This research describes the effect of age, sex, reproductive status and habitat location on serum biochemical analytes in the critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis ssp. asiaeorientalis). Results highlight conservation relevant effects of biologic stages and suggests that without proper management habitat degradation may adversely affect their long-term sustainability.
Keywords: biochemistry, Cetacean, critically endangered, habitat dynamics, hepatic profile, lipid profile, Yangtze Finless Porpoise
Abstract
The objectives of this study were to investigate the effects of habitat and biological state on the physiology of critically endangered wild and semi-natural Yangtze Finless Porpoises (YFPs; Neophocaena asiaeorientalis ssp. asiaeorientalis) by measuring and comparing serum biochemical parameters. A total of 168 YFPs were sampled, 68 living in the semi-natural (Tian-E-Zhou Oxbow) and 98 living in the wild (Poyang Lake, PL) environment. The YFPs in the Tian-E-Zhou Oxbow were sampled from 2002 to 2015 and in the PL from 2009 to 2017. Each population was divided into Juvenile Male, Juvenile Female, Adult Male, Pregnant and Lactating Female life history categories. Overall, with location, 19/33 of the analytes and with season 18/33 of the analytes were significantly different. Similarly, within each location, 15/33 of the analytes changed with time in PL while only 8/33 changed with time in Tian-E-Zhou Oxbow, respectively. Finally, 15/33 of the analytes demonstrated significant differences between the different age and sex groups of animals. In our study, a significant variation, as well as an increasing and decreasing pattern of several parameters in both populations, suggest a worsening ecological environment of both habitats. This study will help in health assessment, improving conservation and management practices, a crucial requisite for biodiversity conservation.
Introduction
Effects of climate change and population growth on global freshwater reserves are increasing to such an extent that in some locations this valuable resource is shrinking, and in some instances, may disappear (Coe and Foley, 2001; Oren et al., 2010). Poyang Lake (PL), the largest freshwater lake in China and an extension of the Yangtze River, is an important habitat for critically endangered Yangtze finless porpoises (YFPs, Neophocaena phocaenoides asiaorientalis; Wang et al., 2013). In addition to significant water loss from a prolonged drought (Mei et al., 2015; Zhang et al., 2016), this crucial habitat is under increasing anthropogenic pressures in the form of sand dredging (Li, 2008), vessel trafficking (Zhang, 2007) and water quality degradation due to Yangtze River watershed alterations (Wu et al., 2004; Mueller et al., 2008). In addition to direct impacts on water quality, habitat fragmentation from these alterations may only increase with future hydroelectric power plant projects (Jiangxi Water Conservancy, 2010). Such changes in the Yangtze River are believed to have played a part in the rapid decrease of the YFP population numbers, which have been declining at the average rate of 13.73% per annum (Mei et al., 2014). These declines have resulted in the latest estimated total YFP population numbers to be around 1000 individuals, with almost half (~450) of the living population in PL (Mei et al., 2014). With the current population trajectory, the YFP may soon join the baiji (Lipotes vexillifer), a previous co-inhabitant of the Yangtze River, in extinction (Turvey et al., 2007).
Worldwide deleterious alterations in habitat quality are believed to be the main cause of almost 40% of all mammalian species experiencing severe population declines (Ceballos et al., 2017; Johnson et al., 2017) with freshwater cetaceans, or river dolphins, appearing to be extremely vulnerable toward these pressures (Turvey et al., 2010; He et al., 2017). Habitat destruction in the biodiversity-rich area around the globe can cause mass extinction, especially for threatened and endemic species (Brooks et al., 2002). Therefore, determining current physiological characteristics of individuals within an at-risk population provides baselines for future investigations on the potential effect of anthropogenic activities on their health and well-being (Schwacke et al., 2009). It is well accepted that habitat quality effects body fitness and physiological functions of the animals living within these locations (Pulliam, 1988; Huey, 1991; Carey, 2005). Therefore, monitoring of physiologic traits can help us to understand and predict organismal and population responses to environmental change and stressors, cause-effect relationships, and specificity of management techniques (Christine et al., 2016).
While the population of YFP in PL represents almost half of the remaining animals living within their historic range, an experimental conservation strategy involving relocation of a small number of animals into a natural ex situ reserve called the Tian-E-Zhou Oxbow (TZO) began in 1990. The use of this reserve, a habitat removed from many of the detrimental anthropogenic activity faced by in situ YFP, if successful over the long-term, would serve as a model for the identification of other natural habitats which could provide shelter for relocated YFP until the natural habitat of the Yangtze River could be sufficiently reclaimed for animal reintroduction. While the use of ex situ populations as a conservation strategy has been successfully implemented in terrestrial mammals, it represents the first application of this model toward a cetacean (O’Brien and Robeck, 2010). The initial success of the ex situ TZO population has been demonstrated by its increased from a net introduction of five animals from the wild since 1990 to a total of 25 in 2010. From 2010 to 2015, due to successful animal breeding, the population exceeds to more than 60 individuals with a net 108% increase in the population (Wang, 2009; 2015). This growing ex situ population, which has been living in a unique environment, may serve as a physiologic control for changes that may have occurred in wild populations of the Yangtze River, Poyang and Dongting Lake system. However, until recently (Nabi et al., 2017a), most efforts at evaluating the health of the TZO ex situ population has focused on their reproductive health and resource management (Wu et al., 2010; Zhao et al., 2010; Zeng et al., 2018). As a result, no information has been published examining the effects of their habitat relocation on their physiology over time. Therefore, the primary objective of our study was to determine if habitat, while controlling for season, had an adverse effect on the YFP health as indicated by differences in serum biochemical parameters within YFP between each location (PL vs. TZO). Secondarily, to determine if indirect evidence exists that habitats have degraded by evaluating changes in YFP serum biochemical profiles within each location over time. And finally, if changes existed between locations, to detect during what age (immature or mature), sex or physiologic state (pregnancy or lactation) they were occurring. The results of this study will help determine the efficacy of establishing ex situ natural reserves and provide evidence for or against the continued expansion of this conservation strategy.
Materials and methods
Animal ethics
The protocols for animal collection and handling were approved under Chinese law and guidelines for wildlife use by the Ministry of Agriculture of China. The Research Ethics Committee of Institute of Hydrobiology, the Chinese Academy of Science reviewed and approved the blood sampling and handling procedures.
Study design
A totals of 168 animals were sampled from TZO (n = 70) and PL (n = 98). Information about animals and sampling dates are summarized in Tables 1 and 2. Data collected in 2002, 2003 and 2015 in the TZO and 2009 and 2015 in the PL have been previously reported (Nabi et al., 2017a, 2018). Animals were grouped based on total body length (Gao and Zhou, 1993) and sex as follows: Juvenile male (JM < 138 cm), adult male (AM > 138 cm), juvenile female (JF < 138 cm) and adult females (AF > 138 cm, Table 1). Adult females were further classified as non-pregnant, lactating females (L) and pregnant non-lactating females (P). No non-pregnant, non-lactating females were sampled. Lactating females were identified based on the presence of milk in the mammary gland and pregnant females (PF) were identified by ultrasonography (LOGIQ Book XP, New York, America) of the reproductive tract.
Table 1:
Basic information of the studied animals in Tian-E-Zhou Oxbow.
| Status | ID | BL (cm) | BW (Kg) | Year/season | Status | ID | BL (cm) | BW (Kg) | Year/season |
|---|---|---|---|---|---|---|---|---|---|
| JM | 02T-M1 | 114 | 23 | 2002 | AM | 06-T-M04 | 164 | 73.2 | |
| JM | 02T-M03 | 129 | 42 | (Fall) | AM | 06-T-M06 | 148 | 65.5 | |
| JM | 04-T-M04 | 123 | 34 | 2004 (summer) | AM | 08-T-M02 | 161 | 68.75 | 2008 (spring) |
| JM | 06-T-M07 | 134 | 42 | 2006 | AM | 08-T-M03 | 161 | 70.5 | |
| JM | 06-T-M08 | 137 | 49.4 | (spring) | AM | 08-T-M07 | 162 | 77 | |
| JM | 08-T-M01 | 133 | 38.6 | 2008 | AM | 08-T-M08 | 160 | 69 | |
| JM | 08-T-M04 | 133 | 39.5 | (spring) | AM | 08-T-M12 | 146 | 49 | |
| JM | 08-T-M06 | 114 | 29.6 | AM | 08-T-M13 | 142 | 53 | ||
| JM | 08-T-M09 | 120 | 33.75 | AM | 10-T-M01 | 166 | 61.3 | 2010 | |
| JM | 08-T-M10 | 134 | 44 | AM | 10-T-M02 | 163 | 69.9 | (Fall) | |
| JM | 08-T-M11 | 118 | 34 | AM | 10-T-M03 | 158 | 70.6 | ||
| JM | 10-T-M09 | 121 | 36.5 | 2010 | AM | 10-T-M04 | 165 | 68.8 | |
| JM | 10-T-M10 | 126 | 35 | (Fall) | AM | 10-T-M06 | 143 | 48.4 | |
| JM | T15M07 | 125 | 31.4 | 2015 | AM | 10-T-M07 | 142 | 47.9 | |
| JM | T15M18 | 133 | 35.2 | (Fall) | AM | 10-T-M08 | 145 | 47.6 | |
| AM | 02T-M01 | 152 | 53 | 2002 | AM | T15M02 | 139 | 35.5 | 2015 |
| AM | 02T-M02 | 158 | 59.05 | (Fall) | AM | T15M05 | 141 | 36.8 | (winter) |
| AM | 02T-M04 | 155 | 57.5 | AM | T15M08 | 148 | 40.8 | ||
| AM | 02T-M05 | 139 | 43 | AM | T15M09 | 160 | 42 | ||
| AM | 02T-M06 | 156 | 55.4 | AM | T15M12 | 151 | 44.5 | ||
| AM | 02T-M07 | 157 | 54.3 | AM | T15M17 | 156 | 46.1 | ||
| AM | 03T-M02 | 141 | 32.7 | 2003 | P | 08-T-F03 | 136 | 59 | 2008 |
| AM | 03T-M03 | 146 | 46.15 | (Fall) | P | 08-T-F05 | 140 | 53.25 | (spring) |
| AM | 03T-M04 | 158 | 57 | P | 08-T-F06 | 148 | 71.5 | ||
| AM | 03T-M05 | 142 | 41.4 | P | 08-T-F07 | 152 | 63.75 | ||
| AM | 03T-M06 | 149 | 50.15 | P | 08-T-F09 | 149 | 67.5 | ||
| AM | 03T-M07 | 157 | 55.1 | P | TEZ 18 | 149.5 | 51.1 | 2015 | |
| AM | 03T-M08 | 154 | 55.8 | P | TEZ 19 | 143 | 55.3 | (winter) | |
| AM | 04-T-M01 | 159 | 59.35 | 2004 | P | TEZ 21 | 147.5 | 53.4 | |
| AM | 04-T-M02 | 147 | 43.45 | (Fall) | P | TEZ 25 | 139 | 45.8 | |
| AM | 04-T-M03 | 147 | 48.45 | L | 10-T-F01 | 148 | 2010 | ||
| AM | 04-T-M05 | 149 | 50.45 | L | 10-T-F03 | 140 | 51.2 | (Fall) | |
| AM | 04-T-M06 | 149 | 48.7 | L | 10-T-F04 | 149 | 56 | ||
| AM | 06-T-M01 | 149 | 50.2 | 2006 | L | 10-T-F09 | 140 | 58 | |
| AM | 06-T-M02 | 158 | 73.2 | (spring) | |||||
| AM | 06-T-M03 | 156 | 55.55 |
Table 2:
Basic information of the studied animals in Poyang Lake.
| Status | ID | BL (cm) | BW (Kg) | Year/season | Status | ID | BL (cm) | BW (Kg) | Year/season |
|---|---|---|---|---|---|---|---|---|---|
| JM | 09PYM006 | 128 | 36.95 | 2009 | AM | 15PY-M09 | 160 | 57.2 | |
| JM | 09PYM008 | 127 | 36 | (winter) | AM | 15PY-M10 | 152 | 53.8 | |
| JM | 09PYM009 | 136 | 39.1 | AM | 15PY-M12 | 138 | 46.8 | ||
| JM | 09PYM017 | 127 | 34.7 | AM | 15PY-M13 | 154 | 51.2 | ||
| JM | 09PYM020 | 125 | 38.1 | AM | 17PYM15 | 145 | 49 | 2017 | |
| JM | 10PYM03 | 127 | 35.1 | 2010 | AM | 17PYM06 | 150 | 45.1 | (spring) |
| JM | 10PYM04 | 114 | 30.9 | (spring) | AM | 17PYM14 | 142 | 49.7 | |
| JM | 11PYM03 | 124 | 39.2 | 2011 | AM | 17PYM07 | 146 | 47.7 | |
| JM | 11PYM04 | 124 | 35.3 | (winter) | JF | 10PYF05 | 128 | 34.8 | 2010 |
| JM | 11PYM06 | 137 | 45.4 | JF | 10PYF07 | 119 | 25.9 | (spring) | |
| JM | 11PYM07 | 113 | 37.1 | JF | 11PYF02 | 127 | 39.3 | 2011 | |
| JM | 11PYM08 | 113 | 35 | JF | 11PYF05 | 124 | 36 | (winter) | |
| JM | 11PYM09 | 124 | 33.6 | JF | 11PYF07 | 118 | 37.3 | ||
| JM | 11PYM11 | 116 | 38 | JF | 11PYF09 | 128 | 39 | ||
| JM | 11PYM14 | 124 | 39.5 | JF | 11PYF10 | 112 | 32.5 | ||
| JM | 11PYM15 | 116 | 39.4 | JF | 11PYF11 | 123 | 32.8 | ||
| JM | 15PY-M01 | 122 | 36.8 | 2015 | JF | 11PYF19 | 119 | 33 | |
| JM | 15PY-M05 | 124 | 24 | (spring) | JF | 11PYF23 | 115 | 36 | |
| JM | 15PY-M07 | 112 | 31.8 | JF | 11PYF14 | 128 | |||
| JM | 15PY-M11 | 129 | 37.6 | JF | 15PY-F04 | 115 | 34.7 | 2015 | |
| JM | 15PY-M14 | 129 | 35.7 | JF | 15PY-F10 | 123 | 40.3 | (spring) | |
| JM | 17PYM02 | 135.2 | 40.4 | 2017 | JF | 15PY-F12 | 127 | 45.7 | |
| JM | 17PYM10 | 128 | 39.2 | (spring) | JF | 15PY-F17 | 125 | 36.3 | |
| JM | 17PYM01 | 130 | 39.4 | JF | 17PYF14 | 122 | 33 | 2017 | |
| JM | 17PYM16 | 132 | 41.9 | JF | 17PYF10 | 118 | 27.7 | (spring) | |
| AM | 09PYM004 | 154 | 53 | 2009 | P | 09PYF002 | 145 | 62.9 | 2009 |
| AM | 09PYM007 | 150 | 53.5 | (winter) | P | 09PYF003 | 144 | 52.2 | (winter) |
| AM | 09PYM010 | 158 | 83.4 | P | 09PYF007 | 152 | |||
| AM | 09PYM011 | 138 | 40.6 | P | 10PYF01 | 138 | 50.6 | 2010 | |
| AM | 09PYM013 | 154 | 52.2 | P | 10PYF04 | 149 | 63.6 | (spring) | |
| AM | 09PYM014 | 150 | 52.4 | P | 10PYF06 | 146 | 62.7 | ||
| AM | 09PYM015 | 153 | 53.4 | P | 11PYF04 | 147 | 66.7 | 2011 | |
| AM | 09PYM016 | 158 | 62.1 | P | 11PYF06 | 140 | 60.4 | (winter) | |
| AM | 09PYM018 | 157 | 48.4 | P | 11PYF08 | 130 | 63.6 | ||
| AM | 09PYM021 | 146 | 46.8 | P | 11PYF13 | 139 | 63 | ||
| AM | 10PYM01 | 149 | 38.1 | 2010 | P | 11PYF15 | 129 | 55 | |
| AM | 10PYM05 | 152 | 47.4 | (spring) | P | 11PYF16 | 137 | 63.5 | |
| AM | 11PYM01 | 168 | 67.2 | 2011 | P | 11PYF18 | 148 | 61.1 | |
| AM | 11PYM02 | 166 | 74.1 | (winter) | P | 11PYF20 | 151 | 72.3 | |
| AM | 11PYM05 | 140 | 49 | P | 11PYF21 | 138 | 69.9 | ||
| AM | 11PYM10 | 149 | 62 | P | 15PY-F22 | 138 | 67.5 | 2015 | |
| AM | 11PYM13 | 146 | 48.6 | P | 15PY-F19 | 134 | 58.7 | (spring) | |
| AM | 11PYM16 | 148 | 51.6 | P | 15PY-F18 | 148 | |||
| AM | 11PYM17 | 150 | 59.1 | P | 15PY-F15 | 134 | 60.2 | ||
| AM | 11PYM20 | 152 | 73.4 | P | 15PY-F05 | 147 | 72.1 | ||
| AM | 15PY-M03 | 153 | 54.4 | 2015 | P | 17PYF11 | 160 | 2017 | |
| AM | 15PY-M04 | 158 | 69.8 | (spring) | P | 17PYF04 | 148 | 62.1 | (spring) |
| AM | 15PY-M06 | 144 | 53.1 | P | 17PYF16 | 140 | 58.9 | ||
| AM | 15PY-M08 | 145 | 49.8 | P | 17PYF02 | 162 |
Animal collection and blood sampling
Both in the PL and TZO, YFPs were captured by using the ‘sound chase and net capture’ method (Hua, 1987). The detailed information of animal chasing, catching, handling and release are explained in detail by Hao et al. (2009). Briefly, for each capture, animals were randomly selected within different geographical areas of the sampling locations for each collection attempt. The methodology during the capture event, the blood sampling procedure and the timing of blood collection were consistent for both populations. Ten ml of blood were drawn from the major vein of tail fluke aseptically using a disposable 10-ml syringe (Gemtier, G/Ø/ L: 21/0.7/31 mm, 201502, Shanghai, China). The blood was then transferred into serum separator and EDTA Vacutainer® tubes (BD Vacutainer, Becton Dickinson, Franklin Lakes, New Jersey, USA), and placed immediately on ice. After centrifugation (Eppendorf AG, 22332, Hamburg, Germany) at 1500 × g for 15 min, the obtained serum was then immediately transferred into cryotubes (Fisher Scientific, Pittsburgh, Pennsylvania, USA), and stored in a liquid nitrogen kettle for transportation to the laboratory for immediate analysis.
Laboratory analyses
The liver function parameters; Indirect Bilirubin (I-BILI), Direct Bilirubin (D-BILI), Total Bilirubin (T-BILI), Total Bile Acid (TBA), Gamma-glutamyl Transferase (GGT), Alkaline Phosphatase (ALP), Aspartate amino Transferase (AST), Alaline amino Transferase (ALT), lipid profile; Ligh Density Lipoprotein cholesterol (LDL-c), High Density Lipoprotein cholesterol (HDL-c), Triglyceride (TG), Total Cholesterol (TC), enzymes; Lactate Dehydrogenase (LDH), Creatine Kinase (CK), Amylase (AMS), Electrolytes; (PO43−, Ca2+, Cl−, Na+, K+, Mg2+, Fe2+) and other biochemical parameters such as Creatinine (Cr), Urea (UA), Blood Urea Nitrogen (BUN), Carbon Dioxide (CO2), Glucose (GLU), Globulin (GLB), Albumin (AlB) and Total Protein (TP) were investigated using a clinical auto-analyzer (Abbott Aeroset System). Before each assay, the auto-analyzer was calibrated.
Statistical analysis
Based on sampling methodology, and for the analysis, animals were considered to have been randomly selected during each collection period and no animal was sampled more than once. Statistical analysis was performed by either STATA (version 14, Stata Corp LP, College Station, TX, USA) or Graph Pad Prism, version 5.01 (Graph Pad Software Inc., San Diego, CA, USA). Prior to analysis, results for each analyte were evaluated for normality by the Shapiro–Wilk-test (STATA) and transformed as appropriate (natural log or sqrt). For the analysis, individual sample results for each analyte were coded for location (0: PL; 1: TZO), group (JM, JF, AM, PF, LF) and season (winter [W]: November through February; Spring [S]: March through May; Summer [Sm]: June through August; Fall [F]: September through October).
A maximum likelihood (ML) general linear model (GLM, identity link, Gaussian family) was used to determine if the dependent variable (analyte concentration) differed between locations (STATA) and between groups combined across both locations. Many of the analytes evaluated are known to be effective by season (Hall et al., 2007; Macchi et al., 2011; Nollens et al., 2018); therefore, season was added as a covariate Post hoc marginal mean comparison between locations, groups and season were then preformed and data was presented as marginal mean and 95% confidence interval. To determine if changes had occurred for each dependent variable within each location over time (2002–15 for TZO and 2009–17 for PL) a linear regression was used with time as a continuous independent variable and group as a covariate (STATA).
An unpaired Student’s t-test was used to compare the analyte concentration within one group from PL to its respective group in TZO using Graph Pad Prism, version 5.01 (Graph Pad Software Inc., San Diego, CA, USA). Results of the t-test between locations were presented as mean ± SEM. For all analyses, significance was defined as P ≤ 0.05.
Results
Overall, the results of the GLM analysis indicated that 58% (19 of 33) of the analytes were significantly different between locations, while, 55% (18 of 33) demonstrated significant seasonal effects (Table 3). However, a complete picture of the seasonal effects could not be determined due to the lack of sampling during the summer months. Across both locations, 46% (15/33) of the analytes demonstrated significant differences between the different age and sex groups of animals (Fig. 1). Finally, the linear regression results indicated that within each location, 46% (15 of 33) of the analytes changed over time in PL while only 24% (8 of 33) changed over time in TZO, respectively (Table 3).
Table 3:
Effect of habitat (PL and TZO) and time (years, 2002 to 2015 for TZO and 2009 to 2017 for PL) while controlling for season and groups (JM, JF, AM, PF, LF) on biochemical analytes (marginal mean, lower 95% CI to higher 95% CI) from YFPs.
| Analyte | Poyang Lake (PL) | Time PLa | Tian-E-Zhou Oxbow (TZO) | Time TZOa | Location | Seasonal Sidak Comparisonc |
|---|---|---|---|---|---|---|
| Coef., P value | Coef., P value | P valueb | ||||
| ALT (U/l) | 31.7, 28.5–35.3 | −0.0476, 0.01 | 39.1, 34.2 to 44.8 | NSD | 0.03 | S < W & F |
| AST (U/l) | 197.2, 185.1–209.4 | −5.973, 0.002 | 218.9, 203.7–234.1 | NSD | 0.05 | S < W & F |
| AST/ALT | 6.3, 5.7–6.9 | NSD | 5.5, 4.8–6.3 | NSD | NSD | NSD |
| GGT (U/l) | 38.0, 35.6–40.6 | −0.0302, 0.008- | 40.3, 36.9–44.0 | NSD | NSD | NSD |
| ALP (U/l) | 130.2, 112.0–151.3 | 0.0532, 0.03 | 170.0, 141.1–204.8 | NSD | NSD | NSD |
| TBA (μmol/l) | 4.16, 3.47–5.0 | NSD | 8.0, 6.39–10.0 | NSD | < 0.0001 | F < W |
| T-BILI (μmol/l) | 3.37, 2.85–3.94 | NSD | 2.71 2.15–3.34 | 0.0562, 0.001 | NSD | NSD |
| D-BILI (μmol/l) | 0.69, 0.52–0.87 | NSD | 1.01, 0.77–1.3 | 0.0514, < 0.001 | 0.06 | S & Sm & F < W |
| I-BILI (μmol/l) | 1.99, 1.16–2.47 | NSD | 1.42, 1.09–1.9 | 0.0604, 0.01 | NSD | NSD |
| TP (g/l) | 76.4, 74.7–78.0 | NSD | 70.3, 68.5–72.3 | NSD | <0.0001 | NSD |
| ALB (g/l) | 43.8, 41.9–45.7 | NSD | 50.6, 48.1–53.2 | −0.762, 0.039 | 0.0002 | W < S |
| GLB (g/l) | 32.8, 30.7–34.9 | NSD | 18.4, 15.7–21.2 | NSD | <0.0001 | S < W |
| ALB/GLB | 1.34, 1.16–1.55 | NSD | 3.53, 2.91–4.30 | NSD | <0.0001 | W < S |
| BUN (mmol/l) | 17.6, 16.8–18.5 | NSD | 18.5, 17.4–19.6 | NSD | NSD | NSD |
| UA (μmol/l) | 48.2, 41.1–56.4 | −0.1024, <0.001 | 41.3, 34.0–50.2 | NSD | NSD | S < W |
| TC (mmol/l) | 5.5, 5.11–5.83 | −0.1997, 0.01 | 6.40, 5.97–6.82 | NSD | 0.004 | NSD |
| TG (mmol/l) | 4.26, 3.44–5.27 | NSD | 2.37, 1.84–3.04 | NSD | 0.002 | NSD |
| HDL-C (mmol/l) | 2.36, 2.21–2.50 | −0.232, <0.001 | 3.21, 3.03–3.39 | NSD | <0.0001 | S & F < W |
| LDL-C (mmol/l) | 1.75, 1.50–2.04 | 0.1128, <0.001 | 1.83, 1.50–2.22 | −0.1442, <0.001 | NSD | W < F |
| HDL-C/LDL-C | 5.81, 4.65–7.26 | −2.175, <0.001 | 8.07, 6.15–10.59 | NSD | NSD | S & F < W |
| CK (U/l) | 90.3, 75.1–106.9 | −1.064, <0.001 | 156.9, 132.0–183.9 | NSD | 0.0001 | S < W |
| LDH (U/l) | 224.0, 203.8–246.2 | 0.067, <0.001 | 233.8, 207.5–263.3 | NSD | NSD | F & W < S |
| AMS (U/l) | 15.6, 11.6–20.1 | 8.577, < 0.001 | 4.57, 2.11–8.0 | NSD | 0.0005 | F < W |
| Glucose (mmol/l) | 7.64, 7.37–7.91 | NSD | 7.99, 7.6–8.38 | NSD | NSD | W < F & S |
| CO2 (mmol/l) | 21.6, 20.3–22.8 | NSD | 26.3, 24.2–28.5 | NSD | 0.002 | NSD |
| Cr (μmol/l) | 75.3, 71.0–80.0 | NSD | 78.1, 72.5–84.2 | NSD | NSD | F < S |
| K+ (mmol/l) | 4.4, 4.2–4.5 | NSD | 4.0, 3.8–4.2 | −0.012, 0.08 | 0.032 | NSD |
| Na+ (mmol/l) | 156.7, 155.8–157.6 | NSD | 152.8, 151.7–154.0 | −0.344, 0.003 | <0.0001 | NSD |
| Cl− (mmol/l) | 108.7, 107.8–109.4 | NSD | 108.3 107.3–109.4 | −0.3345, 0.005 | NSD | W & S < F |
| Ca2+ (mmol/l) | 2.56, 2.53–2.60 | −0.0135, 0.008 | 2.47, 2.42–2.51 | NSD | 0.003 | NSD |
| PO4 | 1.58, 2.45–1.70 | 0.0838, <0.001 | 1.28, 1.12–1.43 | NSD | 0.013 | W & S < F |
| Mg2+ (mmol/l) | 2.26, 2.15–2.37 | 0.0584, 0.003 | 2.06, 1.95–2.18 | NSD | 0.05 | NSD |
| Fe2+ (μmol/l) | 27.6, 23.2–32.3 | NSD | 33.9, 27.4–41.1 | NSD | NSD | NSD |
NSD: Not significantly different (P > 0.05). JM = juvenile male, JF = juvenile female, AM = adult male, PF = pregnant female, LF = lactating female.
aResults of linear regression using time as the independent variable for analyte data (dependent variable, across all years and animal groups) calculated separately for each location.
bSignificance (P ≤ 0.05) determined by location (TZ vs. PL) specific marginal mean (controlled for variance due to group and season) post hoc sidak comparison of the respective biochemical analytes.
cSignificance (P ≤ 0.05) determined for season (W = winter, S = spring, Sm = summer, F = fall) specific marginal mean (controlled for variance due to group and location) post hoc sidak comparison of the respective biochemical analytes. Only significantly different seasons are shown.
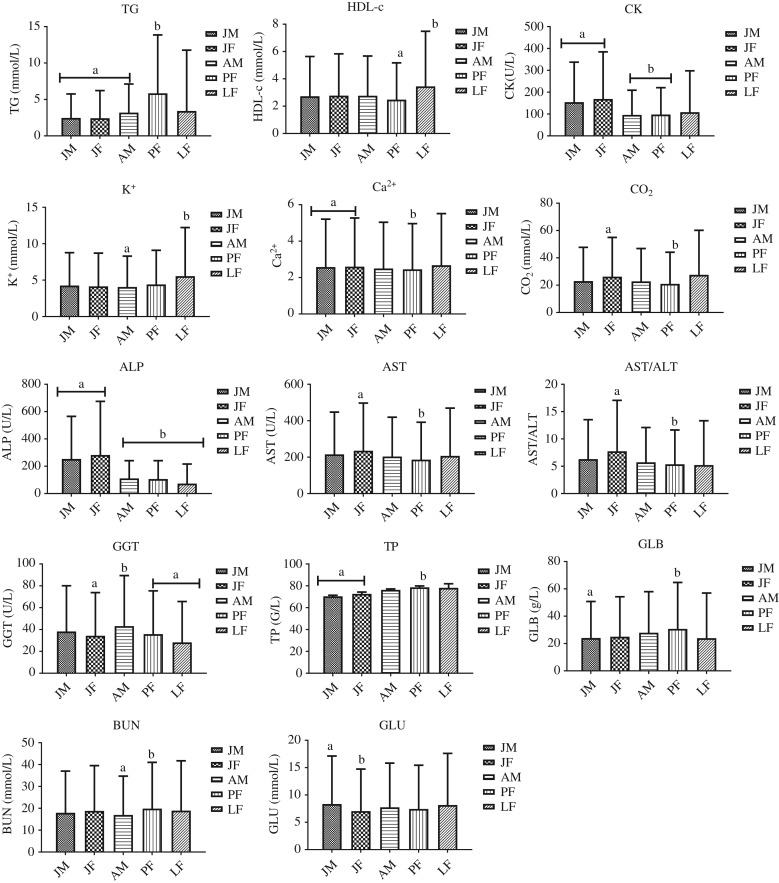
Figure 1:
Overall marginal mean (±95% confidence interval) biochemical analyte comparisons in YFP from different groups (JM = juvenile male, JF = Juvenile female, AM = adult male, PF = non-lactating, pregnant female, LF = non-pregnant, lactating female). Marginal mean concentration for each analyte were controlled for variance due to location (TZO and PL) and season. Each biochemical parameter followed by an alternate letter was significantly different at P ≤ 0.05.
Comparison of biochemical parameters from YFP between each habitat (TZO vs. PL)
Significant increases in the hepatic enzymes (ALT, AST,) and the hepatobiliary system (TBA, D-BILI) [approximate significance, P = 0.06] were detected in the animals located at TZO (Table 3). However, PL animals had increased (P < 0.0001) TP, decreased (P = 0.0002) ALB and almost doubled (P < 0.0001) GLB compared to TZO (Table 3). For TZO animals, TC was increased (P = 0.004) while TG was almost half (P = 0.002) than those observed in PL, but HDL-c was increased (P < 0.0001, Table 3). The AMS was three times higher (P = 0.0005) in PL animals, while CK in TZO YFP was almost double (P = 0.0001) than PL animals. Finally, CO2 was elevated (P = 0.002) while K+, Na+ Ca2+ PO4 and Mg2+ were significantly decreased in TZO animals (Table 3).
Biochemical parameter changes across the years for animals living in PL and TZO YFPs, respectively
Poyang Lake
Significant decreases in the liver related analytes ALT, AST and GGT were detected. No other significant changes were noted for liver related biochemical values (Table 3). The lipid profile indicated a significant decrease in TC, HDL-c and HDL-c/LDL-c, while a significant increase in LDL-c was detected (Table 3). Both the UA and CK concentrations decreased significantly, while LDH and AMS both significantly increased. For electrolytes, Ca+ decreased while Mg2+ and PO4 increased (Table 3).
Tian-E-Zhou Oxbow
Significant changes in the hepatic system were characterized by an increase in T-BILI, D-BILI and I-BILI. Similarly, decreases (P = 0.04) in ALB were noted, however, no changes in liver enzymes over time were detected. A decrease (P < 0.001) in LDL-c concentration was also noted. Finally, a significant decrease in the Na+ and Cl- were noted, with a near significant decrease in K+ (P = 0.08; Table 3).
Comparisons of marginal mean biochemical parameters between groups (JM, JF, AM, PF, LF) combined across both locations
The YFPs showed significant decreases in ALP, CK and increased in TP with age (P < 0.05, Fig. 1). The Ca2+ was non-significantly higher in juveniles (JM, JF) versus AM and significantly higher than PF (Fig. 1). Inter sex differences within adults could not be measured directly since we did not sample any non-pregnant, non-lactating adult females and both pregnancy and lactation are known to affect multiple analytes in killer whales (Robeck and Nollens, 2012). However, within the juveniles, only GLU was significantly increased in JM compared to JF. The GGT was significantly greater in adult males compared to JF, PF and LF (Fig. 1). Pregnant YFP had significantly reduced AST, AST/ALT, CO2, Ca2+ compared to JF, and significantly increased GLB, BUN and TG compared to JM, AM and both juveniles and AM, respectively. The LF had significantly increased HDL-c and K+ compared to PF and AM, respectively (Fig. 1).
Comparisons of biochemical parameters in YFP between each location (TZO vs. PL) within each group (JM, JF, AM, P, L)
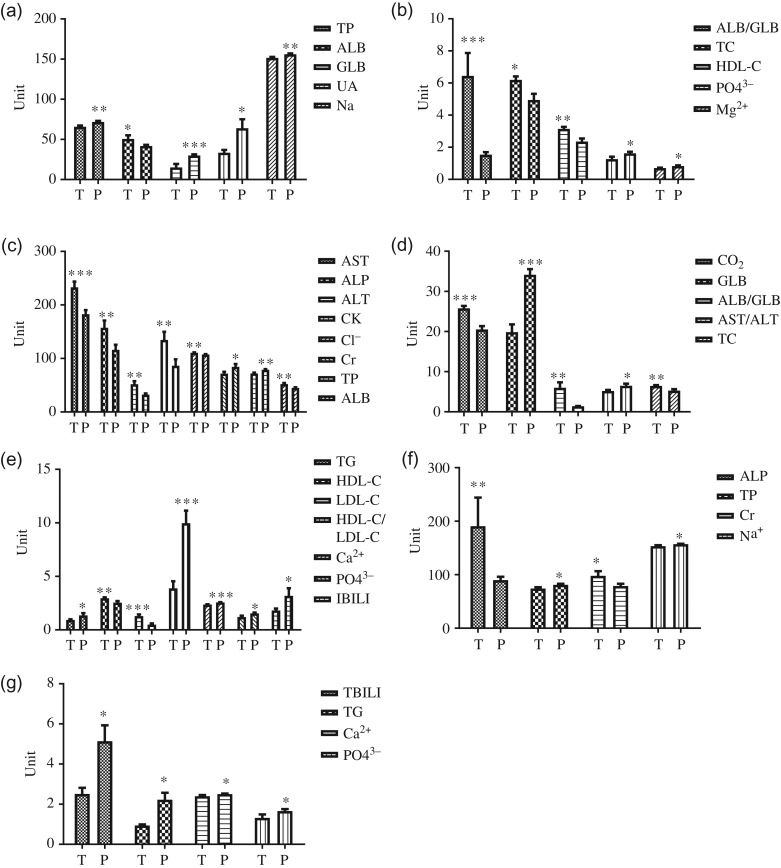
Juvenile males
The overall results of biochemical parameters are summarized in (Fig. 2). Juvenile male (JM) dwelling in the PL showed significantly higher serum level of GLB, Mg2+, Na+, PO43-, TP and UA. In TZO YFPs, serum ALB, ALB/GLB, HDL-C and TC were significantly higher.
Figure 2:
Overall comparison of the biochemical parameters between the Tian-E-Zhou Oxbow (T) and Poyang Lake (P) YFPs. The figures (a, b) compared the biochemical parameters between the T and P juvenile males. The figures (c, d, e) indicate biochemical differences in the adult males between T and P animals. The biochemical differences in pregnant groups are indicated by figures (f, g) between the T and P animals. The significant differences between the two populations are indicated by asterisk at *P < 0.05, **P < 0.01 and ***P < 0.001.
Adult males
For AM living in the TZO, the hepatic enzymes (ALP, ALT, AST), lipid profile (TC, HDL-c, LDL-c) and other biochemical parameters (ALB, ALB/GLB, CK, Cl−, CO2) were significantly higher than the adult males (AM) of PL YFPs. On the other hand, AM living in the PL showed significantly higher serum levels of hepatic enzymes (I-BILI, AST/ALT), lipid profile (TG, HDL-c/LDL-c) and other biochemical parameters (Ca2+, Cr, GLB, PO43−, TP) as shown in (Fig. 2).
Pregnant females
The same as observed in other groups, serum levels of TP, PO43−, Ca2+, Na+ and TG along with TIBILI were significantly higher in the PL YFPs. Only serum ALP and Cr were significantly higher in the TZO individuals (Fig. 2).
Discussion
Biochemical differences between locations and across time
Liver parameters
The hepatobiliary system of the TZO YFPs appeared to be under increased activation as demonstrated by a significant increase in bilirubin (T-BILI, D-BILI and I-BILI) over time and significant increases in liver associated analytes including ALP, AST, ALP, T-BILI, TBA and decreased TP compared to animals located at PL. In addition, during the entire study period, animals at PL had significant decreases in ALT, AST and GGT. Bile acids and bilirubin are typically used as indicators of liver clearance and can be elevated during states of parenchymal liver disease and biliary obstruction (Limdi and Hyde, 2003). Similarly, AST and ALT are typically increased during hepatocellular injury due to multiple clinical etiologies such as viral hepatitis, toxic hepatitis, cholestatic hepatitis and chronic active hepatitis (Rosalki and Mcintyre, 1999; Limdi and Hyde, 2003). Alternatively, significantly higher serum ALT, AST and ALP paired with the significantly increased serum cholesterol concentrations in the TZO animals suggests steatosis (Bayard et al., 2006). Total protein was reduced, albumin, which is produced by the liver and decreased in liver dysfunction, was increased for animals at TZO verses PL. The exact cause for elevated albumin in TZO needs further investigations. Complicating this determination is the fact that cetaceans are known to have heavy reserve capacity for hepatic albumin production. In addition, dehydration can elevate albumin production. Therefore, these factors limit the use of albumin as an early indicator of hepatic disorders (Bossart et al., 2001). The GLB were half and ALB/GLB was double those at TZO verse PL and were the cause of the observed decrease in TP.
Water quality in the TZO has recently been reported as being of reduced quality when compared to PL (Nabi et al., 2017a). We are aware (our unpublished work) that the TZO is primarily influenced by agricultural non-point pollution, natural input, poultry excrement and decayed organic matter pollutants. In addition to pesticides, poultry discharge from local poultry farms have been entering the TZO for over 20 years. Poultry discharge is known to possible contain viruses, bacteria, parasites, veterinary pharmaceuticals and heavy metals (EPA, 1998; University of Iowa and Iowa State Study Group, 2002; Boxall et al., 2003; Wei et al., 2010). All of these toxic chemicals or biologics are metabolized by or can directly affect the liver and may account for the increase in hepatic associated enzymes. Consequently, the significant changes observed in the hepatobiliary system of TZO over time are of important concern.
Lipid profile
In Poyang Lake YFPs, we observed a significant increase in LDL-C, and a significant decrease in TC, HDL-C and HDL-C/LDL-C as compared to TZO, while a significant decrease in LDL-C in the TZO YFPs over time. (Table 3). Variation in the YFPs lipid profile may be affected by changing fisheries resources, overall animal nutritional health, habitat utilization and the reproductive cycle (Pethybridge et al., 2011a, b; Kim et al., 2012; Nabi et al., 2017b). In cetaceans, such as bottlenose dolphins (Tursiops truncates), beluga whales (Delphinapterus leucas) and pantropical spotted dolphins (Stenella attenuata), the effects of diet on the lipid profile has been reported (Asper et al., 1990; Cook et al., 1990; St. Aubin et al., 2013). A significant variation in the lipid profile in response to habitat dynamics may indicated changing prey availability between the two locations (Miller et al., 2012). In the PL, there are ample of evidences that overfishing (Chen et al., 2002; Wei et al., 2007; Li, 2008), illegal fishing, using illegal fish gears (Wang, 2009; Schelle, 2010), and removal of large numbers of fish and shrimps by sand mining machine (Yu et al., 2001), are reducing the availability or diversity of prey for the YFPs. Furthermore, water pollution, acoustic pollution and habitat degradation (Warner, 2008; Chen et al., 2009) have already threatened several fish species within this environment (Chen et al., 2004; Hvistendahl, 2008). Similarly, in the TZO, the increasing population of YFPs (Wang, 2015) combined with possible fish mortality and morbidity by various toxic chemical pollutants in the reserve (Nabi et al., 2017a) deplete or change the availability of prey for the YFPs.
Other enzymes
The significantly higher levels of serum CK and non-significantly higher concentration of LDH in TZO YFPs could be due to rhabdomyolysis linked to capture stress as these animals are occasionally exposed to chasing during capture (Williams and Pulley, 1983; Gasper and Gilchrist, 2005). Despite using the same capture method for both populations, animals in the TZO were apparently more active when compared to the PL (Nabi et al., 2017a). Therefore, the overall significantly higher CK level in the adult male of TZO might be due to the hyper-muscular activities (Paola et al., 2007).
Electrolytes
The significant decrease in serum levels of Na+, K+ and Cl− in TZO as compared to PL, and a significant increase in the serum levels of Mg2+ and PO43− while decrease in the Ca+ levels of PL YFPs over time may reflect endocrine and gastrointestinal conditions (Bossart et al., 2001) of YFPs in response to a changing habitat (Sun et al., 2012; Dong, 2013). Overall, the electrolytes (Ca2+, PO43−, Mg2+, Na+) were significantly higher in the PL YFPs compared to TZO YFPs where only Cl- was significantly higher. In addition to dehydration, hyperaldosteronism, liver and renal dysfunctions elevate Na+ levels in cetacean (Bossart et al., 2001). Similarly, dehydration, renal disease, hypoadrenocorticism and primary hyperparathyroidism increase the serum Ca2+ concentration (Bossart et al., 2001). The Mg2+, PO43− and Cl− levels are affected by renal problems. However, PO43− is also affected by rhabdomyolysis, dietary phosphorus excess, osteolytic bone disease, and hypoparathyroidism and hypercalcemia with normal glomerular filtration (Bossart et al., 2001). While differentiating clinical conditions from normal homeostatic variations in response to dietary, or environmental differences would require further diagnostics, these significant changes are worth noting.
Biochemical profile changes during maturation and reproductive state
Serum ALP was significantly higher in the juveniles compared to the adults. While Ca2+ was also increased in juveniles compared to adults with significant differences found when compared to PF. Both ALP, and Ca2+ have been reported to be increased in juveniles of multiple cetaceans including killer whales, bottlenose dolphins and beluga (Andersen, 1968; St Aubin et al., 2001; Venn-Watson et al., 2011; Nollens et al., 2018). Higher ALP and Ca2+ concentrations in young animals is generally associated with active bone growth (Andersen, 1968; Kovacs, 2001) and has been used to indicate physical maturity in other mammals including finless porpoises (Andersen, 1968). Increased CK in young animals was also observed in killer whales, bottlenose dolphins and pigs (Thorén-Tolling, 1982; Venn-Watson et al., 2007; Nollens et al., 2018). This increase during growth may be an indicator of muscle development in both species, but without isoenzyme identification a similar phenomenon occurring in YFP is only unknown.
Similar to what was observed in killer whales (Robeck and Nollens, 2013), Pregnant YFP had significantly decreased AST, AST/ALT ratio and increased GLB, TG. Liver enzyme decreases were attributed to volume expansion during pregnancy in killer whales and mare (Harvey et al., 2005). However, contrary to our results in YFP whereby BUN increased, this volume expansions also results in increased renal clearance and decreased BUN (Robeck and Nollens, 2013). Therefore, volume expansion may not be as significant in the relatively short gestation of the YFP (~<12 month) as compared to the killer whale (17.5 month, Robeck et al., 2015). Since BUN is considered metabolic waste of protein metabolism, the increase during YFP gestation may simply reflect increase food intake during gestation. The increase in TG were also observed in the killer whale (Robeck and Nollens, 2013) and in humans and horses this change has been attributed to estrogen mediated increase in Very Low Density Lipoprotein (VLDL) (Alvarez et al., 1996; Harvey et al., 2005).
Only HDL-C was elevated during lactation, while this change hints at the well-documented changes in lipid mobilization during lactation in most mammals (Koopman et al., 2002; Struntz et al., 2004), changes in TG, and TC post-partum are commonly observed in other species (Struntz et al., 2004; Robeck and Nollens, 2013). This lack of observed changes in TG and TC during lactation may indicate differences in YFP physiology or is most likely due to extremely small sample set of animals from which detecting significant deviations from the other groups was not possible.
Seasonality in biochemical parameters
While complete evaluation of seasonal changes could not be conducted due to the lack of sampling in the summer months, multiple parameters exhibited significant differences between the remaining three seasons. Serum creatinine in the YFPs showed seasonality with significantly higher levels in spring vs fall suggesting the effects of nutrition as observed in captive and wild bottlenose dolphins (Terasawa et al., 2002; Hall et al., 2007). We are aware (unpublished work) that in both populations of YFPs, prey availability is higher in the spring and summer and therefore these changes could reflect higher intake of prey. Furthermore, increased creatinine concentration during spring and summer months has been attributed to seasonal alteration in muscle mass in bottlenose dolphins (Hall et al., 2007; Macchi et al., 2011). In addition, higher concentrations of creatinine have been associated with increased and prolonged physical exertion (Gasper and Gilchrist, 2005) and this increase in spring for the YFP may be due to the increased socially driven physical activity associated with seasonality of reproduction in both males and females. For YFP, a seasonal spring increase in GLU was also observed and may also indicate metabolic changes in response to increased physical activity. Seasonal changes in activity can also be associated with changes in both demand and types of prey availability. For example, in the polar bear, concentrations of GLU correlate with seasonal changes in the percentage of dietary protein and fat (Bossart et al., 2001). The synthesis of ALB is directly associated with protein intake (Hall et al., 2007), therefore, higher serum ALB and ALB/GLB in spring provides additional support for increased prey consumption during the spring. The increased serum UA concentrations observed in winter for the YFP has also been reported in bottlenose dolphin and could be due to seasonal variation in the nutrient composition of their diet (Hall et al., 2007) as a diet rich in purines can cause hyperuricemia (Villegas et al., 2012). Similarly, seasonal variations in the lipid profile, electrolytes and hepatobiliary parameters of YFPs could be due to changes in the water components, changes in body condition, water temperature or quality, diet, photoperiod and other factors (Domingo-Roura et al., 2001; Terasawa et al., 2002; Sergent et al., 2004; Norman et al., 2013).
Conclusions and future recommendations
In summary, our findings provide indirect evidence for potential changes in fisheries resources in both populations as indicated by a significantly different and changing lipid profiles within and between the two populations. If the cause is from a declining fisheries resources in the TZO, it may be due to pesticides induce fish mortality, morbidity and low productivity. To regulate the Oxbow, fisheries resources and the total population numbers should be closely managed. Similarly, in the PL, illegal fishing and the use of non-selective fishing gears should be strictly avoided. While significant age specific differences were noted, all could be attributed to normal physiologic changes with age that have also been observed in other cetacean species. Across age groups, however, both the populations in general and TZO YFPs specifically showed hepatic dysfunction as indicated by higher hepatobiliary parameters which might be in response to various pollutants such as pesticides and poultry. The TZO also showed differing concentrations of various electrolytes as compared to PL which suggest gastrointestinal and endocrine changes whose etiology requires further investigation. Our findings suggest that YFPs in the TZO have not been removed from the negative effects of escalating anthropogenic activities especially, pesticides pollutions. Therefore, to conserve the YFPs in the TZO, special and immediate attention is required to improve the water quality and control the discharge from agriculture runoffs. In addition, future efforts at quantifying current concentrations of PCBs and other hydrocarbon bioaccumulations in fat and blood in both populations should be prioritized.
Acknowledgements
The authors thank all the member of the Research Group of Conservation Biology of Aquatic Animals of IHB for their cooperation work on the animal capture and blood sampling. This is a SeaWorld technical contribution number 2018-3-F.
Funding
This work was supported by the National Natural Science Foundation of China [no. 31430080].
References
- Alvarez JJ, Montelongo A, Iglesias A, Lasuncion MA, Herrera E (1996) Longitudinal survey on lipoprotein profile, high density lipoprotein subclass, and post heparin lipases during gestation in women. J Lipid Res 37: 299–308. [PubMed] [Google Scholar]
- Andersen S. (1968) Physiological ranges of blood-chemical parameters in captive harbour porpoises, in Pbocoena pbocoena (L). Nord Vet Med 20: 267–278. [Google Scholar]
- Asper ED, Cornell LH, Duffield DA, Odell DK, Joseph BE, Stark BI, Perry CA (1990) Haematology and serum chemistry values in bottlenose dolphins In Leatherwood S, Reeves RR, eds. The Bottlenose Dolphin. Academic Press, San Diego, pp 479–485. [Google Scholar]
- Bayard M, Holt J, Boroughs E (2006) Nonalcoholic fatty liver disease. Am Fam Phys 73: 1961–1968. [PubMed] [Google Scholar]
- Bossart GD, Reidarson TH, Dierauf LA, Duffield DA (2001) Clinical pathology In Dierauf LA, Gulland FMD, eds. Marine mammal medicine, Ed 2 CRC Press Inc, Boca Raton, Florida, pp 383–436. [Google Scholar]
- Boxall ABA, Kolpin DW, Halling-Sorenson B, Tolls J (2003) Are veterinary medicines causing environmental risks? Environ Sci Technol 37: 286–294. [DOI] [PubMed] [Google Scholar]
- Brooks TM, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldfield S, Magin J, et al. (2002) Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol 16: 909–923. [Google Scholar]
- Carey C. (2005) How physiological methods and concepts can be useful in conservation biology. Integr Comp Biol 45: 4–11. [DOI] [PubMed] [Google Scholar]
- Ceballos G, Ehrlich PR, Dirzo R (2017) Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declined. Proc Natl Acad Sci U S A 114: 6089–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DQ, Duan XB, Liu SP, Shi WG (2004) Status and management of fishery resources of the Yangtze River, Kingdom of Cambodia, pp 173–182. http://www.apfic.org/apfic_downloads/pubs_RAP/2004-16.pdf#page=187 (last accessed 25 April 2018).
- Chen DQ, Duan XB, Liu SP, Shi WG, Wang B (2002) On the dynamics of fishery resources of the Yangtze River and its management. Acta Hydrobiol Sin 26: 685–690. (in Chinese). [Google Scholar]
- Chen DQ, Xiong F, Wang K, Chang YH (2009) Status of research on Yangtze fish biology and fisheries. Environ Biol Fishes 85: 337–357. [Google Scholar]
- Christine LM, Steven JC, Erica JC, Jennifer LF, Kevin RH, Kathleen EH, Jason RH, Brent JS, Cory DS, Craig KR, et al. (2016) Success stories and emerging themes in conservation physiology. Conserv Physiol 4: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe MT, Foley JA (2001) Human and natural impacts on the water resources of the Lake Chad basin. J Geophys Res Atmos 106: 3349–3356. [Google Scholar]
- Cook RA, Michael KS, Ellen SD (1990) Circulating levels of vitamin E, cholesterol, and selected minerals in captive and Wild Beluga Whales (Delphinapterus leucas). J Zoo Wildl Med 21: 65–69. [Google Scholar]
- Domingo-Roura X, Newman C, Calafell F, Macdonald DW (2001) Blood biochemistry reflects seasonal nutritional and reproductive constraints in the Eurasian badger (Meles meles). Physiol Biochem Zool 74: 450–460. [DOI] [PubMed] [Google Scholar]
- Dong Y. (2013) Background information of Poyang Lake and Yangtze Finless Porpoises Contingent Valuation of Yangtze Finless Porpoises in Poyang Lake, China. Springer, Netherland, pp 5–36. [Google Scholar]
- EPA US (1998) Environmental Impacts of Animal Feeding Operations. Washington, DC:U.S. Environmental Protection Agency, Office of Water, Standards and Applied Sciences Division. http://www.epa.gov/ostwater/guide/feedlots/envimpct.pdf (last accessed 26 April 2018).
- Gao A, Zhou K (1993) Growth and reproduction of three populations of finless porpoise. Neophocaena phocaenoides, in Chinese waters. Aquat Mamm 19: 3–12. [Google Scholar]
- Gasper MC, Gilchrist JM (2005) Creatine kinase: a review of its use in the diagnosis of muscle disease. Med Health RI 88: 398–404. [PubMed] [Google Scholar]
- Hall AJ, Wells RS, Sweeney JC, Townsend FI, Balmer BC, Hohn AA, Rhinehart HL (2007) Annual, seasonal and individual variation in hematology and clinical blood chemistry profiles in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida. Comp Biochem Physiol A Mol Integr Physiol 148: 266–277. [DOI] [PubMed] [Google Scholar]
- Hao YJ, Zhao QZ, Wu HP, Chen DQ, Gong C, Li L, Wang D (2009) Physiological responses to capture and handling of free-ranging male Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis). Mar Freshwater Behav Physiol 42: 315–327. [Google Scholar]
- Harvey JW, Pate MG, Kivipelto J, Asquith RL (2005) Clinical biochemistry of pregnant and nursing mares. Vet Clin Pathol 34: 248–254. [DOI] [PubMed] [Google Scholar]
- He F, Zarfl C, Bremerich V, Henshaw A, Darwall W, Tockner K, Jänig SC (2017) Disappearing giants: a review of threats to freshwater megafauna. WIREs Water 4: e1208 10.1002/wat2.1208. [DOI] [Google Scholar]
- Hua YY. (1987) Live capture of the Chinese river dolphin Lipotes by the noise of small boats and the seine. Acta Hydrobiol Sin 11: 99–100. [Google Scholar]
- Huey R. (1991) Physiological consequences of habitat selection. Am Nat 137: 91–115. [Google Scholar]
- Hvistendahl M. (2008) China’s Three Gorges Dam: An Environmental Catastrophe? https://www.scientificamerican.com/article/chinas-three-gorges-dam-disaster/. (last accessed 25 April 2018).
- Jiangxi Water Conservancy (2010) The Poyang lake project. http://www.jxsl.gov.cn. (last accessed 2 May 2018).
- Johnson CN, Balmford A, Brook BW, Buettel JC, Galetti M, Guangchun L, Wilmshurst JM (2017) Biodiversity losses and conservation responses in the Anthropocene. Science 356: 270–275. [DOI] [PubMed] [Google Scholar]
- Kim SL, Tinker MT, Estes JA, Koch PL (2012) Ontogenetic and among- Individual variation in foraging strategies of Northeast Pacific white sharks based on stable isotope analysis. PLoS One 7: e45068 10.1371/journal.pone.0045068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman H, Pabst D, McLellan W, Dillaman R, Read A (2002) Changes in blubber distribution and morphology associated with starvation in the harbor porpoise (Phocoena phocoena): evidence for regional differences in blubber structure and function. Physiol Biochem Zool 75: 498–512. [DOI] [PubMed] [Google Scholar]
- Kovacs CS. (2001) Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab 86: 2344–2348. [DOI] [PubMed] [Google Scholar]
- Li T. (2008) Crisis at Poyang Lake. http://www.chinadialogue.net/homepage/show/single/en/1846-Crisis-at-Poyang-Lake. (last accessed 5 May 2108).
- Limdi JK, Hyde GM (2003) Evaluation of abnormal liver function tests. Postgrad Med J 79: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi E, Pezzoli L, Ponzio P (2011) Influence of season on the hematological and serum biochemical values of bottlenose dolphins (Tursiops truncatus) housed in a controlled environment in northern Italy. J Zoo Wild Med 42: 480–484. [DOI] [PubMed] [Google Scholar]
- Mei X, Dai Z, Du J, Chen J (2015) Linkage between three Gorges Dam impacts and the dramatic recessions in China’s largest freshwater lake, Poyang Lake. Sci Rep 5: 18197 10.1038/srep18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Zhang X, Huang SL, Zhao X, Hao Y, Zhang L, Qian Z, Zheng J, Wang K, Wang D (2014) The Yangtze finless porpoise: on an accelerating path to extinction? Biol Conserv 172: 117–123. [Google Scholar]
- Miller CA, Best PB, Perryman WL, Baumgartner MF, Moore MJ (2012) Body shape changes associated with reproductive status, nutritive condition and growth in right whales Eubalaena glacialis and E. australis. Mar Ecol Prog Ser 459: 135–156. [Google Scholar]
- Mueller B, Berg M, Yao ZP, Zhang XF, Wang D, Pfluger A (2008) How polluted is the Yangtze River? Water quality downstream from the Three Gorges Dam. Sci Total Environ 402: 232–247. [DOI] [PubMed] [Google Scholar]
- Nabi G, Hao Y, Zeng X, Jinsong Z, McLaughlin RW, Wang D (2017. a) Hematologic and biochemical differences between two free ranging Yangtze finless porpoise populations: the implications of habitat. PLoS One 12: e0188570 10.1371/journal.pone.0188570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi G, Hao Y, Zeng X, Wang D (2017. b) Assessment of Yangtze Finless Porpoises (Neophocaena asiaorientalis) through biochemical and hematological parameters. Zool Stud 56: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi G, McLaughlin RW, Hao Y, Wang D (2018) The possible effects of high vessel traffic on the physiological parameters of the critically endangered Yangtze Finless Porpoise (Neophocaena asiaeorientalis ssp. asiaeorientalis). Front Physiol. 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollens HK, Robeck TR, Schmitt TL, Croft LL, Osborn S, McBain JF (2018) Effect of age, gender and season on the variation in blood analytes of clinically normal killer whales (Orcinus orca). Vet Clin Path. (manuscript accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SA, Beckett LA, Miller WA, Leger JS, Hobbs RC (2013) Variation in hematologic and serum biochemical values of belugas (Delphinapterus leucas) under managed care. J Zoo Wildl Med 44: 376–388. [DOI] [PubMed] [Google Scholar]
- Oren A, Plotnikov IS, Sokolov S, Aladin NV (2010) The Aral Sea and the Dead Sea: disparate lakes with similar histories. Lakes Reserv Res Manag 15: 223–236. [Google Scholar]
- O’Brien JK, Robeck TR (2010) The value of ex situ cetacean populations in understanding assisted reproductive technology for ex situ and in situ species management and conservation efforts. Int J Comp Psychol 23: 227–248. [Google Scholar]
- Paola B, Nicola M, Francesco ML (2007) Creatine kinase monitoring in sport medicine. Br Med Bull 81–82: 209–230. [DOI] [PubMed] [Google Scholar]
- Pethybridge H, Daley R, Virtue P, Nichols P (2011. a) Diet composition of demersal sharks and chimaeras inferred by fatty acid profiles and stomach content analysis. J Exp Mar Biol Ecol 409: 290–299. [Google Scholar]
- Pethybridge H, Daley R, Virtue P, Nichols PD (2011. b) Lipid (energy) reserves, utilisation and provisioning during oocyte maturation and early embryonic development of deep water chondrichthyans. Mar Biol 158: 2741–2754. [Google Scholar]
- Pulliam RH. (1988) Sources, sinks, and population regulation. Am Nat 132: 652–661. [Google Scholar]
- Robeck TR, Nollens HH (2012) Hematologic and serum biochemical parameters reflect physiological changes during gestation and lactation in killer whales (Orcinus orca). Zoo Biol 32: 497–509. [DOI] [PubMed] [Google Scholar]
- Robeck TR, Nollens HH (2013) Hematological and serum biochemical analytes reflect physiological challenges during gestation and lactation in killer whales (Orcinus orca). Zoo Biol 32: 497–509. [DOI] [PubMed] [Google Scholar]
- Robeck TR, Willis K, Scarpuzzi MR, O’Brien JK (2015) Comparisons of life history parameters between free-ranging and captive killer whale (Orcinus orca) populations for application toward species management. J Mamm 96: 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki SB, Mcintyre N (1999) Biochemical investigations in the management of liver disease Oxford textbook of clinical hepatology, Ed 2 Oxford university press, New York, pp 503–521. [Google Scholar]
- Schelle P. (2010) River dolphins & people: Shared rivers, shared future. WWF International. ISBN: 978-2-940443-09-3.
- Schwacke LH, Hall AJ, Townsend FI, Wells RS, Hansen LJ, Hohn AA, Bossart GD, Fair PA, Rowles TK (2009) Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am J Vet Res 70: 973–985. [DOI] [PubMed] [Google Scholar]
- Sergent N, Rogers T, Cunningham M (2004) Influence of biological and ecological factors on hematological values in wild Little Penguins, Eudyptula minor. Comp Biochem Physiol A Mol Integr Physiol 138: 333–339. [DOI] [PubMed] [Google Scholar]
- St. Aubin DJ, Deguise S, Richard PR, Smith TG, Geraci JR (2001) Hematology and plasma chemistry as indicators of health and ecological status in Beluga Whales, Delphinapterus leucas. Arctic 54: 317–331. [Google Scholar]
- St. Aubin DJ, Forney KA, Chivers SJ, Scott MD, Danil K, Romano TA, Wells RS, Gulland FM (2013) Hematological, serum, and plasma chemical constituents in pantropical spotted dolphins (Stenella attenuata) following chase, encirclement, and tagging. Mar Mamm Sci 29: 14–35. [Google Scholar]
- Struntz DJ, McLellan WA, Disllaman R, Blum J, Kucklick J, Pabst DA (2004) Blubber development in bottlenose dolphins (Tursiops truncatus). J Morphol 259: 7–20. [DOI] [PubMed] [Google Scholar]
- Sun SL, Chen HS, Ju WM, Song J, Li JJ, Ren YJ, Sun J (2012) Past and future changes of stream flow in Poyang Lake Basin, Southeastern China. Hydrol Earth Syst Sci 16: 2005–2020. [Google Scholar]
- Terasawa F, Kitamura M, Fujimoto A, Hayama S (2002) Seasonal changes of blood composition in captive bottlenose dolphins. J Vet Med Sci 64: 1075–1078. [DOI] [PubMed] [Google Scholar]
- Thorén‐Tolling K. (1982) Age dependant variation of serum creatine kinase isoenzyme levels in pigs. Zentralbl Veterinarmed A 29: 420–428. [DOI] [PubMed] [Google Scholar]
- Turvey ST, Pitman RL, Taylor BL, Barlow J, Akamatsu T, Barrett LA, Zhao X, Reeves RR, Stewart BS, Wang K, et al. (2007) First human-caused extinction of a cetacean species? Biol Lett 3: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey ST, Risley CL, Barrett LA, Yujiang H, Wang D (2010) River dolphins can act as a population trend indicator in degrading freshwater systems. PLoS One 7: e37902 10.1371/journal.pone.0037902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Iowa and Iowa State Study Group 2002. Iowa Concentrated Animal Feeding Operations Air Quality Study. Iowa City, IA: The University of Iowa College of Public Health. https://www.public-health.uiowa.edu/ehsrc/CAFOstudy/CAFO_final2-14.pdf. (last accessed 10 May 2018).
- Venn-Watson S, Jensen ED, Ridgway SH (2007) Effects of age and sex on clinicopathologic reference ranges in a healthy managed Atlantic bottlenose dolphin population. J Am Vet Med Ass 231: 596–601. [DOI] [PubMed] [Google Scholar]
- Venn-Watson S, Smith CR, Gomez F, Jensen ED (2011) Physiology of aging among healthy, older bottlenose dolphins (Tursiops truncatus): comparisons with aging humans. J Comp Physiol B 181: 667–680. [DOI] [PubMed] [Google Scholar]
- Villegas R, Xiang YB, Elasy T, Xu WH, Cai H, Cai Q, Linton MF, Fazio S, Zheng W, Shu XO (2012) Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men’s Health Study. Nutr Metab Cardiovasc Dis 22: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. (2009) Population status, threats and conservation of the Yangtze finless porpoise. Chin Sci Bull 54: 3473–3484. [Google Scholar]
- Wang D. (2015) Progress achieved on natural ex situ conservation of the Yangtze finless porpoise. IUCN SSC- Cetacean Specialist Group. http://www.iucn-csg.org/index.php/2015/12/10/progress-achieved-on-natural-ex-situ-conservation-of-the-yangtze-finless-porpoise/ (last accessed 9 May 2018).
- Wang D, Turvey ST, Zhao X, Mei Z (2013) Neophocaena asiaeorientalis ssp. asiaeorientalis The IUCN Red List of Threatened Species. Version 3.1. http://www.iucnredlist.org/details/summary/43205774/0. (last accessed 8 May 2018).
- Warner R. (2008) Protecting the diversity of the depths: environmental regulation of bioprospecting and marine scientific research beyond national jurisdiction. Ocean Yearbook 22: 411–443. [Google Scholar]
- Wei J, Jin Y, Sims T, Kniel KE (2010) Survival of murine norovirus and hepatitis A virus in different types of manure and biosolids. Foodborne Pathog Dis 7: 901–906. [DOI] [PubMed] [Google Scholar]
- Wei Q, Wang D, Wang L (2007) Aquatic biodiversity conservation In Yang GS, Weng LD, Li LF, eds. Yangtze Conservation and Development Report. Changjiang Press, Wuhan, China, pp 90–113. [Google Scholar]
- Williams TD, Pulley LT (1983) Hematology and blood chemistry in the sea otter (Enhydra lutris). J Wildl Dis 19: 44–50. [DOI] [PubMed] [Google Scholar]
- Wu HP, Hao YJ, Yu XY, Xian YJ, Zhao QZ, Chen DQ, Kuang XA, Kou ZB, Feng KK, Gong WM, et al. (2010) Variation in sexual behaviors in a group of captive male Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis): motivated by physiological changes? Theriogenology 74: 1467–1475. [DOI] [PubMed] [Google Scholar]
- Wu JG, Huang JH, Han XG, Gao XM, He FL, Jiang MX, Jiang ZG, Primack RB, Shen ZH (2004) The Three Gorges Dam: an ecological perspective. Front Ecol Environ 2: 241–248. [Google Scholar]
- Yu D, Dong M, Wang J, Zhang X (2001) Population status of Yangtze finless porpoise in the Yangtze River section from Hukou to Nanjing. Acta Theriol Sin 21: 174–179. (in Chinese). [Google Scholar]
- Zeng X, Huang SL, Hao Y, Wang D, Ji J, Deng X, Nabi G (2018) Ultrasonography of mammary glands in finless porpoises (Neophocaena asiaeorientalis) at different reproductive stages. Mar Mam Sci 34: 529–540. [Google Scholar]
- Zhang K. (2007) Poyang Lake: Saving the finless porpoise. http://www.chinadialogue.net/article/show/single/en/839-Poyang-Lake-saving-the-finless-porpoise. (last accessed 26 April 2018).
- Zhang Z, Huang Y, Xu CY, Chen X, Moss EM, Jin Q, Bailey AM (2016) Analysis of Poyang Lake water balance and its indication of river–lake interaction. Springerplus 5: 1555 10.1186/s40064-016-3239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang D, Turvey ST, Taylor B, Akamatsu T (2010) Distribution patterns of Yangtze finless porpoises in the Yangtze River: implications for reserve management. Anim Conserv 16: 509–518. [Google Scholar]