Abstract
An increased number of treatment options has become available for patients with single sided deafness (SSD), who are seeking hearing rehabilitation. For example, bone-conduction devices that employ contralateral routing of sound (CROS), by transmitting acoustic bone vibrations from the deaf side to the cochlea of the hearing ear, are widely used. However, in some countries, cochlear implantation is becoming the standard treatment. The present study investigated whether CROS intervention, by means of a CROS bone-conduction device (C-BCD), affected sound-localization performance of patients with SSD. Several studies have reported unexpected moderate to good unilateral sound-localization abilities in unaided SSD listeners. Listening with a C-BCD might deteriorate these localization abilities because sounds are transmitted, through bone conduction to the contralateral normal hearing ear, and could thus interfere with monaural level cues (i.e. ambiguous monaural head-shadow cues), or with the subtle spectral localization cues, on which the listener has learned to rely on. The present study included nineteen SSD patients who were using their C-BCD for more than five months. To assess the use of the different localization cues, we investigated their localization abilities to broadband (BB, 0.5–20 kHz), low-pass (LP, 0.5–1.5 kHz), and high-pass filtered noises (HP, 3–20 kHz) of varying intensities. Experiments were performed in complete darkness, by measuring orienting head-movement responses under open-loop localization conditions. We demonstrate that a minority of listeners with SSD (5 out of 19) could localize BB and HP (but not LP) sounds in the horizontal plane in the unaided condition, and that a C-BCD did not deteriorate their localization abilities.
Keywords: azimuth, Bone-conduction device, Monaural listening, Sound-localization, Hearing loss, Baha, Spectral cues, SSD
1. Introduction
Single-sided (total) deafness (SSD) severely hampers sound-localization performance, and results in difficulties understanding speech when the talker is at the deaf side (Wazen et al., 2005; Bosman et al., 2003). Several studies have suggested that in children with congenital unilateral hearing loss speech and language development is affected, as well as their academic performance and social interactions (Bess et al., 1986; Wie et al., 2010; Lieu, 2013; Sangen et al., 2017; Van Wieringen et al., 2018). While the treatment of bilateral sensorineural hearing loss is well accepted and standardized, there is little consensus regarding the treatment of patients with unilateral (sensorineural) hearing loss. Differences in treatment are partly due to local regulations, but also to differences in awareness amongst caregivers. In some countries, SSD patients might be eligible for cochlear implantation. A cochlear implant for SSD patients restores bilateral input, which might lead to some form of binaural hearing (Dillon et al., 2017). In other countries, only devices that use contralateral routing of sound (CROS; Zeitler et al., 2012; Peters et al., 2015) are reimbursed. With a conventional CROS hearing aid, sounds are picked up with a microphone at the deaf side. That signal is transmitted electronically to a behind-the-ear hearing aid with open earmould, positioned at the normal-hearing ear. Note that a CROS device does not restore binaural hearing, as it only lifts the head shadow.
A second type of CROS device is based on hearing by bone conduction. A bone-conduction device (BCD) is placed at the deaf side, and the amplified sounds are transmitted transcranially to the contralateral normal-hearing cochlea through bone conduction. Whether or not SSD listeners opt for a CROS intervention might depend on several factors. Perceived limited benefits after a soft-band trial is one of the common reasons to decline implantation (Saroul et al., 2014; Siau et al., 2015). Alternatively, it might be that (congenital) SSD patients are not aware of the disadvantages of unilateral hearing (Lieu, 2004, 2013; Vila and Lieu, 2015), and therefore do not ask for clinical help. However, quantitative data on the percentages of SSD patients who do, or do not, receive treatment are still lacking.
In this study, we focus on sound-localization performance of SSD listeners, listening with and without a BCD as a CROS device (C-BCD). Sound localization in the horizontal plane (azimuth) relies on the neural processing of ILDs (interaural level differences), and ITDs (interaural time differences). SSD patients don't have access to these cues. Localization in the vertical plane (elevation) is determined by high-frequency, spectral pinna cues (Batteau, 1967; Hofman and Van Opstal, 1998), and these monaural cues are still available in the younger (SSD) listeners, who do not yet suffer from presbycusis (Otte et al., 2013; Agterberg et al., 2014). Theoretically, the localization abilities of SSD patients are not expected to improve by C-BCD use, although improvement of sound localization with a BCD has been reported (Monini et al., 2015). Yet, the opposite, a decrement in localization performance of SSD patients equipped with a C-BCD (Grantham et al., 2012), and of patients equipped with a CROS hearing aid (Pedley and Kitterick., 2017), has been demonstrated. Most SSD patients rely on the ambiguous monaural head-shadow cue, which, in combination with non-acoustic information, like familiarity with the sound source, might enable them to localize sounds in familiar environments (Slattery and Middlebrooks, 1994; Van Wanrooij and Van Opstal, 2004; Agterberg et al., 2014). The monaural level cue results from the head-shadow effect (HSE), which is the frequency-specific attenuation of sounds by the head. Because the head-shadow cue is more pronounced for higher frequencies (>3 kHz) than for lower frequencies (<1.5 kHz), broadband noise bursts presented at the deaf side are perceived different as compared to broadband sounds presented at the hearing side (i.e. the head acts as a low-pass filter). Some SSD patients, however, have learned to use the ambiguous monaural level cues and veridical spectral-shape cues of their hearing ear for the unaided localization of sounds in the horizontal plane (Van Wanrooij and Van Opstal, 2004; Agterberg et al., 2014; Firszt et al., 2015).
It is conceivable that the CROS signals from the deaf side could interfere with the ambiguous monaural head-shadow cue, and the subtle veridical spectral-shape cues perceived by the normal functioning ear, and might thus deteriorate existing localization performance in SSD patients without high-frequency hearing loss of the normal-hearing ear (i.e. younger listeners). Grantham et al. (2012) reported reduced localization ability in patients with SSD who were listening with a C-BCD. They suggested that the reduced performance could be caused by the interaction of air-conducted and bone-conducted sounds at the hearing ear, which altered the (unaided) monaural localization. Despite the uncertainty of its potential effects on localization, the C-BCD has become a commonly used treatment offered to SSD patients (Bosman et al., 2003; Niparko et al., 2003; Wazen et al., 2003; Desmet et al., 2014; Kompis et al., 2016). Several studies have indeed reported that a C-BCD does not improve the localization abilities of SSD listeners (e.g. Wazen et al., 2005; Hol et al., 2006; Lin et al., 2006). The present paper investigated, whether a C-BCD would perhaps jeopardize directional hearing based on monaural spectral and/or level cues.
2. Patients and methods
2.1. SSD listeners
Nineteen SSD patients (aged 17–68) were included in the present study. Ten patients were familiar with the sound-localization setup because they had participated in a previous study using the same setup (Agterberg et al., 2014). Nine patients had congenital SSD, and ten patients had acquired SSD. All patients were using their C-BCD for more than 5 months, when evaluated in this study. Seven SSD listeners were deaf on the right side, and twelve on the left side (see Table 1). Patients decided for a C-BCD on a percutaneous titanium implant after a trial period in which they were provided with a conventional CROS hearing aid, and a C-BCD on a headband (Hol et al., 2010). After the trial the patients were fitted with the Baha®-Divino, Intenso or BP-110 (Cochlear), or Ponto Pro Power® or Ponto Pro® sound processor (Oticon Medical). All listeners were tested with microphones in omnidirectional mode and in daily volume setting used by the patients. Functionality of the devices was checked prior to the experiment. The fitting was not further optimized. The patients were tested in the aided (C-BCD on) and unaided (C-BCD off) condition. Test conditions were pseudo-randomly varied. Table 1 lists the characteristics of the patients and indicates which patients have been familiar with the setup.
Table 1.
Characteristics of the listeners with SSD. The response gain, MAE and bias for all SSD listeners in the unaided and aided conditions for BB stimuli. * indicates the ‘good performers’. Subjects who have been familiar with the set-up are indicated in bold and italic. Acq = acquired, Con = congenital, C-BCD = CROS Bone-conduction device, MAE = Mean absolute error, PontP = Ponto Pro Power® sound processor, PontPro = Ponto Pro® sound processor, SSD = Single sided deafness, Time (m) = time of usage in months, y = years.
| SSD Patients | Age (y) | Side HL | Con Acq | BCD | Time (m) | C-BCD Off |
C-BCD On |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gain | MAE | Bias | Gain | MAE | Bias | ||||||
| P1* | 17 | Left | con | BP110 | 8 | 0.90 | 30 | 19 | 0.99 | 23 | 7 |
| P2* | 24 | Right | con | Divino | 15 | 0.79 | 26 | 2 | 0.80 | 24 | −11 |
| P3* | 27 | Right | con | BP110 | 8 | 0.95 | 14 | 1 | 1.10 | 16 | 6 |
| P4 | 29 | Right | con | Divino | 43 | 0.51 | 30 | −29 | 0.57 | 32 | −25 |
| P5 | 33 | Left | con | Intenso | 38 | 0.62 | 54 | 28 | 0.17 | 38 | 48 |
| P6 | 35 | Right | acq | Intenso | 15 | 0.01 | 53 | −67 | 0.45 | 61 | −20 |
| P7 | 35 | Left | con | PontP | 10 | 0.13 | 45 | 21 | 0.20 | 56 | 11 |
| P8 | 37 | Left | con | PontPro | 90 | 0.29 | 42 | 33 | 0.03 | 44 | 33 |
| P9* | 40 | Left | acq | PontPro | 10 | 0.83 | 23 | 4 | 0.98 | 23 | 5 |
| P10 | 43 | Left | acq | Intenso | 116 | −0.02 | 42 | 11 | 0.04 | 40 | 6 |
| P11 | 45 | Right | acq | Divino | 42 | 0.34 | 36 | −28 | 0.35 | 33 | −27 |
| P12 | 45 | Left | acq | Intenso | 27 | 0.02 | 61 | 58 | −0.05 | 60 | 52 |
| P13* | 46 | Left | con | Divino | 50 | 1.10 | 22 | −22 | 1.10 | 33 | 0 |
| P14 | 47 | Left | con | Intenso | 22 | 0.20 | 39 | 60 | 0.38 | 56 | 42 |
| P15 | 47 | Right | acq | Intenso | 31 | 0.01 | 36 | −5 | 0.06 | 37 | −8 |
| P16 | 47 | Left | acq | Intenso | 63 | 0.26 | 36 | 24 | 0.23 | 39 | 14 |
| P17 | 65 | Left | acq | Intenso | 14 | 0.02 | 35 | 15 | 0.13 | 39 | 5 |
| P18 | 67 | Right | acq | BP110 | 6 | 0.02 | 35 | −6 | 0.05 | 37 | −8 |
| P19 | 68 | Left | acq | Intenso | 19 | 0.01 | 59 | 80 | 0.34 | 78 | 39 |
2.2. Stimuli and experimental setup
Broadband noise bursts (BB; 0.5–20 kHz; n = 36 stimuli) and high-pass noise bursts (HP; 3–20 kHz; n = 36 stimuli) were presented at randomly selected sound levels of 45, 55 or 65 dB SPL. All low-pass noise bursts (LP; 0.5–1.5 kHz; n = 12 stimuli) were presented at a level of 55 dB SPL. All stimuli had 150-ms duration and were presented in pseudo-random order within a block of 108 trials. Sounds were digitally generated in Matlab (The Mathworks 7.4), and were delivered through a broadband loudspeaker, moved by a computer-controlled motorized circular hoop with 63 speakers, equally spaced in elevation at 2.5° intervals, which could rotate around a vertical axis at a distance of 0.85 m from the patient's head. Stimulus coordinates for BB and HP stimuli ranged from −85° to +85° in azimuth (resolution 0.1°) and from −30° to +30° in elevation (resolution 2.5°). The LP stimuli were presented only at 0° elevation.
To ensure that patients could only use acoustic information to localize sounds, the tests were performed in a completely dark, sound-attenuated room. For more details of the setup see Agterberg et al. (2014).
2.3. Paradigms
After calibration of the setup, each experimental session started with a brief practice session, in which ten BB stimuli were presented, in order to familiarize the participant with the testing procedures. Subjects were asked to fixate a LED located at 0° azimuth and 0° elevation, and then to trigger the (next) sound burst by pressing a button. After stimulus presentation, the listener had to direct the head-mounted dim laser spot, which was projected onto a small plastic frame at about 40 cm in front of the eyes, as fast and accurately as possible towards the perceived sound direction. Patients were monitored by the experimenter through an infrared camera positioned in the test room, and received no feedback about their performance during the experiments.
2.4. Data analysis
We analysed the azimuth responses (αRESP) separately for each patient and for each stimulus condition (LP, HP and BB noises). For each patient; the mean absolute error (MAE) for the unaided and aided conditions was determined, as well as the best linear fit (αRESP = b + g · αSTIM) of the stimulus-response relationship (pooled across presentation levels, α is the azimuth angle in degrees, b is the response bias in degrees, and g is the dimensionless response gain). We consider the localization bias as a measure for the dominance of the hearing ear, and its systematic change with sound level as a proxy for the use of the (ambiguous) HSE. If the left ear is impaired, the bias is expected to be positive, and largest for the lowest sound level. For the right ear, these effects are reversed. For normal-hearing listeners, the bias remains close to zero for all sound levels.
3. Results
3.1. The C-BCD does not affect the localization performance of SSD listeners
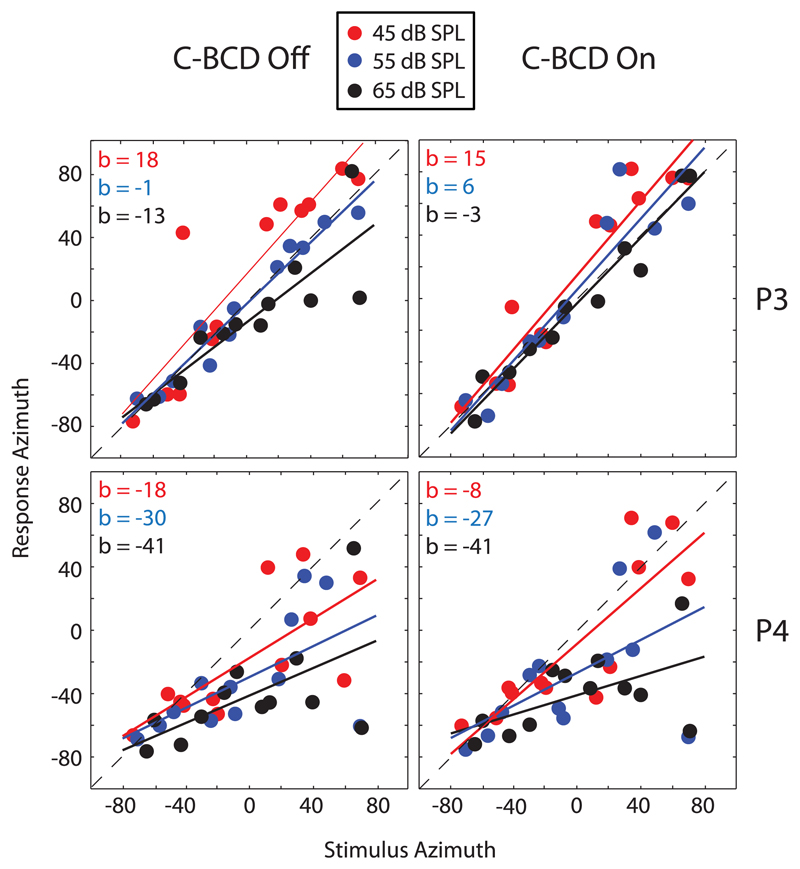
Fig. 1 shows the stimulus-response relations of BB stimuli for patients P3 and P4 for the different sound levels (45, 55 and 65 dB SPL), in both the unaided (left) and aided (right) conditions. Both patients were hearing impaired on the right side. In the unaided condition Patient P3 could localize sounds in the horizontal plane, especially for stimuli with a sound level of 55 dB SPL, and for stimuli of 65 dB SPL in the aided condition. Patient P4 localized most stimuli on the side of the hearing ear (large, intensity dependent, negative bias). Obviously, in both the unaided and aided condition, the spread of data points was larger at the deaf side for both patients. Furthermore, as seen in the unaided condition, low-intensity stimuli were more often perceived towards the deaf side than higher intensities, confirming studies in listeners with perturbed binaural hearing (Van Wanrooij and Van Opstal, 2007). This effect persisted in the aided condition, as the bias systematically decreased with sound level for both patients and both conditions. These examples confirm earlier studies (Van Wanrooij and Van Opstal, 2004), which demonstrated that SSD listeners perceive stimuli with higher sound levels (65 dB SPL) more often in the direction of the hearing ear, because of their use of the HSE.
Fig. 1.
Unaided (C-BCD Off) and aided (C-BCD On) sound-localization responses for SSD listeners P3 and P4 for BB noise bursts. Both listeners are hearing impaired at the right side. Red circles 45 dB SPL (red regression lines). Blue 55 dB SPL (blue regression lines). Black 65 dB SPL (black regression lines). C-BCD = CROS bone-conduction device. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Individual data regarding the age of testing, side of hearing loss, type of BCD, time of usage (in months) response gain, MAE and bias, for the unaided and aided conditions are presented in Table 1. The response gain, MAE and bias are pooled for levels. All results listed were obtained with BB noise bursts. The table shows that the bias for patients with hearing loss at the right side is shifted to the left (negative bias values), and that the bias for patients with hearing loss at the left side is shifted to the right (positive bias values), indicating that these patients perceived the stimuli mainly at their hearing side. Furthermore, the table shows that the gain is higher and the MAE smaller (i.e. better localization performance) for the youngest SSD patients as compared to the gain and MAE values of the oldest SSD patients. This is related to high frequency hearing loss in the hearing ear of older SSD patients (Agterberg et al., 2014). The mean localization bias and mean MAE for the C-BCD off condition, when calculated contralateral to the impaired side, was unchanged when compared with the mean bias and MAE for the C-BCD on condition (bias off/on +24.4° vs. +18.7°, paired t-test, p = 0.43; MAE off/on 38° vs. 40°, paired t-test, p = 0.56). For comparison, in a similar study investigating sound-localization performance of thirteen patients with unilateral conductive hearing loss who were listening with a BCD, the bias for BB stimuli decreased from +32.5° in the unaided condition to +4.1° in the aided condition, and the MAE from 40° to 22°, when listening with the BCD (Agterberg et al., 2012).
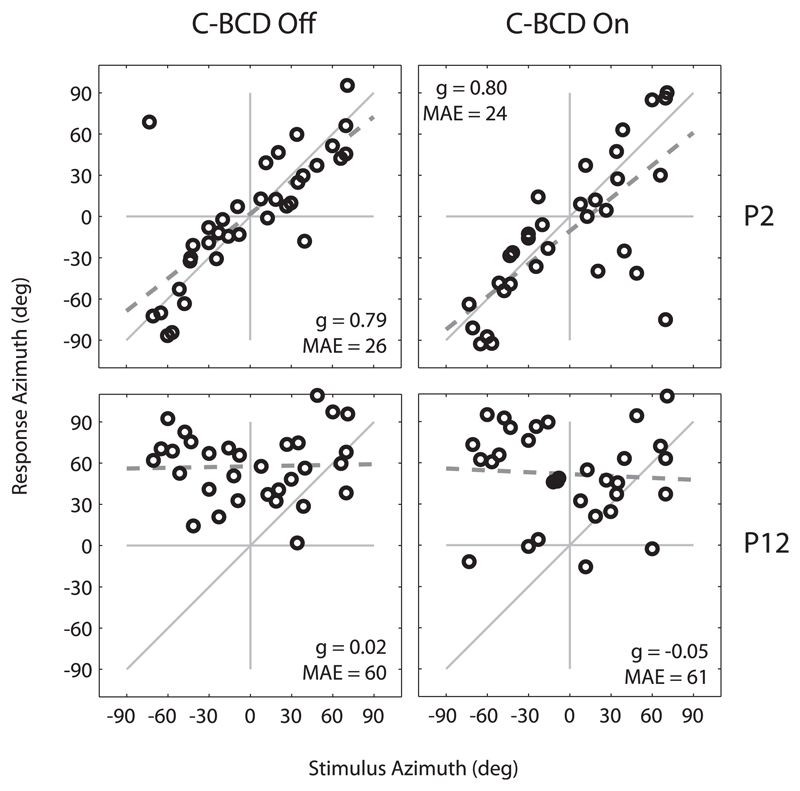
Fig. 2 shows the stimulus-response relation of BB stimuli (pooled for the 3 stimulus levels) for patients P2 and P12. Data are presented for both the unaided (left, C-BCD Off) and aided (right, C-BCD On) conditions. Patient P2 was hearing impaired on the right side, patient P12 on the left side. Patient P2 could localize sounds, especially in the C-BCD off condition (gain 0.79, MAE 26, bias 2°). Patient P12 demonstrates a poor localization performance. Almost all stimuli were perceived on the left hearing side, which resulted in a considerable leftward bias (b = +58° in the C-BCD Off and +52° in the C-BCD On condition).
Fig. 2.
Sound-localization responses for SSD listeners P2 and P12. Responses for the different presentation levels (45, 55 and 65 dB SPL) are pooled and plotted for BB noise bursts in the unaided (C-BCD Off) and aided (C-BCD On) condition. P2 demonstrates a relatively good localization performance while P12 demonstrates poor localization abilities. The dashed line denotes the linear regression fit. C-BCD = CROS bone-conduction device, g = response gain, MAE = Mean absolute error.
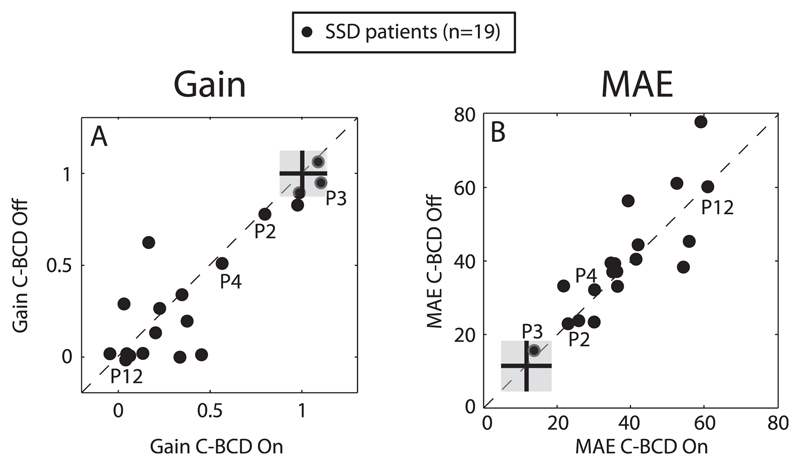
In Fig. 3A for all SSD patients the response gain in the unaided condition (C-BCD On) is plotted against the gain in the aided condition (C-BCD Off), and Fig. 3B shows the MAE results. For normal hearing listeners listening in the unaltered condition, the average azimuth gain is 1, and the average MAE is about 10°. These averaged scores (black cross) ± SD (Grey zone) taken from Agterberg et al. (2014) are indicated in the subfigures (Fig. 3A and B).
Fig. 3.
Response gain (A) and MAE (B) for the unaided (C-BCD Off) condition plotted against the aided (C-BCD On) condition for BB stimuli. Data points from the SSD listeners depicted in Fig. 1 and 2 (P2, P3, P4 and P12), are indicated in the figure. Note the high degree of variation in both aided and unaided localization abilities. Averaged scores (black cross) ± SD (grey zone) for normal hearing listeners are indicated. C-BCD = CROS bone-conduction device, MAE = Mean absolute error.
Note that in both figures most points, especially those with a gain close to one and with a lower MAE, are located near the diagonal. Typically, binaural listeners demonstrate a gain near one, and a MAE of 10°, or less, referred to here as normal sound-localization performance. For the gain, a data point significantly below the diagonal would indicate an improvement in localization performance, for the MAE, a data point significantly above the diagonal indicates an improvement in response accuracy in the aided condition. Separate binomial tests demonstrate no difference between the aided and unaided conditions, for gain (12/19: p = 0.096) and MAE (10/19: p = 0.17). There is a high degree of idiosyncratic variation in localization ability in both the unaided and aided listening conditions, but within-subject variability across these conditions is low.
In accordance with previous research, several patients (like P3 in Fig. 1 and P2 in Fig. 2) could localise sounds quite accurately in the unaided situation, which is based on the effective use of monaural cues (loudness cues (HSE), and spectral cues; Van Wanrooij and Van Opstal 2004; Agterberg et al., 2014). Therefore, the group of patients was divided in a subgroup of patients with a gain above 0.75 (referred to as good performers; n = 5; P1, P2, P3, P9 and P13), and a second subgroup with highly variable responses (poor performers; n = 12). All five SSD patients in the group of ‘good performers’ demonstrated a MAE below 34° in the ‘BCD On’ and ‘BCD Off’ condition (Table 1 and Fig. 3). The mean MAE for the good performers was a factor 2 smaller compared to the mean MAE for the poor performers in both the C-BCD off (MAE good/poor 23 vs. 43, unpaired t-test, p < 0.001) and the C-BCD on condition (MAE good/poor 24 vs. 46, unpaired t-test, p = 0.002).
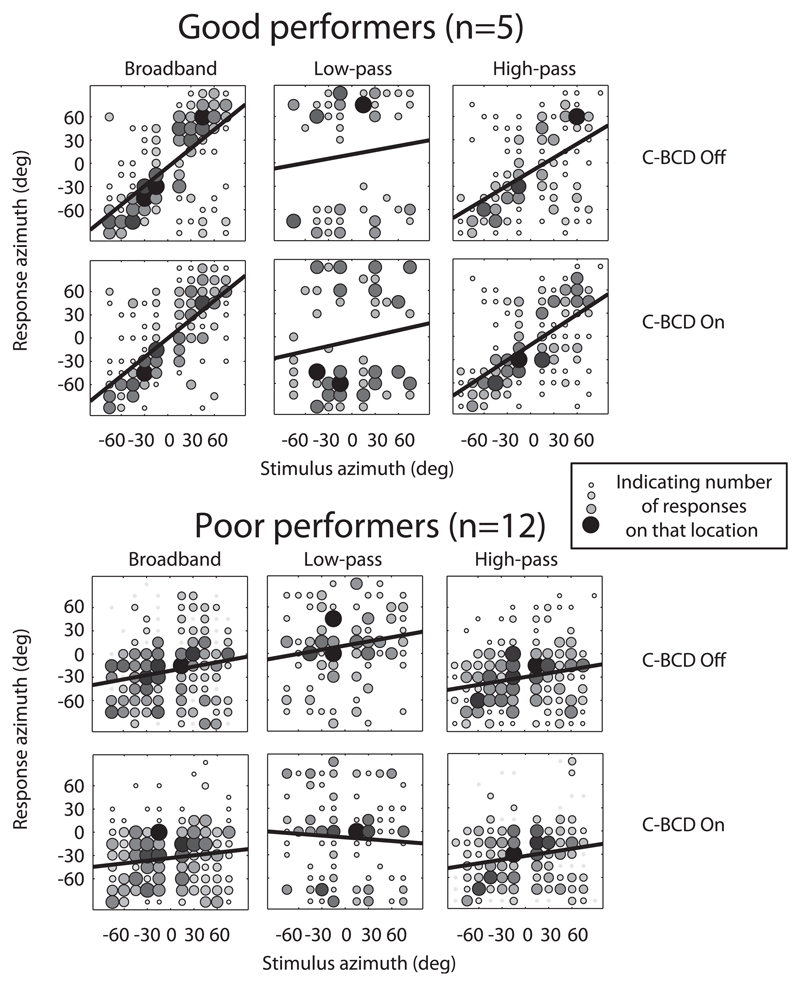
Fig. 4 presents the pooled data of these two subgroups for the BB, LP and HP noise bursts. In this figure, negative azimuth values refer to the side of the normal hearing ear, and positive azimuth values to the deaf side. Considering the group of good performers, we addressed the question whether or not the use of a C-BCD jeopardized the (unaided) localization abilities. The second question, whether or not the use of a C-BCD could improve directional hearing, was studied in both groups. In Fig. 4, the upper row of graphs presents, per subgroup, the unaided data, and the lower row, the aided data. In the group of good performers, in the unaided condition, a significant relation between target azimuth and response azimuth was found for the BB and HP noise bursts. The best-fit regression lines are drawn in the figures. In contrast, the responses to LP noise bursts were not related to target azimuth, but instead were lateralised (perceives at the extreme positions). Multiple regression analysis for the BB stimuli confirmed that these patients used monaural level and spectral cues to localize sounds in azimuth. The target-azimuth gave a larger contribution to their localization performance than the proximal sound level at the hearing ear, and when azimuth localization was poor, responses were more influenced by sound level (data not shown). Poor performers perceived the BB and HP noise bursts predominantly at the normal hearing side (evidenced by the large negative bias and low gain). For LP noise bursts, a noisy response pattern is seen. The localization responses of these patients were predominantly determined by the proximal sound level, determined by correcting the free-field presentation levels for the HSE. The HSE is less pronounced for LP noises than for BB and HP noises (−15 to +15 dB, Van Wanrooij and Van Opstal, 2004). Comparing the aided and unaided graphs of these pooled scores, as presented in the figure, shows no significant differences.
Fig. 4.
Azimuth stimulus-response relationships for BB, LP and HP noise burst pooled for five ‘good performers’ (upper graph) and the ‘poor performers’ (lower graph). Black bold lines denote best-fit regression lines over the pooled data. Gray scale and size of the data points indicates the number of responses on that location. Black indicates a larger number of responses than light gray. C-BCD = CROS bone-conduction device.
4. Discussion
The present study demonstrates that the C-BCD does neither improve nor deteriorate the localization abilities of patients with SSD. Furthermore, our data demonstrate good monaural localization abilities in the horizontal plane of some patients.
Since SSD patients have only one functioning cochlea and the treatment with a C-BCD does not restore binaural hearing, it may not be surprising that sound-localization abilities don't improve after applying a C-BCD. However, these results contrast with previous studies reporting better sound-localization for SSD listeners with a C-BCD (Monini et al., 2015), or with a conventional CROS hearing aid (Leterme et al., 2015). Possibly this difference in results originates from methodological differences in the procedure, and the accuracy of testing sound-localization abilities. Monini et al. (2015) reported an improvement in sound localization with a BCD. In that study, only four speakers, placed 90° apart, were used to score sound-localization abilities. The question is whether these results refer to sound localization, lateralization or discrimination, based on a simple memory task. The study performed by Grantham et al. (2012) reported a decrement in localization abilities for two of the seven SSD patients. They used 33 loudspeakers, placed 5° apart. For one of the two patients, the decrement was observed only when a sentence was presented and head-movements were allowed.
It is promising that sound localization abilities in the ‘good performers’ did not deteriorate with C-BCD use (Fig. 4). This group consisted mainly of younger SSD patients (Table 1, average age of listeners P1, P2, P3, P9 and P13 was 31 years), who were not suffering from high-frequency hearing loss (thresholds 8 kHz < 40 dB HL) in their hearing ear. Therefore, these listeners can detect the difference in spectral shape between a BB sound originating from the deaf side and a BB sound originating from the side of the normal hearing ear, and can potentially benefit from the ambiguous monaural head-shadow cues (Van Wanrooij and van Opstal, 2004; Agterberg et al., 2014). Sound localization in the ‘poor performers’ was mainly affected by the use of ambiguous monaural level cues as has been demonstrated in Agterberg et al. (2014). The observation that the C-BCD did not deteriorate sound-localization abilities corroborates our earlier report indicating that inter-subject variability of SSD can be partly explained by high-frequency hearing loss in the hearing ear (Agterberg et al., 2014), and not by the etiology (e.g., congenital vs. acquired) of the unilateral deafness (supported by Colburn, 1982). A potential explanation for the observation that the C-BCD did not deteriorate sound-localization abilities is that the C-BCD does not provide sufficient transmission of high-frequency sounds, and therefore does not interfere with the spectral information from the unimpaired ear. Alternatively, there is some interference with spectral cues when the C-BCD is on, but the ‘good localizers’ have learned to localize with the altered spectral input. Another widely used option that is used besides the C-BCD, is the treatment with the traditional CROS hearing aid. Recently it has been demonstrated that re-routing of sounds with the CROS hearing aid in patients with SSD, can disrupt the ability to localize sounds on monaural level and spectral cues (Pedley and Kitterick., 2017). This effect was mainly present for stimuli presented from the side of the deaf ear and for stimuli presented from straight ahead. Their results suggest that the signal re-routing when listening with a CROS hearing aid does not entirely disrupt sound localization in the horizontal plane.
The localization of sounds, and the understanding of speech in noise, are important factors in everyday life. These abilities are not only important for communication, but also for perceived safety and comfort. With regard to children, several studies have reported problems for SSD patients at school, problems with language comprehension, and speech production (Lieu, 2004, 2013), and a weaker central representation of the deaf ear in case of a unilateral innervated auditory system during early childhood (Gordon et al., 2015). In contrast to a C-BCD, new treatment options like a cochlear implant, restore bilateral input and could potentially invoke binaural hearing in such children. Cochlear implants might provide more benefit when compared to a C-BCD, and several children with congenital SSD have already been implanted at a young age (Polonenko et al., 2017). However, the performance of children with SSD implanted with a cochlear implant is still inferior compared to normal listeners (Thomas et al., 2017). It remains to be seen whether or not the limited benefit is still appreciated in the long run, and whether or not the CI will be preferred over the C-BCD. Indeed, Thomas et al. (2017) reported non-use of the cochlear implant in several children. Related to this, a study performed by Nelissen et al. (2015) reported that an increasing percentage of children with congenital unilateral conductive hearing loss, stopped using the BCD device, which potential restores the use of binaural cues, within a few years after implantation. Therefore, in order to provide the best possible advice for parents of children with congenital SSD, it is important to increase our understanding of both the limitations and possibilities of the different treatment options.
Acknowledgements
The authors thank Marc van Wanrooij, Chris-Jan Beerendonk and Gunter Windau for their technical support. We thank John Noten, Mieki Verbruggen, Teja Repkes, and Herman Kok for performing audiological tests. This research was funded by the William Demants og Hustra Ida Emilies Fond (16-0042), the Donders Institute for Brain, Cognition and Behaviour and FP7-PEOPLE-2013-ITN Marie Curie Initial Training Network iCare.
Abbreviations
- BB
broadband
- CROS
contralateral routing of sound
- C-BCD
CROS bone-conduction device
- HP
high-pass
- HSE
head-shadow effect
- LP
low-pass
- MAE
mean absolute error
- SSD
single-sided deaf(ness)
References
- Agterberg MJ, Hol MK, Van Wanrooij MM, Van Opstal AJ, Snik AF. Single-sided deafness and directional hearing: contribution of spectral cues and high-frequency hearing loss in the hearing ear. Front Neurosci. 2014;8:188. doi: 10.3389/fnins.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJ, Snik AF, Hol MK, Van Wanrooij MM, Van Opstal AJ. Contribution of monaural and binaural cues to sound localization in listeners with acquired unilateral conductive hearing loss: improved directional hearing with a bone-conduction device. Hear Res. 2012;286:9–18. doi: 10.1016/j.heares.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Batteau D. The role of the pinna in human localization. Proc R Soc Lond B Biol Sci. 1967;168:158–180. doi: 10.1098/rspb.1967.0058. [DOI] [PubMed] [Google Scholar]
- Bess FH, Tharpe AM, Gibler AM. Auditory performance of children with unilateral sensorineural hearing loss. Ear Hear. 1986;7:20–26. doi: 10.1097/00003446-198602000-00005. [DOI] [PubMed] [Google Scholar]
- Bosman AJ, Hol MK, Snik AF, Mylanus EA, Cremers CW. Bone-anchored hearing aids in unilateral inner ear deafness. Acta Otolaryngol. 2003;123:258–260. doi: 10.1080/000164580310001105. [DOI] [PubMed] [Google Scholar]
- Colburn HS. Binaural interaction and localization with various hearing impairments. Scand Audiol Suppl. 1982;15:27–45. [PubMed] [Google Scholar]
- Desmet J, Wouters K, De Bodt M, Van de Heyning P. Long-term subjective benefit with a bone conduction implant sound processor in 44 patients with single-sided deafness. Otol Neurotol. 2014;35:1017–1025. doi: 10.1097/MAO.0000000000000297. [DOI] [PubMed] [Google Scholar]
- Dillon MT, Buss E, Anderson ML, King ER, Deres EJ, Buchman CA, Brown KD, Pillsbury HC. Cochlear implantation in case of unilateral hearing loss: Initial Localization Abilities. Ear Hear. 2017;38:611–619. doi: 10.1097/AUD.0000000000000430. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Reeder RM, Dwyer NY, Burton H, Holden LK. Localization training results in individuals with unilateral severe to profound hearing loss. Hear Res. 2015;319:48–55. doi: 10.1016/j.heares.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K, Henkin Y, Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 2015;136:141–153. doi: 10.1542/peds.2014-3520. [DOI] [PubMed] [Google Scholar]
- Grantham DW, Ashmead DH, Haynes DS, Hornsby BW, Labadie RF, Ricketts TA. Horizontal plane localization in single-sided deaf adults fitted with a bone-anchored hearing aid (Baha) Ear Hear. 2012;33:595–603. doi: 10.1097/AUD.0b013e3182503e5e. [DOI] [PubMed] [Google Scholar]
- Hofman P, Van Opstal AJ. Spectro-temporal factors in two-dimensional human sound localization. J Acoust Soc Am. 1998;103:2634–2648. doi: 10.1121/1.422784. [DOI] [PubMed] [Google Scholar]
- Hol MK, Bosman AJ, Snik AF, Mylanus EA, Cremers CW. Bone-anchored hearing aids in unilateral inner ear deafness: an evaluation of audiometric and patient outcome measurements. Otol Neurotol. 2006;26:999–1006. doi: 10.1097/01.mao.0000185065.04834.95. [DOI] [PubMed] [Google Scholar]
- Hol MK, Kunst SJ, Snik AF, Cremers CW. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. Eur Arch Oto-Rhino-Laryngol. 2010;267:889–896. doi: 10.1007/s00405-009-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompis M, Wilhelm a, Caversaccioa W. Long term benefit of bone anchored hearing systems in single sided deafness. Acta Otolaryngol. 2016;1:1–5. doi: 10.1080/00016489.2016.1261410. [DOI] [PubMed] [Google Scholar]
- Leterme G, Bernardeschi D, Bensemman A, Coudert C, Portal JJ, Ferrary E, Sterkers O, Vicaut E, Frachet B, Bozorg Grayeli A. Contralateral routing of signal hearing aid versus transcutaneous bone conduction in single-sided deafness. Audiol Neuro Otol. 2015;20:251–260. doi: 10.1159/000381329. [DOI] [PubMed] [Google Scholar]
- Lieu JE. Speech-language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg. 2004;130:524–530. doi: 10.1001/archotol.130.5.524. [DOI] [PubMed] [Google Scholar]
- Lieu JE. Unilateral hearing loss in children: speech-language and school performance. B-ENT. 2013;(Suppl. 21):107–115. [PMC free article] [PubMed] [Google Scholar]
- Lin LM, Bowditch S, Anderson MJ, May B, Cox KM, Niparko JK. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol. 2006;27:172–182. doi: 10.1097/01.mao.0000196421.30275.73. [DOI] [PubMed] [Google Scholar]
- Monini S, Musy I, Filippi C, Atturo F, Barbara M. Bone conductive implants in single-sided deafness. Acta Otolaryngol. 2015;135:381–388. doi: 10.3109/00016489.2014.990057. [DOI] [PubMed] [Google Scholar]
- Nelissen RC, Mylanus EA, Cremers CW, Hol MK, Snik AF. Long-term compliance and satisfaction with percutaneous bone conduction devices in patients with congenital unilateral conductive hearing loss. Otol Neurotol. 2015;36:826–833. doi: 10.1097/MAO.0000000000000765. [DOI] [PubMed] [Google Scholar]
- Niparko J, Cox K, Lustig L. Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol. 2003;24:73–78. doi: 10.1097/00129492-200301000-00015. [DOI] [PubMed] [Google Scholar]
- Otte RJ, Agterberg MJ, Van Wanrooij MM, Snik AF, Van Opstal AJ. Age-related hearing loss and ear morphology affect vertical but not horizontal sound-localization performance. J Assoc Res Otolaryngol. 2013;14:261–273. doi: 10.1007/s10162-012-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley AJ, Kitterick PT. Contralateral routing of signals disrupts monaural level and spectral cues to sound localisation on the horizontal plane. Hear Res. 2017;353:104–111. doi: 10.1016/j.heares.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JP, Smit AL, Stegeman I, Grolman W. Bone conduction devices and contralateral routing of sound systems in single-sided deafness. Laryngoscope. 2015;125:218–226. doi: 10.1002/lary.24865. [DOI] [PubMed] [Google Scholar]
- Polonenko MJ, Papsin BC, Gordon KA. Children with single-sided deafness use their cochlear implant. Ear Hear. 2017;38:681–689. doi: 10.1097/AUD.0000000000000452. [DOI] [PubMed] [Google Scholar]
- Sangen A, Royackers L, Desloovere C, Wouters J, van Wieringen A. Single-sided deafness affects language and auditory development - a case-control study. Clin Otolaryngol. 2017;42:979–987. doi: 10.1111/coa.12826. [DOI] [PubMed] [Google Scholar]
- Saroul N, Akkari M, Pavier Y, Gilain L, Mom T. Baha-mediated rehabilitation of patients with unilateral deafness: selection criteria. Audiol Neurootol. 2014;19:85–90. doi: 10.1159/000354272. [DOI] [PubMed] [Google Scholar]
- Siau D, Dhillon B, Andrews R, Green KM. Bone-anchored hearing aids and unilateral sensorineural hearing loss: why do patients reject them? J Laryngol Otol. 2015;129:321–325. doi: 10.1017/S0022215115000602. [DOI] [PubMed] [Google Scholar]
- Slattery WH, III, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hear Res. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Thomas JP, Neumann K, Dazert S, Voelter C. Cochlear implantation in children with congenital single-sided deafnes. Otol Neurotol. 2017;38:496–503. doi: 10.1097/MAO.0000000000001343. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Sound localization under perturbed binaural hearing. J Neurophysiol. 2007;97:715–726. doi: 10.1152/jn.00260.2006. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Contribution of head shadow and pinna cues to chronic monaural sound localization. J Neurosci. 2004;24:4163–4171. doi: 10.1523/JNEUROSCI.0048-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wieringen A, Boudewyns A, Sangen A, Wouters J, Desloovere C. Unilateral congenital hearing loss in children: challenges and potentials. Hear Res. 2018 doi: 10.1016/j.heares.2018.01.010. 2018. [DOI] [PubMed] [Google Scholar]
- Vila PM, Lieu JE. Asymmetric and unilateral hearing loss in children. Cell Tissue Res. 2015;361:271–278. doi: 10.1007/s00441-015-2208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazen JJ, Ghossaini SN, Spitzer JB, Kuller M. Localization by unilateral BAHA users. Otolaryngol. Head Neck Surg. 2005;132:928–932. doi: 10.1016/j.otohns.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Wazen JJ, Spitzer JB, Ghossaini SN, Fayad JN, Niparko JK, Cox K, Brackmann DE, Soli SD. Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngol. Head Neck Surg. 2003;129:248–254. doi: 10.1016/S0194-5998(03)00527-8. [DOI] [PubMed] [Google Scholar]
- Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol. 2010;119:772–781. [PubMed] [Google Scholar]
- Zeitler DM, Snapp HA, Telischi FF, Angeli SI. Bone-anchored implantation for single-sided deafness in patients with less than profound hearing loss. Otolaryngol Head Neck Surg. 2012;147:105–111. doi: 10.1177/0194599812438522. [DOI] [PubMed] [Google Scholar]