0.001 |
| Hypernatremia | 0.306 | 0.110 | 1.358 | 1.094–1.686 | 0.006 |
| Hypochloremia | 0.496 | 0.111 | 1.642 | 1.322–2.039 | <0.001 |
| RDW | 0.379 | 0.074 | 1.461 | 1.263–1.690 | <0.001 |
BNP, B-type natriuretic peptide; RDW, red blood cell distribution width.
Figure 1.
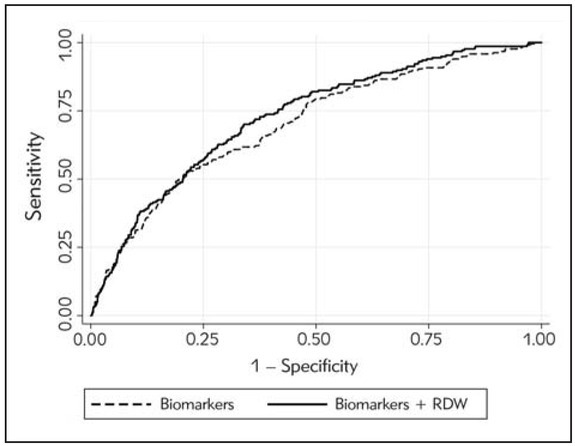
Receiver operating characteristics (ROC) curve analysis of a predictive model for 30-day mortality in patients with acute decompensated heart failure (ADHF). Comparison of a predictive model based on conventional laboratory tests (i.e., B-type natriuretic peptide, creatinine, sodium and chloride), with or without red blood cell distribution width (RDW).
Table III.
Results of net reclassification improvement (NRI) obtained by combining red blood cell distribution width (RDW) with conventional laboratory tests (B-type natriuretic peptide, creatinine, sodium and chloride).
| Conventional Tests | Conventional Tests + RDW | |||
|---|---|---|---|---|
| < 5% | 5–10% | > 10% | ||
| Patients died | ||||
| < 5% | 3 | 1 | 2 | 0 |
| 5–10% | 62 | 6 | 31 | 25 |
| > 10% | 152 | 0 | 14 | 138 |
| Total | 217 | 7 | 47 | 163 |
| Conventional Tests | Conventional Tests + RDW | |||
| Patients survived | < 5% | 5–10% | > 10% | |
| < 5% | 61 | 46 | 10 | 5 |
| 5–10% | 787 | 275 | 372 | 140 |
| > 10% | 639 | 7 | 155 | 477 |
| Total | 1487 | 328 | 537 | 622 |
Bold font, more accurate risk classification; italic font, less accurate risk classification.
which expresses prediction performance gained by adding a specific parameter, was 0.222 (p=0.001). Better reclassification was especially evident in the intermediate risk group, where 40.3% (25/62) of patients who died and 34.9% (275/787) of those who survived were more accurately classified versus 9.6% (6/62) and 17.8% (140/787) of patients whose risk was instead less accurately stratified. Overall, the inclusion of RDW in the model allowed achieving a better risk stratification of 16.9% of the patients admitted to the ED with ADHF (Figure 2).
Figure 2.
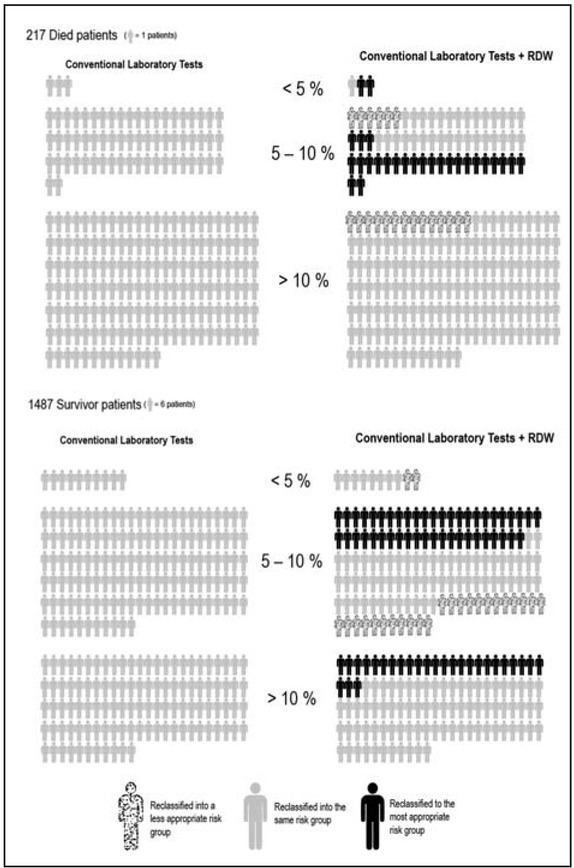
Results of the net reclassification improvement (NRI) after including red blood cell distribution width (RDW) in a predictive model based on conventional laboratory tests (i.e., B-type natriuretic peptide, creatinine, sodium and chloride).
Figure 3 shows the comparison of 30-day cumulative mortality risk after ED assessment among the three risk thresholds after NRI. Notably, patients belonging to the >10% risk category were more likely to die at 30 days than those in lower risk categories (Log Rank Test, p<0.001).
Figure 3.
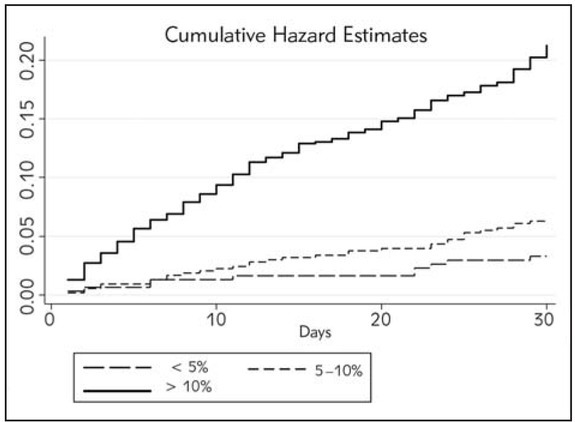
Survival curve analysis after including red blood cell distribution width (RDW) in a predictive model based on conventional laboratory tests (i.e., B-type natriuretic peptide, creatinine, sodium and chloride).
Discussion
Heart failure (HF) is currently diagnosed in over 10% of subjects aged 65 years or older in developed countries (2, 22). The frequent and often life-threatening episodes of ADHF occurring in HF patients need timely therapeutic management and are associated with a remarkably high rate of hospitalization and mortality (2, 23). Like in other potentially lethal conditions that are frequently observed in the ED, timely diagnosis and prognostication are the mainstays for optimizing management of patients with ADHF, and thus lowering the risk of long-term hospitalization and death (24, 25). Nevertheless, consolidated evidence suggests that clinical history, physical examination, laboratory testing and even diagnostic imaging are not accurate enough to timely rule out a diagnosis of ADHF in the ED (24, 26).
Laboratory testing (i.e., ions, hemoglobin, creatinine, BUN, natriuretic peptides) upon ED admission may provide early prognostic information, which can support clinicians in the medical management of ADHF (26, 27). The prognostic role of chloride (28), sodium (29), creatinine and BNP (31, 32) measured at ED presentation has been extensively studied over the last decade. Albeit these tests were found to be associated with severity and risk of cumulative mortality of HF, their diagnostic accuracy was far below satisfactory, especially for predicting medium-term outcomes (26, 33, 34, 35).
The results of our study show that combination of RDW with some conventional tests such as BNP, creatinine, sodium and chloride, may improve 30-day prognostication of ADHF patients in the ED. More specifically, NRI showed that implementation of RDW in the clinical practice management of ADHF would definitely help to improve the prognostic accuracy provided by other routine laboratory tests. Notably, the role of RDW in HF has been critically acclaimed during the past decade, but definitive conclusions have been lacking.
The RDW value in ADHF, as well as in other acute cardiovascular disorders, is associated with disease severity (36, 37). Section et al. first demonstrated that RDW value >14.5% was independently associated with medium and long term survival in a prospective cohort of 707 patients (36). These results were confirmed by Van Kimmenade et al. (38) who showed that increased RDW was associated with 1-year mortality irrespective of BNP value and inflammatory state in 205 patients with ADHF. More recently, the evidence that RDW may be a significant prognostic factor in ADHF patients has been strengthened by the publication of studies with larger sample size and including specific patients’ populations (e.g., African Americans, subjects with diabetes or cardiovascular disease) (39, 40, 41). Notably, Sotiropoulos et al. (42) recently carried out a prospective study in patients hospitalized for ADHF and with preserved left ventricular ejection fraction (LVEF), showing that high RDW values were associated with 1-year all-cause mortality. This concept has been recently reinforced by demonstrating that RDW variations (i.e., DeltaRDW) during the acute phase of hospitalization were better markers of adverse prognosis in patients with ADHF than the baseline values (43, 44).
Taken together, the results of our study demonstrate that routine assessment of RDW upon ED presentation may improve the risk assessment of patients with ADHF, improving especially the risk classification for 30-day mortality provided by other conventional laboratory tests. In particular, NRI analysis allowed to estimate that combination of RDW with BNP, creatinine, sodium and chloride could improve risk prediction in as many as 17% of patients admitted to the ED with ADHF, thus providing more accurate information for timely and appropriate patient management.
Our study, however, has some limitations. First, the retrospective design introduces a possible bias in the collection of clinical information, although the large sample size and the systematic and accurate exclusion of unclear records may have probably mitigated this drawback. Then, some clinical variables (e.g., echo cardiographic assessment of ventricular function) which are significantly associated with disease severity, were unavailable in our investigation. Notably, the main scope of our study was to assess the prognostic efficiency of conventional laboratory tests (with or without RDW). Therefore, future reclassification studies, including additional useful parameters such as echocardiography, may be advisable to verify whether or not risk stratification of ADHF patients may be further improved.
Conclusions
ADHF is an extremely severe condition still characterized by an inefficient diagnostic approach and high mortality rate (26). Nevertheless, our results show that the prognostic assessment of ADHF patients in the ED can be significantly improved by combining RDW with other conventional laboratory tests.
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259. doi: 10.1016/j.jacc.2012.12.038. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munir MB, Sharbaugh MS, Thoma FW, Nisar MU, Kam - ran AS, Althouse AD. Trends in hospitalization for congestive heart failure, 1996-2009. Clin Cardiol. 2017;40:109. doi: 10.1002/clc.22638. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646. doi: 10.1161/CIRCRESAHA.113.300268. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castello LM, Molinari L, Renghi A, Peruzzi E, Capponi A, Avanzi GC. Acute decompensated heart failure in the emergency department: identification of early predictors of outcome. Medicine (Baltimore) 2017;96:e7401. doi: 10.1097/MD.0000000000007401. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassus JP, Siirilä-Waris K, Nieminen MS, Tolonen J, Tarv - asmäki T, Peuhkurinen K. Long-term survival after hospitalization for acute heart failure - differences in prog - nosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458. doi: 10.1016/j.ijcard.2012.09.128. et al. –. [DOI] [PubMed] [Google Scholar]
- 6.McIlvennan CK, Allen LA. Outcomes in acute heart failure: 30-day readmission versus death. Curr Heart Fail Rep. 2014;11:445. doi: 10.1007/s11897-014-0215-7. –. [DOI] [PubMed] [Google Scholar]
- 7.Sperry BW, Ruiz G, Najjar SS. Hospital readmission in heart failure, a novel analysis of a longstanding problem. Heart Fail Rev. 2015;20:251. doi: 10.1007/s10741-014-9459-2. –. [DOI] [PubMed] [Google Scholar]
- 8.Demissei BG, Postmus D, Cleland JG, O'Connor CM, Metra M, Ponikowski P. Plasma biomarkers to predict or rule out early post-discharge events after hospitalization for acute heart failure. Eur J Heart Fail. 2017;19:728. doi: 10.1002/ejhf.766. et al. –. [DOI] [PubMed] [Google Scholar]
- 9.Novack V, Pencina M, Zahger D, Fuchs L, Nevzorov R, Jotkowitz A. Routine laboratory results and thirty day and one-year mortality risk following hospitalization with acute decompensated heart failure. PLoS One. 2010;5:e12184. doi: 10.1371/journal.pone.0012184. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill SA, Booth RA, Santaguida PL, Don-Wauchope A, Brown JA, Oremus M. Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: a systematic review of the evidence. Heart Fail Rev. 2014;19:421. doi: 10.1007/s10741-014-9447-6. et al. –. [DOI] [PubMed] [Google Scholar]
- 11.Meijers WC, de Boer RA, van Veldhuisen DJ, Jaarsma T, Hillege HL, Maisel AS. Biomarkers and low risk in heart failure. Data from COACH and TRIUMPH. Eur J Heart Fail. 2015;17:1271. doi: 10.1002/ejhf.407. et al. –. [DOI] [PubMed] [Google Scholar]
- 12.de Boer RA, Daniels LB, Maisel AS, Januzzi JL. State of the Art: Newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559. doi: 10.1002/ejhf.273. Jr. –. [DOI] [PubMed] [Google Scholar]
- 13.Danese E, Lippi G, Montagnana M.. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. 2015;7:E402. doi: 10.3978/j.issn.2072-1439.2015.10.04. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230. doi: 10.1016/j.cardfail.2009.11.003. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai R, Uemura Y, Okumura T, Takemoto K, Uchikawa T, Koyasu M. Impact of red blood cell distribution width on non-cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J Cardiol. 2017;70:591. doi: 10.1016/j.jjcc.2017.03.010. et al. –. [DOI] [PubMed] [Google Scholar]
- 16.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC, Eur J Heart Fail 2016; 2016;18:891. doi: 10.1002/ejhf.592. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F. Management of Acute Coronary Syn - dromes in Patients Presenting without Persistent STSegment Elevation of the European Society of Car - diology. 2015 ESC Guidelines for the management of acute co ronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Mana ge ment of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J 2016; 37:267. doi: 10.1093/eurheartj/ehv320. et al. –. [DOI] [PubMed] [Google Scholar]
- 18.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation in - cidence in the community. Circulation. 2010;121:200. doi: 10.1161/CIRCULATIONAHA.109.882241. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB Sr, D’Agostino RB. Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157. doi: 10.1002/sim.2929. Jr. –. [DOI] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128. doi: 10.1097/EDE.0b013e3181c30fb2. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15- year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64:851. doi: 10.1016/j.jacc.2014.03.061. et al. –. [DOI] [PubMed] [Google Scholar]
- 22.Kahraman A, Emre Mutlu E, Aldag M. ADMA, SDMA and l-arginine may be novel targets in pharmacotherapy for complications due to cardiopulmonary bypass. J Med Biochem. 2017;36:8. doi: 10.1515/jomb-2016-0025. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miró Ò, Rossello X, Gil V, Martín-Sánchez FJ, Llorens P, Herrero-Puente P. ICA-SEMES Research Group. Predicting 30-Day Mortality for Patients With Acute Heart Failure in the Emergency Department: A Cohort Study. Ann Intern Med. 2017;167:698. doi: 10.7326/M16-2726. et al. –. [DOI] [PubMed] [Google Scholar]
- 24.Gungor ZB, Sipahioglu N, Sonmez H, Ekmekci H, Toprak S, Ayaz G, Bayram C, Gurel Mutlu T, Ulutin T, Sipahioglu F, Ilerigelen B. Endothelial dysfunction markers in low cardiovascular risk individuals: comparison of males and females. J Med Biochem. 2017;36:62. doi: 10.1515/jomb-2016-0030. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong YW, Fonarow GC, Mi X. Mills RM, Curtis LH. Early intravenous heart failure therapy and outcomes among older patients hospitalized for acute decompensated heart failure: findings from the Acute Decompensated Heart Failure Registry Emergency Module (ADHERE-EM) Am Heart J. 2013;166:349. doi: 10.1016/j.ahj.2013.05.014. Peacock WF 4th. et al. –. [DOI] [PubMed] [Google Scholar]
- 26.Rahko PS. Acute Heart Failure in the Emergency Depart - ment: What Is the Prognosis? 21; Ann Intern Med. 2017;167:744. doi: 10.7326/M17-2389. –. [DOI] [PubMed] [Google Scholar]
- 27.De Denus S, White M, Tardif JC, Bourassa MG, Racine N, Levesque S. Temporal increases in subclinical levels of inflammation are associated with adverse clinical outcomes in patients with left ventricular dysfunction. J Card Fail. 2006;12:353. doi: 10.1016/j.cardfail.2006.02.014. et al. –. [DOI] [PubMed] [Google Scholar]
- 28.Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M. Prognostic Role of Serum Chloride Levels in Acute Decompensated Heart Failure. 11; J Am Coll Cardiol. 2015;66:659. doi: 10.1016/j.jacc.2015.06.007. et al. –. [DOI] [PubMed] [Google Scholar]
- 29.Amin A, Chitsazan M, Shiukhi Ahmad Abad F, Taghavi S, Naderi N. On admission serum sodium and uric acid levels predict 30 day rehospitalization or death in patients with acute decompensated heart failure. ESC Heart Fail. 2017;4:162. doi: 10.1002/ehf2.12135. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, Di Capua P. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987. doi: 10.1016/j.jacc.2005.11.084. et al. –. [DOI] [PubMed] [Google Scholar]
- 31.Noveanu M, Breidthardt T, Potocki M, Reichlin T, Tweren - bold R, Uthoff H. Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and long-term outcome in acute decompensated heart failure. Crit Care. 2011;15:R1. doi: 10.1186/cc9398. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Israr MZ, Heaney LM, Takaoka M, Squire IB, Ng LL. Prognostic Role of Molecular Forms of B-Type Natriuretic Peptide in Acute Heart Failure. Clin Chem. 2017;63:880. doi: 10.1373/clinchem.2016.265140. –. [DOI] [PubMed] [Google Scholar]
- 33.Lam LL, Cameron PA, Schneider HG, Abramson MJ, Muller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcomes in patients with acute dyspnea in the emergency setting. Annals of internal medicine. 2010;153:728. doi: 10.7326/0003-4819-153-11-201012070-00006. –. [DOI] [PubMed] [Google Scholar]
- 34.Trinquart L, Ray P, Riou B, Teixeira A. Natriuretic peptide testing in EDs for managing acute dyspnea: a metaanalysis. Am J Emerg Med. 2011;29:757. doi: 10.1016/j.ajem.2010.02.026. –. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez MB, Schwartz RS, Asher CR, Navas EV, Totfalusi V, Buitrago I. Predictors of 30-day readmission in patients hospitalized with decompensated heart failure. Clin Cardiol. 2013;36:542. doi: 10.1002/clc.22180. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson CE, Dalzell JR, Bezlyak V, Tsorlalis IK, Myles RC, Spooner R. Red cell distribution width has incremental prognostic value to B-type natriuretic peptide in acute heart failure. Eur J Heart Fail. 2009;11:1152. doi: 10.1093/eurjhf/hfp157. et al. –. [DOI] [PubMed] [Google Scholar]
- 37.Pascual-Figal DA, Bonaque JC, Redondo B, Caro C, Man zano-Fernandez S, Sánchez-Mas J. Red blood cell distribution width predicts long-term outcome re - gardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009;11:840. doi: 10.1093/eurjhf/hfp109. et al. –. [DOI] [PubMed] [Google Scholar]
- 38.van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Fail. 2010;12:129. doi: 10.1093/eurjhf/hfp179. Jr. –. [DOI] [PubMed] [Google Scholar]
- 39.Xanthopoulos A, Giamouzis G, Tryposkiadis K, Para ske - vo poulou E, Paraskevopoulou P, Karagiannis G. A simple score for early risk stratification in acute heart failure. Int J Cardiol. 2017;230:248. doi: 10.1016/j.ijcard.2016.12.131. et al. –. [DOI] [PubMed] [Google Scholar]
- 40.Dai Y, Konishi H, Takagi A, Miyauchi K, Daida H. Red cell distribution width predicts short- and long-term outcomes of acute congestive heart failure more effectively than hemoglobin. Exp Ther Med. 2014;8:600. doi: 10.3892/etm.2014.1755. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang Y, Kang JS, Yu JX, Yin SJ, Cong XF. Differences in the predictive value of red cell distribution width for the mortality of patients with heart failure due to various heart diseases. J Geriatr Cardiol. 2015;12:647. doi: 10.11909/j.issn.1671-5411.2015.06.001. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sotiropoulos K, Yerly P, Monney P, Garnier A, Regamey J, Hugli O. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Fail. 2016;3:198. doi: 10.1002/ehf2.12091. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhlestein JB, Lappe DL, Anderson JL, Muhlestein JB, Budge D, May HT. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30-day outcomes. Int J Lab Hematol. 2016;38:328. doi: 10.1111/ijlh.12490. et al. –. [DOI] [PubMed] [Google Scholar]
- 44.Turcato G, Zorzi E, Prati D, Ricci G, Bonora A, Zannoni M. Early in-hospital variation of red blood cell distribution width predicts mortality in patients with acute heart failure. Int J Cardiol. 2017;243:306. doi: 10.1016/j.ijcard.2017.05.023. et al. –. [DOI] [PubMed] [Google Scholar]


