Summary
Background
Recent studies have highlighted the role of anti-Müllerian hormone (AMH) in numerous ovarian disorders. Polycystic Ovary Syndrome (PCOS) is one of the major causes of infertility in Egypt. Several reports have linked PCOS with vitamin D deficiency. This investigation illustrates the possibility of using serum AMH for PCOS diagnosis in infertile Egyptian females, determines the variables affecting it and correlates it with serum 25(OH)D, testosterone, dyslipidemia and anthropometric parameters.
Methods
All parameters were assessed either with ELISA or colorimetrically in 53 infertile PCOS women and 17 age matched apparently healthy controls diagnosed according to Rotterdam consensus.
Results
Serum AMH, total testosterone, triacylglycerol (TG) levels and BMI were significantly higher in PCOS group compared to healthy controls (p=0.0239, p=0.0381, p=0.0457, and p=0.0067, respectively), while serum 25(OH)D levels and HDL-cholesterol (HDL-C) were significantly lower (p=0.0397 and p=0.0443, respectively). No significant correlation existed between AMH and 25(OH) D, BMI and dyslipidemia markers. AMH was found to have a significant negative correlation with age and a highly significant positive one with total testosterone in PCOS group (r=-0.303, p=0.027 and r=0.370, p=0.008, respectively). In the receiver operating characteristic curve of AMH, the cut-off value was 42.63 pmol/L with a specificity of 59% and a sensitivity of 82%. Multivariate regression analysis showed total testosterone to be the only determinant for AMH (β=0.381 and p=0.038).
Conclusions
There should be a future trend of using AMH as a diagnostic marker for PCOS in Egyptian females. The variation in serum AMH levels is determined by total testosterone.
Keywords: anti-Müllerian hormone, polycystic ovary syndrome, vitamin D, fertility, hyperandrogenemia
Kratak sadržaj
Uvod
Nedavne studije ukazale su na ulogu antimilerovog hormona (AMH) u mnogim oboljenjima jajnika. Sindrom policističnih jajnika (SPJ) jedan je od glavnih uzroka neplodnosti u Egiptu. Nekoliko izveštaja povezalo je SPJ sa nedostatkom vitamina D. Ovaj rad ilustruje mogućnost korišćenja serumskog AMH-a za postavljanje dijagnoze SPJ kod neplodnih Egipćanki, određuje varijable koje na njega utiču i uspostavlja korelacije sa serumskim 25(OH)D, testosteronom, dislipidemijom i antropometrijskim parametrima.
Metode
Svi parametri određeni su ili pomoću ELISA testa ili kolorimetrijski kod 53 neplodne Egipćanke sa SPJ i 17 na vodno zdravih kontrolnih subjekata odgovarajuće starosne dobi prema dijagnozi koja odgovara roterdamskom sporazumu.
Rezultati
Serumski AMH, ukupni testosteron, nivoi triacilglicerola (TG) i ITM bili su značajno viši u grupi sa SPJ u poređenju sa zdravim subjektima (P=0,0239, P=0,0381, P=0,0457 i P=0,0067), dok su nivoi 25(OH)D i HDL-holesterola (HDL-C) u serumu bili značajno niži (P=0,0397 i P=0,0443). Nije nađena značajna korelacija između AMH-a i 25(OH)D, ITM i markera dislipidemije. Pokazalo se da je u grupi sa SPJ AMH bio u značajnoj negativnoj korelaciji sa starosnom dobi i veoma značajnoj pozitivnoj korelaciji sa ukupnim testosteronom (r=-0,303, P=0,027 i r=0,370, P=0,008). U ROC analizi za AMH, cut-off vrednost bila je 42,63 pmol/L uz specifičnost 59% i osetljivost 82%. Analiza multivarijantne regresije pokazala je da je ukupni testosteron jedina determinanta za AMH (β=0,381 i p=0,038).
Zaključak
Trebalo bi nastaviti sa korišćenjem AMH-a kao dijagnostičkog markera za SPJ u Egiptu. Varijacije u serumskim nivoima AMH-a determiniše ukupni testo steron.
Ključne reči: antimilerov hormon, sindrom policističnih jajnika, vitamin D, fertilitet, hiperandrogenemija
Introduction
Polycystic Ovary Syndrome (PCOS) is considered a dangerous threat to women of reproductive age with a prevalence of 6–16% all over the world. PCOS is a complex endocrine disease that could be diagnosed according to the Rotterdam consensus; by the presence of two of the following criteria: oligo-and/or anovulation, clinical and/or biochemical features of hyperandrogenism or polycystic ovaries on performing ultrasound investigations. Nevertheless, there are other common features of PCOS such as obesity, insulin resistance (IR) and dyslipidemia (1).
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein in nature. It originates from transforming growth factor β-superfamily (2). Serum AMH is generally produced from the granulosa cells of preantral and small antral follicles. This starts from birth up till menopause. Thus, serum AMH level reflects the ovarian reserve (3).
Recent studies have highlighted that vitamin D deficiency is a pandemic phenomenon affecting not only countries of high latitude, having shorter hours of sunlight, but also sunny countries of low latitude such as Egypt (4, 5). Serum vitamin D levels are evaluated by measuring serum 25(OH)D due to its longer half-life (6). Vitamin D receptor (VDR), mediating vitamin D actions, is mainly expressed in ovarian tissues, endometrium, fallopian epithelial cells, decidua and placenta (7, 8, 9). Therefore, vitamin D deficiency is found to contribute to the pathogenesis of female reproductive system disorders. Furthermore, it has a negative impact on female fertility as in PCOS (10).
Regarding dyslipidemia and PCOS, several investigations have revealed that in non-diabetic and non-hypertensive PCOS females, the levels of triacylglycerols (TG) and non-HDL-cholesterol (HDL-C) were twice as high compared to control group (11). Moreover, a recent meta-analysis expressed the same pattern of dyslipidemia in PCOS females (12). However, few studies have focused on the female Egyptian population. Therefore, the aim of this present study is to illustrate the possibility of using serum AMH for PCOS diagnosis in infertile Egyptian females, determine the variables affecting its levels and to evaluate its correlation with 25(OH)D, total testosterone, dyslipidemia and anthropometric markers.
Materials and Methods
Study population
This current investigation is a cross-sectional study which included 53 PCOS females aged from 17 to 39 with primary or secondary infertility. The diagnosis of PCOS was made according to Rotterdam criteria (13). Two out of the three following conditions are required to confirm a diagnosis of PCOS: oligo-and/or anovulation (defined by the presence of oligomenorrhea or amenorrhea), clinical and/or biochemical features of hyperandrogenism (defined by having clinical hirsutism (Ferriman-Gallwey score ≥ 6), acne or alopecia and/or elevated androgens) or polycystic ovaries on ultrasound (having > 12 follicles in each ovary, measuring from 2–9 mm and/or ovarian volume > 10 mL). Patients and control subjects were recruited from the outpatient clinics of different hospitals all over the governorates of Egypt. Patients diagnosed with Cushing syndrome, androgen secreting tumors, congenital adrenal hyperplasia and hyperprolactinemia were excluded from the study.
The control group comprised 17 apparently healthy females aged from 19 to 35. All control females had regular cycles ranging from 25 to 35 days and had no ovarian gynecological disorders or endocrine abnormalities.
The present study was performed after obtaining informed consent from all subjects and the approval of the ethics committee and the Review Board at the German University in Cairo and in accordance with The Code of Ethics of the Declaration of Helsinki.
Laboratory procedures
All blood samples were taken on a random day of the menstrual cycle. Weight and height were measured on the day of blood sampling and were used to calculate BMI. Serum was separated and samples were stored at –80 °C until time of analysis.
Serum AMH and 25(OH)D were measured using AMH Gen II ELISA (Beckman Coulter, Inc., USA) and DRG 25-OH Vitamin D (total) ELISA (DRG Instruments GmbH, Germany), respectively. Serum total testosterone was measured by immunoassay (Architect 2nd Generation, Abbott Diagnostics, USA). TG, HDL-C and total cholesterol (TC) were determined using enzymatic colorimetric assay (Diamond Diagnostics, D-P international, Egypt). LDL-Cholesterol (LDL-C) was calculated from TG and HDL-Cl values using the Friedewald formula (14).
Statistical analysis
The subjects’ characteristics were compared using the Student's t-test and the Mann-Whitney U test, as appropriate. Spearman Coefficient was used to determine the correlation between AMH and the various parameters. Multiple backward regression analysis was performed to evaluate the preferential effect of the different studied variables on AMH level. Cut-off values of serum AMH levels to predict its use for PCOS diagnosis, were analyzed using Receiver Operating Characteristic (ROC) curve. The area under curve (AUC) was calculated. The yield values were from 0.5 (no predictive power) to 1.0 (perfect prediction). The optimal cut-off was determined using Yoden’s Index: maximum [sensitivity – (1-specificity)] (15). Statistical significance was set at p≤ 0.05. All data were expressed as mean ± standard deviation (S.D.). Statistical analysis was performed using SPSS Statistics for Windows, version 24.0 (IBM Corp, Armonk, New York, USA) and Graph Pad Prism for Windows, version 5.00 (Graph Pad Software, San Diego, California, USA).
Results
Results of different variables measured in both the PCOS and control group
There was no significant difference in age between the PCOS and control groups, nor was there a difference in serum levels of LDL-cholesterol or TC. In contrast, BMI, AMH, total testosterone and TG were significantly higher in PCOS females in comparison to control group. On the other hand, 25(OH)D and HDL-cholesterol were significantly lower in PCOS when compared to control as shown in Table I.
Table I.
Comparison between age, BMI, serum AMH, serum 25(OH)D, serum total testosterone and dyslipidemia markers in PCOS and control groups.
| Variable | PCOS (n = 53) | Control (n = 17) | p-value |
|---|---|---|---|
| Age (years) | 25.96 ± 5.70 | 26.24 ± 4.90 | 0.8580 |
| BMI (kg/m2) | 29.94 ± 5.20 | 25 ± 4.7 | 0.0067** |
| AMH (pmol/L) | 51.27 ± 32.5 | 30.42 ± 19.21 | 0.0239* |
| Total testosterone (nmol/L) | 1.35 ± 0.45 | 1.04 ± 0.31 | 0.0381* |
| 25(OH)D (nmol/L) | 31.32 ± 14.85 | 48.65 ± 27.30 | 0.0397* |
| TG (mmol/L) | 1.51 ± 0.77 | 1.13 ± 0.39 | 0.0457* |
| HDL-C (mmol/L) | 1.09 ± 0.44 | 1.32 ± 0.51 | 0.0443* |
| LDL-C (mmol/L) | 3.56 ± 1.63 | 3.53 ± 1.32 | 0.8800 |
| Total cholesterol (mmol/L) | 5.36 ± 1.62 | 5.37 ± 1.19 | 0.8210 |
Distribution of Vitamin D across different BMI cut-offs in PCOS and control group
In this present study, it was observed that in the normal BMI category, the mean vitamin D level was 39.05 nmol/L in PCOS group while in control, it was 52.38 nmol/L. Moreover, in the overweight category, the mean vitamin D was 33.85 nmol/L in PCOS group versus 32.70 nmol/L in control group. Finally, in the obese group the mean vitamin D level was 26.50 nmol/L in PCOS group, in contrast to 30.33 nmol/L in controls. Hence, it was observed that within the PCOS group, as BMI increased, the mean serum vitamin D decreased. A similar trend was observed in the control group, as shown in Table II.
Table II.
Mean 25(OH)D levels at different BMI ranges in PCOS and control groups.
| BMI (kg/m2) cut-off points | Classification | Mean 25(OH)D (nmol/L) | |
|---|---|---|---|
| PCOS (n = 53) | Controls (n = 17) | ||
| 18.5–24.99 kg/m2 | Normal | 39.05 (16%) | 52.38 (68.75%) |
| 25–29.9 kg/m2 | Overweight | 32.88 (48%) | 32.70 (12.5%) |
| ≥ 30 kg/m2 | Obese | 26.50 (36%) | 30.33 (18.75%) |
Legend: Results are expressed as mean ± standard deviation (S.D.). % expresses the number of normal, overweight and obese subjects in relation to the total number of subjects in both PCOS and control groups, respectively.
Correlation between anthropometric parameters, total testosterone, 25(OH)D and dyslipidemia markers
In the current investigation, by using Spearman correlation coefficient, a significant negative correlation was found between serum AMH and age in PCOS group (r = -0.303, p = 0.027). Furthermore, serum AMH showed highly significant positive correlation with serum total testosterone in PCOS group (r = 0.37, p = 0.008). Finally, no correlation existed between AMH and 25(OH)D and dyslipidemia markers, as shown in Table III.
Table III.
Correlations between AMH and different variables measured in control group and PCOS groups.
| AMH Control | AMH PCOS | |||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Age | -0.149 | 0.569 | -0.303* | 0.027 |
| BMI | 0.503* | 0.047 | -0.031 | 0.883 |
| 25(OH)D | -0.553 | 0.062 | -0.136 | 0.343 |
| Total testosterone | 0.187 | 0.541 | 0.370** | 0.008 |
| TG | 0.292 | 0.256 | -0.057 | 0.688 |
| HDL-C | 0.209 | 0.422 | 0.345 | 0.103 |
| LDL-C | -0.059 | 0.823 | 0.143 | 0.309 |
| Total cholesterol | 0.132 | 0.613 | 0.218 | 0.117 |
Legend: ** represent significance at p ≤ 0.01 * represents significance at p ≤ 0.05. P-values: represent the significance of these correlations in control and PCOS groups, respectively.
Regression analysis models
Multivariate backward regression model was performed in the PCOS group to determine the preferential effect of the different studied variables on AMH level. AMH was considered the dependent variable. On the other hand, age, BMI, total testosterone, 25(OH)D, TG, HDL-C and LDL-C were considered as independent variables. It was deduced that total testosterone was the only determinant for AMH variation. It was shown that 14.5% variability in serum AMH levels could be explained by total testosterone (β = 0.381, p = 0.038). Other parameters were not significantly related.
ROC Curve
An ROC curve was constructed to test the ability of AMH to be used as a diagnostic tool for PCOS diagnosis. The AUC was 0.634 with 95% CI (0.5 – 0.798). The optimal cut-off point was found to be 42.63 pmol/L (Sensitivity 59%, Specificity 82%) as shown in Figure 1
Figure 1.
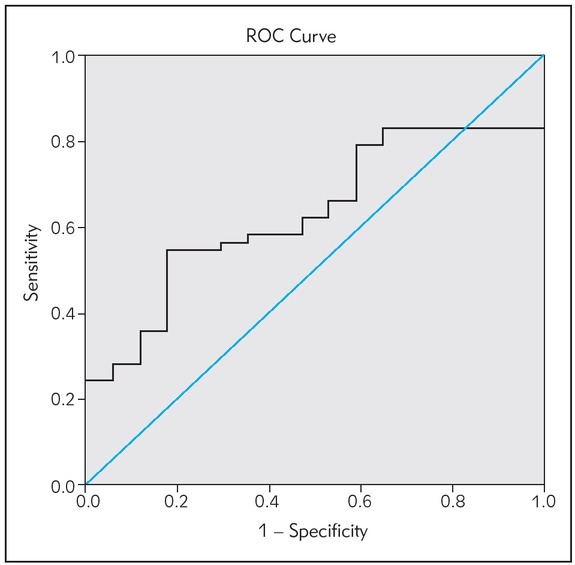
The ROC curve of AMH as a diagnostic tool for PCOS diagnosis.
Discussion
PCOS is a prominent underlying cause of infertility in females. Moreover, women with PCOS are at high risk of type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, cardiovascular diseases, depression and sleep apnea, so the search for new biomarkers is crucial to counteract these huge complications. Hence, our study aims to examine the potential of using serum AMH as a biomarker of PCOS, to explore its determinants and to test its correlation with serum 25(OH)D, total testosterone, dyslipidemia and anthropometric parameters in Egyptian infertile females with PCOS. In the present investigation, it was clear that BMI was significantly higher in PCOS group. These results are consistent with the study of Lego et al. (16). However, these results alone are not adequate to establish a causal relationship between high BMI and PCOS diagnosis (17). In addition, PCOS is found in lean individuals as well as obese in different populations (18). Nevertheless, obesity contributes to the pathogenesis of PCOS, and could lead to the exacerbation of PCOS symptoms. It causes IR and hyperinsulinemia, which in turn leads to a decrease in hepatic sex hormone binding globulin (SHBG) production and subsequent hyperandrogenism (19). Moreover, insulin stimulates ovarian androgen production in theca cells, by binding to its own receptor (20). Furthermore, this excessive androgen production could lead to acne, alopecia and hirsutism which are major features of PCOS (21).
Previous studies have well correlated dyslipidemia with PCOS. In the present study, serum HDL-C was significantly lower in PCOS group compared to control, whereas serum TG were significantly higher. These results agree with Brunzell et al. (22) who observed the same pattern of dyslipidemia in PCOS. Moreover, they indicated that this pattern was similar to that observed with IR. On the other hand, several studies have shown that dyslipidemia in PCOS is independent of BMI. This lipid profile persisted when comparing subjects of same age and BMI (23, 24, 25). In this study, no significant difference was found between PCOS and controls concerning serum LDL-C and serum TC. These results are similar to those observed by Pirwany et al. (26).
In the present investigation, serum AMH was found to be significantly higher in PCOS group. This increase in PCOS has been previously reported in several studies done by Pigny et al. (27), Lie et al. (28) and Laven et al. (29). The increase in AMH could be attributed to the increase in the number of preantral and antral follicles. This is caused either by the slow growth of follicles mediated by the increase in AMH (30) or due to signaling abnormalities in the follicles (31). Moreover, the level of AMH in serum was found to reflect the severity of PCOS symptoms such as hirsutism, anovulation and hyperandrogenism (29, 32, 33). Further more, on correlating age and AMH in different study groups, it was found that serum AMH was negatively correlated with age. This negative correlation reflects the decrease in ovarian reserve observed with in crease in age. This was also confirmed in the AMH dynamics model constructed by Kelsey et al. (34). The model showed that serum AMH starts to increase at birth, and reaches a peak at 25 years, then decreases gradually till reaching undetectable levels at time of menopause at the age 45–50 years.
In the present study, it was observed that total testosterone serum levels were significantly higher in PCOS compared to controls. These results are consistent with those of Tehrani et al. (35) and Skałba et al. (36). Hyperandrogenism observed in PCOS has more than one etiology. First, increased thickness of thecal layers observed in PCOS leads to excessive steroidogenic expression and activity (37). Second, elevated AMH has an inhibitory effect on FSH-induced aromatase activity. This leads to a decrease in the conversion of testosterone to estrogen, and an increase in testosterone levels (38). This effect of AMH explains the correlation found in our study between AMH and total testosterone in PCOS group. Furthermore, in the regression model, total testosterone explained the variability in serum AMH levels by 14.5% and was the only determinant of AMH. This agrees with the study of Begway et al. (39). They proposed that hyperandrogenism may contribute to the increase of small antral follicles and hence increasing AMH secretion in PCOS.
Several studies have reported that 25(OH)D is essential to maintain normal reproductive function in females. In the present study, serum 25(OH)D was significantly lower in the PCOS group compared to control. This is consistent with the fact that vitamin D deficiency affects sunny countries such as Egypt (4). Moreover, this significant decrease in vitamin D in the PCOS group agrees well with the study conducted by Hahn et al. (40). They correlated the decrease in 25(OH)D with features of PCOS such as obesity, dyslipidemia and levels of AMH and total testosterone (40). Furthermore, within the PCOS group, as BMI increased, the mean vitamin D decreased and a similar trend was observed in control group. This observed 25(OH)D deficiency trend in PCOS could be attributed to the higher BMI observed in PCOS group, rather than to PCOS itself. This inverse correlation could be explained by the fact that vitamin D is fat soluble and gets sequestered in adipose tissue, lowering its serum levels (6).
On the other hand, no statistically significant correlation was found between serum 25(OH)D and serum AMH in either of the study groups. These results are consistent with those of Cappy et al. (41) and Mumford et al. (42). Cappy et al. (41) demonstrated no significant difference in serum AMH levels before and after 25(OH)D supplementation in the PCOS group or in normal ovarian reserve group. Further more, Mumford et al. (42) found that vitamin D was not associated with fertility or AMH in women with proved fertility. These results suggest that vitamin D supplementation might be of little benefit in improving AMH which is considered the clinical marker of ovarian reserve. Moreover, in a large retrospective study by Pearce et al. (43), including 340 subjects of which 58 PCOS patients and 282 normovulatory controls, there was no association between seasonal fluctuations in vitamin D and AMH levels in either of the studied groups. Nevertheless, in an interventional study, Dennis et al. (44) observed that the seasonal variation of 25(OH)D serum levels was comparable to a seasonal variation in serum AMH. In another prospective study (n = 49), vitamin D supplementation lead to 36% decrease of AMH into normal levels in women with PCOS. This could be due to the fact that 25(OH)D improves the PCOS diagnosis, rather than altering the expression of AMH (45).
In order to investigate the use of AMH as a potential diagnostic tool of PCOS, the ROC curve was constructed. The AUC was found to be 0.634 with 95% CI (0.5–0.798). The cut-off was determined to be 42.63 pmol/L (sensitivity = 59%, specificity = 82%). Comparable values for AMH cut-offs were reported by other studies. Wiweko et al. (46) reported an AUC of 0.87 and cutoff of 31.77 pmol/L (sensitivity = 76.1%, specificity = 74.6%). This study included 71 PCOS women and 71 controls and the results were obtained using the Beckman Coulter Gen II ELISA. Furthermore, Dewailly et al. (47) defined a threshold of 35.7 pmol/L (AUC = 0.949, sensitivity = 81%, specificity = 92%) in a study including 240 patients, using 2nd generation AMH-EIA ELISA from Beckman Coulter. The major differences from the present study are either the number of subjects recruited, their race and ethnicity and the different assays used to measure serum AMH. These results collectively emphasize the strength of AMH as a possible biomarker for PCOS diagnosis (32, 33, 48). Moreover, serum AMH could also be superior to transvaginal ultrasonography, as it doesn’t depend on neither the operator’s skills, nor the technological advancement of the specific device used (49). Thus, serum AMH measurement represents a more standardized method for diagnosing PCOS.
In Egyptian PCOS females,
There should be a future trend of using AMH as a potential tool for PCOS diagnosis instead of it being abandoned for this long time.
Serum AMH and serum total testosterone are positively correlated.
Furthermore, the variation in AMH could be attributed to total testosterone levels as demonstrated by regression analysis.
AMH and 25(OH)D are not significantly correlated in either PCOS or control groups.
Acknowledgements
No acknowledgements to be made and no funding source to be acknowledged.
List of abbreviations
- AMH
Anti-Müllerian hormone
- SHBG
sex hormone binding globulin
- IR
Insulin resistance
- LDL-C
LDLCholesterol
- PCOS
Polycystic Ovary Syndrome
- ROC
Receiver Operating Characteristic
- S.D
Standard Deviation
- TC
Total cholesterol
- TG
Triacylglycerols
- VDR
Vitamin D receptor
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Lauritsen MP, Bentzen JG, Pinborg A, Loft A, Forman JL, Thuesen LL. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti- Müllerian hormone. Hum Reprod. 2014;29(4):791–801. doi: 10.1093/humrep/det469. [DOI] [PubMed] [Google Scholar]
- 2.Rajpert-DeMeyts E, Jørgensen N, Graem N, Müller J, Cate RL, Skakkebaek N.. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–44. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 3.Kevenaar ME, Meerasahib MF, Kramer P, van deLang- Born BM, de Jong FH, Groome NP. Serum anti- Müllerian hormone levels reflect the size of the primordial follicle pool in mice. Endo. 2006;147:3228–34. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- 4.Botros RM, Sabry IM, Abdelbaky RS, Eid YM, Nasr MS, Hendawy LM.. Vitamin D deficiency among healthy Egyptian females. Endocrinol Nutr. 2015;62(7):314–21. doi: 10.1016/j.endonu.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361–8. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. The D-lightful vitamin D for health. J Med Biochem. 2013;32:1–10. [Google Scholar]
- 7.Parikh G, Varadinova M, Suwandhi P. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. 2010:42754–7. doi: 10.1055/s-0030-1262837. [DOI] [PubMed] [Google Scholar]
- 8.Zarnani AH, Shahbazi M, Salek-Moghaddam A, Zareie M, Tavakoli M, Ghasemi J. Vitamin D3 receptor is expressed in the endometrium of cycling mice throughout the estrous cycle. Fertil Steril. 2010;93:2738–43. doi: 10.1016/j.fertnstert.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 9.Avila E, Dìaz L, Halhali A, Larrea F. Regulation of 25- hydroxyvitamin D3 1 alpha-hydroxylase,1,25-dihydroxyvitamin D3 24-hydroxylase and vitamin D receptor gene expression by 8-bromo cyclic AMP in cultured human syncytiotrophoblast cells. J Steroid Biochem Mol Biol. 2004;89–90(1–5):115–9. doi: 10.1016/j.jsbmb.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 10.Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract. 2013;67:225–35. doi: 10.1111/ijcp.12031. [DOI] [PubMed] [Google Scholar]
- 11.Perovic Blagojevic I, Eror T, Pelivanovic J, Jelic S, Kotur- Stevuljevic J, Ignjatovic S.. Women with polycystic ovary syndrome and risk of cardiovascular disease. J Med Biochem. 2017;36:259–69. doi: 10.1515/jomb-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wild RA, Rizzo M, Clifton S, Carmina E. Dyslipidemia in PCOS: systemic review and meta-analysis. Fertil Steril. 2011;95(3):1073–9. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Rotterdam EA. Revised. consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 2003;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H. Validation of the Friedewald Equation for Evaluation of Plasma LDL-Cholesterol. J Clin Biochem Nutr. 2008;43(1):1–5. doi: 10.3164/jcbn.2008036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins NJ, Schisterman EF. The Inconsistency of »Optimal« Cutpoints Obtained using Two Criteria based on the Receiver Operating Characteristic Curve. Am J Epidemiol. 2006;163(7):670–5. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legro RS. Obesity and PCOS: Implications for diagnosis and treatment. Semin Reprod Med. 2012;30(6):496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fauser BC, Tarlatzis BC, Rebar RW. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Nestler JE, Powers LP, Matt DW. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–9. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 20.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–31. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 21.Conway GS, Honour JW, Jacobs HS. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol. 1989;30:459–70. doi: 10.1111/j.1365-2265.1989.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 22.Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. Am J Med. 2003;115:24S–8. doi: 10.1016/j.amjmed.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Dunaif A, Segal KR, Futterweit W, Dobrjansky. A profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 24.Wild RA. Dyslipidemia in PCOS. Steroids. 2012;77:295–9. doi: 10.1016/j.steroids.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–13. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 26.Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf) 2001;54(4):447–53. doi: 10.1046/j.1365-2265.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- 27.Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti- Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(3):941–5. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- 28.Lie F, Schipper I, de Jong FH, Themmen AP, Visser JA, Laven JS. Serum anti-Müllerian hormone and inhibin B concentrations are not useful predictors of ovarian response during ovulation induction treatment with recombinant follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril. 2011;96(2):459–63. doi: 10.1016/j.fertnstert.2011.05.084. [DOI] [PubMed] [Google Scholar]
- 29.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89(1):318–23. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 30.Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10:107–17. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 31.Maciel GA, Baracat EC, Benda JA. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5321–7. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 32.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240–5. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 33.Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20(7):1814–9. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- 34.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-Mullerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9(39):1–7. doi: 10.1186/1477-7827-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skałba P, Cygal A, Madej P, Dąbkowska-Huć A, Sikora J, Martirosian G. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158(2):254–9. doi: 10.1016/j.ejogrb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol. 2004;63:51–60. doi: 10.1016/j.jri.2004.01.010. Strauss JF3rd. [DOI] [PubMed] [Google Scholar]
- 38.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–85. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 39.Begawy AF, El-Mazny AN, Abou-salem NA, El-Taweel NE. Anti–Müllerian hormone in polycystic ovary syndrome and normo-ovulatory women: Correlation with clinical, hormonal and ultrasonographic parameters. Middle East Fertility Society Journal. 2010;15:253–8. [Google Scholar]
- 40.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 41.Cappy H, Giacobinib P, Pignyc P, Bruyneela A, Leroy-Billiarda M, Dewaillya D. Low vitamin D3 and high anti-Müllerian hormone serum levels in the polycystic ovary syndrome (PCOS): Is there a link? Ann Endocrinol (Paris) 2016;77(5):593–9. doi: 10.1016/j.ando.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Mumford SL, Silver R, Sjaarda LA, Galai N, Stanford J, Lynch A. Vitamin D and Ovarian Reserve and Fecund ability among Women with Proven Fecundity [abstract] The FASEB Journal. 2016;30(1):290. [Google Scholar]
- 43.Pearce K, Gleeson K, Tremellen K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum Reprod. 2015;30(9):2171–7. doi: 10.1093/humrep/dev167. [DOI] [PubMed] [Google Scholar]
- 44.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum Anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–5. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 45.Irani M, Seifer D, Minkoff H, Merhi Z. Vitamin D supplementation appears to normalize serum AMH levels in vitamin D deficient premenopausal women. Fertil Steril. 2013;100(3):S338. [Google Scholar]
- 46.Wiweko B, Maidarti M, Priangga MD, Shafira N, Fernando D, Sumapraja K. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. Journal of assisted reproduction and genetics. 2014;31:1311–6. doi: 10.1007/s10815-014-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–9. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 48.Köninger A, Koch L, Edimiris P, Enekwe A, Nagarajah J, Kasimir Bauer S. Anti-Mullerian Hormone: an indicator for the severity of polycystic ovarian syndrome. Arch Gynecol Obstet. 2014;290:1023–30. doi: 10.1007/s00404-014-3317-2. [DOI] [PubMed] [Google Scholar]
- 49.Dewailly D, Alebi M, Duhamel A, Stojanović N.. Using cluster analysis to identify a homogeneous subpopulation of women with polycystic ovarian morphology in a population of non-hyperandrogenic women with regular menstrual cycles. Hum Reprod. 2014;29(11):2536–43. doi: 10.1093/humrep/deu242. [DOI] [PubMed] [Google Scholar]


