Abstract
Sex differences in the mechanical properties of different musculoskeletal tissues and their impact on tendon function and disease are becoming increasingly recognized. Tendon mechanical properties are influenced by the presence or absence of sex hormones and these effects appear to be tendon- or ligament-specific. The objective of this study was to determine how sex and hormone differences in rats affect supraspinatus tendon and muscle properties. We hypothesized that male supraspinatus tendons would have increased cross-sectional area but no differences in tendon material properties or muscle composition when compared to supraspinatus tendons from female or ovariectomized (OVX) female rats. Uninjured supraspinatus tendons and muscles from male, female, and OVX female rats were collected and mechanical and histological properties were determined. Our analysis demonstrated decreased dynamic modulus and increased hysteresis and cross-sectional area in male tendons. We found that male tendons exhibited decreased dynamic modulus (during low strain frequency sweep and high strain fatigue loading), increased hysteresis, and increased cross-sectional area compared to female and OVX female tendons. Despite robust mechanical differences, tendon cell density and shape, and muscle composition remained unchanged between groups. Interestingly, these differences were unique compared to previously reported sex differences in rat Achilles tendons, which further supports the concept that the effect of sex on tendon varies anatomically. These differences may partially provide a mechanistic explanation for the increased rate of acute supraspinatus tendon ruptures seen in young males.
Keywords: mechanics, injury, fatigue, gender, shoulder, supraspinatus, rotator cuff, skeletal muscle, sex differences, orthopedics
Introduction
Our appreciation of sex differences in musculoskeletal tissues and their effect on normal tissue properties is becoming increasingly recognized [1–9]. Functional and anatomical sex differences in the shoulder are present, with males having increased size of both bony [10,11] and tendinous [11,12] structures as well as differences in proprioception [13] and neurologic activation of shoulder and neck musculature, which may impact the way the joint responds to repetitive activities [14]. It is not known if sex differences extend to tissue-level properties of the rotator cuff. An understanding of whether sex differences in tissue-level properties of the supraspinatus tendon exist will provide valuable information about how shoulder mechanics differs across sex and permit more informed investigation into sex differences in tendon homeostasis.
It is well established that tendons may be influenced by the presence or absence of hormones [1–3,7,15]. Human tenocytes are known to express estrogen receptors [16,17] and it has been shown that estrogen deficiency decreases proteoglycan expression [18], tendon metabolism [19], tendon healing [9], tenocyte viability [20], and tendon laxity [21]. Studies investigating the mechanical properties of various tendons and ligaments in different hormonal environments have found evidence of altered function across sex [1,2]. The precise physiologic response of tendons and ligaments to sex hormones is debated, with studies demonstrating a range of changes in response to estrogen, including diminished adaptive response to exercise in females [22] or increases in collagen expression [23], while others have found no differences in cell proliferation or collagen expression [24].
Interestingly, the effects of sex hormones on musculoskeletal tissues clinically appear to be tendon- or ligament-specific as well as sex-specific. For example, up to 84% of Achilles tendon ruptures occur in males [4] and previous studies done in rats demonstrate decreased modulus of the male Achilles tendon, which may explain the clinical disparity in rupture rates [3]. The increased incidence of Achilles tendon ruptures in males may also be related to sex differences in gastrocnemius muscle fiber diameter [4] or differences in peak plantar flexion moment during running with increased tendon loading [25,26]. Similar to the Achilles tendon, quadriceps ruptures also occur four to eight times more often in men [8]. Conversely, anterior cruciate ligament (ACL) tears occur more commonly in young women [27]. However, studies assessing mechanical properties have been inconclusive with some noting increased ACL laxity [7] and changes in the mechanical properties of the patellar tendon [5] during the female menstrual cycle, while others have found no differences with hormonal cycles [28] or following ovariectomy [29]. This may be related to an increased quadriceps-to-hamstring strength ratio in females [30] or sex differences in knee joint anatomy and biomechanics [31–35]. These biomechanical differences are also seen when comparing pre- and postpubertal females, further implicating sex hormones in these injuries [36], although this has not been consistently demonstrated [31].
With respect to the shoulder, sex has not been demonstrated to be a clear risk factor for rotator cuff tears in most clinical studies [37–40], although epidemiological reports are conflicting [6,37,41]. Female athletes have been found to have increased posterior shoulder laxity [42]; however, others have not found sex differences in the mechanical properties of the glenohumeral capsule [43]. Decreased estrogen levels in postmenopausal women have been proposed to increase the rate of asymptomatic degenerative rotator cuff tears [44]. Males, however, have also been found to have an increased rate of acute full thickness supraspinatus tears compared to females [6]. Ultimately, the effects that sex hormones have on the mechanical properties of the supraspinatus tendon are unknown.
The objective of this study was to investigate how sex and hormone differences in rats affect supraspinatus tendon and muscle properties. We hypothesized that male supraspinatus tendons would have increased cross-sectional area but no differences in tendon material properties and muscle composition compared to females. We also assessed similarities across tendons by relating our findings in the supraspinatus tendon to recently reported sex differences in the Achilles tendon [3]. Due to the clinical differences in the rates of Achilles rupture and supraspinatus tears across sex, we expected that the sex differences seen in the Achilles tendon would differ from the supraspinatus tendon.
Methods
Animals.
Shoulders were collected from 60 adult male (n = 20), female (n = 20), and ovariectomized female (OVX) (n = 20) Sprague-Dawley rats at approximately 5 months of age (OVX was performed 6 weeks prior to sacrifice in relevant subgroup) that had been previously sacrificed and frozen. Achilles tendons had been previously harvested from these male, female, and OVX rats in an IACUC approved study [3] at the University of Pennsylvania. Right supraspinatus tendons (n = 12/group) were allocated to fatigue testing and left supraspinatus tendons (n = 12/group) were used for ramp-to-failure testing. Supraspinatus muscle samples (n = 8/group) were harvested from separate adult male, female, and OVX Sprague-Dawley rats (n = 8/group) for fiber analysis. Tendons and muscles were prepared, tested, and analyzed in a blinded fashion.
Mechanical Testing.
Supraspinatus–humerus complexes were isolated and prepared for mechanical testing, as described [45]. Briefly, muscle was fine dissected from the tendon and Verhoeff's stain lines were placed at the bony insertion site and 8 mm distally. Tendon cross-sectional area was measured using a custom laser device [46]. Humeri were secured in polymethyl methacrylate and cyanoacrylate was used to secure the tendon between two pieces of sandpaper, leaving an 8 mm gage length (Fig. 1). A custom fixture was used to secure the potted samples in an Instron ElectroPuls E3000 (Norwood, MA) affixed with a 250 N load cell. Samples were submerged in a 37 °C 1× phosphate buffered saline bath and preloaded to 0.1 N prior to undergoing one of two protocols. Left supraspinatus tendons underwent a ramp to failure to assess quasi-static properties while right supraspinatus tendons underwent a second protocol that measured stress relaxation, viscoelastic, and fatigue properties:
Fig. 1.
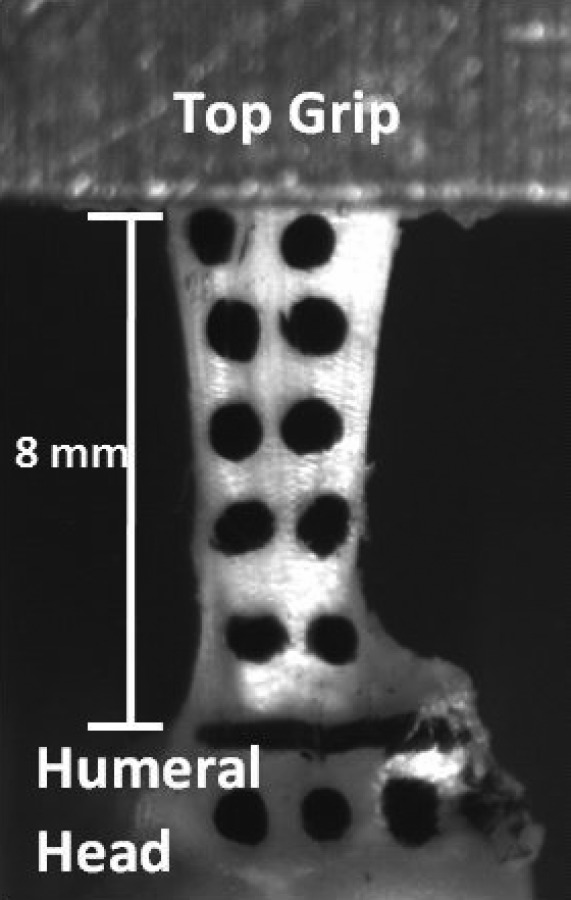
Pretest supraspinatus tendon. Tendons were secured in custom grips prior to undergoing ramp to failure or fatigue tensile testing. Verhoeff's stain was used to delineate the junction between the humeral head and tendon as well as 8 mm distally (concealed by grip). Dots were applied to the humeral head and tendon to permit optical tracking.
-
(1)
Protocol 1: preconditioning followed by a ramp to failure at 0.1% strain/s [3,47].
-
(2)
Protocol 2 [3]: preconditioning, stress relaxation to 6% strain, a low strain dynamic frequency sweep (10 cycles at 0.1, 1, 5, and 10 Hz), followed by fatigue testing until failure (tendons were cycled between 7% and 40% max stress at 2 Hz) (Supplemental Fig. 1 is available under the “Supplemental Data” tab for this paper on the ASME digital collection) [3,48–50].
The loads selected for each group were based on the average maximum stress during ramp to failure testing. This was done to ensure that tendons in different groups were tested at the same stress. To permit comparisons between groups, sex-specific fatigue protocols were designed such that male tendons cycled between 2 and 11 N and female/OVX tendons cycled between 2 and 7 N during fatigue loading.
Force–displacement data were used to calculate failure load, percent relaxation, transition strain (the strain at the intersection of the toe and linear regions of the stress–strain curve as determined from a bilinear fit of tensile loading data), maximum stress, and toe and linear modulus and stiffness. Stain dots placed at the tendon midsubstance and insertion and images were captured throughout testing for optical strain measurement. The viscoelastic parameter dynamic modulus (the ratio of the amplitudes of the stress and strain sinusoids) was calculated during both frequency sweep phase of the test, |E|freq, and during fatigue loading, |E|fat. |E|fat was estimated between 7 and 40% maximum stress, which was the linear region of the load displacement curve [51–53]. The phase angle δ was calculated at steady-state at a given frequency as the product of the constant frequency f and the delay between the nth strain peak and the corresponding nth stress peak, termed “(tε − tσ)” (Eq. 1). The measured values for tan δ were calculated by this equation at all frequencies tested (Supplemental Fig. 2 is available under the “Supplemental Data” tab for this paper on the ASME digital collection).
| (1) |
Fig. 2.
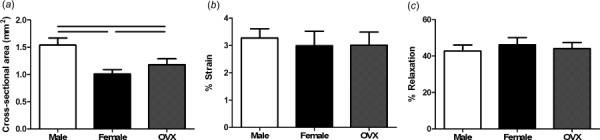
Supraspinatus quasi-static and viscoelastic properties. Male tendons were significantly larger than female and OVX tendons and OVX tendons were significantly larger than female tendons (a). There were no significant differences in transition strain (b) or percent relaxation (c) across sex. Data represented as mean and standard deviation. Significant differences are indicated by solid bars (p < 0.05/3) and trends are indicated by dashed lines (p < 0.1/3).
Peak strain, hysteresis, and laxity (measure of tendon slack length) were calculated from force–displacement data acquired during fatigue testing. Laxity is a measure of tendon slack length defined as the percentage change in nonrecoverable tendon length compared to its pretest length measured continuously throughout cyclic loading, calculated by Eq. (2). L0 is the initial length of the specimen and Ls is the length at any given point during fatigue loading measured at a constant force. Although all samples had identical testing protocols in our study, this parameter is dependent on the testing protocol applied
| (2) |
Tendon and Muscle Sample Collection.
For samples assigned to histological analysis, the supraspinatus muscle, tendon, and humeral head were collected en bloc at the time of sacrifice, as described [45]. The supraspinatus muscle body was excised and embedded in optimal cutting temperature compound, frozen with N-methylbutane in liquid nitrogen, and stored at −80 °C. Tendon–bone complexes were fixed in 4% paraformaldehyde and processed using standard paraffin techniques [45]. Fresh frozen axial sections (10 μm) of the muscle belly were collected and stored at −80 °C prior to immunofluorescent staining for muscle fiber analysis.
Tendon Histology.
Tendon-bone samples designated for histological assessment (n = 8/group) were sagittally sectioned (7 μm) and stained with Hematoxylin-Eosin or Safranin-O/Fast Green using previously established protocols. The tendon insertion and midsubstance were imaged and cell shape and cell density were calculated using commercial software (bioquant osteo II).
Muscle Histology.
Muscle sections were thawed at room temperature for 10 minutes and stained for muscle fiber analysis using established protocols [45]. Sections were blocked with 4% BSA in phosphate buffered saline and were then incubated with primary antibodies against laminin (1:100, L9393, Sigma Aldrich; St. Louis, MO), myosin heavy chain (MyHC) type 1 (1:100, BA-D5), MyHC type 2a (1:100, SC-71), and MyHC type 2b (1:100, BF-F3). Anti-MyHC antibodies were developed by Stefano Schiaffino and were obtained from the Developmental Studies Hybridoma Bank (University of Iowa) [54]. Visualization of primary antibodies was achieved with fluorescent secondary antibodies (antirabbit IgM Alexa 488, 1:100 goat antimouse IgG2b DyLight 405, 1:400 goat antimouse IgG1 Alexa 488, and 1:500 goat antimouse IgM Alexa 546, respectively). Expression of MyHC2x was determined by characterization of unstained fibers. Coverslips were mounted with ProLong Gold Antifade. Images from the superficial and deep regions of the muscle were obtained and imaging planes were used to calculate the Feret diameter. Fiber size and fiber type distribution were characterized using the matlab smash application [55].
Statistical Analysis.
Data were tested for normality and one way ANOVAs with posthoc Student's t-tests with Bonferroni corrections (α = 0.05/3) were used to evaluate differences between groups for mechanical and immunohistochemical properties. Kruskal–Wallis tests with posthoc Dunn's tests were employed for non-normal data sets. All data are presented as mean ± standard deviation.
Results
Mechanical Properties.
Cross-sectional area was significantly increased in male supraspinatus tendons compared to both female and OVX tendons, and OVX tendons were significantly larger than female tendons (Fig. 2(a)). There were no significant differences in transition strain or percent relaxation (Figs. 2(b) and 2(c)). All groups had similar toe or linear moduli (Fig. 3(a)); however, male tendons had increased stiffness at toe and linear strain levels (Fig. 3(b)). Regional differences in modulus across groups were not identified and male and OVX tendons were significantly stiffer than female tendons (data not shown). |E|freq was decreased in male tendons compared to female and OVX tendons (Fig. 4(a)) and there were few differences in tan(δ) across sex (Fig. 4(b)). |E|fat was also decreased in male tendons compared to female and OVX tendons (Fig. 5(a)) and kfat was decreased in female tendons compared to other groups (Fig. 5(b)). Compared to other groups, male tendons had increased hysteresis throughout testing (Fig. 5(c)) but no change in laxity at 50% of the total number of cycles to failure during fatigue loading (Fig. 5(d)). Data sets that were not normally distributed were tan(δ) at 5 and 10 Hz. All other data were normally distributed.
Fig. 3.
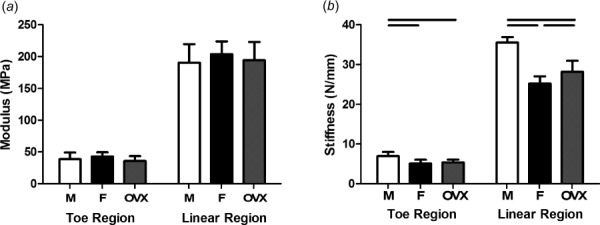
Toe and linear modulus and stiffness. There were no differences in toe or linear moduli across groups (a). Male supraspinatus tendons were stiffer than female and OVX tendons in toe and linear regions (b). Data represented as mean and standard deviation, n = 12/group. Significant differences are indicated by solid bars (p < 0.05/3) and trends are indicated by dashed lines (p < 0.1/3).
Fig. 4.
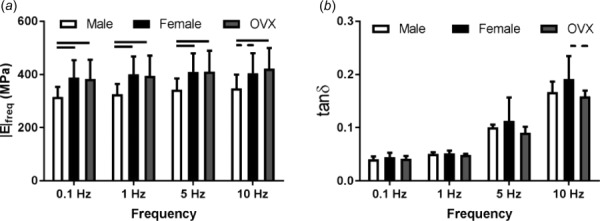
Dynamic properties. Male supraspinatus tendons had significantly decreased |E|freq than female and OVX tendons (a). There was a trend toward an increase in tan(δ) in females compared to OVX at 10 Hz (b). Non-normal data sets include tan(δ) for females and OVX rats at 5 Hz and males at 10 Hz. Data represented as mean and standard deviation, n = 12/group. Significant differences are indicated by solid bars (p < 0.05/3) and trends are indicated by dashed lines (p < 0.1/3).
Fig. 5.
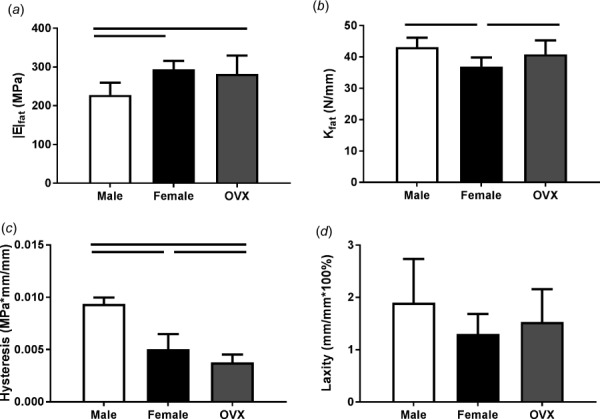
Fatigue properties. Male supraspinatus tendons had significantly decreased |E|fat than female and OVX tendons (a) while females had decreased kfat compared to other groups (b). Hysteresis was greater in male tendons than female and OVX tendons (c). There were no significant differences in laxity at 50% fatigue life (d). Data represented as mean and standard deviation, n = 12/group. Significant differences are indicated by solid bars (p < 0.05/3) and trends are indicated by dashed lines (p < 0.1/3).
Muscle and Tendon Histology.
Average muscle fiber size was decreased in females compared to males (Figs. 6(a)–6(c)). Average muscle fiber size was increased in the superficial region of muscle compared to the deep region in all groups (Supplemental Fig. 3 is available under the “Supplemental Data” tab for this paper on the ASME digital collection). In all groups, type 2a fibers in deep muscle had increased diameter compared to type 2a fibers located superficially. There were no significant differences in the distribution of fiber type in superficial and deep regions of muscle across sex (Figs. 6(d) and 6(e)). In all groups, there was a greater proportion of type 2a and type 2x fibers and a smaller proportion of type 2b fibers in deep regions of muscle compared to superficial regions. There were no significant differences in tendon cell density or cell shape (Figs. 7(a)–7(c)).
Fig. 6.
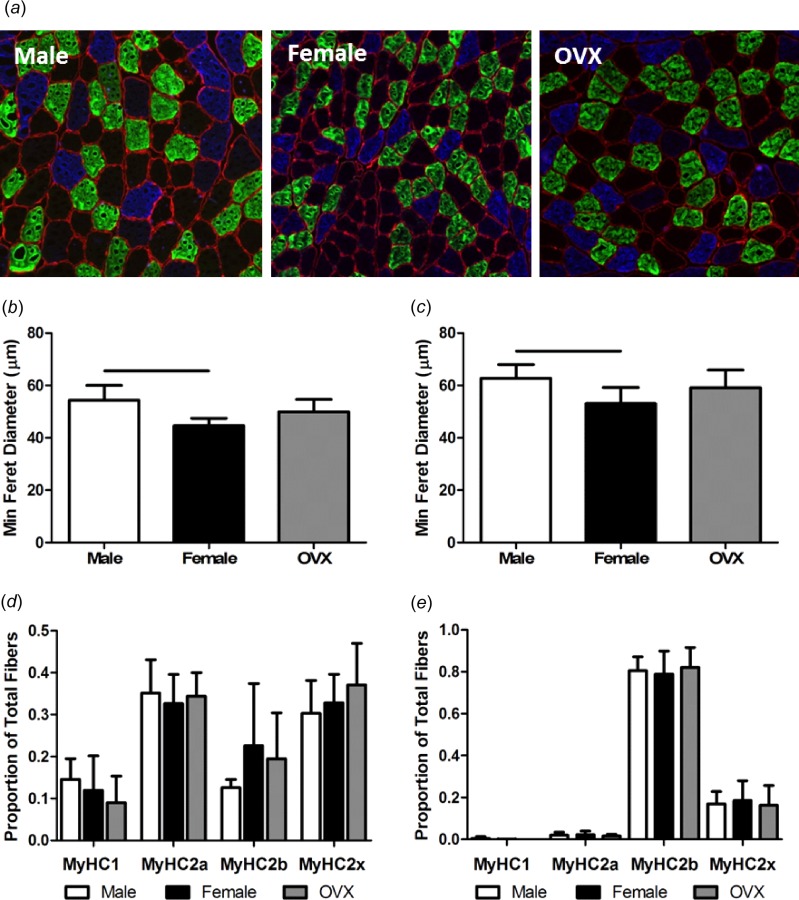
Supraspinatus muscle fiber characterization. Male, female, and OVX fresh frozen muscle sections were stained for laminin (cell borders/red), MyHC type 2a (light gray/green), MyHC type 2b (dark gray/blue), MyHC type 2x (black/unstained), and MyHC type 1 fibers (not pictured) (a). Male fibers had increased average size compared to female fibers in deep (b) and superficial (c) muscle. There were no differences in regional distribution of fiber type across sex in deep (d) or superficial (e) muscle. Data represented as mean and standard deviation, n = 8/group. Significant differences are indicated by solid bars (p < 0.05/3) and trends are indicated by dashed lines (p < 0.1/3).
Fig. 7.
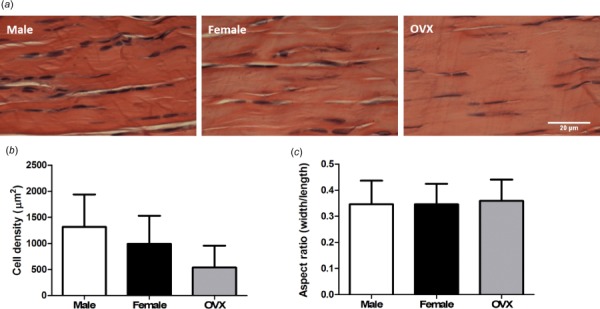
Supraspinatus tendon histology. Supraspinatus tendons from male, female, and OVX (a) rats are pictured. There were no significant differences in cell density (b) or cell shape (c). Data represented as mean and standard deviation, n = 8/group.
Discussion
The objective of this study was to investigate whether the supraspinatus tendon exhibits differences in mechanical and immunohistochemical properties across sex and to relate them to previously measured sex differences in the Achilles tendon. Clinically, males and females exhibit differences in shoulder anatomy and muscle function but differences in tendon function have not been established. Contrary to our hypothesis, differences in the mechanical properties of the supraspinatus were seen across sex.
The decreased dynamic modulus calculated during fatigue loading and increased hysteresis of male supraspinatus tendons compared to females suggests that they respond differently to cyclic loading and are less able to resist deformation under stress. Previous work [3] showed that the quasi-static and dynamic moduli of the male Achilles tendon are about 50% of the female Achilles tendon. Interestingly, results revealed a tendon-specific response to sex. Unlike male supraspinatus tendons that are larger, but maintain similar tendon material properties compared to female supraspinatus tendons, male Achilles tendons display decreased material properties compared to female Achilles tendons [3]. This supports the clinical discrepancy in sex on Achilles injuries unlike the supraspinatus tendon [4,56]. Further mechanistic evaluation comparing these tendons is required in future studies. The ability of male supraspinatus tendons to behave similarly to female and OVX tendons when loaded quasi-statically but not under fatigue loading also suggests that mechanical properties of the supraspinatus tendon not only differ across sex, but are also influenced by the way in which the tendon is loaded. This further indicates that sex differences in tendons may be affected by factors other than their hormonal environment. Interestingly, the cross-sectional area and stiffness of tendons from ovariectomized females was greater than that of normal female tendons, which implies that ovarian hormone withdrawal affects tendon structure. Other studies have also noted changes in estrogen deficient tendons and ligaments [2] including decreased tensile strength of the ACL [57], reduced collagen turnover [9], increased fiber diameter [58], and decreased inflammatory response after injury [9].
Tendon-specific variation in mechanical properties was determined by comparing these data to previously established sex-specific Achilles tendon data determined from the same animals [3]. Interestingly, the sex differences seen in the supraspinatus tendon were distinct from those seen in the Achilles tendon (Fig. 8). Male supraspinatus tendons had higher stiffness while male Achilles tendons had lower elastic moduli when compared to female supraspinatus and Achilles tendons, respectively. In contrast to quasi-static properties, male supraspinatus and Achilles tendons both exhibited significantly smaller |E|freq and |E|fat than female tendons, although the magnitude of this difference was greater in Achilles tendons. Both supraspinatus and Achilles tendons exhibited similar patterns of sex differences in cross-sectional area, hysteresis, and kfat and no sex differences in tan(δ). This suggests that tendon mechanical properties are tendon-specific as well as sex-specific.
Fig. 8.
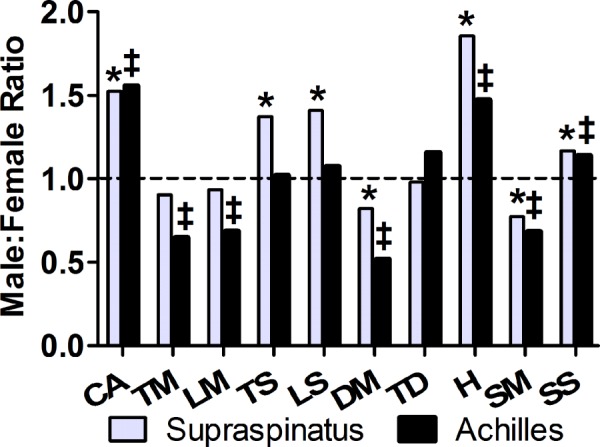
Tendon-specific sex differences in mechanical properties. Ratio of male to female means were taken for each parameter. Data that were significantly different between male and female supraspinatus or Achilles tendons are indicated by * and ‡, respectively. There were significant differences in dynamic and fatigue mechanical properties in both supraspinatus and Achilles tendons. Male supraspinatus tendons had higher stiffness while male Achilles tendons had lower elastic moduli compared to female tendons. No direct comparisons were made between supraspinatus and Achilles tendon property male:female ratio. Achilles tendon data referenced here has been previously published [3]. Dotted line represents a ratio of 1.0. CA: cross-sectional area, TM: toe modulus, LM: linear modulus, TS: toe stiffness, LS: linear stiffness, DM: |E|freq (1Hz), TD: tan(δ), H: hysteresis, SM: |E|fat, SS: kfat.
In contrast to the Achilles tendon, most clinical and epidemiological reports do not find sex to be a significant risk factor for rotator cuff tears [37,39], although this remains controversial. One study found a higher overall incidence of rotator cuff tears in females (90/100,000 versus 83/100,000), with middle aged females (age 40–54) having an increased incidence of tears compared to males, while young males (age 25–34) experienced more tears than females of the same age [41]. Others have found males to have an increased incidence of acute full thickness rotator cuff tears [6]. Our findings are in agreement with this, but unlike the Achilles tendon [3,4], the effect of the decreased modulus of male tendons may be difficult to capture in clinical studies. This may be because the supraspinatus tendon is not subjected to the same physiological loads as the tendons and ligaments of the lower extremity. Rapid eccentric loading of the Achilles tendon at loads that exceed the tendon's ultimate strength may result in rupture [59,60]. Reports of similar acute traumatic ruptures of the supraspinatus tendon are uncommon even in athletes [61], which suggests that the strength of the rotator cuff muscles is insufficient to rupture a tendon that has not been weakened by chronic degeneration. The supraspinatus tendon also experiences different strains in conjunction with the rest of the rotator cuff at various abduction angles [62,63] and different regions of the supraspinatus tendon have unique biomechanical properties [64,65], making it unlikely that a single uniaxial force vector will rupture the entire tendon. This study focused on the biomechanical and histological properties of the supraspinatus tendon and muscle; however, sex differences in neuromuscular control of the shoulder [13,14], shoulder strength [66], and glenoid and cuff anatomy [10–12] may independently affect the rate of rotator cuff tears.
Decreased estrogen levels in postmenopausal women may increase the rate of asymptomatic rotator cuff tears [44]. This may be related to decreased healing potential in estrogen deficient tendons [9]. In this study, ovarian hormone withdrawal was found to increase cross-sectional area, stiffness, and hysteresis, however, no differences in histological parameters were seen. Although it is possible that the time between ovariectomy and sacrifice was too short to detect more subtle changes in tendon function, this time frame was appropriate based on prior studies done in ovariectomized rodents [3,67].
Clinically, males and females often exhibit different injuries and this study provides additional insight into potential reasons for this. We used male, female, and ovariectomized rats to assess the role that both sex and ovarian hormone deprivation have on tendon mechanical properties. The inclusion of muscle fiber analysis helps define how the tendon-muscle unit differs across sex. Directly measuring muscle force in future studies would allow us to establish a direct connection between muscle fiber characteristics and tendon mechanical properties. Nevertheless, this study is not without limitations. First, the incidence of supraspinatus tears is greatest during late adulthood and these rats were not aged. By assessing the tendon at this time point, we established baseline mechanical properties independent of age-related tendon degeneration. Second, sex hormone levels were not tested and phase of menstrual cycle was not controlled for in female rats. Some studies indicate that the mechanical properties of other tendons do not fluctuate with the normal menstrual cycle, however, this remains controversial and this specific data is not available for the supraspinatus tendon [1,5,7,28,68–70]. Third, animals were selected using the average weight at 5 months of age for male, female, and OVX Sprague-Dawley rats. This may result in small differences in tissue structure-function relationships based on increased variation in the precise age of the rats, however, it decreased variability in tendon and muscle size within groups. Finally, the frequency sweep protocol was organized such that low frequencies were tested prior to high ones, therefore, the history effects of the slow cycles cannot be entirely deconvoluted from the fast cycles. This protocol, however, has been previously validated and facilitates comparisons with prior work [71,72]. Male and female rat supraspinatus tendons have significantly different material properties, which are different than those seen in the Achilles tendon, supporting the notion that sex differences are tendon-specific and may influence the types of injuries that men and women experience. Future work will study the rate and extent to which male and female rats recover after rotator cuff injury and repair in order to better assess the impact that these sex differences have on injury processes and tendon healing.
Acknowledgment
This study was funded by the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders, the NIH/NIAMS, and NSF GRFP. The authors thank Dr. Snehal Shetye for his contributions.
Contributor Information
K. A. Bonilla, McKay Orthopaedic Laboratory, , University of Pennsylvania, , Philadelphia, PA 19104.
A. M. Pardes, McKay Orthopaedic Laboratory, , University of Pennsylvania, , Philadelphia, PA 19104.
B. R. Freedman, McKay Orthopaedic Laboratory, University of Pennsylvania, , Philadelphia, PA 19104;; John A. Paulson School of Engineering, and Applied Sciences, , Harvard University, , Cambridge, MA 02138; Wyss Institute for Biologically, Inspired Engineering, , Harvard University, , Cambridge, MA 02115
L. J. Soslowsky, McKay Orthopaedic Laboratory, , University of Pennsylvania, , Stemmler Hall, 3450 Hamilton Walk, , Philadelphia, PA 19104 , e-mail: soslowsk@upenn.edu.
Funding Data
Foundation for the National Institutes of Health (P30 AR069619, R01AR064216S1, and T32AR007132).
References
- [1]. Oliva, F. , Piccirilli, E. , Berardi, A. C. , Frizziero, A. , Tarantino, U. , and Maffulli, N. , 2016, “ Hormones and Tendinopathies: The Current Evidence,” Br. Med. Bull., 117(1), pp. 39–58. 10.1093/bmb/ldv054 [DOI] [PubMed] [Google Scholar]
- [2]. Frizziero, A. , Vittadini, F. , Gasparre, G. , and Masiero, S. , 2014, “ Impact of Oestrogen Deficiency and Aging on Tendon: Concise Review,” Muscles, Ligaments Tendons J., 4(3), pp. 324–328https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4241423/#__ffn_sectitle. [PMC free article] [PubMed] [Google Scholar]
- [3]. Pardes, A. M. , Freedman, B. R. , Fryhofer, G. W. , Salka, N. S. , Bhatt, P. R. , and Soslowsky, L. J. , 2016, “ Males Have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model,” Ann. Biomed. Eng., 44(10), pp. 2901–2910. 10.1007/s10439-016-1635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Vosseller, J. T. , Ellis, S. J. , Levine, D. S. , Kennedy, J. G. , Elliott, A. J. , Deland, J. T. , Roberts, M. M. , and O'Malley, M. J. , 2013, “ Achilles Tendon Rupture in Women,” Foot Ankle Int., 34(1), pp. 49–53. 10.1177/1071100712460223 [DOI] [PubMed] [Google Scholar]
- [5]. Pearson, S. J. , Burgess, K. E. , and Onambélé, G. L. , 2011, “ Serum Relaxin Levels Affect the In Vivo Properties of Some but Not All Tendons in Normally Menstruating Young Women,” Exp. Physiol., 96(7), pp. 681–688. 10.1113/expphysiol.2011.057877 [DOI] [PubMed] [Google Scholar]
- [6]. Aagaard, K. E. , Abu-Zidan, F. , and Lunsjo, K. , 2015, “ High Incidence of Acute Full-Thickness Rotator Cuff Tears,” Acta Orthop., 86(5), pp. 558–562. 10.3109/17453674.2015.1022433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Lee, H. , Petrofsky, J. S. , Daher, N. , Berk, L. , Laymon, M. , and Khowailed, I. A. , 2013, “ Anterior Cruciate Ligament Elasticity and Force for Flexion During the Menstrual Cycle,” Med. Sci. Monit., 19, pp. 1080–1088. 10.12659/MSM.889393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Trobisch, P. D. , Bauman, M. , Weise, K. , Stuby, F. , and Hak, D. J. , 2009, “ Histologic Analysis of Ruptured Quadriceps Tendons,” Knee Surg. Sports Traumatol. Arthroscopy, 18(1), pp. 85–88. 10.1007/s00167-009-0884-z [DOI] [PubMed] [Google Scholar]
- [9]. Circi, E. , Akpinar, S. , Balcik, C. , Bacanli, D. , Guven, G. , Akgun, R. C. , and Tuncay, I. C. , 2009, “ Biomechanical and Histological Comparison of the Influence of Oestrogen Deficient State on Tendon Healing Potential in Rats,” Int. Orthop., 33(5), pp. 1461–1466. 10.1007/s00264-009-0778-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Merrill, A. , Guzman, K. , and Miller, S. L. , 2008, “ Gender Differences in Glenoid Anatomy: An Anatomic Study,” Surg. Radiol. Anat., 31(3), pp. 183–189. 10.1007/s00276-008-0425-3 [DOI] [PubMed] [Google Scholar]
- [11]. Tang, C.-T. , and Simpson, S. , 2016, “ Shoulder,” Sex Differences in Sports Medicine, Casey E., Rho M., and Press J. M., eds., Demos Medical Publishing, New York, pp. 31–44. [Google Scholar]
- [12]. Karthikeyan, S. , Rai, S. B. , Parsons, H. , Drew, S. , Smith, C. D. , and Griffin, D. R. , 2014, “ Ultrasound Dimensions of the Rotator Cuff in Young Healthy Adults,” J. Shoulder Elbow Surg., 23(8), pp. 1107–1112. 10.1016/j.jse.2013.11.012 [DOI] [PubMed] [Google Scholar]
- [13]. Vafadar, A. K. , Côté, J. N. , and Archambault, P. S. , 2015, “ Sex Differences in the Shoulder Joint Position Sense Acuity: A Cross-Sectional Study,” BMC Musculoskeletal Disord., 16(1), pp. 273–280. 10.1186/s12891-015-0731-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Fedorowich, L. , Emery, K. , Gervasi, B. , and Côté, J. N. , 2013, “ Gender Differences in Neck/Shoulder Muscular Patterns in Response to Repetitive Motion Induced Fatigue,” J. Electromyogr. Kinesiol., 23(5), pp. 1183–1189. 10.1016/j.jelekin.2013.06.005 [DOI] [PubMed] [Google Scholar]
- [15]. Wojtys, E. M. , Huston, L. J. , Boynton, M. D. , Spindler, K. P. , and Lindenfeld, T. N. , 2002, “ The Effect of the Menstrual Cycle on Anterior Cruciate Ligament Injuries in Women as Determined by Hormone Levels,” Am. J. Sports Med., 30(2), pp. 182–188. 10.1177/03635465020300020601 [DOI] [PubMed] [Google Scholar]
- [16]. Bridgeman, J. T. , Zhang, Y. , Donahue, H. , Wade, A. M. , and Juliano, P. J. , 2010, “ Estrogen Receptor Expression in Posterior Tibial Tendon Dysfunction: A Pilot Study,” Foot Ankle Int., 31(12), pp. 1081–1084. 10.3113/FAI.2010.1081 [DOI] [PubMed] [Google Scholar]
- [17]. Liu, S. H. , Al-Shaikh, R. , Panossian, V. , and Nelson, S. D. , 1996, “ Primary Immunolocalization of Estrogen and Progesterone Target Cells in the Human Anterior Cruciate Ligament,” J. Orthop. Res., 14(4), pp. 526–533. 10.1002/jor.1100140405 [DOI] [PubMed] [Google Scholar]
- [18]. Huisman, E. S. , Andersson, G. , Scott, A. , Reno, C. R. , Hart, D. A. , and Thornton, G. M. , 2014, “ Regional Molecular and Cellular Differences in the Female Rabbit Achilles Tendon Complex: Potential Implications for Understanding Responses to Loading,” J. Anat., 224(5), pp. 538–547. 10.1111/joa.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Torricelli, P. , Veronesi, F. , Pagani, S. , Maffulli, N. , Masiero, S. , Frizziero, A. , and Fini, M. , 2013, “ In Vitro Tenocyte Metabolism in Aging and Oestrogen Deficiency,” Age (Dordrecht), 35(6), pp. 2125–2136. 10.1007/s11357-012-9500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Aydin, A. , Kenar, H. , Atmaca, H. , Alici, T. , Gacar, G. , Muezzinoglu, U. S. , and Karaoz, E. , 2013, “ The Short- and Long- Term Effects of Estrogen Deficiency on Apoptosis in Musculoskeletal Tissues: An Experimental Animal Model Study,” Arch. Iran. Med., 16(5), pp. 271–276. [PubMed] [Google Scholar]
- [21]. Dehghan, F. , Muniandy, S. , Yusof, A. , and Salleh, N. , 2014, “ Sex-Steroid Regulation of Relaxin Receptor Isoforms (RXFP1 & RXFP2) Expression in the Patellar Tendon and Lateral Collateral Ligament of Female WKY Rats,” Int. J. Med. Sci., 11(2), pp. 180–191. 10.7150/ijms.6283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Westh, E. , Kongsgaard, M. , Bojsen-Moller, J. , Aagaard, P. , Hansen, M. , Kjaer, M. , and Magnusson, S. P. , 2008, “ Effect of Habitual Exercise on the Structural and Mechanical Properties of Human Tendon, In Vivo, in Men and Women,” Scand. J. Med. Sci. Sports, 18(1), pp. 23–30. 10.1111/j.1600-0838.2007.00638.x [DOI] [PubMed] [Google Scholar]
- [23]. Lee, C.-Y. , Liu, X. , Smith, C. L. , Zhang, X. , Hsu, H.-C. , Wang, D.-Y. , and Luo, Z.-P. , 2004, “ The Combined Regulation of Estrogen and Cyclic Tension on Fibroblast Biosynthesis Derived From Anterior Cruciate Ligament,” Matrix Biol., 23(5), pp. 323–329. 10.1016/j.matbio.2004.07.004 [DOI] [PubMed] [Google Scholar]
- [24]. Seneviratne, A. , Attia, E. , Williams, R. J. , Rodeo, S. A. , and Hannafin, J. A. , 2004, “ The Effect of Estrogen on Ovine Anterior Cruciate Ligament Fibroblasts: Cell Proliferation and Collagen Synthesis,” Am. J. Sports Med., 32(7), pp. 1613–1618. 10.1177/0363546503262179 [DOI] [PubMed] [Google Scholar]
- [25]. Magrum, E. , Statuta, S. , Hryvniak, D. , and Wilder, R. , 2016, “ Running,” Sex Differences in Sports Medicine, Casey E., Rho M., and Press J. M., eds., Demos Medical Publishing, New York, pp. 161–167. [Google Scholar]
- [26]. Greenhalgh, A. , and Sinclair, J. , 2014, “ Comparison of Achilles Tendon Loading Between Male and Female Recreational Runners,” J. Human Kinet., 44(1), pp. 155–159. 10.2478/hukin-2014-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Beynnon, B. D. , Vacek, P. M. , Newell, M. K. , Tourville, T. W. , Smith, H. C. , Shultz, S. J. , Slauterbeck, J. R. , and Johnson, R. J. , 2014, “ The Effects of Level of Competition, Sport, and Sex on the Incidence of First-Time Noncontact Anterior Cruciate Ligament Injury,” Am. J. Sports Med., 42(8), pp. 1806–1812. 10.1177/0363546514540862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Burgess, K. E. , Pearson, S. J. , and Onambélé, G. L. , 2010, “ Patellar Tendon Properties With Fluctuating Menstrual Cycle Hormones,” J. Strength Cond. Res., 24(8), pp. 2088–2095. 10.1519/JSC.0b013e3181aeb12b [DOI] [PubMed] [Google Scholar]
- [29]. Wentorf, F. A. , Sudoh, K. , Moses, C. , Arendt, E. A. , and Carlson, C. S. , 2006, “ The Effects of Estrogen on Material and Mechanical Properties of the Intra- and Extra-Articular Knee Structures,” Am. J. Sports Med., 34(12), pp. 1948–1952. 10.1177/0363546506290060 [DOI] [PubMed] [Google Scholar]
- [30]. Ahmad, C. S. , Clark, M. , Heilmann, N. , Schoeb, J. S. , Gardner, T. R. , and Levine, W. N. , 2005, “ Effect of Gender and Maturity on Quadriceps-to-Hamstring Strength Ratio and Anterior Cruciate Ligament Laxity,” Am. J. Sports Med., 34(3), pp. 370–374. 10.1177/0363546505280426 [DOI] [PubMed] [Google Scholar]
- [31]. Sigward, S. M. , Pollard, C. D. , Havens, K. L. , and Powers, C. M. , 2012, “ Influence of Sex and Maturation on Knee Mechanics During Side-Step Cutting,” Med. Sci. Sports Exercise, 44(8), pp. 1497–1503. 10.1249/MSS.0b013e31824e8813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Wojtys, E. M. , Ashton-Miller, J. A. , and Huston, L. J. , 2002, “ A Gender-Related Difference in the Contribution of the Knee Musculature to Sagittal-Plane Shear Stiffness in Subjects With Similar Knee Laxity,” J. Bone Jt. Surg., 84A(1), pp. 10–16. 10.2106/00004623-200201000-00002 [DOI] [PubMed] [Google Scholar]
- [33]. Ro, D. H. , Lee, D. Y. , Moon, G. , Lee, S. , Seo, S. G. , Kim, S. H. , Park, I. W. , and Lee, M. C. , 2016, “ Sex Differences in Knee Joint Loading: Cross-Sectional Study in Geriatric Population,” J. Orthop. Res., 35(6), pp. 1283–1289 10.1002/jor.23374. [DOI] [PubMed] [Google Scholar]
- [34]. Kerrigan, D. C. , Todd, M. K. , and Croce, U. D. , 1998, “ Gender Differences in Joint Biomechanics During Walking: Normative Study in Young Adults,” Am. J. Phys. Med. Rehabil., 77(1), pp. 2–7. 10.1097/00002060-199801000-00002 [DOI] [PubMed] [Google Scholar]
- [35]. Murshed, K. A. , Çiçekcibaşi, A. E. , Karabacakoğlu, A. , Şeker, M. , and Ziylan, T. , 2005, “ Distal Femur Morphometry: A Gender and Bilateral Comparative Study Using Magnetic Resonance Imaging,” Surg. Radiol. Anat., 27(2), pp. 108–112. 10.1007/s00276-004-0295-2 [DOI] [PubMed] [Google Scholar]
- [36]. Hewett, T. E. , Myer, G. D. , and Ford, K. R. , 2004, “ Decrease in Neuromuscular Control About the Knee With Maturation in Female Athletes,” J. Bone Jt. Surg., 86A(8), pp. 1601–1608. 10.2106/00004623-200408000-00001 [DOI] [PubMed] [Google Scholar]
- [37]. Yamamoto, A. , Takagishi, K. , Osawa, T. , Yanagawa, T. , Nakajima, D. , Shitara, H. , and Kobayashi, T. , 2010, “ Prevalence and Risk Factors of a Rotator Cuff Tear in the General Population,” J. Shoulder Elbow Surg., 19(1), pp. 116–120. 10.1016/j.jse.2009.04.006 [DOI] [PubMed] [Google Scholar]
- [38]. Keener, J. D. , Steger-May, K. , Stobbs, G. , and Yamaguchi, K. , 2010, “ Asymptomatic Rotator Cuff Tears: Patient Demographics and Baseline Shoulder Function,” J Shoulder Elbow Surg., 19(8), pp. 1191–1198. 10.1016/j.jse.2010.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Yamaguchi, K. , Ditsios, K. , Middleton, W. D. , Hildebolt, C. F. , Galatz, L. M. , and Teefey, S. A. , 2006, “ The Demographic and Morphological Features of Rotator Cuff Disease: A Comparison of Asymptomatic and Symptomatic Shoulders,” J. Bone Jt. Surg., 88A(8), pp. 1699–1704. 10.2106/JBJS.E.00835 [DOI] [PubMed] [Google Scholar]
- [40]. Milgrom, C. , Schaffler, M. , Gilbert, S. , and van Holsbeeck, M. , 1995, “ Rotator-Cuff Changes in Asymptomatic Adults,” J. Bone Jt. Surg. Br., 77(2), pp. 296–298https://online.boneandjoint.org.uk/doi/abs/10.1302/0301-620X.77B2.7706351. [PubMed] [Google Scholar]
- [41]. White, J. J. E. , Titchener, A. G. , Fakis, A. , Tambe, A. A. , Hubbard, R. B. , and Clark, D. I. , 2014, “ An Epidemiological Study of Rotator Cuff Pathology Using the Health Improvement Network Database,” Bone Jt. J., 96B(3), pp. 350–353. 10.1302/0301-620X.96B3.32336 [DOI] [PubMed] [Google Scholar]
- [42]. McFarland, E. G. , Campbell, G. , and McDowell, J. , 1996, “ Posterior Shoulder Laxity in Asymptomatic Athletes,” Am. J. Sports Med., 24(4), pp. 468–471. 10.1177/036354659602400410 [DOI] [PubMed] [Google Scholar]
- [43]. Voycheck, C. A. , Rainis, E. J. , McMahon, P. J. , Weiss, J. A. , and Debski, R. E. , 2010, “ Effects of Region and Sex on the Mechanical Properties of the Glenohumeral Capsule During Uniaxial Extension,” J. Appl. Physiol., 108(6), pp. 1711–1718. 10.1152/japplphysiol.01175.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Abate, M. , Schiavone, C. , Di Carlo, L. , and Salini, V. , 2014, “ Prevalence of and Risk Factors for Asymptomatic Rotator Cuff Tears in Postmenopausal Women,” Menopause, 21(3), pp. 275–280. 10.1097/GME.0b013e31829638e3 [DOI] [PubMed] [Google Scholar]
- [45]. Rooney, S. I. , Baskin, R. , Torino, D. J. , Vafa, R. P. , Khandekar, P. S. , Kuntz, A. F. , and Soslowsky, L. J. , 2016, “ Ibuprofen Differentially Affects Supraspinatus Muscle and Tendon Adaptations to Exercise in a Rat Model,” Am. J. Sports Med., 44(9), pp. 2237–2245. 10.1177/0363546516646377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Favata, M. , 2006, Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair, University of Pennsylvania, Pennsylvania, PA. [Google Scholar]
- [47]. Pardes, A. M. , Beach, Z. M. , Raja, H. , Rodriguez, A. B. , Freedman, B. R. , and Soslowsky, L. J. , 2017, “ Aging Leads to Inferior Achilles Tendon Mechanics and Altered Ankle Function in Rodents,” J. Biomech., 60, pp. 30–38. 10.1016/j.jbiomech.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Freedman, B. R. , Salka, N. S. , Morris, T. R. , Bhatt, P. R. , Pardes, A. M. , Gordon, J. A. , Nuss, C. A. , Riggin, C. N. , Fryhofer, G. W. , Farber, D. C. , and Soslowsky, L. , 2017, “ Temporal Healing of Achilles Tendons After Injury in Rodents Depends on Surgical Treatment and Activity,” J. Am. Acad. Orthop. Surg., 25(9), pp. 635–647. 10.5435/JAAOS-D-16-00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Fryhofer, G. W. , Freedman, B. R. , Hillin, C. D. , Salka, N. S. , Pardes, A. M. , Weiss, S. N. , Farber, D. C. , and Soslowsky, L. J. , 2016, “ Postinjury Biomechanics of Achilles Tendon Vary by Sex and Hormone Status,” J. Appl. Physiol. (1985), 121(5), pp. 1106–1114. 10.1152/japplphysiol.00620.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Freedman, B. R. , Fryhofer, G. W. , Salka, N. S. , Raja, H. A. , Hillin, C. D. , Nuss, C. A. , Farber, D. C. , and Soslowsky, L. J. , 2017, “ Mechanical, Histological, and Functional Properties Remain Inferior in Conservatively Treated Achilles Tendons in Rodents: Long Term Evaluation,” J. Biomech., 56, pp. 55–60. 10.1016/j.jbiomech.2017.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Freedman, B. R. , Sarver, J. J. , Buckley, M. R. , Voleti, P. B. , and Soslowsky, L. J. , 2014, “ Biomechanical and Structural Response of Healing Achilles Tendon to Fatigue Loading Following Acute Injury,” J. Biomech., 47(9), pp. 2028–2034. 10.1016/j.jbiomech.2013.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Freedman, B. R. , Gordon, J. A. , Bhatt, P. R. , Pardes, A. M. , Thomas, S. J. , Sarver, J. J. , Riggin, C. N. , Tucker, J. J. , Williams, A. W. , Zanes, R. C. , Hast, M. W. , Farber, D. C. , Silbernagel, K. G. , and Soslowsky, L. J. , 2016, “ Nonsurgical Treatment and Early Return to Activity Leads to Improved Achilles Tendon Fatigue Mechanics and Functional Outcomes During Early Healing in an Animal Model,” J. Orthop. Res., 34(12), pp. 2172–2180. 10.1002/jor.23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Fung, D. T. , Wang, V. M. , Laudier, D. M. , Shine, J. H. , Basta-Pljakic, J. , Jepsen, K. J. , Schaffler, M. B. , and Flatow, E. L. , 2009, “ Subrupture Tendon Fatigue Damage,” J. Orthop. Res., 27(2), pp. 264–273. 10.1002/jor.20722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Schiaffino, S. , Gorza, L. , Sartore, S. , Saggin, L. , Ausoni, S. , Vianello, M. , Gundersen, K. , and Lømo, T. , 1989, “ Three Myosin Heavy Chain Isoforms in Type 2 Skeletal Muscle Fibres,” J. Muscle Res. Cell Motil., 10(3), pp. 197–205. 10.1007/BF01739810 [DOI] [PubMed] [Google Scholar]
- [55]. Smith, L. R. , and Barton, E. R. , 2014, “ SMASH–Semi-Automatic Muscle Analysis Using Segmentation of Histology: A MATLAB Application,” Skeletal Muscle, 4(1), p. 21. 10.1186/2044-5040-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Freedman, B. R. , Gordon, J. A. , and Soslowsky, L. J. , 2014, “ The Achilles Tendon: Fundamental Properties and Mechanisms Governing Healing,” Muscles Ligaments Tendons J., 4(2), pp. 245–255https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4187594/. [PMC free article] [PubMed] [Google Scholar]
- [57]. Slauterbeck, J. , Clevenger, C. , Lundberg, W. , and Burchfield, D. M. , 1999, “ Estrogen Level Alters the Failure Load of the Rabbit Anterior Cruciate Ligament,” J. Orthop. Res., 17(3), pp. 405–408. 10.1002/jor.1100170316 [DOI] [PubMed] [Google Scholar]
- [58]. Hansen, M. , Kongsgaard, M. , Holm, L. , Skovgaard, D. , Magnusson, S. P. , Qvortrup, K. , Larsen, J. O. , Aagaard, P. , Dahl, M. , Serup, A. , Frystyk, J. , Flyvbjerg, A. , Langberg, H. , and Kjaer, M. , 2009, “ Effect of Estrogen on Tendon Collagen Synthesis, Tendon Structural Characteristics, and Biomechanical Properties in Postmenopausal Women,” J. Appl. Physiol., 106(4), pp. 1385–1393. 10.1152/japplphysiol.90935.2008 [DOI] [PubMed] [Google Scholar]
- [59]. Shepherd, J. H. , and Screen, H. R. C. , 2013, “ Fatigue Loading of Tendon,” Int. J. Exp. Pathol., 94(4), pp. 260–270. 10.1111/iep.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Komi, P. , Fukashiro, S. , and Järvinen, M. , 1992, “ Biomechanical Loading of the Achilles Tendon During Normal Locomotion,” Clin. Sports Med., 11(3), pp. 521–531. [PubMed] [Google Scholar]
- [61]. Kannus, P. , and Natri, A. , 1997, “ Etiology and Pathophysiology of Tendon Ruptures in Sports,” Scand. J. Med. Sci. Sports, 7(2), pp. 107–112https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0838.1997.tb00126.x. [DOI] [PubMed] [Google Scholar]
- [62]. Andarawis-Puri, N. , Kuntz, A. F. , Ramsey, M. L. , and Soslowsky, L. J. , 2010, “ Effect of Glenohumeral Abduction Angle on the Mechanical Interaction Between the Supraspinatus and Infraspinatus Tendons for the Intact, Partial-Thickness Torn, and Repaired Supraspinatus Tendon Conditions,” J. Orthop. Res., 28(7), pp. 846–851. 10.1002/jor.21068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Bey, M. J. , Ramsey, M. L. , and Soslowsky, L. J. , 2002, “ Intratendinous Strain Fields of the Supraspinatus Tendon: Effect of a Surgically Created Articular-Surface Rotator Cuff Tear,” J. Shoulder Elbow Surg., 11(6), pp. 562–569. 10.1067/mse.2002.126767 [DOI] [PubMed] [Google Scholar]
- [64]. Nakajima, T. , Rokuuma, N. , Hamada, K. , Tomatsu, T. , and Fukuda, H. , 1994, “ Histologic and Biomechanical Characteristics of the Supraspinatus Tendon: Reference to Rotator Cuff Tearing,” J. Shoulder Elbow Surg., 3(2), pp. 79–87. 10.1016/S1058-2746(09)80114-6 [DOI] [PubMed] [Google Scholar]
- [65]. Itoi, E. , Berglund, L. J. , Grabowski, J. J. , Schultz, F. M. , Growney, E. S. , Morrey, B. F. , and An, K.-N. , 1995, “ Tensile Properties of the Supraspinatus Tendon,” J. Orthop. Res., 13(4), pp. 578–584. 10.1002/jor.1100130413 [DOI] [PubMed] [Google Scholar]
- [66]. Elert, J. , Sterner, Y. , Nyberg, V. , and Gerdle, B. , 2000, “ Lack of Gender Differences in the Ability to Relax Between Repetitive Maximum Isokinetic Shoulder Forward Flexions: A Population-Based Study Among Northern Swedes,” Eur. J. Appl. Physiol., 83(4–5), pp. 246–256. 10.1007/s004210000300 [DOI] [PubMed] [Google Scholar]
- [67]. Boyd, S. K. , Davison, P. , Müller, R. , and Gasser, J. A. , 2006, “ Monitoring Individual Morphological Changes Over Time in Ovariectomized Rats by In Vivo Micro-Computed Tomography,” Bone, 39(4), pp. 854–862. 10.1016/j.bone.2006.04.017 [DOI] [PubMed] [Google Scholar]
- [68]. Bryant, A. L. , Clark, R. A. , Bartold, S. , Murphy, A. , Bennell, K. , Hohmann, E. , Marshall-Gradisnik, S. , Payne, C. , and Crossley, K. M. , 2008, “ Effects of Estrogen on the Mechanical Behavior of the Human Achilles Tendon In Vivo,” J. Appl. Physiol., 105(4), pp. 1035–1043. 10.1152/japplphysiol.01281.2007 [DOI] [PubMed] [Google Scholar]
- [69]. Burgess, K. E. , Pearson, S. J. , and Onambélé, G. L. , 2009, “ Menstrual Cycle Variations in Oestradiol and Progesterone Have No Impact on In Vivo Medial Gastrocnemius Tendon Mechanical Properties,” Clin. Biomech., 24(6), pp. 504–509. 10.1016/j.clinbiomech.2009.03.011 [DOI] [PubMed] [Google Scholar]
- [70]. Kubo, K. , Miyamoto, M. , Tanaka, S. , Maki, A. , Tsunoda, N. , and Kanehisa, H. , 2008, “ Muscle and Tendon Properties During Menstrual Cycle,” Int. J. Sports Med., 30(2), pp. 139–143. 10.1055/s-0028-1104573 [DOI] [PubMed] [Google Scholar]
- [71]. Lujan, T. J. , Underwood, C. J. , Jacobs, N. T. , and Weiss, J. A. , 2009, “ Contribution of Glycosaminoglycans to Viscoelastic Tensile Behavior of Human Ligament,” J. Appl. Physiol. (1985), 106(2), pp. 423–431. 10.1152/japplphysiol.90748.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Dunkman, A. A. , Buckley, M. R. , Mienaltowski, M. J. , Adams, S. M. , Thomas, S. J. , Satchell, L. , Kumar, A. , Pathmanathan, L. , Beason, D. P. , Iozzo, R. V. , Birk, D. E. , and Soslowsky, L. J. , 2013, “ Decorin Expression Is Important for Age-Related Changes in Tendon Structure and Mechanical Properties,” Matrix Biol., 32(1), pp. 3–13. 10.1016/j.matbio.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


