Summary
Background
Acute lymphoblastic leukemia is the most common childhood malignancy. Optimal use of anti leukemic drugs has led to less toxicity and adverse reactions, and a higher survival rate. Thiopurine drugs, including 6-mercaptopurine, are mostly used as antileukemic medications in the maintenance phase of treatment for children with acute lymphoblastic leukemia. For those patients, TPMT genotype- tailored 6-mercaptopurine therapy is already implemented in the treatment protocols. We investigated the role of TPMT, ITPA, ABCC4 and ABCB1 genetic variants as predictors of outcome and 6-mercaptopurine induced toxicity during the maintenance phase of treatment in pediatric acute lymphoblastic leukemia.
Methods
Sixty-eight children with acute lymphoblastic leukemia were enrolled in this study. Patients have been treated according to ALL IC-BFM 2002 or ALL IC-BFM 2009 protocols. Toxicity and adverse events have been monitored via surrogate markers (off-therapy weeks, episodes of leu - ko penia and average 6-mercaptopurine dose) and a prob- abilistic model was employed to predict overall 6-mercaptopurine related toxicity.
Results
We confirmed that patients with acute lymphoblastic leukemia that carry inactive TPMT allele(s) require 6- mercaptopurine dose reduction. ITPA and ABCC4 genetic variants failed to show an association with 6-mercapto - purine induced toxicity during the maintenance phase. Carriers of ABCB1 variant allele experienced greater hepatotoxicity. The probabilistic model Neural net which considered all the analysed genetic variants was assessed to be the best prediction model. It was able to discriminate ALL patients with good and poor 6-mercaptopurin tolerance in 71% of cases (AUC=0.71).
Conclusions
This study contributes to the design of a panel of pharmacogenetic markers for predicting thiopurineinduced toxicity in pediatric ALL.
Keywords: childhood acute lymphoblastic leukemia (ALL), 6-mercaptopurine (6-MP), TPMT, ITPA, ABCC4, ABCB1
Kratak sadržaj
Uvod
Akutna limfoblastna leukemija je najčešća maligna bolest dečjeg doba. Optimalna upotreba antileukemijskih lekova dovela je do smanjenja toksičnosti i neželjenih događaja, kao i do povećanja stope preživljavanja. Tiopurinski lekovi, uključujući 6-merkaptopurin, predstavljaju najčešće korišćene antileukemijske lekove u lečenju dece obolele od akutne limfoblastne leukemije, koji se koriste u toku faze održavanja. Personalizovana terapija 6-merkaptopurinom, prilagođena TPMT genotipu svakog pacijenta, već je implementirana u terapijske protokole. Cilj ove studije je bio da ispita ulogu varijanti u genima TPMT, ITPA, ABCC4 i ABCB1 kao prediktora ishoda i toksičnosti uzrokovane 6-merkaptopurinom u toku faze održavanja u lečenju pedijatrijske akutne limfoblastne leukemije.
Metode
U studiju je bilo uključeno 68 dece obolele od akutne limfoblastne leukemije. Pacijenti su lečeni primenom ALL IC-BFM 2002 ili ALL IC-BFM 2009 protokola. Toksičnost i neželjeni događaji su posmatrani i beleženiupotrebom surogat markera (broj nedelja bez terapije, broj epizoda leukopenije i prosečna doza 6-merkaptopurina) a probabilistički modeli su korišćeni za predikciju ukupne toksičnosti uzrokovane primenom 6-merkaptopurina.
Rezultati
Studija je potvrdila da pacijenti oboleli od akutne limfoblastne leukemije koji imaju neaktivni TPMT alel zahtevaju redukciju doze 6-merkaptopurina. Varijante u genima ITPA i ABCC4 nisu bile povezane sa toksičnošću koja je uzrokovana primenom 6-merkaptopurina u toku faze održavanja. Nosioci varijante u genu ABCB1 su ispoljili veću hepatotoksičnost. Probabilistički model Neural net kojim su posmatrane sve analizirane genske varijante se pokazao kao najbolji predikcioni model. Primenom ovog modela bilo je moguće razlikovati ALL pacijente sa lošom i dobrom tolerancijom 6-merkaptopurina u 71% slučajeva (AUC=0,71).
Zaključak
Ova studija doprinosi dizajniranju panela farmakogenetskih markera koji bi se koristili za predikcijutoksičnosti uzrokovane primenom tiopurinskih lekova kod dece obolele od akutne limfoblastne leukemije.
Introduction
Acute lymphoblastic leukemia (ALL) is a malignant clonal disease of the hematopoietic tissue. It is the most common cancer in childhood, accounting for approximately 30% of all pediatric malignancies. The optimal use of known antileukemic drugs in the context of multicentric clinical trials and improvements in the field of supportive therapy have led to higher survival rates (1).
Thiopurine drugs are mostly used as immuno-suppressive and anticancer therapy for hematological malignancies, inflammatory bowel disease and renal transplant patients. Excessive accumulation of their active metabolites inside hematopoietic cells leads to increased toxicity. On the contrary, increased efflux of active thiopurine metabolites can lead to diminished efficacy of the therapy (2). 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG) are key immunosuppressive thiopurine medications in the therapy protocols for childhood ALL. The role of 6-MP is especially important during the maintenance phase of treatment (1).
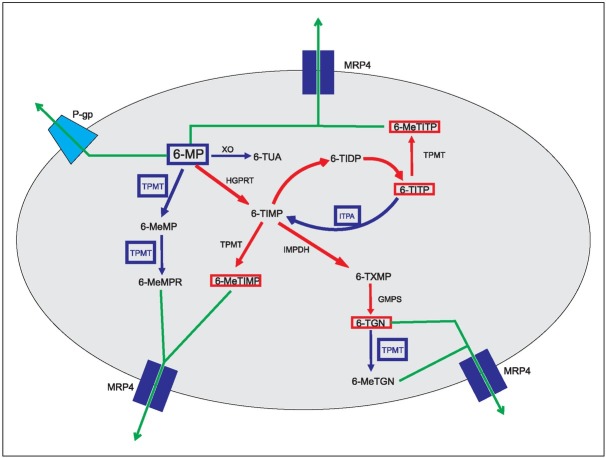
Single nucleotide polymorphisms (SNPs) in the genes encoding proteins involved in 6-MP metabolism, cellular transport and mechanism of action can influence drug efficacy and toxicity (Figure 1) (3). Thiopurine S-methyltransferase (TPMT) is an important enzyme that inactivates thiopurine drugs. It is well known that individuals carrying two inactive TPMT alleles (homozygous TPMT deficiency) experience severe myelosuppression when treated with conventional doses of thiopurine drugs. Heterozygous deficient TPMT carriers (one inactive TPMT allele) show moderate to severe myelosuppression in contrast to homozygous carriers of the wild-type TPMT allele (TPMT*1) and have a lower risk of myelosuppression when on conventional 6-MP doses (4). SNPs in the inosine triphosphate pyrophosphatase (ITPA) gene, which modulates the ITPase activity, have been shown to influence the accumulation of the above-mentioned toxic thiopurine metabolites. Thus, carriers of these SNPs are prone to severe myelosuppression (5, 6, 7, 8). The Multidrug Resistance Protein 4 (MRP4) belongs to the family of ATP-binding cassette transporters and serves as a transmembrane efflux-pump for various organic anions. The physiological role of MRP4 protein includes the detoxification of drugs and various endogenous toxic molecules (7). MRP4 protein ex ports methylated 6-MP nucleotides – among other mole cules – from cells, thus protecting them from 6-MP toxicity. The ABCC4 (ATP-binding cassette sub-family C member 4) gene that encodes for MRP4 protein is highly polymorphic, with more than 20 missense genetic variants. Less functional ABCC4 alleles may be responsible for more severe myelosuppression during 6-MP treatment. Furthermore, more active ABCC4 alleles could be responsible for 6-MP resistance (9). Notably, nucleotide T in ABCC4 c.*1372 T>G has been pointed-out by Lopez et al. (10) in a GWAS study as the genetic variant that results in decreased ABCC4 expression and thus, 6-MP induced toxicity. The ATP-binding cassette sub-family B member 1 (ABCB1) gene encodes for P-glycoprotein, an ATP-de pendent transmembrane efflux-pump. It trans ports xenobiotics out of cells, but also prevents the entry of toxic metabolites (11). Some tumours that are resistant to chemotherapy have ABCB1 over-expressed. In more than half of the relapsed acute myeloid leukemia incidents, P-glycoprotein is more active than in the non-relapsed cases, thus making it a suitable candidate marker of poor prognosis. P-glycoprotein serves as an efflux-pump for various chemotherapeutics used in the treatment of childhood ALL, such as doxorubicin, etoposide, vincristine, and 6-MP (12). The overexpression of ABCB1 could be responsible for therapy failure or relapse (13) while the reduced activity of P-glycoprotein can lead to more severe 6-MP toxicity.
Figure 1.
Schematic representation of 6-mercaptopurine metabolism.
Blue lines represent inactivation pathways; red lines represent pathways that generate active metabolites; green lines represent elimination (efflux) out of the cell. 6-MP: 6-mercaptopurine, XO: xanthine oxidase, 6-TUA: 6-thiouric acid, TPMT: thiopurine S methyltransferase, 6-MeMP: 6-methylmercaptopurine, 6-MeMPR: 6-methylmercaptopurine ribonucleotide, HGPRT: hypoxanthine phosphoribosyl transferase, 6-TIMP: 6-thioinosine 5’-monophosphate, 6-TIDP: 6-thioinosine 5’-diphosphate, 6-TITP: 6-thioinosine 5’-triphosphate, 6-MeTITP: 6-methylthioinosine 5’-triphosphate, ITPA: inosine triphosphate pyrophosphatase, 6-MeTIMP: 6-methylthioinosine 5’-monophosphate, IMPDH: inosine mono phosphate dehydrogenase, 6-TXMP: 6-thioxanthosine 5’-mono phosphate, GMPS: guanosine monophosphate synthetase, 6-TGN: 6-thioguanine nucleotide, 6-MeTGN: 6-methylthio guanine nucleotide, MRP4: multidrug resistance protein 4, P-gp: P-glycoprotein.
Herein, we aim to explore the role of TPMT, ITPA, ABCC4 and ABCB1 genetic variants in the occurrence and intensity of 6-MP induced toxicity during the maintenance phase of childhood ALL treatment.
Materials and Methods
Sixty-eight children with ALL, diagnosed and treated at University Children’s Hospital, Belgrade in the period between January 2003 and January 2013 were enrolled in this study. Informed consent has been obtained from parents or legal guardians. The study has been approved by the Ethics Committee of University Children’s Hospital, University of Belgrade. Children have been treated according to either ALL IC-BFM 2002 or ALL IC-BFM 2009 protocols, depending on the time of diagnosis: those diagnosed before April 1, 2010 were treated according to the ALL IC BFM 2002 protocol, whereas those diagnosed after that date were treated according to the currently used protocol (14). Patients were classified into risk groups based on their risk for relapse (standard risk group – SR, intermediate risk group – IR and high risk group – HR) according to the current protocol. All patients have received maintenance therapy that included daily oral 6-MP (50 mg/m2) and weekly oral methotrexate (20 mg/m2). The maintenance phase lasted between 13 and 17 months (60–77 weeks). The target white blood cell (WBC) count was monitored to be between 2.0 and 3.0 × 109/L. Patients had regular check-ups every 2 weeks during the entire maintenance phase. If the WBC count fell below 2.0 × 109/L, the 6-MP dose was reduced in 25% weakly increments. Instead, if the WBC count increased above 3.0 × 109/L, the 6-MP dose was also increased in 25% weakly increments. If the WBC count was below 1.0 × 109/L, 6-MP administration was discontinued, until WBC recovery. The TPMT genotype was determined for all patients before the beginning of the maintenance therapy and 6-MP doses were adjusted accordingly (4, 15). Toxicity and adverse events during 6-MP treatment have been monitored using surrogate markers (off-therapy weeks, episodes of leukopenia and average dose, calculated considering 50 mg/m2 as 100%). Side effects, such as hepatotoxicity (elevated levels of transaminases), rash, acute pancreatitis and flu-like symptoms, have also been recorded for all patients.
The analysis of TPMT c.460G>A (rs1800460), TPMT c.719A>G (rs1142345), ITPA c.94C>A (rs1127354), ABCC4 c.*1372T>G (rs9516519) and ABCB1 c.2677G>T (rs2032582) was performed by PCR-RFLP in the Laboratory for Molecular Biomedicine, Institute of Molecular Genetics and Genetic Engineering, University of Belgrade. In particular, the TPMT, ITPA and ABCB1 genetic variants of interest were analysed as described previously (18). The region of ABCC4 gene that contains c.*1372 T>G was amplified using 5’-GCTTTTTAAGGCT-TCACTCAAT-AAAACAGC-3’ as the forward primer and 5’-GTGTC-ACCTCCCTGAAATTGC-3’ (reverse primer). Restriction digestion with PvuII enzyme followed by 3% agarose gel electrophoresis was used to distinguish T nucleo tide (118 + 29 bp) from G nucleotide carriers (147 bp). Since both TPMT c.460 G>A and TPMT c.719 A>G cause major decreases in TPMT enzyme activity, presence of any or both SNPs was considered to have the same effect. Genotyping for ITPA, ABCC4 and ABCB1 was performed retrospectively, using bone marrow samples taken on the day of diagnosis.
To explore any association of our genotyping findings and toxicity, Mann-Whitney test, Student t-test, Kruskal-Wallis test and ANOVA were used when appropriate.
In order to predict 6-MP induced toxicity in ALL patients based on the studied variants in TPMT, ITPA, ABCB1 and ABCC4 genes, probabilistic classifier models were designed using Elastic net, Neural net and Random forest machine learning algorithms. These models were used to discriminate patients whose mean dose of 6-MP was below (poor tolerance) or above average (good tolerance). Repeated 10-fold cross-validation method was applied to avoid model overfitting. The area under the ROC curve (AUC) values were used to assess performance of the classifying models. AUC values range from 0.5 (random classifications) to 1.0 (perfect classifications) (19). R software (v.3.4.3) was used to apply predictive algorithms and measure their performance using following packages: glmnet (20), nnet (21), random-Forest (22), ROCR (23), PresenceAbsence (24).
Results
A total number of 68 patients with ALL have been evaluated in this study. Median age at diagnosis was 5.2 years, ranging from 11 months up to 17.6
years. Minor predominance of male patients was observed (56%). Patient characteristics are summarized in Table I. The genotype frequencies (%) for the TPMT, ABCC4, ITPA and ABCB1 genomic variants of interest are shown in Table II. Average and median duration of maintenance therapy for patients was both 16 months, ranging from 9 to 21 months. The median dose and average dose of 6-MP during maintenance therapy was approximately 100% and 101%, respectively. The lowest 6-MP dose administered was 34% and the highest 197%. Ten patients with ALL (15%) have spent more than 10% of time skipping therapy due to leukopenia. Only 21 patients (30.5%) had no episodes of leukopenia during maintenance therapy. The remaining 47 patients had at least one episode of leukopenia. Hepatotoxicity of various degrees was detected in 45 patients (66%) at some point during the maintenance phase of therapy.
Table I.
Patients characteristics.
| (No=68 patients) Age | Years | |
|---|---|---|
| Average | 6.5 | |
| Median | 5.2 | |
| Range | 0.9–17.6 | |
| Gender | N° | % |
| Male | 38 | 56 |
| Female | 30 | 44 |
| Immunophenotype | ||
| B lineage | 58 | 85 |
| T lineage | 10 | 15 |
| Molecular genetics | ||
| Negative | 47 | 69 |
| BCR/ABL | 3 | 4 |
| MLL/AF4 | 2 | 3 |
| TEL/AML1 | 14 | 21 |
| E2A/PBX1 | 2 | 3 |
| Applied protocol | ||
| ALL IC BFM 2002 | 39 | 57 |
| ALL IC BFM 2009 | 29 | 43 |
| Prednisolone response | ||
| Good response | 61 | 90 |
| Poor response | 7 | 10 |
| Day 33 BM | ||
| M1 | 67 | 98 |
| M2 | 0 | 0 |
| M3 | 1 | 2 |
| Risk group | ||
| Standard risk | 11 | 16 |
| Intermediate risk | 47 | 69 |
| High risk | 10 | 15 |
| Outcome | ||
| CR | 59 | 87 |
| Relapse | 5 | 7 |
| Death due to relapse | 4 | 6 |
Good prednisolone response: <1000 leukoblasts per mm3, poor prednisolone response: >1000 leukoblasts per mm3, BM: bone marrow, M1:<5% leukoblasts in BM, M2: ≥5% <25% leukoblasts, M3: ≥25% leukoblasts, CR: complete remission
Table II.
Genotype frequency of genetic variants in TPMT, ABCC4, ITPA and ABCB1 genes in childhood ALL patients and surrogate myelotoxicity parameters and hepatotoxicity.
| Gene | n (%) | Median average dose of 6-MP % | P-value | Longer than 10% off-therapy | P-value | Average number of leukopenic episodes | P-value | Hepatotoxicity n (%) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| TPMT | |||||||||
| TPMT*1/TPMT*1 | 64 (94.2) | 111.0 | 10 | 2.3 | 42 (65.6) | ||||
| Variant allele† carriers | 4 (5.8) | 72.0 | 0.003 | 0 | 0.521 | 3.5 | 0.155 | 3 (75) | 0.584 |
| ABCC4 c.*1372T>G | |||||||||
| TT – wild type | 41 (61.0) | 104 | 7 | 2.6 | 27 (65.8) | ||||
| TG | 24 (35.0) | 95 | 0.836†† | 3 | 0.729†† | 1.8 | 0.165†† | 16 (66.7) | 0.945†† |
| GG | 3 (4.0) | 89 | 0 | 2 | 2 (66.7) | ||||
| ITPA c.94C>A | |||||||||
| CC – wild type | 63 (93.0) | 100 | 9 | 2.3 | 40 (63.5) | ||||
| CA | 5 (7.0) | 91 | 0.814†† | 1 | 0.560†† | 2.2 | 0.737†† | 5 (100) | 0.159†† |
| AA | 0 | / | / | / | 0 | ||||
| ABCB1 c.2677G>T | |||||||||
| GG – wild type | 29 (43.0) | 99 | 5 | 2.5 | 15 (51.7) | ||||
| GT | 26 (26.0) | 100 | 0.843†† | 2 | 0.733†† | 2.5 | 0.613†† | 21 (80.7) | 0.030†† |
| TT | 13 (19.0) | 95 | 3 | 1.5 | 9 (69.2) | ||||
Detected TPMT variant alleles include TPMT*3A (c.460A and c.719G in cis) and TPMT*3C (c.719G)
Dominant genetic model was used.
TPMT
The median average dose of 6-MP was 72% in the group of patients with ALL that carried at least one TPMT variant allele, c.460A or c.719G, and was found to be significantly lower (p=0.003) than that of the ALL patients carrying wild type TPMT (111%). In TPMT positive patients with ALL, there were no patients that spent more than 10% of time skipping therapy and the number of episodes of leukopenia was not higher than that of the patients carrying wild type TPMT (Table II). The absence of common therapy complications in TPMT positive patients is most likely a result of the fact that 6-MP doses were adjusted according to the TPMT genotype (determined for all patients before beginning of maintenance therapy).
ITPA
Five patients (7%) were carriers of the ITPA c.94CA allele and their median average dose for 6-MP was 91%. The patients with ALL carrying wild type ITPA had a median average dose of 100%. No statistically significant difference was noted between those groups and analysis failed to show any relationship between the ITPA c.94CA genotype and in creased 6-MP toxicity using all three surrogate markers for myelotoxicity (Table II). Also, we could not establish any association among the variant ITPA alleles and increased occurrence of hepatotoxicity (Table II).
ABCC4
The ABCC4 c.*1372TT genotype (wild type) was detected in 61% of our patients with ALL, where heterozygote and homozygote carriers of variant allele represented 35% and 4% of the cohort, respectively. In ABCC4 c.*1372GG and MRP4 c.*1372TG genotype carriers, the median average doses of 6-MP were 95% versus 104% in wild type allele carriers. Using all three surrogate markers for myelotoxicity, we did not confirm that ABCC4 variant allele carriers experience greater 6-MP induced toxicity during the maintenance phase of therapy nor correlation with increased hepatotoxicity (Table II).
ABCB1
The ABCB 1c.2677TT genotype was detected in 19% of our patients and the ABCB 1c.2677GT genotype in 38% of the cohort (Table II). The median average dose of 6-MP in wild type, heterozygote and homozygote carriers of variant alleles was 99%, 100% and 95%, respectively. Neither homozygote nor heterozygote carriers of ABCB1 variant alleles experienced greater 6-MP induced myelotoxicity during treatment (Table II). However, patients that were variant allele carriers showed increased frequency of hepatotoxicity (p=0.030).
Pharmacogenetic predictive model for 6-MP induced toxicity in treatment of childhood ALL
Eleven patients with ALL, carriers of wild type TPMT alleles, could tolerate the same or even lower average dose of 6-MP (similarly to) as the carriers of TPMT variant alleles. Among these 11 patients, there were 4 heterozygous carriers of ABCC4 variant allele, ABCC4c.*1372TG, as well as 4 heterozygotes, ABCB1 c.2677GT, and 4 homozygotes, ABCB1 c.2677TT, for ABCB1 variant allele. This finding suggests that, besides TPMT variant alleles, the above-mentioned genetic variants should be considered as additional pharmacogenetic markers towards patient-tailored treatment of childhood ALL.
Different prediction models (Elastic net, Neural net and Random forest) were used to classify patients whose mean dose of 6-MP for the entire duration of maintenance therapy was below average (poor tolerance) or above average (good tolerance). After evaluating performance of these prediction models using AUC values as measurement of classification accuracy, it was found that the best predictive model performance was achieved when all analyzed genetic variants (TPMT c.460G>A, TPMT c.719A>G, ITPA c.94C>A, ABCC4 c.*1372T>G and ABCB1 c.2677G>T) were used, even though not all genetic variants showed association with 6-MP tolerance. Out of 3 predictive algorithms, Neural net algorithm showed the best performance for 6-MP tolerance prediction: AUC = 0.71 [95% CI: 0.59–0.83]. This means that according to our results, this model can accurately discriminate between pairs of ALL patients that have good and poor tolerance in 71% of cases.
Discussion
This is the first study of the effect of pharmacogenetic variants in ABCC4, ITPA and ABCB1 genes in Serbian childhood ALL patients. Pharmacogenetic variants in TPMT gene have already been studied in the Serbian childhood ALL patients (15). The ITPA variant genotype frequency in our group of patients was 7.0%. The genotype frequency of the same variant, ITPA c.94CA, in Malaysian children with ALL has been shown to be 28% (25). It was also shown that carriers of ITPA variant alleles were at increased risk of developing fever and liver toxicity during 6-MP treatment in Malaysian patients (25). In particular, carriers of ITPA variant alleles have a higher incidence of febrile neutropenia, but only if 6-MP treatment has been already modified according to patients’ TPMT genotype (26). An increased intracellular concentration of 6-MP toxic metabolites was also monitored in patients carrying ITPA variant alleles (27). However, some studies did not confirm any correlation among ITPA variant alleles and 6-MP toxicity (28). Our results did not support the findings of others (27, 28, 29) that ITPA variant allele can be an independent predictor of 6-MP myelotoxicity or other adverse reactions, like flu-like symptoms, rash and pancreatitis, to thiopurine drugs. In our study group, those adverse reactions were not detected.
Studies in animal models have shown that low ABCC4 expression was responsible for thiopurine sensitivity and increased myelotoxicity, whereas high gene expression could be responsible for treatment failure (9). Also, ABCC4 variants were correlated to a decreased event free survival in children with ALL (30). Today, available data on myelotoxicity and outcome correlation with ABCC4 genetic variants are scarce. Herein, the frequency of ABCC4 c.*1372TG and ABCC4 c.*1372GG in our group was 34.7% and 4.3%, respectively. No association of ABCC4 c.*1372 T>G variant with myelotoxicity was found.
Genotype frequencies of ABCB 1c.2677GT and ABCB1 c.2677TT carriers in Korean children with ALL were 36% and 9%, respectively. In our patient group the same genotype frequencies were 38% and 19%. Also, the same study has failed to show correlation of variant ABCB1 alleles with increased myelotoxicity or outcome (2). Our results have not confirmed that ABCB1 variant allele could be an independent predictor of myelotoxicity. Increased occurrence of hepatotoxicity in our patients that were variant allele carriers is probably related to methotrexate use, rather than 6-MP (10). Gregers et al. have explored polymorphisms in ABCB1 gene in relation to myelotoxicity and hepatotoxicity and failed to show correlation of ABCB 1c.2677GT and ABCB1 c.2677TT variants with hepatotoxicity, but did reveal correlation with increased myelotoxicity which was not the case in our study (31). Our study failed to demonstrate univariate association of any analysed genetic marker, except in TPMT gene, with 6-MP induced toxicities.
One of the aims of this study was to develop a prediction model based on genetic data which could potentially estimate 6-MP tolerance. To the best of our knowledge, no study has dealt with 6-MP tolerance or toxicity by employing predictive modeling with selected genetic markers as input variables. We used 3 classification algorithms and the best prediction model was assessed using Neural net which could discriminate ALL patients that have good and poor tolerance in 71% of cases. This model took into account all analysed genetic variants, not only those that showed significant association in univariate analysis, because liberal inclusion of variables could still contribute to model performance (32).
In our study group, we did not have all the possible combinations for the investigated genetic markers. This is a consequence of the relatively small cohort of patients we could collect in Serbia. Anyway, the probabilistic model predicts that not only variants in TPMT gene, but also variants in other genes relevant for 6-MP metabolism and transport (ITPA, ABCC4 and ABCB 1) could be used as predictors of 6-MP induced toxicity during the maintenance phase of treatment in pediatric ALL. We suggest that TPMT c.460G>A, TPMT c.719A>G, ITPA c.94C>A, ABCC4 c.*1372T>G and ABCB1 c.2677G>T variants serve as markers towards patient stratification, identifying those patients at a higher risk of developing myelotoxicity and therefore, discontinuing therapy.
Children with ALL suffer from a rare, but highly curable disease. However, there is still around 10% of patients with complications and unfavourable outcome. As such, a therapeutic strategy that is efficacious and continuous (devoid of side effects) is of fundamental importance. This strategy has to include a treatment approach which is personalized, based on the individual genomic profile of each patient. Association studies on the pharmacogenomic profile of patients and data on the toxicity of drugs are the most promising directions on the road to personalized medicine. The high throughput methodology, such as next generation sequencing, would facilitate analysis of all known genes relevant for 6-MP metabolism and transport in each childhood ALL patient. The study of association of these genomic data and the occurrence and intensity of 6-MP induced toxicity during the maintenance phase of childhood ALL treatment, would enable the design of a panel of pharmacogenetic markers for predicting thiopurine-induced toxicity in pediatric ALL.
Acknowledgement
This research has been supported by grant III41004 from the Ministry of Education and Science, Republic of Serbia.
Footnotes
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Pui C, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120(6):1. doi: 10.1182/blood-2012-05-378943. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H, Kang HJ, Kim HJ, Jang MK, Kim NH, Oh Y. Pharmacogenetic Analysis of Pediatric Patients with Acute Lymphoblastic Leukemia: A Possible Association between Survival Rate and ITPA Polymorphism. PLoS One. 2012;7(9):1. doi: 10.1371/journal.pone.0045558. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paugh SW, Stocco G, Evans WE. Pharmacogenomics in pediatric leukemia. Curr Opin Pediatr. 2010;22:703. doi: 10.1097/MOP.0b013e32833fde85. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui C-H, Yee SW. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387. doi: 10.1038/clpt.2010.320. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stocco G, Franca R, Verzegnassi F, Londero M, Rabusin M, Decorti G. Multilocus genotypes of relevance for drug metabolizing enzymes and therapy with thio - purines in patients with acute lymphoblastic leukemia. Front Genet. 2013;3:1. doi: 10.3389/fgene.2012.00309. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipkova M, Franz J, Abe M, Klett C, Wieland E, Andus T. Association between adverse effects under azathioprine therapy and inosine triphosphate pyrophosphatase activity in patients with chronic inflammatory bowel disease. Ther Drug Monit. 2011;33(3):321. doi: 10.1097/FTD.0b013e31821a7c34. –. [DOI] [PubMed] [Google Scholar]
- 7.Abla N, Chinn L, Nakamura T, Liu L. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325(3):859. doi: 10.1124/jpet.108.136523. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azimi F, Mortazavi Y, Alavi S, Khalili M, Ramazani A. Frequency of ITPA gene polymorphisms in Iranian patients with acute lymphoblastic leukemia and prediction of its myelosuppressive effects. Leuk Res. 2015;39(10):1048. doi: 10.1016/j.leukres.2015.06.016. –. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy P, Schwab M, Takenaka K, Nachagari D, Morgan J, Leslie M. Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Res. 2008;68:4983. doi: 10.1158/0008-5472.CAN-07-6790. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Lopez E, Ballesteros J, Piñan MA, Sanchez de Toledo J, Garcia de Andoin N, Garcia-Miguel P. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Geno - mics. 2013;23:53. doi: 10.1097/FPC.0b013e32835c3b24. et al. –. [DOI] [PubMed] [Google Scholar]
- 11.Balamurugan S, Sugapriya D, Shanthi P, Thilaka V, Venkatadesilalu S, Pushpa V. Multidrug resistance 1 gene expression and AgNOR in childhood acute leukemias. Indian J Hematol Blood Transfus. 2007;23:73. doi: 10.1007/s12288-008-0002-2. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai X, Wang H, Zhu X, Miao H, Qian X, Li J. Gene polymorphisms of ABC transporters are associated with clinical outcomes in children with acute lymphoblastic leukemia. Arch Med Sci. 2012;8:659. doi: 10.5114/aoms.2012.30290. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plasschaert SLA, De Bont ESJM, Boezen M, Vander Kolk DM, Daenen SMJG, Faber KN. Expression of multidrug resistance-associated proteins predicts prognosis in childhood and adult acute lymphoblastic leuke - mia. Clin Cancer Res. 2005;11(24):8661. doi: 10.1158/1078-0432.CCR-05-1096. et al. –. [DOI] [PubMed] [Google Scholar]
- 14.Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol 2014; 32(3):174. doi: 10.1200/JCO.2013.48.6522. et al. –. [DOI] [PubMed] [Google Scholar]
- 15.Dokmanovic L, Urosevic J, Janic D, Jovanovic N, Petru - cev B, Tosic N. Analysis of thiopurine S-methyltransferase polymorphism in the population of Serbia and Montenegro and mercaptopurine therapy tolerance in childhood acute lymphoblastic leukemia. Dec; Ther Drug Monit. 2006;28(6):800. doi: 10.1097/01.ftd.0000249947.17676.92. et al. –. [DOI] [PubMed] [Google Scholar]
- 16.Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH. Molecular diagnosis of thiopurine Smethyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126(8):608. doi: 10.7326/0003-4819-126-8-199704150-00003. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Penna G, Allegra A, Alonci A, Aguennouz M, Garufi A, Cannavò A. MDR-1 polymorphisms (G2677T and C3435T) in B-chronic lymphocytic leukemia: an impact on susceptibility and prognosis. Med Oncol. 2010;28(4):1549. doi: 10.1007/s12032-010-9561-9. et al. –. [DOI] [PubMed] [Google Scholar]
- 18.Vucicevic K, Jakovljevic V, Colovic N, Tosic N, Kostic T, Glumac I, Pavlovic S, Karan-Djurasevic T, Colovic M. Association of bax expression and bcl2/bax ratio with clinical and molecular prognostic markers in chronic lymphocytic leukemia. J Med Biochem. 2016;35:150. doi: 10.1515/jomb-2015-0017. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theodorson E. Quality assurance in clinical chemistry: a touch of statistics and a lot of common sense. J Med Biochem. 2016;35:103. doi: 10.1515/jomb-2016-0012. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1. –. [PMC free article] [PubMed] [Google Scholar]
- 21.Venables WN, Ripley BD. Modern Applied Statistics With S. Vol. 12. New York: Springer; 2002. 4th edn. [Google Scholar]
- 22.Liaw A, Wiener M. Classification and regression by random forest. R News. 2002;2:18. –. [Google Scholar]
- 23.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinfor matics. 2005;21(20):7881. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EA, Moisen G. PresenceAbsence: An R Pack - age for Presence-Absence Model Analysis. Journal of Statistical Software. 2008;23(11):1. –. [Google Scholar]
- 25.Wan Rosalina WR, Teh LK, Mohamad N, Nasir A, Yusoff R, Baba AA. Polymorphism of ITPA 94C>A and risk of adverse effects among patients with acute lymphoblastic leukaemia treated with 6-mercaptopurine. J Clin Pharm Ther. 2012;37:237. doi: 10.1111/j.1365-2710.2011.01272.x. et al. –. [DOI] [PubMed] [Google Scholar]
- 26.Stocco G, Cheok MH, Crews KR, Dervieux T, French D, Pei D. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercapto - purine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009;85(2):164. doi: 10.1038/clpt.2008.154. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam de Beaumais T, Fakhoury M, Medard Y, Azo - ugagh S, Zhang D, Yakouben K. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011;71:575. doi: 10.1111/j.1365-2125.2010.03867.x. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawwa AF, Collier PS, Millership JS, McCarthy A, Dempsey S, Cairns C. Population pharmacokinetic and pharmacogenetic analysis of 6-mercaptopurine in paediatric patients with acute lymphoblastic leuka e - mia. Br J Clin Pharmacol. 2008;66:826. doi: 10.1111/j.1365-2125.2008.03281.x. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Hadithy a. FY, de Boer NKH, Derijks LJJ, Escher JC, Mulder CJJ, Brouwers JRBJ. Thiopurines in inflammatory bowel disease: Pharmacogenetics, therapeutic drug monitoring and clinical recommendations. Dig Liver Dis. 2005;37:282. doi: 10.1016/j.dld.2004.09.029. –. [DOI] [PubMed] [Google Scholar]
- 30.Ansari M, Sauty G, Labuda M, Gagné V, Laverdière C, Moghrabi A. Polymorphisms in multidrug resistance- associated protein gene 4 is associated with outcome in childhood acute lymphoblastic leukemia. Blood. 2009;114:1383. doi: 10.1182/blood-2008-11-191098. et al. –. [DOI] [PubMed] [Google Scholar]
- 31.Gregers J, Gréen H, Christensen IJ, Dalhoff K, Schroe - der H, Carlsen N. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. The Pharmacogenomics Journal. 2015;15:372. doi: 10.1038/tpj.2014.81. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans DM, Visscher PM, Wray NR. Harnessing the in - formation contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum Mol Genet. 2009;18(18):3525. doi: 10.1093/hmg/ddp295. –. [DOI] [PubMed] [Google Scholar]