Summary
Background
Oxidative stress has been associated with cervical cancer. Our aim was to examine lipid peroxidation and the extent of oxidative stress in women diagnosed with different stages of cervical cancer in order to evaluate its potential role in the evolution of cancer.
Methods
We measured the concentration of thiobarbituric acid reactive substances, activities of antioxidative enzymes and 8-hydroxy-2-deoxyguanosine in 153 subjects. Enzymatic activity as well as TBARS concentration were measured spectrophotometrically, while 8-OHdG was determined by gas chromatography-mass spectrometry. PPatients were categorized: group II H-SIL; group III FIGO Ia-Ib and group IV FIGO IIa-IV.
Results
Our results showed highly significant increase in the level of lipid peroxidation in group IV when com pared to the control group, group II and group III (p<0.001). Activity of superoxide dismutase was also significantly higher in group IV when compared to control group (p<0.01), group II (p<0.01) and group III (p<0.05). Activity of catalase was also significantly higher in group IV when compared to control group (p<0.005), group II (p<0.005) and group III (p<0.05). Activity of glutathione-S-transferase was also significantly higher in group IV when compared to control group (p<0.05), group II (p<0.05) and group III (p<0.05). Activities of glutathione peroxidase and glutathione reductase showed no significant differences among the groups. Level of 8-OHdG was significantly higher in group IV than in the other groups (p<0.01).
Conclusions
It can be concluded that oxidative stress is possibly involved in the pathogenesis of cervical cancer, demonstrated by increased lipid peroxidation and an altered antioxidant defense system and higher levels of 8-OHdG.
Keywords: 8-OHdG, catalase, glutathione peroxidase, glutathione reductase, glutathione-S-transferase, super oxide dismutase
Kratak sadržaj
Uvod
Utvrđeno je da postoji veza između oksidativnog stresa i cervikalnog karcinoma. Naš cilj bio je da ispitamo lipidnu peroksidaciju i nivo oksidativnog stresa kod žena kojima je dijagnostikovan cervikalni karcinom u različitim fazama da bi se otkrila njegova eventualna uloga u razvoju karcinoma.
Metode
Merili smo koncentraciju reaktivnih supstanci tiobarbiturne kiseline, aktivnosti antioksidativnih enzima i 8-hidroksi-2-deoksigvanozina kod 153 ispitanice. Enzimska aktivnost kao i koncentracija TBARS merene su spektrofotometrijski, dok je 8-OhdG određen pomoću gasne hromatografije – masene spektrometrije. Pacijentkinje su podeljene u kategorije: grupu II H-SIL; grupu III FIGO Ia-Ib i grupu IV FIGO IIa-IV.
Rezultati
Naši rezultati pokazali su vrlo značajan porast nivoa lipidne peroksidacije u grupi IV u poređenju s kontrolnom grupom, grupom II i grupom III (p<0,001). Aktivnost superoksid-dismutaze bila je takođe značajno viša u grupi IV u poređenju s kontrolnom grupom (p<0,01), grupom II (p<0,01) i grupom III (p<0,01). Aktivnost katalaze bila je isto značajno viša u grupi IV u poređenju s kontrolnom grupom (p<0,005), grupom II (p<0,005) i grupom III (p<0,05). Aktivnost glutation-S-transferaze je takođe bila značajno viša u grupi IV u poređenju s kontrolnom grupom (p<0,05), grupom II (p<0,05) i grupom III (p<0,05). Aktivnosti glutation peroksidaze i glutation reduktaze nisu ukazale na značajne razlike između grupa. Nivo 8-OHdG bio je značajno viši u grupi IV nego u ostalim grupama (p<0,01).
Zaključak
Može se zaključiti da oksidativni stres potencijalno učestvuje u patogenezi cervikalnog karcinoma, na šta ukazuju povišena lipidna peroksidacija i promene su sistemu antioksidativne zaštite i povišen nivo 8-OHdG.
Ključne reči: 8-OHdG, katalaza, glutation peroksidaza, glutation reduktaza, glutation-S-transferaza, superoksid dismutaza
Introduction
Cervical cancer is the second most common cancer in women, and the seventh overall (1). Risk factors for cervical cancer include: early age at first sexual intercourse, number of sexual partners, malnutrition, smoking, long time use of oral contraceptives and, most importantly, Human Papilloma Virus infection (2). Recent data revealed the role of oxidative stress in cervical cancer (3).
The development of cervical cancer at first begins with precancerous lesions, either low squamous intraepithelial lesions (L-SIL) or the more advanced high squamous intraepithelial lesion (H-SIL). These lesions can develop further into a neoplasia (4).
Reactive oxygen species (ROS) tend to pair with adjacent molecules, which can be lipids, proteins and DNA (5). On the other hand, antioxidants serve as scavengers of ROS. In a healthy system, ROS and anti oxidants are in a state of balance. However, when the quantity of ROS exceeds that of antioxidants, the system enters a state of oxidative stress (6). Antioxidative enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST), maintain redox balance. Their activity can be used to estimate the oxidative defense mechanism strength. Antioxidative enzymes inhibit both the initiation and promotion of carcinogenesis (7).
As a result of interaction between the ROS and lipids in membranes, the process of lipid peroxidation leads toward decreased fluidity, increased permeability and inactivation of membranes, which may be one of the possible reasons for the progression of the cervical carcinoma. A degradation product of lipid peroxidation called malonodialdehyde (MDA) is highly cytotoxic and acts as a tumor promoter and a carcinogenesis inductor (8, 9, 10).
8-OHdG is one of the most abundant oxidatively modified lesions in DNA and it represents a marker of oxidative stress. Among the bases, guanine is the most susceptible DNA target. 8-OHdG may be induced by hydroxyl radical or singlet oxygen. It is a mutagenic lesion, which can be mispaired with adenine. In the next round of replication, wrongly paired adenine will pair with thymidine, therefore causing G:C- > T:A transversion (11).
The aim of our study was to assess the activity of the enzymes included in the antioxidant defense of the organism and to determine the levels of lipid peroxidation in the blood and plasma, as well as the level of 8-OHdG in women with different stages of cervical cancer and in healthy women. Very few studies have investigated any correlations between free radical damage and different stages of cervical cancer as defined by the FIGO staging/grading system. Therefore, the present study was designed to evaluate the possible involvement of oxidative stress during the progression of cervical carcinoma from the premalignant to the malignant state.
Materials and Methods
Study groups
The present study included 153 subjects. Out of 153 subjects, 32 were controls. Inclusion criteria for the control group were: no cervical pathology (confirmed with healthy PAP smear) nor any other cancer, no previous interventions on the cervix, older than 18 years, signed written consent and negative pregnancy test. The 121 patients were newly diagnosed patients with HSIL or cervical cancer. Inclusion criteria for the patient groups were: histological confirmation of patient belonging to a defined group, older than 18 years, signed written consent, negative pregnancy test, ECOG 0-1, negative for hepatitis C and B, and HIV virus. Exclusion criteria for the patient groups were: previous history of cancer, relapse, previous interventions on the cervix, previous radio- or chemotherapy, acute inflammatory processes in the body. Patients were categorized according to their stages as follows: group II H-SIL (37 patients), settled for conization as treatment; group III FIGO Ia-Ib (39 patients), treated with radical hysterectomy and group IV FIGO IIa-IV (45 patients), treated with concomitant chemo-radio -therapy.
The protocol of informed consent was approved by the Ethical Committee of the Oncology Institute of Vojvodina. Samples were collected from December 2013 to April 2016.
Blood collection and erythrocyte lysate preparation
Five milliliter blood samples were drawn by venipuncture into EDTA tubes. An amount of 400 μL of blood was used for the analysis of GPx. One mL of blood samples was centrifuged for 10 min at 3000 rpm. After removal of plasma, red blood cells were washed thrice with physiologic saline. Hemolysates were prepared by the addition of ice-cold distilled deionized water to the erythrocytes up to 4 mL, and used for the analysis of SOD, CAT, GR and GST.
The remaining blood samples were centrifuged for 10 min at 3000 rpm, and plasma samples were separated for the analysis of MDA.
Laboratory analyses
All chemicals used in this study were of analytical grade and purchased from Sigma Aldrich. Patients’ blood was analyzed on Beckman Coulter’s automated hematology analyzer. Concentration of MDA and activity of antioxidative enzymes were measured on an Agilent 8453 UV-visible spectrophotometar, Biochem Analysis UV/Vis SW. The concentration of 8-OHdG was determined by gas chromatography with mass detection (GC-MS) using the Agilent GC 7890A, 5975C VL MSD device. The 8-OHdG identification was performed using commercial mass spectrometric libraries (Fiehn.L and NIST8.L) and confirmed by the use of the AMDIS software package and characteristic ions m/z 383 (T); 368 (Q1) and 311 (Q2). (T-target; Q1 and Q2-qualifier ions).
Superoxide dismutase
Total (Cu-Zn and Mn) SOD activity measuring method was based on the xanthine/xanthine oxidase system (12). One unit of SOD was defined as the enzyme amount causing 50% inhibition in the cytochrome C reduction rate and the results were expressed as U/g Hgb.
Catalase
CAT activity was measured by the method of Aebi (13) after dilution of the sample with 50 mmol/L phosphate buffer, pH 7.00, just before the measurements. The reaction mixture was 50 mmol/L phosphate buffer pH 7.00, 30% H2O2, and 10 μL erythrocyte lysate. The reduction rate of H2O2 was followed at 240 nm for 3 min at 25 °C. CAT activity was expressed in U/g Hgb.
Glutathione reductase
Glutathione reductase (GR) was determined by measuring the reduction rate of oxidized glutathione (GSSG) with NADPH as a suitable enzyme substrate at 340 nm (14). Activity of GR was defined as nmol of NADPH/min/g Hgb.
Glutathione peroxidase
GPx activity was determined in erythrocyte lysate after dilution (14). The reaction mixture was 1 mol/L Tris buffer, pH 8.00, containing 5 mmol/L of Na2EDTA, 0.1 mol/L reduced glutathione (GSH), 2 mmol/L NADPH, 250 IU/mL of glutathione reductase (GR). Reaction mixture was incubated for 10 min at 37 °C. Then the reaction was initiated with 7 mmol/L T-butylhydroper oxide. Formation rate of oxidized glutathione (GSSG) and concomitant oxidation of NADPH to NADP+ were monitored spectroscopically at 340 nm. Activity of GSH-Px was defined as nmol of NADPH/min/g Hgb.
Glutathione-S-transferase
Determination of glutathione-S-transferase (GST) was based on conjugation of the –SH group of reduced glutathione with 1-chloro-2,4-dinitrobenzene (CDNB) (14). The measurement of GST enzyme activity was performed with 20 mmol/L GSH as the first, and 25 mmol/L CDNB as the electrophilic second substrate in 0.5 mol/L potassium phosphate buffer, pH 6.50. Absorbance of the conjugate CDNB-glutathione was measured at 340 nm. Activity of GST was expressed as nmol of CDNB-glutathione conjugate/min/g Hgb.
Thiobarbituric acid reactive substance
Lipid peroxidation was estimated by the formation of thiobarbituric acid reactive substances (TBARS), as described by Draper et al. (15). The samples were deproteinised with 15% trichloroacetic acid and then treated with 0.375% thiobarbituric acid. The mixture was heated in a boiling water bath for 15 minutes. It was then cooled to room temperature, centrifuged at 3500 rpm for 10 min and the developed pink color was measured at 535 nm. The values are expressed as nmol of MDA/L.
8-OHdG
Three mL of urine sample was centrifuged at 3500 rpm, and then 1 mL of supernatant was taken for the analysis. After the column has been conditioned, 1 mL of sample was injected in the column. Afterwards, colon was washed and centrifuged at 3500 rpm. Analyte was eluated with methanol. Be fore the analysis, eluate was quantitatively transferred in GC-inserts and evaporated and after re-dissolution derivatized. Derivatization was carried out by dissolving the residue in 20 μL of acetonitrile (HPLC grade) and adding 20 μL of bis-trimethylsilyl trifluoroacetamide (BSTFA) and letting sit for at least 30 minutes at room temperature, after which it was analyzed by GC-MS (EI ionization). The values are expressed as concentration of 8-OH2dG/mg creatitine.
Statistical analysis
The statistical analysis included parametric and nonparametric methods: Student's t-test, analysis of variance, Mann Whitney test, Kruskal Walis test, and the chi-square test. For variables with normal distribution, comparison between groups was performed by analysis of variance, followed by, if necessary, Duncan’s multiple comparison test. For variables that do not have normal distribution, comparison between groups was performed using the Kruskal Wallis test, followed by, if necessary, multiple comparison test of medium ranges. P-values at the level of 0.05 were considered statistically significant. For statistical analysis, Microsoft Excel 2007 was used with Statistica 13 software package (StatSoft Inc., Tulsa, OK, USA), University License University of Novi Sad.
Sample size for the detection of effect size of 0.33 in variance analysis with four groups for the lipid peroxidation, SOD, CAT, GST, GPx and GR variables for the statistical significance level of 0.05 and the statistical power of 0.8 is 104. The effect size was obtained on the basis of the predicted relationship between the values of explained and the residual variance from 0.1 to 0.9. Sample size calculation was done using G-Power 3.1.9.2
Canonical discriminant analysis (CDA) was used for assessment of discrimination between the examined groups of patients based on the values obtained for markers of oxidative stress, while the determination of resemblance between the groups was performed by hierarchical cluster analysis based on Mahalanobius distances.
Results
Characteristics of the patients and controls are given in the Table I.
Table I.
Characteristics of the patients and controls.
| Characteristics | Number | Mean age±SD |
|---|---|---|
| Total Count | 153 | 44.6±12.8 |
| Group I | 32 | 33.6±11.3 |
| Group II | 37 | 40.5±10.2 |
| Group III | 39 | 48.9±10.2 |
| Group IV | 45 | 52.2±10.9 |
| FIGO Stage | Percentage | |
| H-SIL | 37 | 30.6 |
| Ia1 | 2 | 1.6 |
| Ia2 | 1 | 0.8 |
| Ib1 | 19 | 15.7 |
| Ib2 | 11 | 9.1 |
| IIa | 3 | 2.5 |
| IIa2 | 3 | 2.5 |
| IIb | 31 | 25.6 |
| IIIb | 9 | 7.4 |
| IVa | 3 | 2.5 |
| IV | 2 | 1.6 |
| Histopathological features | ||
| Planocellular | 78 | 66.0 |
| Adenosquamous | 6 | 5.1 |
| Lymphovascular invasion | ||
| Absent | 16 | 41.0 |
| Present | 23 | 28.9 |
| Treatment modalities | ||
| Conization | 37 | 30.6 |
| Radical hysterectomy | 39 | 32.2 |
| Radiotherapy | 45 | 37.2 |
Lipid peroxidation
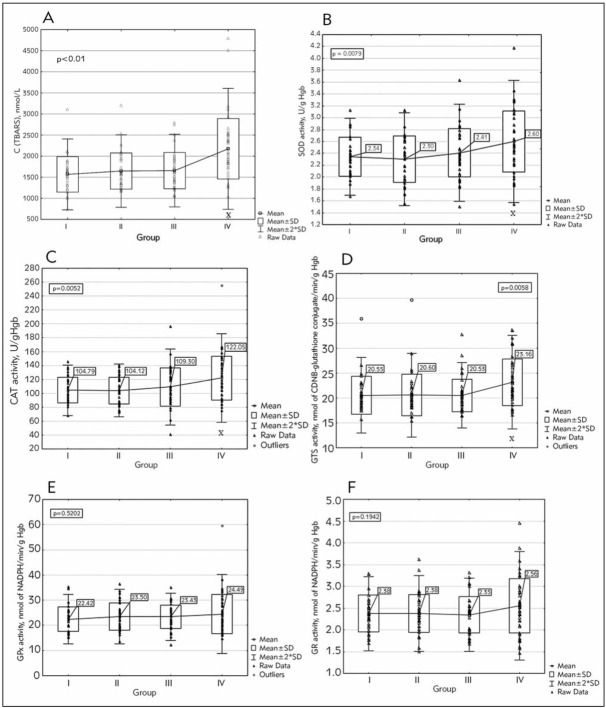
Data obtained in this study demonstrated moderate elevation of lipid peroxidation in group II and III, with a tendency to increase with the extent of severity of the lesion, while it increased significantly in patients with locally advanced disease (Figure 1A). Statistically significant differences are between the control group and the group with advanced disease (p<0.001) as well as between the second and fourth (p<0.001) and third and fourth groups (p<0.001).
Figure 1.
(A) Level of lipid peroxidation, Activity of (B) SOD (C) CAT (D) GST (E) GPx (F) GR in cervical cancer patients I – control group; II – HSIL; III – FIGO Ia-Ib; IV – FIGO IIa-IV; Kruskal Wallis test used. X p<0.01 vs other groups
SOD activity
Tumor progression is followed by an increase in SOD activity resulting in a significant increase when the fourth group was compared with the other three groups. Statistically significant differences are between the control group and group with advanced disease (p<0.01) as well as between the second and fourth (p<0.01) and third and fourth group (p<0.05) (Figure 1B).
CAT activity
It is obvious from Figure 1C that the activity of CAT is significantly increased in patients with advanced disease comparing to the other three groups. Progression of the disease is followed by an increase in CAT activity. Statistically significant differences are between the control group and group with advanced disease (p<0.005) as well as between the second and fourth (p<0.005) and third and fourth group (p<0.05).
GST activity
Statistically significant differences are between the control group and group with advanced disease (p<0.05) as well as between the second and fourth (p<0.05) and third and fourth group (p<0.05) (Figure 1D). It is evident from Figure 1D that there is a highly significant difference in GST activity between the group of patients with advanced disease and all the other examined groups. Progression of the disease is followed by an increase in GST activity, but without a significant difference, when those three groups were compared.
GPx activity
There were no significant differences in the activities of GPx, although an increasing pattern was observed between the group of patients with advanced disease and other groups (Figure 1E).
GR activity
There were no significant differences in the activities of GP, although an increasing pattern was observed between the group of patients with advanced disease and other groups (Figure 1F).
Level of 8-OHdG
We observed statistically significant differences between the control group and group with advanced disease (p<0.01) and evident, but not statistically significant differences between the second and fourth and third and fourth group (Figure 2).
Figure 2.
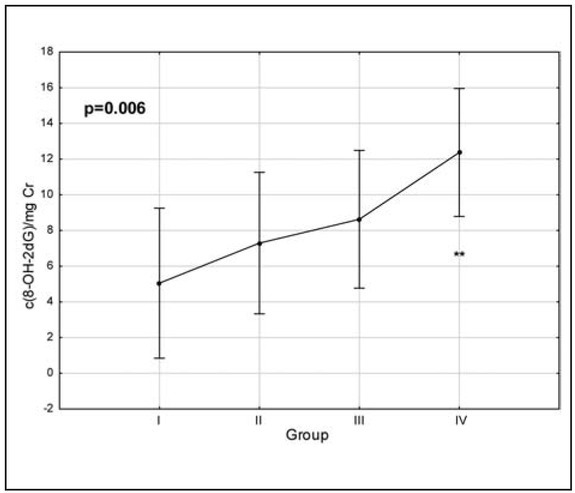
Level of 8-OHdG in cervical cancer patients. I – control group; II – HSIL; III – FIGO Ia-Ib; IV – FIGO IIa- IV; Kruskal Wallis test used. **p<0.01 vs control group (I)
Canonical discriminant analysis was applied to the variables describing the markers of oxidative stress in patients. The results have shown that the first two discriminant functions describe more than 98% of discriminations between the groups. Furthermore, in a term of the first discriminant function, separation of different groups occurs as a result of determined lipid peroxidation level, while in the case of second discriminant function, separation of groups occurs as a result of determined levels of superoxide dismutase (Table II). The position of the evaluated patients in the space defined by the first two canonical axes suggests no possible separation of groups based on the levels of determined oxidative stress in patients because of the high variability of the obtained results (Figure 3A). However, it can be concluded that patients belonging to the first three groups are positioned mostly in the positive part of CA1 as a result of lower levels of determined lipid peroxidation. On the other hand, a large number of patients belonging to the group IV is localized in the negative part of CA1 as a result of higher levels of LP. Performed Hierarchical cluster analysis Figure 3B) reveals the resemblance of groups I, II and III based on the determined levels of oxidative stress, as well as a segregation of group IV as a separate cluster.
Table II.
Loadings of the first two canonical axes.
| Variable | CA1 | CA2 |
|---|---|---|
| LP | -0.842325 | 0.138900 |
| SOD | -0.207943 | -0.759528 |
| CAT | -0.182681 | -0.435075 |
| GST | -0.153091 | 0.640474 |
| GPX | 0.162314 | 0.062417 |
| GR | -0.041366 | 0.375422 |
| Eigenval | 0.368662 | 0.024604 |
| Cum.Prop | 0.921399 | 0.982892 |
Figure 3A.
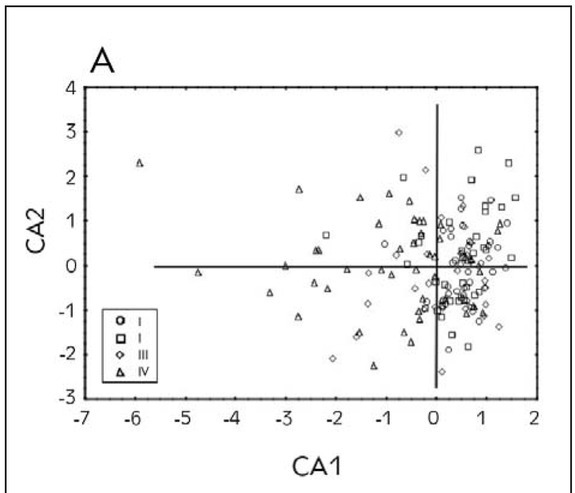
The position of the evaluated patients in the space defined by first two canonical axes.
Figure 3B.
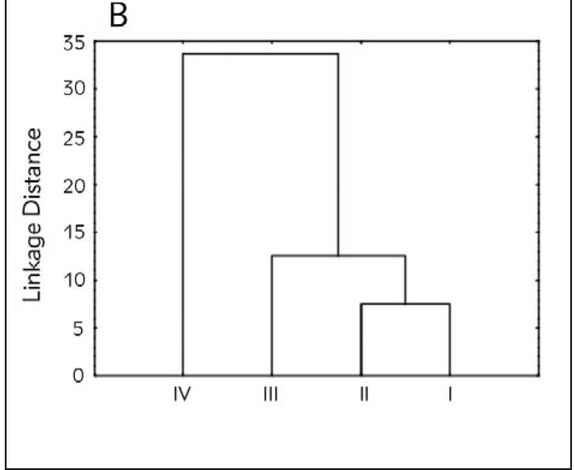
Cluster analysis diagram.
Discussion
Chronic inflammation due to cervical trauma, bacteria and viruses, especially human papilloma virus (HPV) is one of the predominant factors in the etiopathogenesis of cervical cancer. Chronic inflammation results in the activation of the monocyte/ macro phage system which produces high levels of ROS. Sustained high levels of ROS cause genetic damage to the cervical epithelium, leading to transformation of cells and initiation of cervical cancer. Subsequent steps, such as promotion and progression of these initiated cells, are also related to the oxidant/antioxidant milieu in humans. Thus, it is possible that HPV infection induces an oxidative stress in cervical cancer (16).
The present study showed that erythrocyte TBARS levels, as markers of oxidative stress, were higher in all the examined groups than in control, and were significantly elevated in women with advanced cervical cancer. A number of studies show similar results (9, 10, 17, 18, 19, 20). On the other hand, a study by Beevi SS et al. (21) showed no significant differences in MDA levels according to the stage of the disease. Authors that reported higher MDA values in patients with CIN claimed oxidative injury as a prominent factor even in the early steps of carcinogenesis (10, 22). The fact that oxygen radical production, which elevates lipid peroxidation, increases with the progression of the disease may indicate that patients with advanced cervical carcinoma have a wider extent of cellular membrane degeneration than the patients in lower stages (22). Therefore, lipid peroxidation may be one possible cause of cervical cancer progression. It has also been suggested that an increase in lipid peroxidation would cause degeneration of tissues and that lipid peroxidation formed at the primary sites would be transferred through the circulation to other tissues and provoke damage by propagating the process of lipid peroxidation (23).
Antioxidant enzyme activities have shown different patterns in different types of cancer (24). Studies which have shown lower levels of antioxidant enzymes hypothesize that they have been more utilized in the presence of the disease. Other studies, showing higher activity levels, explain that the enzymes are more active as the disease progresses, since there is a bigger lesion to be »repaired«.
Superoxide dismutase (SOD) plays an important role in protection against lipid peroxidation. It is present in high amounts in erythrocytes, and catalyzes the dismutation of superoxide anions into oxygen and hydro gen peroxide. SOD increases its activity compensatively when there is excessive production of superoxide anions. Inflammation in the cervix may contribute to high levels of SOD, which may lead to increased intracellular hydrogen peroxide, thereby creating an environment favorable to DNA damage and the promotion of cancer. Results from our study showed that SOD activity in blood was increased in all examined patient groups compared to healthy subjects, with statistical significance for patients in the advanced disease group. Demirci et al. (25) also showed an in crease in SOD activity in cervical cancer patients, compared to the control group. Decreased SOD activity was reported by Balasubramaniyan et al. (20) in cervical tissues (16) and by Srivastava et al. (26) in blood. They proposed the relation of prolonged exposure to oxidative stress and consumption of activated enzymes. Chiou and Hu (10), Bhuvaraharamurthy et al. (19), Manoharan et al. (27) associated it with the damage to the enzyme caused by ROS, or deficiency of trace elements like zinc and manganese which are cofactors for SOD.
Increased generation of ROS such as O2 and H2O2 can also induce CAT. Changes in CAT are closely correlated with alterations in SOD activity. An increase in CAT activities has been reported in breast cancer, tumor cell lines, uterine cancer as well as cervical cancer (28, 29, 30, 31). Our results lend credence to these reports. One possible explanation may be that as tumor burden increases, CAT activity increases in order to defend cells from the increasing oxidative stress caused by tumor environment. Chiou et al. (10) reported that changes in antioxidant activity may be due to the type or extent of tissue damage. Reduced CAT activity was found in the study of Balasubramaniyan et al. (20) in the cervical tissues and in several studies in erythrocytes, in those cases, following the decrease of SOD (16, 21, 27, 32).
GPx catalyzes the conversion of glutathione (GSH) to its oxidized form, glutathione disulfide (GSSG), while GR catalyzes the reduction of GSSG to GSH, regenerating it again (33, 34). They are secondary antioxidant enzymes and play a prominent role in the protection of cells against many cytotoxic and carcinogenic chemicals. GST activity is increased in most human cancers and may serve as a marker for neoplasms (21, 35).
Increased activity of GST has been demonstrated in tumors of the brain and breast as well as in carcinoma of the uterine cervix. Authors of these studies argued that increase in GSH concentration is necessary to restore the sufficient concentration of antioxidants and to stimulate the scavenger enzymes indispensable to counteract the damaging actions of free radicals (20). We observed a significant increase in GST activity which could be considered as an adaptive response to oxidative stress.
Several studies reported lower activity of GPx and GR in patients than in healthy controls (18, 20). Di Ilio et al. (36) reported higher GPx activity in human breast tumor tissue than in non-tumor tissue (37). GPx levels are increased mainly due to the response of increased free radical production. Since the H2O2 is usually detoxified with the help of GPx and CAT, it can be expected that CAT and GPx are also higher. However, results from our study showed that activity of GPx and GR did not vary with worsening of the disease. Blunt and Fridovich have found that GPx activity may be inactivated in oxidative stress conditions by superoxide anions (36, 38). In our study activity of SOD was increased, which in turn lead to higher levels of superoxide anions that could inactivate GPx. Since there is not much utilization of GSH, there is no activity of GR as well, so these two enzymes didn’t have the impact on oxidative defense as the others.
When 8-OHdG level was assessed in human cervical cells and was related to the presence of human papilloma virus (HPV) infection and precancerous lesions, the analysis showed significant differences in the 8-OHdG content among normal, low-grade (LSIL) and high-grade squamous intraepithelial lesions (HSIL). In the comparison of the three groups, statistically significant differences were detected between normal SIL and HSIL and between LSIL and HSIL, whereas no statistically significant difference was found between normal SIL and LSIL. Concerning HPV status, no significant difference was detected in 8-OHdG levels between HPV+ and HPV– subjects. These results are clear evidence that significant differences exist in 8-OHdG content between normal and dysplastic cells and that oxidative DNA damage might play an important role in cervical carcinogenesis (39). On the other hand, in the study that compared urinary 8-OHdG in patients with SIL and squamous cell carcinoma of the cervix with normal controls, there were no significant changes in any studied disease status as compared with control (40). In our study, level of 8-OHdG tended to increase with the higher stage of the disease, suggesting its role in cervical carcinogenesis. Similarly, Sgambato et al. (4) observed pro gressive significant increase in the levels of 8-OHdG from LSIL to HSIL to invasive carcinomas. They suggested that alteration of this parameter at early stages of the process might help to predict patients at high risk of progression (4).
Conclusion
Increased levels of antioxidative enzymes may represent the adaptive mechanism of the cell, since the excessive ROS production mediated by tumor burden may increase the transcription of genes coding for these antioxidative enzymes. Higher values of SOD, CAT and GST in patients with locally advanced cervical carcinoma could also be explained by in creased gene expression and/or upregulation of these enzymes as a response to oxidative damage. Oxidative stress increases significantly in malignancy since cancer cells themselves produce oxidants which was confirmed in our study by the increased level of lipid peroxidation. Increased rate of free radical production frequently elicits an increase in the level of antioxidant enzymes under a high rate of free radicals input. Taken together, altered antioxidative enzymes activity, higher lipid peroxidation and higher level of 8-OHdG reflect the oxidative stress in cervical carcinoma, and this becomes more pronounced in advanced stages due to the increased tumor burden.
Acknowledgment
The Ministry of Science and Technological Development, Republic of Serbia (Grant Number OI 172058) supported this research work.
List of abbreviations
- CAT,
catalase
- GPx,
glutathione peroxidase
- GR,
glutathione reductase
- GST,
glutathione-S-transferase
- SOD,
superoxide dismutase
- 8-OHdG,
8-hydroxy-2-deoxyguanosine
- TBARS,
thiobarbituric acid reactive substances.
Footnotes
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69. doi: 10.3322/caac.20107. –. [DOI] [PubMed] [Google Scholar]
- 2.Jiang B, Xiao S, Khan MA, Xue M. Defective antioxidant systems in cervical cancer. Tumour Biol. 2013;34(4):2003. doi: 10.1007/s13277-013-0804-1. –. [DOI] [PubMed] [Google Scholar]
- 3.Notani PN. Global variation in cancer incidence and mortality. Curr Sci. 2001;81(5):465. –. [Google Scholar]
- 4.Sgambato A, Zannoni GF, Faraglia B, Camerini A, Tar - quini E, Spada D. Decreased expression of the CDK in hibitor p27Kip1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gy - necol Oncol. 2004;92(3):776. doi: 10.1016/j.ygyno.2003.12.008. et al. –. [DOI] [PubMed] [Google Scholar]
- 5.Rice-Evans C, Burdon R.. Free radical-lipid interactions> and their pathological consequences. Prog Lipid Res. 1993;32(1):71. doi: 10.1016/0163-7827(93)90006-i. –. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44. doi: 10.1016/j.biocel.2006.07.001. –. [DOI] [PubMed] [Google Scholar]
- 7.Gutteridge JM, Halliwell B. Antioxidants: Molecules, me - dicines, and myths. Biochem Biophys Res Commun. 2010;393(4):561. doi: 10.1016/j.bbrc.2010.02.071. –. [DOI] [PubMed] [Google Scholar]
- 8.Ozderin OY, Akpinar MY, Topcuoglu C, Kayaçetin E. The role of oxidative stress in the etiopathogenesis of gluten-sensitive enteropathy disease. J Med Biochem. 2017;36:243. doi: 10.1515/jomb-2017-0017. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolanjiappan K, Manoharan S, Kayalvizhi M. Measure - ment of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin Chim Acta. 2002;326(1):143. doi: 10.1016/s0009-8981(02)00300-5. –. [DOI] [PubMed] [Google Scholar]
- 10.Chiou JF, Hu ML. Elevated lipid peroxidation and disturbed antioxidant enzyme activities in plasma and eryth - rocytes of patients with uterine cervicitis and myoma. Clin Biochem. 1999;32(3):189. doi: 10.1016/s0009-9120(98)00110-6. –. [DOI] [PubMed] [Google Scholar]
- 11.Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem. 2006. p. 373. p. [DOI] [PubMed]
- 12.McCord JM, Fridovich I. Superoxide dismutase. An en - zymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049. –. [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121. doi: 10.1016/s0076-6879(84)05016-3. –. [DOI] [PubMed] [Google Scholar]
- 14.Weissman SM. Red Cell Metabolism. A Manual of Bio - chemical Methods. 2nd Edition. Yale J Biol Med. 1976;49(3):310. –. [Google Scholar]
- 15.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421. doi: 10.1016/0076-6879(90)86135-i. –. [DOI] [PubMed] [Google Scholar]
- 16.Grace Nirmala J, Narendhirakannan RT. Detection and genotyping of high-risk HPV and evaluation of anti-oxidant status in cervical carcinoma patients in Tamil Nadu State, India--a case control study. Asian Pac J Cancer Prev. 2011;12(10):2689. –. [PubMed] [Google Scholar]
- 17.Manju V, Kalaivani Sailaja J, Nalini N. Circulating lipid peroxidation and antioxidant status in cervical cancer patients: a case-control study. Clin Biochem. 2002;35(8):621. doi: 10.1016/s0009-9120(02)00376-4. –. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Kim JW, Ko YS, Koo JE, Chung HY, Lee-Kim YC. Changes in Lipid Peroxidation and Antioxidant Trace Ele - ments in Serum of Women With Cervical Intra epithelial Neoplasia and Invasive Cancer. Nutr Cancer. 2003;47(2):126. doi: 10.1207/s15327914nc4702_3. –. [DOI] [PubMed] [Google Scholar]
- 19.Bhuvarahamurthy V, Balasubramanian N, Govindasamy S. Effect of radiotherapy and chemoradiotherapy on circulating antioxidant system of human uterine cervical carcinoma. Mol Cell Biochem. 1996;158(1):17. doi: 10.1007/BF00225878. –. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramaniyan N, Subramanian S, Govindasamy S. Status of antioxidant systems in human carcinoma of uterine cervix. Cancer Lett. 1994;87(2):187. doi: 10.1016/0304-3835(94)90221-6. –. [DOI] [PubMed] [Google Scholar]
- 21.Beevi SS, Rasheed MH, Geetha A. Evidence of oxidative and nitrosative stress in patients with cervical squamous cell carcinoma. Clin Chim Acta. 2007;375(1-2):119. doi: 10.1016/j.cca.2006.06.028. –. [DOI] [PubMed] [Google Scholar]
- 22.Lee GJ, Chung HW, Lee KH, Ahn HS. Antioxidant Vita - mins and Lipid Peroxidation in Patients with Cervical Intraepithelial Neoplasia. J Korean Med Sci. 2005;20(2):267. doi: 10.3346/jkms.2005.20.2.267. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Rajappa M, Satyam A, Sharma M. Oxidant/ anti-oxidant dynamics in patients with advanced cervical cancer: correlation with treatment response. Mol Cell Biochem. 2010;341(1):65. doi: 10.1007/s11010-010-0437-2. –. [DOI] [PubMed] [Google Scholar]
- 24.Hristozov D, Gadjeva V, Vlaykova T, Dimitrov G. Evaluation of oxidative stress in patients with cancer. Arch Physiol Biochem. 2001;109(4):331. doi: 10.1076/apab.109.4.331.4248. –. [DOI] [PubMed] [Google Scholar]
- 25.Demirci S, Ozsaran Z, Celik HA, Aras AB, Aydin HH. The interaction between antioxidant status and cervical cancer: a case control study. Tumori. 2011;97(3):290. doi: 10.1177/030089161109700306. –. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava S, Natu SM, Gupta A, Pal KA, Singh U, Agarwal GG. Lipid peroxidation and antioxidants in different stages of cervical cancer: Prognostic significance. Indian J Cancer. 2009;46(4):297. doi: 10.4103/0019-509X.55549. et al. –. [DOI] [PubMed] [Google Scholar]
- 27.Manoharan S, Kolanjiappan K, Kayalvizhi M. Enhanced lipid peroxidation and impaired enzymic antioxidant activities in the erythrocytes of patients with cervical carcinoma. Cell Mol Biol Lett. 2004;9(4a):699. –. [PubMed] [Google Scholar]
- 28.Kumaraguruparan R, Subapriya R, Viswanathan P, Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2002;325(1-2):165. doi: 10.1016/s0009-8981(02)00292-9. –. [DOI] [PubMed] [Google Scholar]
- 29.Chung-man Ho J, Zheng S, Comhair SA, Farver C, Erzu - rum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61(23):8578. –. [PubMed] [Google Scholar]
- 30.Wozniak B, Mila-Kierzenkowska C, Kedziora-Korna towska K, Drewa T, Drewa G, Wozniak A. Influence of the management of cervical carcinoma on the activity of cata lase and glutathione peroxidase in erythrocytes. Eur J Gynaecol Oncol. 2007;28(6):461. et al. –. [PubMed] [Google Scholar]
- 31.Mila-Kierzenkowska C, Kedziora-Kornatowska K, Woz - niak A, Drewa T, Wozniak B, Drewa S. The effect of brachytherapy on antioxidant status and lipid peroxidation in patients with cancer of the uterine cervix. Cell Mol Biol Lett. 2004;9(3):511. et al. –. [PubMed] [Google Scholar]
- 32.Sharma A, Rajappa M, Saxena A, Sharma M. Antioxidant status in advanced cervical cancer patients undergoing neoadjuvant chemoradiation. Br J Biomed Sci. 2007;64(1):23. doi: 10.1080/09674845.2007.11732751. –. [DOI] [PubMed] [Google Scholar]
- 33.Bhabak KP, Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res. 2010;43(11):1408. doi: 10.1021/ar100059g. –. [DOI] [PubMed] [Google Scholar]
- 34.Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6(2):289. doi: 10.1089/152308604322899350. –. [DOI] [PubMed] [Google Scholar]
- 35.Niitsu Y, Takahashi Y, Saito T, Hirata Y, Arisato N, Maru - yama H. Serum glutathione-S-transferase-pi as a tumor marker for gastrointestinal malignancies. Cancer. 1989;63(2):317. doi: 10.1002/1097-0142(19890115)63:2<317::aid-cncr2820630219>3.0.co;2-p. et al. –. [DOI] [PubMed] [Google Scholar]
- 36.di Ilio C, Sacchetta P, del Boccio G, la Rovere G, Federici G. Glutathione peroxidase, glutathione S-transferase and glutathione reductase activities in normal and neoplastic human breast tissue. Cancer Lett. 1985;29(1):37. doi: 10.1016/0304-3835(85)90120-x. –. [DOI] [PubMed] [Google Scholar]
- 37.Polidoro G, Di Ilio C, Sacchetta P, Del Boccio G, Federici G. Isoelectric focusing of brain cortex GSH S-transferase activity in mammals: evidence that polymorphism is absent in man. Int J Biochem. 1984;16(7):741. doi: 10.1016/0020-711x(84)90184-8. –. [DOI] [PubMed] [Google Scholar]
- 38.Terzi S, Dursun E, Yılmaz A, Cos¸kun ZÖ, Ozgur A, Celiker M, Demirci M. Oxidative stress and antioxidant status in patients with bell’s palsy. J Med Biochem. 2017;36:18. doi: 10.1515/jomb-2016-0033. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romano G, Sgambato A, Mancini R, Capelli G, Giovag - noli MR, Flamini G. 8-hydroxy-2 -deoxyguanosine in cervical cells: correlation with grade of dysplasia and human papillomavirus infection. Carcinogenesis. 2000;21(6):1143. et al. –. [PubMed] [Google Scholar]
- 40.Looi ML, Mohd Dali AZ, Md Ali SA, Wan Ngah WZ, Mohd Yusof YA. Oxidative damage and antioxidant status in patients with cervical intraepithelial neoplasia and carcinoma of the cervix. Eur J Cancer Prev. 2008;17(6):555. doi: 10.1097/CEJ.0b013e328305a10b. –. [DOI] [PubMed] [Google Scholar]