ABSTRACT
Immune-to-brain communication has been studied in a variety of experimental models. Crucial insights into signalling and mechanisms were previously revealed in studies investigating fever induction pathways. The scientific community has primarily focused on neuronal and humoral pathways in the manifestation of this response. Emerging evidence has now shown that immune-to-brain signalling via immune cells is pivotal for normal brain function and brain pathology. The present manuscript aims to provide a brief overview on the current understanding of how immune cells signal to the brain. Insights are summarized on the potential physiological significance of some immune cells signalling from the periphery to the brain. A particular focus is laid on the role of neutrophil granulocytes. As such, IL-1β expressing neutrophil granulocytes have been shown to transfer inflammatory information to the brain and contribute to prolonged behavioural changes due to septic encephalopathy in rats during severe systemic inflammation induced by the bacterial component and TLR4 agonist lipopolysaccharide. Modulation of immune cell recruitment to the brain is discussed by various confounding factors including sleep, exercise, the nutritional status e.g. obesity, leptin and omega 3 fatty acids, and psychological or inflammatory stressors. The physiological significance of immune cell mediated communication between the immune system and the brain is highlighted by the fact that systemic inflammatory insults can exacerbate ongoing brain pathologies via immune cell trafficking. New insights into mechanisms and mediators of immune cell mediated immune-to-brain communication are important for the development of new therapeutic strategies and the better understanding of existing ones.
Abbreviations: ACTH: adrenocorticotropic hormone; BBB: blood–brain barrier; BBI: blood–brain interface; CD: cluster of differentiation; CINC: cytokine-induced neutrophil chemoattractant; CRH: corticotropin releasing hormone; CVOs: circumventricular organs; CXCR: chemokine receptor; DAPI: 40:6-diamidino-2-phenylindole dilactate; DHA: docosahexaenoid acid; ICAM: intracellular adhesion molecule; IL: interleukin; i.p.: intraperitoneal; i.v.: intravenous; KC: keratinocytes-derived chemokine; LPS: lipopolysaccharide; MIP: macrophage inflammatory protein; MS: multiple sclerosis; NFκB: nuclear factor kappa B; NF-IL6: nuclear factor IL-6; PCTR: protectin conjugates in tissue regeneration; PG: prostaglandin; p.i.: post injection; PVN: paraventricular nucleus; ra: receptor antagonist; STAT3: signal transducer and activator of transcription 3; TIMP: tissue inhibitors of metalloproteinases; TLR: toll-like receptor; TNFα: tumor necrosis factor alpha.
KEYWORDS: Immune-to-brain communication, immune cell trafficking, neutrophil granulocytes, macrophages, systemic inflammation, leptin, inflammatory transcription factors, extravasation, cytokines
Introduction
The discovery that tissue transplantation into the brain does not elicit graft rejection reinforced the assumption of the brain as an immune privileged organ [1,2]. It took several decades to establish that the brain is somewhat privileged in its integrated interaction with the immune system. This mode of communication follows some very specific rules such as delayed and tightly controlled immune cell recruitment and the protection of neurons from potential toxic circulating substances. The skull does not enable much flexibility and, thus, inflammatory accumulation of fluid (i.e. oedema in the brain) is associated with life threatening consequences depending on the brain structures involved. Even though the bidirectional interaction of the immune system with the brain has been studied for a long time, knowledge on mechanisms significantly expanded only when cytokines, mediators of the immune system, were characterized [3]. Immune-to-brain communication has been reviewed in depth in previous manuscripts [3–6]. Therefore, underlying mechanisms will only be briefly introduced for the unfamiliar reader. The pathways that transfer information between the immune system and the central nervous system (Figure 1) include:
- So-called humoral i.e. plasma mediators including cytokines [7] or lipid mediators such as prostaglandin (PG)E2 [8].
- These can signal via brain structures with a leaky blood-brain barrier (BBB) i.e. circumventricular organs (CVOs) [9] to convey the information to the brain.
- In addition, circulating mediators like the pro- or anti-inflammatory cytokines interleukin (IL)-6, tumor necrosis factor (TNF)α, and IL-1β or IL-10, and IL-1 receptor antagonist (ra), respectively, can be transported through the BBB into the brain to alter brain function [13];
Recruitment of peripheral immune cells (reviewed here)
Figure 1.
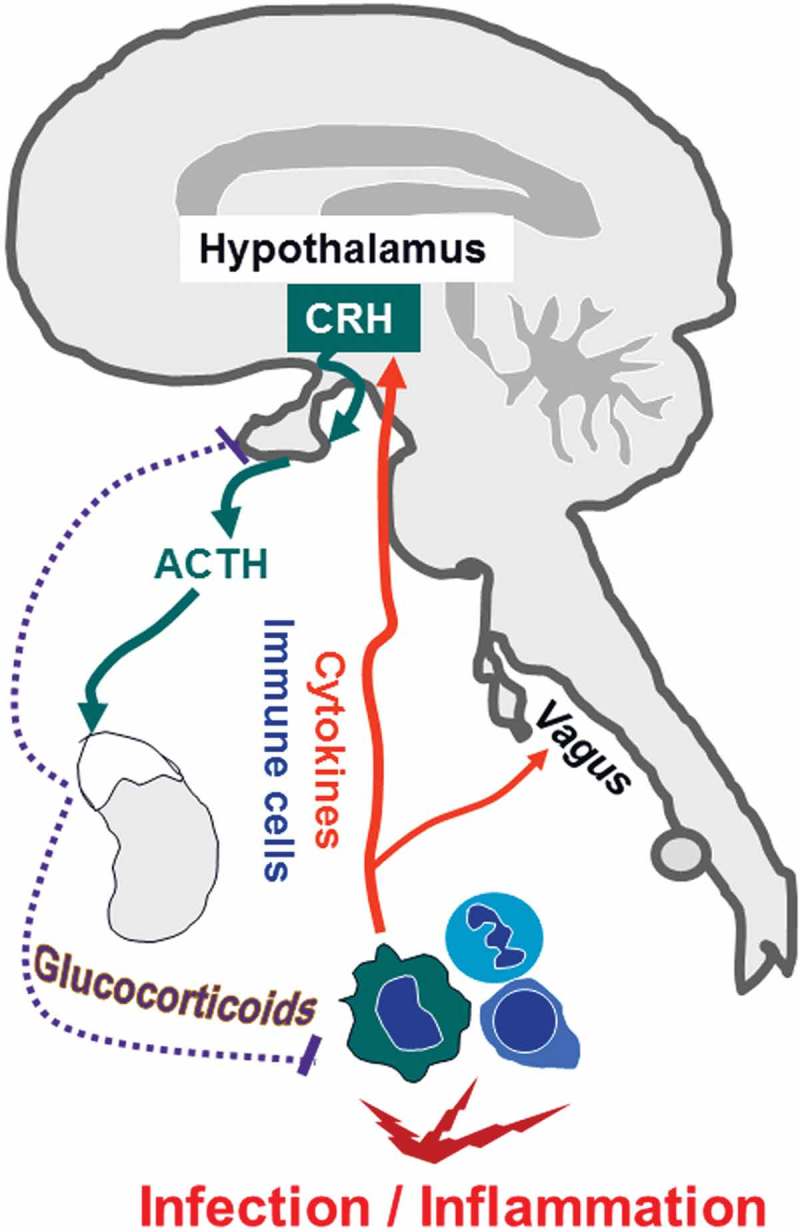
Simplified schematic illustration of immune-to-brain communication. Infection and inflammation stimulate immune cells to produce cytokines. These activate neuronal sensory afferences for example of the vagus nerve. Moreover, cytokines and immune cells directly act on the brain i.e. endothelial cells, brain structures with a leaky blood-brain barrier, namely, the circumventricular organs, meninges and the choroid plexus. Subsequently, brain-controlled sickness responses develop. In the paraventricular nucleus of the hypothalamus (PVN), corticotropin releasing hormone (CRH) is produced and released to stimulate the release of adrenocorticotropic hormone (ACTH) derived from the anterior pituitary into the circulation. ACTH induces an increase in glucocorticoids from the adrenal cortex. This hypothalamus-pituitary-adrenal-axis represents one of the endogenous feedback mechanisms to dampen systemic inflammation. Glucocorticoids are known to exhibit some of their effects dampening the activated immune system by inhibition of inflammatory transcription factors (partially adapted and modified from [112,113]).
Immune cells actually play an increasingly recognized role in the normal brain. For example, dendritic cells have recently been shown to migrate from the brain into the cervical lymph nodes and modulate regulatory T-cell function with relevance for brain autoinflammatory diseases [18]. Yet, the amount of immune cell trafficking into the healthy brain parenchyma is rather low and tends to be higher in meninges, the choroid plexus or the cerebrospinal fluid [19]. Here, we aim to provide a brief overview on the emerging significance of immune cell trafficking for signalling from the periphery to the brain with special focus on potential effects of inflammatory or psychological stressors and neutrophil granulocytes.
A role for immune cells in immune-to-brain communication
Significant invasion of immune cells into the brain is a hallmark of several brain pathologies like stab trauma [20], bacterial meningitis [21], cerebral alterations following experimental multiple trauma [22], and diseases including multiple sclerosis (MS) [23] or viral encephalitis [24,25] and septic encephalopathy induced by a high dose of lipopolysaccharide (LPS, i.v., 5mg/kg, 4h post injection [p.i.]) [15]. Immune cells are recruited to the brain and locally release mediators that contribute to the inflammatory process and modulate brain function [5]. Such processes are often accompanied by leakiness of the BBB, which was initially believed to be insurmountable for immune cells [2]. However, immune cell trafficking does not necessitate opening of the BBB; also transcellular movement of immune cells does occur in the absence of changes to the barrier function of tight junctions [26,27]. Recently, the term BBB has even been challenged and blood-brain interface (BBI) suggested as a replacement since it can better account for most of the actual functions that deal with protection of the brain’s inner milieu and its communication with other body compartments [19,28]. Obviously, communication only works when both partners respond to each other. Indeed, brain cells can for example express MHC molecules to interact with lymphocytes [29]. Such interaction is further illustrated by a few examples on selected immune cell types and their role in immune-to-brain communication:
Lymphocytes
Autoreactive T-lymphocytes are for instance involved in the pathogenesis of MS and are recruited to the CNS [30]. However, T-cells also patrol within the healthy brain as a component of brain physiology and homeostasis [23,31]. It has even been demonstrated that T-cells contribute to hippocampus-dependent spatial learning tasks in the Morris water maze paradigm by the release of IL-4 [32]. Moreover, T-cells can also have some supportive modulatory function by orchestrating the removal of debris in order to terminate local neurotoxic inflammatory responses in the brain [33].
Monocytes
Rivest and colleagues proposed that bone marrow derived monocytes will regularly and continuously replenish brain microglial cells in the healthy brain to maintain integrity [34]. However, the radiation protocols applied in these studies to eliminate these cells were accompanied by confounding leakiness of the BBB and such a scenario seems to only be of relevance in cases of a compromised integrity of the BBB during disease [35] or after radiation [31]. Otherwise microglia replenishment stems from functional local brain intrinsic sources [36]. Recently, macrophages have been recognized as important immune-to-brain signals during repeated social defeat models in rodents [37]. Such stressors engage specific neuronal circuits within the hypothalamus, the prefrontal cortex, the hippocampus and the amygdala [38]. Neuronal activity leads to microglial and brain endothelial activation i.e. expression of chemokines and endothelial adhesion molecules [e.g. intracellular adhesion molecule (ICAM)1], respectively [39]. Simultaneously, stress increases myelopoiesis and enhances the number of monocytes but also neutrophil granulocytes in the circulation via the release of catecholamines [40,41] by the sympathetic nervous system [42]. Subsequently, young “inflammatory” IL-1β expressing monocytes are recruited to the brain and primarily reside in the perivascular space, meninges or choroid plexus and interact with the brain endothelium [37,39]. They activate brain endothelial cells via an IL-1β dependent mechanism in the same brain structures that show neuronal activity during stress and contribute to repeated social defeat stress-induced anxiety [39]. Similarly, spleen-to-brain recruitment of monocytes (48h) and T-cells (96h) can exacerbate stroke [43] in a noradrenergic dependent manner [44]. Moreover, peripheral bile duct ligation-induced liver inflammation has been demonstrated to induce monocyte recruitment to the brain via a TNFα dependent mechanism 10 days after the insult [45] and TNFα secreting macrophages that are recruited to the brain in cholestatic mice may be associated with the sickness response like fatigue that occurs during cholestasis [46].
Recently, we have been able to show higher numbers of CD68-postive macrophages in the posterior pituitary lobe of aged rats, which were partially nuclear factor IL-6 activated during systemic LPS-induced (i.p., 100µg/kg, 24h p.i.) inflammation [47]. These results suggested that immune cell recruitment during aging could contribute to the higher inflammatory status of the aged brain. Indeed, depending on the immune stimulus (IL-1β versus LPS, each i.v. at 2µg/kg, 2-4h p.i.) perivascular cells seem to participate in the activation or inhibition of the hypothalamus-pituitary-adrenal axis [48], one of the negative feedback mechanisms in immune-to-brain communication (see also Figure 1).
In general, the physiological significance of macrophage recruitment to the brain remains only partially understood but pertains to amplification and modulation of brain inflammatory responses and subsequent behavioural or homeostatic consequences.
Neutrophil granulocytes
Earlier studies by Perry and colleagues investigated the recruitment of immune cells to the brain during inflammation (e.g. into the brain, 0.02µg/0.2µg/2µg LPS, 100/50,000 units IL-1β or 104 units TNFα, per mouse) and revealed that neutrophil granulocytes and monocytes show age dependent delayed invasion into the brain (2 ~ 72h p.i. for LPS and 4-24h p.i. for cytokines) with lower numbers than in peripheral organs [49–52]. We further revealed that neutrophil recruitment to the brain during systemic inflammation induced by the toll-like receptor 4 agonist LPS (i.p., 2.5mg/kg) is time controlled (3h, 24h, 48h p.i.) and region specific [53]. Brain sites that show prominent staining for neutrophil granulocytes in saline perfused mouse brains include the ventral lining of the hypothalamus, areas surrounding ventricles, meninges, the hemisphere commissure, a broader distribution in small and large vessels of the brain stem and specific brain structures like the paraventricular nucleus (PVN; see Figure 2). Indeed, neutrophil specific chemokines like cytokine-induced neutrophil chemoattractant (CINC)1 can be induced in specific brain regions like the PVN for example by immobilization stress [54]. Such CINC1-induction in the PVN might also explain why neutrophils get recruited to this brain structure during systemic LPS-induced (i.p., 2.5mg/kg) inflammation ~ 24-48h after stimulation [53]. The adhesion molecule ICAM1 is already significantly increased in this brain structure as early as 4.5 hours after stimulation (Figure 2(c)), which is the next step after chemokine action for attachment of neutrophils to the brain vasculature, and which we observed in the perfused brains (Figure 2(c,d)). Similarly to macrophages, brain recruited neutrophils show IL-1β immunoreactivity [55,56], which most likely represents one of the mediators, and contributes to alterations of brain function by immune-to-brain signalling. Interestingly, the IL-1 type I receptor is also crucial for recruitment of leukocytes to the brain [57].
Figure 2.
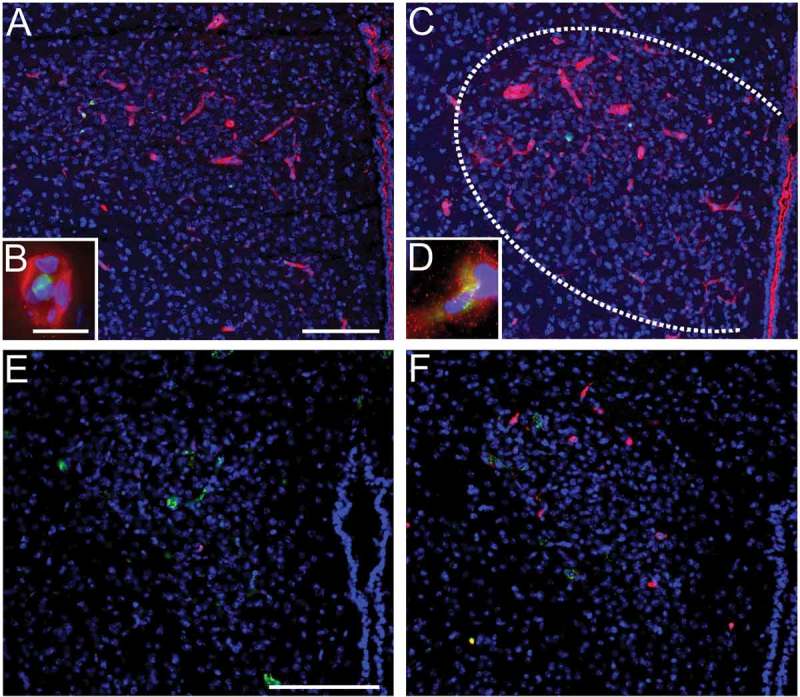
LPS-induced systemic inflammation increase ICAM1-immunoreactivity and induces the recruitment of neutrophil granulocytes to the brain in the paraventricular nucleus (PVN). LPS (5 mg/kg i.p., 4.5h) induces ICAM1-immunoreactivity (red, a-d) in the PVN (c) compared to controls (a). Close association of ICAM1-immunoreactivity with myeloperoxidase (green) staining (a-d) is depicted in insets (b, d). In addition, LPS-stimulation (2.5 mg/kg 24h) increased the number of Ly-6B.2 alloantigen (clone 7/4) stained neutrophil granulocytes in the PVN (red, f) compared to controls (e). Von Willebrand factor (green, e-f) depicts brain vasculature. Blue DAPI staining visualizes the surrounding tissue (blue, a-f). Scale bars represent 100µm and 10µm in insets. The previously unpublished microphotographs were adapted from our own previous studies [15,53].
Several studies investigated the functional significance of neutrophil invasion to brain pathology. As such, inhibition of neutrophil recruitment by neutralizing antibodies against the chemokine receptor CXCR2, which is for example activated by CINC1, ameliorated the disease in an animal model for atypical autoimmune encephalomyelitis [58]. Others investigated the role for neutrophil granulocytes during spinal cord injury and found mixed results; detrimental for tissue regeneration but also beneficial for promoting functional recovery [59] most likely depending on the timing during programing of the inflammatory process [60]. Interestingly, induction of neutropenia by a neutralizing antibody during severe LPS-induced systemic inflammation (i.p., 2.5mg/kg) revealed that such IL-1β-expressing neutrophils contribute to the induction and/or maintenance of depression-like behaviours 48h after LPS-stimulation [56]. Thus, in addition to macrophages [39,45] neutrophils are also important in transmitting inflammatory information to the brain specifically during systemic inflammation [56].
Models of systemic inflammation can exacerbate ongoing brain pathologies via immune-cell mediated immune-to-brain signalling
Systemic inflammation can trigger and worsen pre-existing brain pathologies and accompanying sequelae. For example, systemic LPS-induced inflammation (i.p., 0.5mg/kg) has been shown to enhance monocyte recruitment (4h p.i) and microglia activation (4h, 24h p.i.) in socially defeated animals, which lead to an enhanced sickness response i.e. exaggerated weight loss (48h, 72h p.i.) and prolonged social withdrawal (24h p.i.) [61]. Experimental influenza infection in mice was shown to increase trafficking of T-lymphocytes, monocytes and neutrophil granulocytes to the brain and may exacerbate MS [62]. During a stroke, where neutrophils seem to be preferentially recruited from the nearby skull bone marrow [63], LPS-induced systemic inflammation (i.p. 100µg/kg, 24h p.i.) potentiates the acute phase via an IL-1β and neutrophil dependent mechanism [64,65]. In such a stroke model, macrophage depletion reduced subsequent ischemic brain injury [66]. Clinical data also suggests a higher risk of stroke during systemic inflammation e.g. influenza, infection of the urinary tract or pneumonia [64]. Moreover, chronic neurodegeneration in a model of prion disease is exacerbated by systemic LPS stimulation (i.p., 500µg/kg) 18h after injection [67]. Osteoarthritis seems to accelerate a model of Alzheimer’s disease in mice [68] or ulcerative colitis exacerbates LPS-induced (2µg/mouse, one week p.i.) damage in the substantia nigra as a model for Parkinson’s disease where macrophage depletion ameliorated the response [69]. Thus, systemic inflammation can affect chronic neurodegeneration [70] partially related to immune-cell trafficking to the brain. Even low grade inflammation as observed during obesity [71] has been shown to be accompanied by the recruitment of macrophages to the brain, which may contribute to the brain inflammatory response in obesity [72]. As already mentioned, IL-1β represents an important mediator involved in the recruitment of leukocytes to the brain. For example, chronic systemic adenovirus induced expression of IL-1β aggravated central neuroinflammation and was associated with enhanced neutrophil invasion [73]. Importantly, not all types of inflammation seem to have detrimental influences. Indeed, Quan and colleagues revealed a new model of inflammation with potential beneficial or protective effects termed euflammation. Animals that were pretreated with repeated subthreshold infectious challenges in the periphery (i.e. euflammation) showed lower cytokine expression in the brain and reduced cytokine levels in circulation (3h p.i.) as well as morphological alteration of microglia (24h p.i.) after a systemic LPS- or Escherichia coli-challenge (i.p., 250µg/kg or 100 × 107 colony forming units) [74]. Overall, better understanding of immune-to-brain communication will help to improve our knowledge about underlying mechanisms. New therapeutic approaches can be developed and risk assessments improved for the exaggerated effects of peripheral inflammation on ongoing brain pathologies.
Neutrophil granulocytes and immune-to-brain communication
As already mentioned, neutrophil granulocytes increase in the circulation after psychological stress or peripheral inflammation. However, additional factors are needed for induction of neutrophil extravasation. This process involves the attraction and activation of neutrophils by a concentration gradient of chemokines. Neutrophil specific chemokines in rodents include:
CXCLl also called CINC1 and keratinocytes-derived chemokine (KC),
CXCL2 namely macrophage inflammatory protein (MIP)-2 [53,75] or
CXCL8 (IL-8) in humans.
Brain endothelial cells are activated by cytokines and chemokines to express a vast amount of adhesion molecules including ICAM1, which is a prerequisite for neutrophil diapedesis into the brain tissue. Indeed, ICAM1 deficiency dramatically reduced the number of infiltrating neutrophils during LPS-induced (i.p., 0.5mg/kg, 4 times every 12 hours) systemic inflammation [76]. For further migration of these immune cells to occur they will need to gain entry through the tight network of extracellular matrices for which a variety of metalloproteinases are important. Reflecting the high relevance of the whole process some feedback control is governed by tissue inhibitors of metalloproteinases (TIMP) like TIMP1, which is involved in leukocyte infiltration into the brain [77]. Last but not least brain recruited neutrophils exhibit modulatory effects on the brain by their known immune cell functions that also involve the aforementioned production of cytokines including IL-1β but also IL-1ra [78] and IL-8 [79]. Moreover, proteases and the release of decondensated DNA from neutrophil granulocytes that were recruited to the brain play a role in the inflammatory processes in the brain [80]. However, aggregated neutrophil extracellular traps can also contribute to the resolution of neutrophilic inflammation such as by degrading IL-1β [81]. Similarly to the pro-inflammatory M1 phenotype of macrophages and the anti-inflammatory phenotype of M2 macrophages, N1 and N2 neutrophils have been described (for more details on neutrophil granulocytes see [82]).
Modulation of immune cell trafficking
Exciting research by Evans and colleagues revealed that fever-range thermal stress regulates ICAM-1-dependent lymphocyte extravasation in high endothelial venules of lymphoid organs by IL-6 trans signalling via its soluble receptor [83]. Thus, body core temperature can modulate accessibility of immune cells for immune cell trafficking. A recent study showed that neutrophil recruitment to the brain and apoptosis could be attenuated 24h after striatal IL-1β injection (100ng/animal) by β2-adrenergic stimulation with clenbuterol pre-treatment [84]. Moreover, adrenergic signalling not only alters immune cell recruitment and migration [3,39] but also plasma glucose concentration. Interestingly, the availability of glucose in the circulation can modulate immune-cell trafficking [85] depending on circadian changes and the distribution of energy resources [86]. For example, acute hyperglycaemia induced by dextrose injection dramatically enhanced brain neutrophil recruitment in a rat brain ischemia model, which could exacerbate brain injury [87]. Related to similar mechanisms of regulation (e.g. involvement of glucocorticoids and noradrenalin and changes in circulating glucose levels), exercise and sleep are connected to changes in leukocyte trafficking. Reallocation of energy fuels to the immune system contributes to sleep recovery function [86]. Sleep and circadian rhythms regulate the circulating and reserve pools of leukocytes [88] and even one night of sleep loss is sufficient to increase the number of circulating neutrophils the following morning [89]. Exercise for example mobilizes neutrophil granulocytes, which show increased expression of activation markers (CD11b) and may enhance the redistribution and destruction of old immune cells [90]. Underlying mechanism of exercise on immune cell trafficking involve changes in adhesion molecules [90] and chemokine receptor expression [91]. As a result, previous exercise reduced neutrophil infiltration to the injury side were accompanied by attenuated secondary damage in a model of severe traumatic brain injury [92].
The regulation of extravasation is orchestrated by differential transcriptional regulation in the brain and in immune cells, examples include cytokines, chemokines, adhesion molecules and metalloproteinases. Pivotal inflammatory transcription factors involved in this response include nuclear factor (NF)κB, signal transducer and activator of transcription (STAT)3 and NF-IL6 [5]. STAT3 has been shown to play a role in neutrophil recruitment in other organ systems other than the brain [93] and in neutrophil function [94]. ICAM1 expression can be induced by STAT3- or NFκB- [95] and modulated by NF-IL6-dependant pathways [96]. In a model of acute brain injury hepatic NFκB activation was necessary for the recruitment of neutrophil granulocytes to the brain [97]. Our own experiments using NF-IL6 deficient mice revealed that neutrophil recruitment to the brain during systemic LPS-induced inflammation (i.p., 2.5mg/kg) was transiently reduced by NF-IL6 deficiency most likely due to reduced CXCL-1 expression 8h after stimulation [98]. Moreover, neutrophil invasion was reduced overall in NF-IL6 deficient mice during ischemic brain injury [99]. In addition, wild type but not NF-IL6 deficient animals showed LPS-stimulated increased numbers of perivascular macrophages in the subfornical organ [98]. NF-IL6 and NF-kB also play important roles for cytokine/chemokine induction within neutrophils like for IL-8 expression [100]. Overall, strategies to inhibit these transcription factors remain potential therapeutic targets for modulation of immune-cell recruitment.
Using STAT3-immunohistochemistry as a brain cell activation marker during systemic LPS-induced (i.p., 50 µg/kg, 3h p.i.) inflammation, we were able to show for the first time that the appetite and energy expenditure regulating, cytokine-like hormone leptin can directly act on the blood brain interface along large and small blood vessels, surrounding the ventricles, in the meninges and in specific brain structures including the ventromedial preoptic area [101]. This astonishing observation and the fact that others have shown direct action of leptin on neutrophils via the short form of its receptor [102,103] led us hypothesize that leptin might be involved in neutrophil recruitment during LPS-induced systemic inflammation [104]. Indeed, we revealed a contribution of leptin to LPS-induced (i.p., 2.5mg/kg, 24h p.i.) neutrophil recruitment to the brain via modulation of IL-β, ICAM and chemokine expression [53]. A subsequent study confirmed these results and discovered that acute neutralisation of endogenous circulating leptin during severe LPS-induced (i.p., 2.5mg/kg, 48h p.i.) inflammation or depletion of neutrophil granulocytes prevented long term behavioural changes as a consequence of septic encephalopathy [56].
Efferent and/or afferent vagus nerve stimulation has emerged as a potential anti-inflammatory tool to treat brain pathologies [105,106]. Recently, we revealed that efferent electrical stimulation of the vagus nerve might exhibit some of its anti-inflammatory effects via inhibition of interaction between neutrophil granulocytes and ICAM1 in the brain vasculature during severe septic-like LPS-induced (i.v., 5mg/kg, 4h p.i.) inflammation [15]. Indeed, attenuated neurovascular coupling and loss of brain function during early LPS-induced (i.v., 5mg/kg, 4h p.i.) septic encephalopathy [107] are both stabilized by vagus nerve stimulation as investigated by somatosensory evoked potentials and evoked flow velocity response [15,108]. Interestingly, LPS-induced increases in circulating leptin levels were reduced by vagus nerve stimulation [15]. Thus, as discussed above, lower plasma leptin might represent an underlying mechanism on how neutrophil recruitment to the brain is altered by vagus nerve stimulation [53]. Gautron and colleagues just established that perivascular cells express the leptin receptor with potential implications for immune cell trafficking to and within the brain [109].
Since recent years, specialized proresolving lipid mediators, metabolites derived from omega 3 fatty acids like docosahexaenoid acid (DHA), have been shown to regulate leukocyte trafficking [110]. Macrophage infiltration and phagocytosis was enhanced and neutrophil recruitment inhibited for example by DHA derived protectin conjugates in tissue regeneration (PCTR) during microbial-induced peritonitis [111].
Conclusion
In conclusion, immune cell mediated immune-to-brain communication has evolved to be more than a bystander effect of brain inflammation but a crucial signalling pathway that is not yet completely understood. We follow the exciting new evidence that macrophages and in particular neutrophil granulocytes can transfer inflammatory information to the brain and modulate brain function in the frame of homeostasis and pathophysiology. We believe that better understanding of underlying mechanisms will enable us to improve current therapeutic strategies. Advances in the management of risk factors like systemic inflammation on ongoing brain pathologies can be achieved. Finally, we seek to increase knowledge on the modulatory capacities of a variety of factors like the nutritional status as reflected by leptin, omega 3 fatty acids or obesity, sleep disturbances or psychological and inflammatory stressors on immune-to-brain signalling and brain pathologies.
Acknowledgments
We thank D. Marks for excellent technical assistance to prepare Figure 2(a-d). G. Luheshi and W. Inoue were involved in the development of the projects on neutrophil recruitment and leptin.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. PMID: 18865105. [PMC free article] [PubMed] [Google Scholar]
- [2].Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. PMID: 345773. [PubMed] [Google Scholar]
- [3].Dantzer R. Neuroimmune Interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477–504. PMID: 29351513; PMCID: PMC5866360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–238. PMID: 21334376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rummel C. Inflammatory transcription factors as activation markers and functional readouts in immune-to-brain communication [Review]. Brain Behav Immun. 2016;54:1–14. PMID: 26348582. [DOI] [PubMed] [Google Scholar]
- [6].D’Mello C, Swain MG. Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr Top Behav Neurosci. 2017;31:73–94. PMID: 27677781. [DOI] [PubMed] [Google Scholar]
- [7].Conti B, Tabarean I, Andrei C, et al. Cytokines and fever. Front Biosci. 2004;9:1433–1449. PMID: 14977558. [DOI] [PubMed] [Google Scholar]
- [8].Steiner AA, Ivanov AI, Serrats J, et al. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006;4(9):e284 PMID: 16933973; PMCID: PMC1551923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roth J, Harre EM, Rummel C, et al. Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci. 2004;9:290–300. PMID: 14766367. [DOI] [PubMed] [Google Scholar]
- [10].Rummel C, Voss T, Matsumura K, et al. Nuclear STAT3 translocation in guinea pig and rat brain endothelium during systemic challenge with lipopolysaccharide and interleukin-6. J Comp Neurol. 2005;491(1):1–14. PMID: 16127698. [DOI] [PubMed] [Google Scholar]
- [11].Rummel C, Sachot C, Poole S, et al. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1316–26. PMID: 16809483. [DOI] [PubMed] [Google Scholar]
- [12].Eskilsson A, Mirrasekhian E, Dufour S, et al. Immune-induced fever is mediated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J Neurosci. 2014;34(48):15957–15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241–248. PMID: 8963753. [DOI] [PubMed] [Google Scholar]
- [14].Goehler LE, Gaykema RP, Hansen MK, et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85(1–3):49–59. PMID: 11189026. [DOI] [PubMed] [Google Scholar]
- [15].Schweighofer H, Rummel C, Roth J, et al. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Intensive Care Med Exp. 2016;4(1):19 PMID: 27357828; PMCID: PMC4927529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rummel C, Barth SW, Voss T, et al. Localized vs. systemic inflammation in guinea pigs: a role for prostaglandins at distinct points of the fever induction pathways? Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R340–R347. PMID: 15831768. [DOI] [PubMed] [Google Scholar]
- [17].Ross G, Roth J, Storr B, et al. Afferent nerves are involved in the febrile response to injection of LPS into artificial subcutaneous chambers in guinea pigs. Physiol Behav. 2000;71(3–4):305–313. PMID: 11150562. [DOI] [PubMed] [Google Scholar]
- [18].Mohammad MG, Tsai VW, Ruitenberg MJ, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124(3):1228–1241. PMID: 24569378; PMCID: PMC3934177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Galea I, Perry VH. The blood-brain interface: a culture change. Brain Behav Immun. 2018;68:11–16. PMID: 29107155. [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Moges H, Bharucha Y, et al. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp Neurol. 2007;203(1):168–184. PMID: 16996058. [DOI] [PubMed] [Google Scholar]
- [21].Kim KS. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci. 2003;4(5):376–385. PMID: 12728265. [DOI] [PubMed] [Google Scholar]
- [22].Vogt N, Herden C, Roeb E, et al. Cerebral alterations following experimental multiple trauma and hemorrhagic shock. Shock. 2018;49(2):164–173. PMID: 28682946. [DOI] [PubMed] [Google Scholar]
- [23].Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113(4):477–485. PMID: 16550326. [DOI] [PubMed] [Google Scholar]
- [24].Andrews DM, Matthews VB, Sammels LM, et al. The severity of murray valley encephalitis in mice is linked to neutrophil infiltration and inducible nitric oxide synthase activity in the central nervous system. J Virol. 1999;73(10):8781–8790. PMID: 10482632; PMCID: PMC112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ludlow M, Kortekaas J, Herden C, et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131(2):159–184. PMID: 26659576; PMCID: PMC4713712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Engelhardt B. Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ. 2006;43:259–280. PMID: 17068976. [DOI] [PubMed] [Google Scholar]
- [27].Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109(2):181–190. PMID: 15549331. [DOI] [PubMed] [Google Scholar]
- [28].Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nature Rev Drug Discov. 2016;15(4):275–292. PMID: 26794270. [DOI] [PubMed] [Google Scholar]
- [29].McAllister AK, van de Water J. Breaking boundaries in neural-immune interactions. Neuron. 2009;64(1):9–12. PMID: 19840540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bartholomaus I, Kawakami N, Odoardi F, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462(7269):94–98. PMID: 19829296. [DOI] [PubMed] [Google Scholar]
- [31].Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64(1):110–122. PMID: 19840553; PMCID: PMC2834890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080. PMID: 20439540; PMCID: PMC2867291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ron-Harel N, Cardon M, Schwartz M. Brain homeostasis is maintained by “danger” signals stimulating a supportive immune response within the brain’s borders. Brain Behav Immun. 2011;25(5):1036–1043. PMID: 21182929. [DOI] [PubMed] [Google Scholar]
- [34].Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9(6):429–439. PMID: 19461673. [DOI] [PubMed] [Google Scholar]
- [35].Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. PMID: 18026096. [DOI] [PubMed] [Google Scholar]
- [36].Ajami B, Bennett JL, Krieger C, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. PMID: 18026097. [DOI] [PubMed] [Google Scholar]
- [37].Wohleb ES, McKim DB, Sheridan JF, et al. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2015;8:447 .PMID: 25653581; PMCID: PMC4300916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wohleb ES, Hanke ML, Corona AW, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. PMID: 21525267; PMCID: PMC3160240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McKim DB, Weber MD, Niraula A, et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23(6):1421–1431. PMID: 28373688; PMCID: PMC5628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dhabhar FS, Malarkey WB, Neri E, et al. Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: a tale of three hormones–curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. PMID: 22727761; PMCID: PMC3412918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Powell ND, Sloan EK, Bailey MT, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574–16579. PMID: 24062448; PMCID: PMC3799381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hanke ML, Powell ND, Stiner LM, et al. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26(7):1150–1159. PMID: 22841997; PMCID: PMC3506115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seifert HA, Hall AA, Chapman CB, et al. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7(4):1017–1024. PMID: 23054371; PMCID: PMC3518577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ajmo CT Jr., Collier LA, Leonardo CC, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218(1):47–55. PMID: 19371742; PMCID: PMC2720830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29(7):2089–2102. PMID: 19228962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kerfoot SM, D’Mello C, Nguyen H, et al. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology. 2006;43(1):154–162. PMID: 16374849. [DOI] [PubMed] [Google Scholar]
- [47].Koenig S, Bredehoft J, Perniss A, et al. Age dependent hypothalamic and pituitary responses to novel environment stress or lipopolysaccharide in rats. Front Behav Neurosci. 2018;12:55 .PMID: 29615881; PMCID: PMC5868128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Serrats J, Schiltz JC, Garcia-Bueno B, et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65(1):94–106. PMID: 20152116; PMCID: PMC2873837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Andersson PB, Perry VH, Gordon S. The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues. Neuroscience. 1992;48(1):169–186. PMID: 1584421. [DOI] [PubMed] [Google Scholar]
- [50].Andersson PB, Perry VH, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med. 1992;176(1):255–259. PMID: 1613459; PMCID: PMC2119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Anthony DC, Bolton SJ, Fearn S, et al. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120(Pt 3):435–444. PMID: 9126055. [DOI] [PubMed] [Google Scholar]
- [52].Lawson LJ, Perry VH. The unique characteristics of inflammatory responses in mouse brain are acquired during postnatal development. Eur J Neurosci. 1995;7(7):1584–1595. PMID: 7551185. [DOI] [PubMed] [Google Scholar]
- [53].Rummel C, Inoue W, Poole S, et al. Leptin regulates leukocyte recruitment into the brain following systemic LPS-induced inflammation. Mol Psychiatry. 2010;15(5):523–534. PMID: 19773811. [DOI] [PubMed] [Google Scholar]
- [54].Sakamoto Y, Koike K, Kiyama H, et al. A stress-sensitive chemokinergic neuronal pathway in the hypothalamo-pituitary system. Neuroscience. 1996;75(1):133–142. PMID: 8923529. [DOI] [PubMed] [Google Scholar]
- [55].Proescholdt MG, Chakravarty S, Foster JA, et al. Intracerebroventricular but not intravenous interleukin-1beta induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience. 2002;112(3):731–749. PMID: 12074914. [DOI] [PubMed] [Google Scholar]
- [56].Aguilar-Valles A, Kim J, Jung S, et al. Role of brain transmigrating neutrophils in depression-like behavior during systemic infection. Mol Psychiatry. 2014;19(5):599–606. PMID: 24126927. [DOI] [PubMed] [Google Scholar]
- [57].Ching S, He L, Lai W, et al. IL-1 type I receptor plays a key role in mediating the recruitment of leukocytes into the central nervous system. Brain Behav Immun. 2005;19(2):127–137. PMID: 15664785. [DOI] [PubMed] [Google Scholar]
- [58].Stoolman JS, Duncker PC, Huber AK, et al. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J Immunol. 2014;193(2):564–570. PMID: 24928987; PMCID: PMC4091641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Neirinckx V, Coste C, Franzen R, et al. Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation. 2014;11:150 PMID: 25163400; PMCID: PMC4174328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Saghazadeh A, Rezaei N. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother. 2017;92:128–139. PMID: 28535416. [DOI] [PubMed] [Google Scholar]
- [61].Wohleb ES, Fenn AM, Pacenta AM, et al. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505 PMID: 22386198; PMCID: PMC3368999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Blackmore S, Hernandez J, Juda M, et al. Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2017;114(30):E6107–E6116. PMID: 28696309; PMCID: PMC5544260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Herisson F, Frodermann V, Courties G, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018. PMID: 30150661 DOI: 10.1038/s41593-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McColl BW, Allan SM, Rothwell NJ. Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans. 2007;35(Pt5):1163–1165. PMID: 17956302. [DOI] [PubMed] [Google Scholar]
- [65].McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158(3):1049–1061. PMID: 18789376. [DOI] [PubMed] [Google Scholar]
- [66].Ma Y, Li Y, Jiang L, et al. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice. J Neuroinflammation. 2016;13:38 PMID: 26873581; PMCID: PMC4752808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cunningham C, Wilcockson DC, Campion S, et al. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. PMID: 16207887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kyrkanides S, Tallents RH, Miller JN, et al. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. J Neuroinflammation. 2011;8:112 PMID: 21899735; PMCID: PMC3179730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Villaran RF, Espinosa-Oliva AM, Sarmiento M, et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease. J Neurochem. 2010;114(6):1687–1700. PMID: 20584104. [DOI] [PubMed] [Google Scholar]
- [70].Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–167. PMID: 17220915. [DOI] [PubMed] [Google Scholar]
- [71].Rummel C, Bredehoft J, Damm J, et al. Obesity impacts fever and sickness behavior during acute systemic inflammation. Physiology. 2016;31(2):117–130. PMID: 26889017. [DOI] [PubMed] [Google Scholar]
- [72].Buckman LB, Hasty AH, Flaherty DK, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. PMID: 23831150; PMCID: PMC3858467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Murta V, Farias MI, Pitossi FJ, et al. Chronic systemic IL-1beta exacerbates central neuroinflammation independently of the blood-brain barrier integrity. J Neuroimmunol. 2015;278:30–43. PMID: 25595250. [DOI] [PubMed] [Google Scholar]
- [74].Liu X, Nemeth DP, Tarr AJ, et al. Euflammation attenuates peripheral inflammation-induced neuroinflammation and mitigates immune-to-brain signaling. Brain Behav Immun. 2016;54:140–148. PMID: 26812118; PMCID: PMC4828265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27(16):4403–4412. PMID: 17442825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bohatschek M, Werner A, Raivich G. Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp Neurol. 2001;172(1):137–152. PMID: 11681847. [DOI] [PubMed] [Google Scholar]
- [77].Crocker SJ, Whitmire JK, Frausto RF, et al. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006;169(6):2104–2116. PMID: 17148673; PMCID: PMC1762490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].McColl SR, Paquin R, Menard C, et al. Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med. 1992;176(2):593–598. PMID: 1386877; PMCID: PMC2119339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gainet J, Chollet-Martin S, Brion M, et al. Interleukin-8 production by polymorphonuclear neutrophils in patients with rapidly progressive periodontitis: an amplifying loop of polymorphonuclear neutrophil activation. Lab Invest. 1998;78(6):755–762. PMID: 9645766. [PubMed] [Google Scholar]
- [80].Allen C, Thornton P, Denes A, et al. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA [Research support, Non-U.S. Gov’t]. J Immunol. 2012;189(1):381–392. PMID: 22661091; PMCID: PMC3381844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511–517. PMID: 24784231. [DOI] [PubMed] [Google Scholar]
- [82].Cuzzocrea S. Neutrophils In: Singer J-M, editor. Inflammation: from molecular and cellular mechanisms to the clinics. Forst Edition ed. Vol. 10 Wiley-VCH Verlag GmbH & Co. KGaA; 2018, p. 253–272. [Google Scholar]
- [83].Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7(12):1299–1308. PMID: 17086187. [DOI] [PubMed] [Google Scholar]
- [84].Griffin EW, Yssel JD, O’Neill E, et al. The beta2-adrenoceptor agonist clenbuterol reduces the neuroinflammatory response, neutrophil infiltration and apoptosis following intra-striatal IL-1beta administration to rats. Immunopharmacol Immunotoxicol. 2018;40(2):99–106. PMID: 29303018. [DOI] [PubMed] [Google Scholar]
- [85].Kinoshita K, Kraydieh S, Alonso O, et al. Effect of posttraumatic hyperglycemia on contusion volume and neutrophil accumulation after moderate fluid-percussion brain injury in rats. J Neurotrauma. 2002;19(6):681–692. PMID: 12165130. [DOI] [PubMed] [Google Scholar]
- [86].Straub RH, Cutolo M, Buttgereit F, et al. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases [Review]. J Intern Med. 2010;267(6):543–560. PMID: 20210843. [DOI] [PubMed] [Google Scholar]
- [87].Lin B, Ginsberg MD, Busto R, et al. Hyperglycemia triggers massive neutrophil deposition in brain following transient ischemia in rats. Neurosci Lett. 2000;278(1–2):1–4. PMID: 10643786. [DOI] [PubMed] [Google Scholar]
- [88].Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. PMID: 20398008. [DOI] [PubMed] [Google Scholar]
- [89].Lange T, Born J. The immune recovery function of sleep - tracked by neutrophil counts. Brain Behav Immun. 2011;25(1):14–15. PMID: 20832467. [DOI] [PubMed] [Google Scholar]
- [90].Shephard RJ. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports med. 2003;33(4):261–284. PMID: 12688826. [DOI] [PubMed] [Google Scholar]
- [91].Barry JC, Simtchouk S, Durrer C, et al. Short-term exercise training alters leukocyte chemokine receptors in obese adults. Med Sci Sports Exerc. 2017;49(8):1631–1640. PMID: 28319586. [DOI] [PubMed] [Google Scholar]
- [92].de Castro MRT, Ferreira APO, Busanello GL, et al. Previous physical exercise alters the hepatic profile of oxidative-inflammatory status and limits the secondary brain damage induced by severe traumatic brain injury in rats. J Physiol. 2017;595(17):6023–6044. PMID: 28726269; PMCID: PMC5577552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jones MR, Quinton LJ, Simms BT, et al. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis. 2006;193(3):360–369. PMID: 16388483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Panopoulos AD, Zhang L, Snow JW, et al. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108(12):3682–3690. PMID: 16888100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wung BS, Ni CW, Wang DL. ICAM-1 induction by TNFalpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J Biomed Sci. 2005;12(1):91–101. PMID: 15864742. [DOI] [PubMed] [Google Scholar]
- [96].Manzel LJ, Chin CL, Behlke MA, et al. Regulation of bacteria-induced intercellular adhesion molecule-1 by CCAAT/enhancer binding proteins. Am J Respir Cell Mol Biol. 2009;40(2):200–210. PMID: 18703796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Campbell SJ, Anthony DC, Oakley F, et al. Hepatic nuclear factor kappa B regulates neutrophil recruitment to the injured brain. J Neuropathol Exp Neurol. 2008;67(3):223–230. PMID: 18344913. [DOI] [PubMed] [Google Scholar]
- [98].Schneiders J, Fuchs F, Damm J, et al. The transcription factor nuclear factor interleukin 6 mediates pro- and anti-inflammatory responses during LPS-induced systemic inflammation in mice. Brain Behav Immun. 2015;48:147–164. PMID: 25813145. [DOI] [PubMed] [Google Scholar]
- [99].Kapadia R, Tureyen K, Bowen KK, et al. Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. J Neurochem. 2006;98(6):1718–1731. PMID: 16899075. [DOI] [PubMed] [Google Scholar]
- [100].Cloutier A, Guindi C, Larivee P, et al. Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J Immunol. 2009;182(1):563–571. PMID: 19109189. [DOI] [PubMed] [Google Scholar]
- [101].Rummel C, Inoue W, Sachot C, et al. Selective contribution of interleukin-6 and leptin to brain inflammatory signals induced by systemic LPS injection in mice. J Comp Neurol. 2008;511(3):373–395. PMID: 18803240. [DOI] [PubMed] [Google Scholar]
- [102].Montecucco F, Bianchi G, Gnerre P, et al. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann N Y Acad Sci. 2006;1069:463–471. PMID: 16855174. [DOI] [PubMed] [Google Scholar]
- [103].Bruno A, Conus S, Schmid I, et al. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174(12):8090–8096. PMID: 15944317. [DOI] [PubMed] [Google Scholar]
- [104].Aguilar-Valles A, Inoue W, Rummel C, et al. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2015;96:124–134. PMID: 25582291. [DOI] [PubMed] [Google Scholar]
- [105].Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018;11:203–213. PMID: 29844694; PMCID: PMC5961632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Komegae EN, Farmer DGS, Brooks VL, et al. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun. 2018;73:441–449. PMID: 29883598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rosengarten B, Hecht M, Auch D, et al. Microcirculatory dysfunction in the brain precedes changes in evoked potentials in endotoxin-induced sepsis syndrome in rats. Cerebrovasc Dis. 2007;23(2–3):140–147. PMID: 17124395. [DOI] [PubMed] [Google Scholar]
- [108].Mihaylova S, Killian A, Mayer K, et al. Effects of anti-inflammatory vagus nerve stimulation on the cerebral microcirculation in endotoxinemic rats [Research support, Non-U.S. Gov’t]. J Neuroinflammation. 2012;9:183 .PMID: 22830560; PMCID: PMC3425315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yuan X, Caron A, Wu H, et al. Leptin receptor expression in mouse intracranial perivascular cells. Front Neuroanat. 2018;12:4 .PMID: 29410615; PMCID: PMC5787097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Dalli J, Serhan CN. Immunoresolvents signaling molecules at intersection between the brain and immune system. Curr Opin Immunol. 2018;50:48–54. PMID: 29154174; PMCID: PMC5869050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ramon S, Dalli J, Sanger JM, et al. The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious Inflammation. Am J Pathol. 2016;186(4):962–973. PMID: 26878209; PMCID: PMC4822332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6(4):318–328. PMID: 16557263; PMCID: PMC1783839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Besedovsky HO, Del Rey A, Klusman I, et al. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40(4–6):613–618. PMID: 1659887. [DOI] [PubMed] [Google Scholar]


