ABSTRACT
Various post-exercise strategies have been proposed to accelerate recovery during periods of training. However, the effects of water immersion (WI) temperature on recovery amid multiple daily exercise bouts are not well investigated. PURPOSE: To evaluate the effects of cold and warm water immersion temperatures between acute exercise bouts vs. no WI recovery on running performance. METHODS: Nine recreationally trained men (age: 24.0 ± 6.0 years old) participated in four experimental sessions using a crossover design. Each experimental session consisted of unilateral eccentric knee flexion exercise and 90 min of treadmill running at 70% of peak oxygen consumption followed by 15 min of WI at 15°C, 28°C or 38°C or passive recovery seated at room temperature (CON). Four hours following WI or CON, subjects completed a 5 km running time trial. Rectal temperature (Trec), heart rate, and excess post-exercise oxygen consumption (EPOC) were measured. RESULTS: Statistical analyses indicated that time trial performance was not affected by post-exercise recovery by WI (P > .05). The magnitude-based inferences indicated that 15°C (+ 3.6 ± 7.8%) likely and 28°C (+ 3.2 ± 7.5%) possibly improved recovery compared with CON, while the effect of 38°C (- 0.1 ± 12.3%) on recovery was unclear. During WI, heart rate and rectal temperature were not different from CON, but EPOC was higher in 15°C and 28°C compared to CON. Trec in 15°C was lower than CON from the 15th min post WI. EPOC was also greater in 15°C post WI compared to CON. CONCLUSION: WI at 15°C and 28°C following acute exercise likely and possibly, respectively, improved subsequent 5 km running time trial performance. We speculate that the faster recovery in core temperature post-exercise may underlie these improvements in recovery.
KEYWORDS: Excess Post-Exercise Oxygen Consumption, recovery, rectal temperature, self-pace running
Introduction
During periods of training or competition, athletes and physically active individuals may engage in multiple daily performances, placing a demand on recovery from the initial bout. Inadequate recovery amid repeated bouts of physical performance may compromise training adaptations, increase injury risk, or impair competitive performance [1]. Thus, optimizing recovery represents a strategy to tolerate greater training loads, facilitate training outcomes, as well as maximize athletic performance. Many post-exercise strategies have been employed as a means to enhance recovery (reviewed by Wilcock et al. [2]). Water immersion (WI) is a commonly practiced strategy amongst the athletic population; however, its efficacy is currently unclear including its effect on physical performance in the proximal hours following acute strenuous exercise.
The mechanisms of the proposed benefits of WI are thought to be mediated by the effects of hydrostatic pressure which may alter hemodynamics, tissue metabolism, inflammation, and perceived fatigue [2–5]. During WI, hydrostatic pressure results in an increased displacement of fluid from the periphery toward the central cavity, yielding greater stroke volume, total peripheral vascular resistance, and cardiac output, potentially allowing for enhanced oxygen and metabolite transport [6,7]. Exercise-induced inflammation may elevate pro-inflammatory cytokine release and signaling [8–10] while edema may reduce myocyte oxygen delivery [2]. WI also promotes fluid shifts between tissue compartments that may improve reabsorption of interstitial fluids and reduce edema and swelling [2,5]. Additionally, WI exerts a buoyant force that reduces neuromuscular activation [11,12] potentially facilitating relaxation of postural muscles and contributing to reduced perceptions of fatigue [3,4]. Further, temperature used during WI may also produce distinct effects including influencing peripheral vasodilation and constriction [13,14], capillary and tissue permeability [2,15], subsequent stiffness [16], swelling, and inflammation [15], and neuromuscular activity [2,17,18]; however, little data are available pertaining to performance outcomes. Evidence from contemporary meta-analyses [19,20] suggests WI using cold temperatures (i.e. 5–15°C) may reduce delayed onset muscle soreness and potentially attenuate muscle damage and promote recovery of muscle power. However, these effects were noted in the latter post-exercise period (i.e. 24–96 h) rather than the proximal hours following an acute bout of exercise.
Data relating to the performance effects of WI between successive exercise bouts performed on the same day are not conclusive with less known about the effects of different water temperature. Vaile and coworkers [21] reported that 15 mins of WI at three different temperatures (10°C, 15°C, and 20°C) between cycling bouts separated by 40 mins maintained cycling performance (15 min at 75% peak power, followed immediately by a 15 min time trial) in well-trained males. Yeargin et al. [22] observed that WI at 14°C following 90 min of running in heat resulted in improved 2-mile time trial performance in heat acclimated subjects. It is worth noting these studies, however, were performed in hot and warm environments (34 and 27°C, respectively). Several studies have investigated the effects of WI on repeated performance in temperate conditions. Using well-trained males, Dunne et al. [23] showed that 15 min of WI at 8°C and 15°C between two running bouts resulted in enhanced performance in the second bout compared to no-WI. Similarly, Crampton and colleagues [24] reported longer time to exhaustion in the second repeated bout of cycling exercise after 30 min of WI at 15°C compared to WI at 34°C and contrast water therapy (alternating 2.5 min intervals of 8°C and 40°C) in well-trained male triathletes. Roswell et al. [25] also reported that compared with WI at 34°C, WI at 10ºC is more effective for enhancing cycling interval training performance after intensive interval-running training. The authors suggested that WI at 10ºC may have improved subsequent cycling training performance by reducing perception of fatigue.
While the majority of these data suggest cold water immersion may promote enhanced performance in multiple bouts completed on the same day, the second sessions were performed within an hour following the initial bout. Data involving longer recovery periods between bouts are needed as athletes may often engage in “two-a-days” during phases of training with bouts in the morning and another later in the day or may have multiple competitive events separated by several hours. Identifying an efficacious water temperature to elicit optimal recovery is needed also. Therefore, the purpose of this study was to evaluate the effect of WI using cold (15°C), temperature (28°C), or warm (38°C) water temperatures following an acute bout of eccentric resistance leg exercise and prolonged moderate-intensity running on 5 km running time trial (TT) performance separated by 4 h. We hypothesized that WI at 15°C would result in higher running speed and lower time to complete the 5 km self-pace time trial compared to control, 28°C, or 38°C 4 h after an exercise bout.
Methods
Experimental approach to the problem
To test the hypothesis above, an exercise protocol consisting of eccentric localized and prolonged moderate intensity running exercise were developed to cause fatigue and reduce performance after 4 hours recovery (pilot test). 5 km running time trial (TT) performance was compared among three water immersion temperatures (15°C, 28°C, and 38°C) versus a non-water immersion condition. Heart rate, rectal temperature and excessive post exercise oxygen consumption were also compared.
Subjects
Nine recreationally trained, non-smoking, young healthy men (24 ± 6 years-old; 177 ± 7 cm height; 71.8 ± 13.2 kg body mass; 10.4 ± 3.8% of body fat and 54.7 ± 3.7 ml/kg/min of peak oxygen consumption) participated in this study. All subjects were informed of experimental procedures and the risks associated with participation and signed an informed consent form. The Internal Human Research Ethics Committee Review Board approved the study (# 057/2010).
Experimental design
This study included 3 preliminary sessions (a maximal graded exercise performance test, a familiarization trial, and a baseline time trial) followed by 4 randomized experimental sessions. An overview of the experimental sessions is presented in Figure 1. All sessions were conducted in controlled temperate environment (20 ± 2.0 ºC and 70 ± 10.0% relative humidity).
Figure 1.
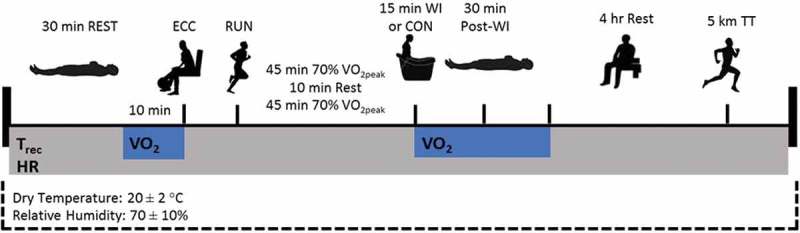
Overview of experimental trial protocol.
Volunteers were instructed to arrive at the laboratory in the morning (~ 8:00 am). They were previously instructed to refrain from exhaustive exercise, alcohol and caffeine consumption during the previous 24 hours. All volunteers recorded their food intake the night prior to and the day of the first experimental session. They were instructed to repeat their food ingestion for the remaining experimental sessions. They were also asked to ingest 500 mL of water 2 hours before arriving at the laboratory.
Familiarization and baseline measurements
In the first preliminary session, percent body fat was calculated using the sum of seven site skin fold [26]. All subjects completed a maximal graded exercise performance test using a treadmill ramp protocol test to determine individual peak oxygen consumption (VO2peak). Briefly, the VO2peak test consisted of 1-minute stages with incremental increases in speed and/or grade until the subject reached volitional fatigue. VO2peak was defined as the highest 30-second average, and maximal heart rate (HR) attained was recorded using a telemetric HR transmitter strap and watch (S810i series TM, Polar, USA). To meet our criteria for VO2peak, the subject had to attain at least two of the following criteria: a respiratory equivalent ratio greater than 1.15, a HR within 5% of age-predicted maximal HR (220-age), or a plateau of oxygen consumption (≤ 150 mLO2/kg/min) with increase in work intensity [27]. Breath-by-breath VO2 was measured with a portable metabolic analyzer (K4b2, Cosmed, Italy). The calibration of airflow, volumes, and both the O2 and CO2 occurred immediately before each test as recommended by the manufacturer.
In the second preliminary session, leg extensor strength through dynamic contraction was assessed by measuring the maximum amount of weight lift for one repetition (1-RM) using a seated knee extension machine (Master, Brasil). Subjects first warmed-up by completing 5–10 repetitions at 40–60% of their estimated 1-RM. Following a 1-min rest, the subject performed 3–5 repetitions at 60–80% of their estimated 1-RM. Subjects then rested 3 min before attempting with 100% of their estimated 1-RM. Approximately 5% increments were repeated until the volunteers could not complete 1 lift with proper form. Participants were seated in the knee extension machine with both legs flexed at 90° knee joint angle and 100° hip flexion. The concentric phase started by the subject extending the knee until full extension (0° knee joint angle), and the eccentric phase returning to the 90° knee joint angle. The machine’s roller pad (lever arm) was adjusted to be at the same level the medial malleoli. The knee and hip joint-angle positions were checked using a goniometer. Standard verbal encouragement was used during the 1-RM trials. Using this procedure, the 1-RM was obtained within 3–5 trials [28].
After the 1 RM test, the participants rested for 30–40 min, and then completed a 5-km self-paced familiarization run, as fast as possible, on the treadmill that the experimental tests would occur. The participants started running at 8 km/h with a 0% slope for 3 min (warm-up), and thereafter, they could adjust the speed on anytime during the 5–km time trial. This procedure was performed to habituate the participants with the experimental procedures before starting the trials.
The third preliminary visit was used as the 5-km time trial baseline session in which subjects completed a self-pace 5-km time trial. The participants did not receive any information about their speed or time, but at every kilometer reached, they were informed of the distance covered.
Experimental procedures
At least 36 hours after the last preliminary sessions, each volunteer was required to complete 4 randomized crossover experimental sessions separated by at least 5 days in order to ensure adequate recovery between experimental sessions.
Upon arrival to the laboratory, a urine sample was collected. The volunteers were considered hydrated if their urine specific gravity (hand held clinical refractometer, model 301, Biobrix, Brazil) was lower than 1.030 (Armstrong et al. 1998; Oppliger et al. 2005). If urine specific gravity was higher than 1.030, the subject was scheduled to perform the experimental session on another day. Subjects then were instrumented with a HR transmitter strap and wrist unit, a portable metabolic analyzer, and a rectal thermistor (YSI 400 series, YSI, Ohio, USA).
Participants were then laid in a supine position for 30 min in a quiet room (REST) and resting heart rate, oxygen consumption, and rectal temperature (Trec) were collected (Figure 1). Following the rest period, participants completed an exercise session, consisting of eccentric exercise (ECC – 3 series of 10 repetitions of unilateral knee flexion for both legs at 100% of 1RM measured during the concentric phase), immediately followed by moderate-intensity continuous running (RUN) on a motorized treadmill (2 bouts of 45 min at 70% of VO2 peak with 10 min of rest between bouts). The combination of the localized eccentric exercise and prolonged moderate-intensity running was used to induce skeletal muscle damage and metabolic fatigue, respectively. Although muscle soreness and swelling do not become apparent until 24 to 48 h after the eccentric exercise [29], mean peak torque is reduced immediately after exercise [30]. The unilateral approach was to guarantee that both legs were fatigued individually.
Immediately after the exercise, volunteers were randomly assigned to 15 min of recovery by WI at 15, 28 or 38°C, or control (CON), followed by 30 min of rest (Post-WI). After the EPOC measurements, subjects received standard diet based on their energy expenditure and dehydration during the exercise sessions. It was used the exercise intensity during running and converted to metabolic equivalents to calculate the energy expenditure. The meal consisted of 65% of carbohydrates, 20% of lipids, and 15% proteins. Water was provided to replace the difference in body weight pre and post the exercise session. Subjects remained at rest in the lab, awake for a period of 4 hours, prior to 5-km time trial. They were allowed to read, watch movies and access the internet.
Post- exercise recovery
During WI, volunteers were seated within a plastic drum and submersed to the xiphoid process level with the arms out of the water wearing only shorts. Desired water temperature was achieved using a thermal resistance (38°C and 28°C) or by means of crushed ice (15°C). Water was circulated every 3 min to maintain a uniform temperature and verified with a digital thermometer (YSI 4600, Ohio, USA). These 3 water temperatures were chosen to change Trec while remaining tolerable to all volunteers. In the CON condition participants remained seated in the plastic drum tank for 15 min, without water. Immediately after the 15 min initial recovery volunteers were dried and rested in supine position covered in a blanket for an additional 30 min.
5-km time trial
The self-paced 5-km time trial occurred 4 hours following the end of WI. During the test, subject’s speed selection, time of each km completed, and total time of completion were recorded. No information about the speed or time was provided to the subjects during the time trial. To calculate the average running speed the following equation was used:
Equation 1
S = d/t
Where S = running speed (in kilometers per hour); d = distance (5 km), and t = total time taken to run the 5 km, in hours.
Physiological measurements
Trec was recorded in the last 10 minutes of rest, during 90 min of RUN, 15 min WI, 30 min Post-WI, and every km of the TT via a rectal probe inserted approximately 12 cm beyond the anal sphincter connected to a telethermometer (YSI 4600 series, YSI, Ohio, USA).
HR was measured every 5 seconds by a HR sensor (Polar, RS800CX) attached to the chest of subjects and registered by the receiver RS800CX in the last 10 minutes of rest, during RUN, WI, Post-WI, and TT. Data from the HR receiver were transferred via infrared device for the software Polar ProTrainer 5 (version 5.40.172) on a computer for later analysis. HR was analyzed every 10 min using the last 10 minutes of rest and, every 5 min during RUN, WI and post-WI, and every km during TT.
For Excess Post-Exercise Oxygen Consumption (EPOC) measurement: VO2 was measured breath by breath by open circuit spirometry during WI (15 minutes) and post-WI period (30 minutes). Values obtained during the last 10 minutes of rest were averaged and used as baseline (Short and Sedlock, 1997). EPOC was determined by calculating the area under the curve subtracting resting VO2 from total VO2 using Prism software for Windows (GraphPad, Prism version 5.0, USA) [31].
Statistical analysis
A repeated measure two-way ANOVA was used to probe for differences in Trec and HR between 15, 28 and 38°C vs. CON followed by post hoc Tukey with significance level of p < 0.05. The analyzes was performed using instantaneous Trec and HR measurement taken every 5 min at different time points in the experiment. A one-way ANOVA was used to compare differences in TT time of completion and average running speed between 15, 28 or 38°C vs. CON followed by Tukey’s post hoc. Time trial performance data were also analyzed for practical significance using magnitude-based inferences [32]. We used this approach because traditional statistical analyses often do not indicate the magnitude of an effect, which is typically more relevant to athletic performance than statistically significant effect. Statistical significance was adopted as p ≤ 0.05. Results are presented as mean ± standard deviation.
Results
Time trial performance
The reduction in the 5 km time trial performance at 4 h after the eccentric and prolonged running exercises was 9.3 ± 8.7% (13.3 ± 1.6 km•h−1 for the preliminary TT versus 12.0 ± 1.3 km•h−1 for the CON TT; P = 0.015). This result suggests that the eccentric and prolonged running exercises were efficient to induce reduction in running performance.
Statistical analyses indicated that time trial performance was not affected by post-exercise recovery by WI (Figure 2). There was no significant difference in the average speed of the test (P = 0.90, Figure 2(a)), neither in the average speed of each kilometer covered (P = 0.79, Figure 2(b)). The magnitude-based inferences indicated that 15°C (+ 3.6 ± 7.8%) likely and 28°C (+ 3.2 ± 7.5%) possibly improved recovery compared with CON, while the effect of 38°C (- 0.1 ± 12.3%) on recovery was unclear Table 1.
Figure 2.
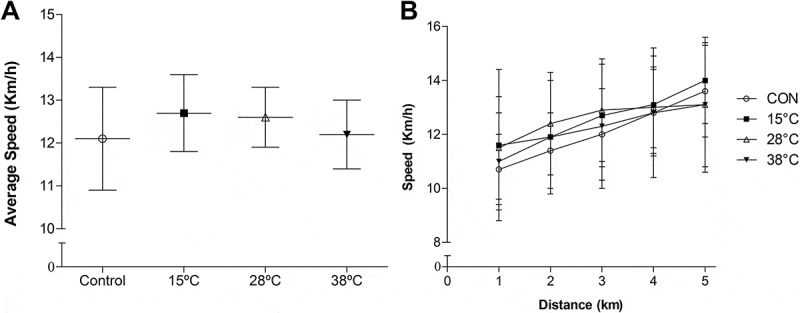
Effect of post-exercise recovery by water immersion on the average speed of the 5-km time trial (a) and of each kilometer covered during the 5-km time trial (b). Data expressed as mean ± SD. CON, control. n = 9.
Table 1.
Magnitude-based inferences for the average speed during the 5 km time trial running in WI at 15°C, 28°C, 38°C or CON trials.
| 15°C versus CON | 28°C versus CON | 38°C versus CON | |
|---|---|---|---|
| Probability inference (% – positive/trivial/negative) |
80/18/2 | 72/25/3 | 42/42/17 |
| Practical inference | Likely beneficial | Possibly beneficial | Unclear |
Rectal temperature
No differences in Trec were observed at rest over time (P = 0.995) or between conditions (P = 0.918). During RUN Trec increased over time (P < 0.001), but it was not different between experimental conditions (P = 0.894; Figure 3(a)). Trec reduced during the 15 min WI period over time (P < 0.001), but there was no difference between experimental conditions (P = 0.187; Figure 3(b)). However, Trec reduced overtime in the 30 min after WI (P < 0.001) and it was lower in WI at 15°C, starting at min 15, compared to CON (P < 0.001; Figure 3(c)). Trec increased over time (P < 0.001), but not between experimental conditions during TT (P = 0.306; Figure 3(d)).
Figure 3.
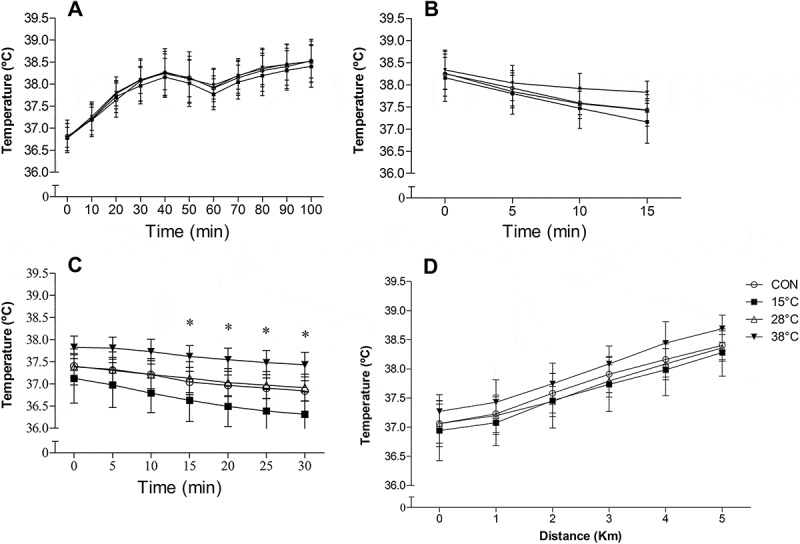
Rectal temperature during RUN (a), recovery by WI (b), post WI (c) and at each kilometer covered during the 5-km time trial (d). Data expressed as mean ± SD. CON, control. n = 9. *p < .05, 15°C compared to CON, Two-way ANOVA, Tukey’s post hoc.
Heart rate
No differences in HR were observed at min 20–30 during rest over time (P = 0.097) or between experimental conditions (P = 0.830). During RUN HR increased over time (P < 0.0001) but was not different between any experimental condition (P = 0.987; Figure 4(a)). However, HR decrease overtime (P < 0.0001) and was significantly elevated (P = 0.0010) in 38°C compared to CON during WI from min 5 (Figure 4(b)). During Post WI, HR did not change overtime (P = 0.107) and was not different between CON and WI conditions (P > 0.05). During TT HR increased over time (P < 0.0001), but no differences were observed between experimental conditions (P = 0.301; Figure 4(c,d)).
Figure 4.
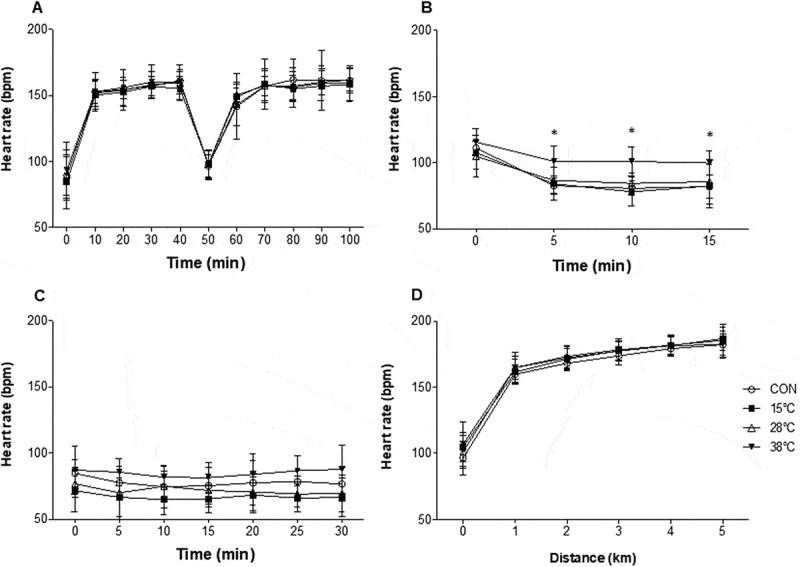
Heart rate during RUN (a), recovery by WI (b), post WI (c) and at each kilometer covered during the 5-km time trial (d). Data expressed as mean ± SD. CON, control. n = 9. *p < .05, 38°C compared to CON, Two-way ANOVA, Tukey’s post hoc.
EPOC
EPOC was modified by post exercise recovery using WI (Figure 5). During WI, EPOC was higher at 15°C (P = .004) and 28°C (P = .002) compared to CON (Figure 5(a)), but this difference was not sustained during the 30 min after WI (Figure 5(b)).
Figure 5.
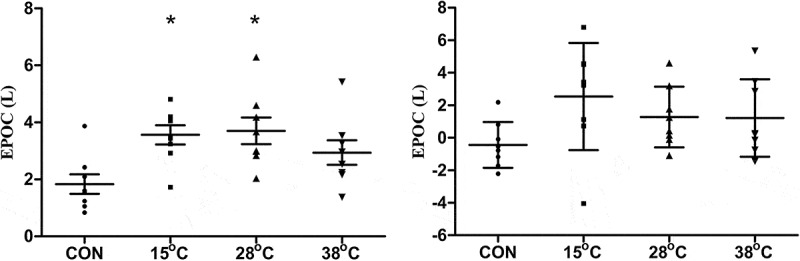
Excess post-exercise oxygen consumption in liters during (a) and after (b) WI or CON recovery conditions. Data expressed as mean ± SD. CON, control. n = 8. *P < .05, compared to CON, One-way ANOVA, Tukey’s post hoc.
Discussion
The purpose of this study was to investigate the effectiveness of three different WI temperatures on running performance following 4 h of recovery from an acute bout of eccentric resistance leg exercise followed by prolonged moderate-intensity running. To date, little evidence is available on the effectiveness of WI in multiple daily performances (over 2-hour recovery). The main finding was that 15 min of WI at 15°C likely (+ 3.6 ± 7.8%) and 28°C (+ 3.2 ± 7.5%) possibly improve subsequent 5 km TT performance. This possible improvement in running performance induced by WI at 15°C and 28°C may be explained by faster recovery of core temperature.
Short-term recovery enhancements of performance in exercise performed in hot environments have been noted following cold WI via a reduction in CNS mediated fatigue and reducing cardiovascular strain. Fifteen minutes of WI at 10°C, 15°C, and 20°C following acute cycling exercise resulted in maintenance of performance vs. no WI in a hot environment (34°C) 40 minutes following WI [21]. And WI at 14°C following 90-min running enhanced 2 mile time trial performance in the heat (27°C) when performed immediately following WI [22]. Our investigation was performed in a temperate environment (20°C; 70% humidity) and Trec did not reach high core temperature (< 39°C), thus not invoking a physiological stressor in which the beneficial responses of cold WI temperatures may manifest as those elicited in the aforementioned studies. Additionally, the 4-hour recovery allowed core temperature return to pre-exercise levels before the 5-km time trial. During the 5-km time trial, core temperature did not reach high values (< 38.5°C) and may not be implicated in the development of central fatigue. This may suggest the performance effects observed might not have been due to protective effects against hyperthermia during 5-km time trial, but potentially resulting from the acute hemodynamic and/or metabolic effects during WI following an exercise session.
There have been many proposed mechanisms involved with WI in the facilitation of short-term recovery, many of which center around hemodynamic and local tissue and whole-body temperatures change. Hydrostatic pressure may shunt fluid from the periphery toward the central cavity allowing for greater oxygen and metabolite transport [6,7], precipitating a return to homeostasis. In the present study, it was not observed any difference in HR responses 15 minutes after WI or during the TT. Although HR is not a measurement of venous return or cardiac output, it may indicate changes in hemodynamic flow. Further, our group has shown previously that WI at 15°C induces greater parasympathetic reactivation compared with no WI [33], but its effects were not evident 4 hours after an exercise bout. Similarly, we did not see any effect of WI on heart rate measurements during the 5-km time trial. Therefore, the acute changes in heart rate with WI does not explain the response in the 5-km time trial.
Rectal temperature did not change after the acute bout of exercise during the 15 minutes of WI at 15, 28 and 38°C. Trec is reliable and valid index for monitoring core temperature, but has a delayed response compared to other methods, such as esophageal temperature. Gagnon et al. [34] reported that Trec increased consistently during intense exercise in the heat (up to 39.5°C) and decreased consistently when participants were aggressively cooled during cold-water immersion (2°C) up to the clavicles. However, Trec remained elevated after approximately 60% of total immersion time (average of 14 minutes) and it was higher than esophageal temperature. Therefore, this lack of effect during WI on Trec may be justified by the use of rectal thermistors. The effects of WI were observed 15 minutes after WI. WI at 15°C was capable to reduce core temperature, but WI at 28 and 38°C did not affect rectal temp.
This faster reduction in core temperature during WI at 15°C may have attenuated physiological responses, such as reduced skeletal muscle temperature, metabolism and blood flow [35,36]. After a short bout of high-intensity interval training, Broatch et al. [37] reported a decrease in skeletal muscle temperature (approximately three degrees Celsius) with cold water immersion (10.3 ± 0.2°C) compared to thermoneutral water immersion. A reduced tissue metabolism and microvascular perfusion with cold water immersion have also been reported previously [38]. The reduction in skeletal muscle temperature and metabolism may have spared glycogen utilization. However, this hypothesis has been challenged by previous studies that found no change [39,40] or decrease [41,42] in the restoration of muscle glycogen. Recently Cheng et al. [41] reported that mean power output during all-out arm-cycling exercise was better preserved after warming the triceps brachii to 38°C than cooling to 15°C. The authors also used intact single mouse muscle fibers and showed that cooling impaired muscle glycogen resynthesis. It is important to highlight that the authors did not observe any increase in core temperature with exercise and heating or cooling techniques. Therefore, it is not clear the effects of skeletal muscle tissue heating and cooling on muscle glycogen synthesis.
EPOC occurs due to elevated respiratory rate, heart rate, ATP and CP restoration, lactate removal and temperature regulation [43]. We hypothesized that post exercise WI at 15°C could reduce EPOC and indicate faster metabolic recovery. Also, a faster recovery could be beneficial for the running performance during the 5-km time trial. However, EPOC was significantly elevated during WI and Post-WI at 15°C and 28°C which may be attributed to cold-induced increase of thermogenesis in skeletal muscle via mitochondrial uncoupling [44]. Another reason for the elevated EPOC may suggest an enhanced rate of muscular recovery (i.e. phosphagen and ATP repletion, restoration of myoglobin O2, removal of metabolites) and thus may benefit physical performance as noted in our study. Yeung et al. [45] observed that cold water immersion (12–15°C) increases muscle oxygenation after maximal dynamic knee extension and flexion contractions both concentrically. The authors speculate that cold water causes greater reperfusion and an increase in oxygen delivery to the muscle. Lastly, cold water induces elevations in circulating hormones, such as epinephrine and norepinephrine, that may contribute to higher EPOC during 15°C and 28°C WI. It is not clear if the elevated EPOC would reflect an enhanced rate of recovery. Further studies are required to support this suggestion.
While the underlying explanation for the performance effects of WI in our study may possibly due to physiological or metabolic changes, a possible placebo effect cannot be excluded. Expectation of an intervention promoting a positive effect has been demonstrated to enhance an athlete’s performance [46]. Broatch et al. [37] showed that a recovery WI placebo (dissolvable skin cleanser added to water at 34.7°C) administered after an acute high-intensity interval training session was superior in the recovery of muscle strength over 48 h as compared with temperate water (34.7°C) and was as effective as cold-WI (10.3°C). The authors speculated that the physiological benefits surrounding cold water immersion are at least partly placebo related. Further, subjects in the Rowsell et al. [25] reported cold WI to be a more effective and preferable recovery method than thermoneutral WI and that they believed that it would benefit performance, suggesting a placebo effect may have impacted performance. It is possible subjects in our study may have held a bias that WI, particularly that of cold temperature (15°C), is beneficial for performance given its popular use amongst athletic populations and may have promoted 5 km TT performance. However, this study did not acquire any measurement of perceptual parameters, such as rating of perceived exertion, rating of perceived recovery, mood and pain scales to support the current results
Another possible explanation for the facilitated recovery may be related to an increased reabsorption of interstitial fluid and attenuation of edema and local tissue inflammation [2,5]. Water temperature, particularly cold temperatures (5–15°C) may also exert distinct effects including reducing muscle damage and delayed onset muscle soreness and promote recovery of muscle power 24–96 hr post exercise (Leeder, 2012) [20]. However, the physiological and performance effects of distinct water temperatures used in WI within shorter periods between exercise sessions are less known. While a likely beneficial effect was observed in circulating concentrations of IL-10, no differences between IL-6, creatine kinase, lactate dehydrogenase, myoglobin, or fatty acid-binding protein were found between WI temperatures of 10°C and 34°C when completed between exercise bouts separated by 9 hrs. While it may be plausible our WI intervention attenuated inflammation and muscle damage, no such markers were measured in our study; moreover, with scarce data speaking to that potential effect, it is highly speculative that inflammation and muscle damage were altered by WI in the present study.
No markers of muscle damage (i.e. creatine kinase, lactate dehydrogenase, etc.) or exercise stress (i.e. lactate, markers of oxidative stress or inflammation, etc.) were measured. Thus, it may be imprudent to assume the exercise protocol was successful in promoting substantive physiological stress in which subsequent performance would be impaired or, if so, the potential therapeutic effects of WI would be incurred. Also, with the exception of HR, no hemodynamic responses were measured, therefore it is unknown whether the WI protocol elicited any of the proposed hemostatic effects that underlie many of the proposed mechanisms of the beneficial responses to WI. Another shortcoming, our sample was composed of very physically active and trained individuals using a laboratory approach and the results may not be directly transferred to athletes during “real world” exercise training or competition.
Conclusion
WI at 15°C and 28°C following acute exercise likely and possibly, respectively, improved subsequent 5 km running time trial performance. It also suggests that WI at 38°C should be discouraged. Further inquiry is warranted in order to fully elucidate the efficacy of WI as a post-exercise recovery practice and justify its common practice. Coaches and athletes may consider using water immersion at 15°C as a recovery strategy in multiple daily performances (e.g. training or competition twice a day).
Funding Statement
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [PNPD-2455/2011], Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [CDS-APQ 00908-08], and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [445096/2014-4] funding agencies.
Abbreviations
- WI:
water immersion
- TT:
time trial
- VO2peak:
peak oxygen consumption
- HR:
heart rate
- 1-RM:
1-repetition maximum
- Trec:
rectal temperature
- ECC:
eccentric exercise
- REST:
30 min rest in a quiet room
- RUN:
moderate-intensity continuous running
- CON:
control
- Post-WI:
30 min of rest post water immersion
- EPOC:
Excess Post-Exercise Oxygen Consumption
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Kibler WB, Chandler TJ, Stracener ES.. Musculoskeletal adaptations and injuries due to overtraining. Exerc Sport Sci Rev. 1992;20:99–126. [PubMed] [Google Scholar]
- [2].Wilcock IM, Cronin JB, Hing WA.. Physiological response to water immersion: a method for sport recovery? Sports Med. 2006;36(9):747–765. [DOI] [PubMed] [Google Scholar]
- [3].Kuligowski LA, Lephart SM, Giannantonio FP, et al. Effect of whirlpool therapy on the signs and symptoms of delayed-onset muscle soreness. J Athl Train. 1998;33(3):222–228. [PMC free article] [PubMed] [Google Scholar]
- [4].Nakamura K, Takahashi H, Shimai S, et al. Effects of immersion in tepid bath water on recovery from fatigue after submaximal exercise in man. Ergonomics. 1996;39(2):257–266. [DOI] [PubMed] [Google Scholar]
- [5].Mishra DK, Fridén J, Schmitz MC, et al. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am. 1995;77(10):1510–1519. [DOI] [PubMed] [Google Scholar]
- [6].Wilcock IM, Cronin JB, Hing WA.. Water immersion: does it enhance recovery from exercise?. Int J Sports Physiol Perform. 2006;1(3):195–206. [DOI] [PubMed] [Google Scholar]
- [7].Johansen LB, Jensen TU, Pump B, et al. Contribution of abdomen and legs to central blood volume expansion in humans during immersion. J Appl Physiol. 1997;83(3):695–699. [DOI] [PubMed] [Google Scholar]
- [8].Prestes J, de Ferreira CK, Dias R, et al. Lymphocyte and cytokines after short periods of exercise. Int J Sports Med. 2008;29(12):1010–1014. [DOI] [PubMed] [Google Scholar]
- [9].Freidenreich DJ, Volek JS.. Immune responses to resistance exercise. Exerc Immunol Rev. 2012;18:8–41. [PubMed] [Google Scholar]
- [10].Lancaster GI, Febbraio MA.. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35(6):262–269. [DOI] [PubMed] [Google Scholar]
- [11].Pöyhönen T, Avela J.. Effect of head-out water immersion on neuromuscular function of the plantarflexor muscles. Aviat Space Environ Med. 2002;73(12):1215–1218. [PubMed] [Google Scholar]
- [12].Pöyhönen T, Keskinen KL, Hautala A, et al. Human isometric force production and electromyogram activity of knee extensor muscles in water and on dry land. Eur J Appl Physiol Occup Physiol. 1999;80(1):52–56. [DOI] [PubMed] [Google Scholar]
- [13].Bonde-Petersen F, Schultz-Pedersen L, Dragsted N.. Peripheral and central blood flow in man during cold, thermoneutral, and hot water immersion. Aviat Space Environ Med. 1992;63(5):346–350. [PubMed] [Google Scholar]
- [14].Weston CF, O’Hare JP, Evans JM, et al. Haemodynamic changes in man during immersion in water at different temperatures. Clin Sci (Lond). 1987;73(6):613–616. [DOI] [PubMed] [Google Scholar]
- [15].Coté DJ, Prentice WE Jr., Hooker DN, et al. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther. 1988;68(7):1072–1076. [DOI] [PubMed] [Google Scholar]
- [16].Eston R, Peters D.. Effects of cold water immersion on the symptoms of exercise-induced muscle damage. J Sports Sci. 1999;17(3):231–238. [DOI] [PubMed] [Google Scholar]
- [17].Meeusen R, Lievens P.. The use of cryotherapy in sports injuries. Sports Med. 1986;3(6):398–414. [DOI] [PubMed] [Google Scholar]
- [18].Sauls J. Efficacy of cold for pain: fact or fallacy?. Online J Knowl Synth Nurs. 1999;6:8. [PubMed] [Google Scholar]
- [19].Machado AF, Ferreira PH, Micheletti JK, et al. Can water temperature and immersion time influence the effect of cold water immersion on muscle soreness? A systematic review and meta-analysis. Sports Med. 2016;46(4):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leeder J, Gissane C, van Someren K, et al. Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br J Sports Med. 2012;46(4):233–240. [DOI] [PubMed] [Google Scholar]
- [21].Vaile J, Halson S, Gill N, et al. Effect of cold water immersion on repeat cycling performance and thermoregulation in the heat. J Sports Sci. 2008;26(5):431–440. [DOI] [PubMed] [Google Scholar]
- [22].Yeargin SW, Casa DJ, McClung JM, et al. Body cooling between two bouts of exercise in the heat enhances subsequent performance. J Strength Cond Res. 2006;20(2):383–389. [DOI] [PubMed] [Google Scholar]
- [23].Dunne A, Crampton D, Egaña M.. Effect of post-exercise hydrotherapy water temperature on subsequent exhaustive running performance in normothermic conditions. J Sci Med Sport. 2013;16(5):466–471. [DOI] [PubMed] [Google Scholar]
- [24].Crampton D, Donne B, Warmington SA, et al. Cycling time to failure is better maintained by cold than contrast or thermoneutral lower-body water immersion in normothermia. Eur J Appl Physiol. 2013;113(12):3059–3067. [DOI] [PubMed] [Google Scholar]
- [25].Rowsell GJ, Reaburn P, Toone R, et al. Effect of run training and cold-water immersion on subsequent cycle training quality in high-performance triathletes. J Strength Cond Res. 2014;28(6):1664–1672. [DOI] [PubMed] [Google Scholar]
- [26].Jackson AS, Pollock ML.. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. [DOI] [PubMed] [Google Scholar]
- [27].Yoon BK, Kravitz L, Robergs R.. VO2max, protocol duration, and the VO2 plateau. Med Sci Sports Exerc. 2007;39(7):1186–1192. [DOI] [PubMed] [Google Scholar]
- [28].Verdijk LB, van Loon L, Meijer K, et al. One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci. 2009;27(1):59–68. [DOI] [PubMed] [Google Scholar]
- [29].Proske U, Allen TJ.. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33(2):98–104. [DOI] [PubMed] [Google Scholar]
- [30].Bowers EJ, Morgan DL, Proske U.. Damage to the human quadriceps muscle from eccentric exercise and the training effect. J Sports Sci. 2004; 22 (11–12): 1005–1014. [DOI] [PubMed] [Google Scholar]
- [31].Jacobsen DJ, Bailey BW, LeCheminant JD, et al. A comparison of three methods of analyzing post-exercise oxygen consumption. Int J Sports Med. 2005;26(1):34–38. [DOI] [PubMed] [Google Scholar]
- [32].Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. [DOI] [PubMed] [Google Scholar]
- [33].de Oliveira Ottone V, de Castro Magalhães F, de Paula F, et al. The effect of different water immersion temperatures on post-exercise parasympathetic reactivation. PLoS One. 2014;9(12):e113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gagnon D, Lemire BB, Jay O, et al. Aural canal, esophageal, and rectal temperatures during exertional heat stress and the subsequent recovery period. J Athl Train. 2010;45(2):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Broatch JR, Petersen A, Bishop DJ.. The influence of post-exercise cold-water immersion on adaptive responses to exercise: a review of the literature. Sports Med. 2018;48(6):1369–1387. [DOI] [PubMed] [Google Scholar]
- [36].Bongers CC, Hopman MT, Eijsvogels TM.. Cooling interventions for athletes: an overview of effectiveness, physiological mechanisms, and practical considerations. Temperature. 2017;4(1):60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Broatch JR, Petersen A, Bishop DJ.. Postexercise cold water immersion benefits are not greater than the placebo effect. Med Sci Sports Exerc. 2014;46(11):2139–2147. [DOI] [PubMed] [Google Scholar]
- [38].Ihsan M, Watson G, Lipski M, et al. Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc. 2013;45(5):876–882. [DOI] [PubMed] [Google Scholar]
- [39].Slivka DR, Dumke CL, Tucker TJ, et al. Human mRNA response to exercise and temperature. Int J Sports Med. 2012;33(2):94–100. [DOI] [PubMed] [Google Scholar]
- [40].Gregson W, Allan R, Holden S, et al. Postexercise cold-water immersion does not attenuate muscle glycogen resynthesis. Med Sci Sports Exerc. 2013;45(6):1174–1181. [DOI] [PubMed] [Google Scholar]
- [41].Cheng AJ, Willis SJ, Zinner C, et al. Post-exercise recovery of contractile function and endurance in humans and mice is accelerated by heating and slowed by cooling skeletal muscle. J Physiol. 2017;595(24):7413–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tucker TJ, Slivka DR, Cuddy JS, et al. Effect of local cold application on glycogen recovery. J Sports Med Phys Fitness. 2012;52(2):158–164. [PubMed] [Google Scholar]
- [43].Gaesser GA, Brooks GA.. Metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc. 1984;16(1):29–43. [PubMed] [Google Scholar]
- [44].Wijers SL, Schrauwen P, Saris WH, et al. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One. 2008;3(3):e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yeung SS, Ting KH, Hon M, et al Effects of cold water immersion on muscle oxygenation during repeated bouts of fatiguing exercise: a randomized controlled study. Medicine (Baltimore). 2016;95(1):e2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McClung M, Collins D.. “Because I know it will!”: placebo effects of an ergogenic aid on athletic performance. J Sport Exerc Psychol. 2007;29(3):382–394. [DOI] [PubMed] [Google Scholar]


