Donor availability has expanded considerably since work by Luznik et al demonstrated hematopoietic cell transplant (HCT) with bone marrow (BM) grafts from haploidentical (haplo) donors and post-transplantation cyclophosphamide (PTCy) was a safe and effective treatment protocol (1). Several studies demonstrated commensurate outcomes between haplo-HCT with PTCy and HLA matched unrelated donor (MUD) HCT (2–4). Previously, donor specific anti-HLA antibodies (DSA) were not considered significant in HCT due to the high degree of HLA matching in traditional donors. However, several recent studies found that DSAs are highly correlated with graft failure, including one cohort of haplo-HCT with BM grafts and PTCy (5–11). Moreover, one study suggested that DSA with ability to fix complement may play a role in the pathogenesis of graft failure in DSA positive patients (11).
We employ a modified version of this protocol utilizing G-CSF mobilized peripheral blood stem cell (PBSC) grafts (4). The use of PBSCs is associated with significantly higher CD34+ dose than BM grafts (12). Data from animal models suggests that high cell doses can overwhelm DSAs and allow engraftment (13). Further, previous studies have been performed in cohorts with some patients undergoing desensitization prior to transplant. Therefore, we undertook a retrospective analysis focusing on the period before standardized treatment of DSAs was implemented at our institution.
Our cohort included adult patients undergoing PBSC haplo-HCT with PTCy at Washington University School of Medicine (WUSM) between July 2009 and January 2015. Patients were excluded if their DSA status could not be determined. Anti-HLA antibody testing was performed using the LABScreen Single Antigen Kit (One Lambda, Canoga Park, CA). A mean fluorescence intensity (MFI) of 2000 was used as the cutoff for clinical relevance (10). Test results were compared to donor HLA typing (HLA-A, B, C, DR and DQ) to identify DSAs. Testing was performed either prior to transplant or retrospectively using banked pre-transplant serum. Starting in November 2014, routine DSA screening was added to our standard pre-transplant workup. Patients with DSAs were also retrospectively screened complement fixing DSA using the C1q Screen kit (One Lambda, Canoga Park, CA) (11). The study was approved by Washington University Human Research Protections Office.
Neutrophil engraftment (NE), platelet engraftment (PE), mixed chimerism (MC) and acute and chronic GvHD were assessed as previously described (4). Graft failure was defined as survival to day +28 and death without engraftment or <5% donor chimerism on day +28 or later. Delayed engraftment was defined as no neutrophil recovery at day +28. Patients who died prior to day +28 without neutrophil recovery were excluded from the analysis of engraftment delay or failure. Treatment related mortality (TRM) was defined as any death occurring before day +28 and any death without relapse following day +28. Baseline risk was classified using the Disease Risk Index (DRI) (14). Pre-transplant comorbidity was scored using the Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI) (15).
Overall survival (OS) was analyzed using the Kaplan-Meier method and log-rank test. Cumulative incidence functions were used to analyzed NE, PE, relapse, TRM, aGvHD and cGvHD. All confidence intervals are 95%. Multivariable analysis of OS was performed using a Cox Proportional Hazards (CPH) model. It was designed to produce an adjusted hazard ratio (aHR) associated with untreated DSA. Model variables were pre-specified based on prior literature and met the proportional hazards assumption per log-minus-log plots and time-dependent covariate analysis. Cumulative incidence analysis was performed in R v. 3.2.3 using the cmprsk package. All other analyses were performed using SPSS v. 23 (IBM Corp., Armonk, NY).
During the study period, 89 patients underwent PBSC haplo-HCT at WUSM. Thirty-one patients were tested for anti-HLA antibodies for non-transplant indications, 9 retrospectively via banked specimens and 26 for pre-transplant screening. Twenty-three patients could not be assessed and were consequently excluded. Patient demographics are summarized in Table 1.
Table 1:
Patient Demographics. All columns are n (%) unless otherwise specified
| Characteristic | No DSA (n = 54) | DSA (n = 12) | Total (n = 66) |
|---|---|---|---|
| Female Gender1 | 21 (39) | 11 (92) | 32 (47) |
| Age at Transplant (years)2 | 51 (12 – 72) | 50.5 (26 – 67) | 51 (12 – 72) |
| HCT-CI Score2 | 3 (0 – 8) | 3.5 (1 – 7) | 3 (0 – 8) |
| Disease Status at Transplant | |||
| Remission | 29 (54) | 4 (33) | 33 (50) |
| Active | 23 (43) | 7 (58) | 31 (45) |
| N/A (Aplastic Anemia) | 2 (4) | 1 (8) | 3 (5) |
| Myeloablative Conditioning | 19 (35) | 5 (42) | 24 (36) |
| CD3+ Cell Dose (107/kg)2 | 19.62 (0.24 – 68.52) | 11.30 (0 –23.59) | 17.87 (0 – 68.52) |
| CD34+ Cell Dose (106/kg)2 | 5.00 (1.64 – 13.34) | 5.01 (2.71 – 14.24) | 5.00 (1.64 – 14.24) |
| Match Grade | |||
| 5/10 | 32 (59) | 8 (67) | 40 (61) |
| 6/10 | 14 (26) | 3 (25) | 17 (26) |
| 7/10 | 4 (7) | 0 (0) | 4 (6) |
| 8/10 | 1 (2) | 0 (0) | 1 (2) |
| 9/10 | 2 (4) | 0 (0) | 2 (3) |
| 4/6 | 0 (0) | 1 (8) | 1 (2) |
| Sex Mismatch | 29 (54) | 7 (58) | 36 (55) |
| ABO Mismatch | 29 (54) | 7 (58) | 36 (55) |
| Prior Transplant | 15 (28) | 3 (25) | 18 (27) |
| CMV Serostatus | |||
| D+/R+ | 16 (30) | 4 (33) | 20 (30) |
| D−/R+ | 13 (24) | 5 (42) | 18 (27) |
| D−/R− | 17 (31) | 2 (17) | 19 (29) |
| D+/R− | 8 (15) | 1 (8) | 9 (14) |
| Diagnosis | |||
| AML | 37 (69) | 8 (67) | 45 (68) |
| ALL | 7 (13) | 1 (8) | 8 (12) |
| MDS | 5 (9) | 0 (0) | 5 (8) |
| Lymphoma | 2 (4) | 1 (8) | 3 (5) |
| Aplastic Anemia | 2 (4) | 1 (8) | 3 (5) |
| CML | 1 (2) | 1 (8) | 2 (3) |
| Donor Relationship | |||
| Parent | 12 (22) | 1 (8) | 13 (20) |
| Sibling | 22 (41) | 7 (58) | 29 (44) |
| Child | 19 (35) | 4 (33) | 23 (35) |
| Other | 1 (2) | 0 (0) | 1 (2) |
Statistically significant difference between DSA
Thirty-two patients had anti-HLA antibodies and 12 had DSAs. Eight patients had DSAs to HLA class I antigens, 2 patients to class II antigens and 2 patients to both classes. Women were significantly more likely than men to have a DSA (34% vs. 3%, RR: 12.4, CI: 1.70 – 90.79). Most women (84%) were parous, but parity was not associated with DSA (37% vs. 20%, RR: 1.85, CI: 0.30 – 11.44). Parous women receiving transplants from their children was not a risk factor for DSA (RR: 0.67, CI: 0.24 – 1.82). Prior HCT, ABO mismatch and sex mismatch were also not associated with DSA.
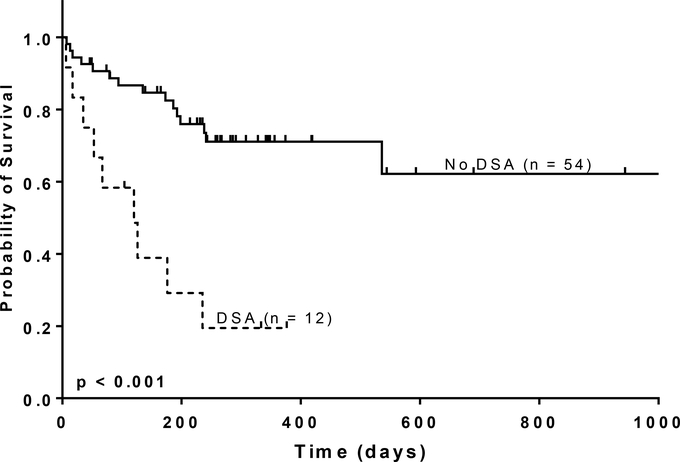
At a median follow-up of 238 days (range: 7 – 1734), 36% (24/66) of patients were deceased, including 75% (9/12) of patients with DSAs. The primary cause of death among patients with DSAs was relapse (5), non-engraftment (1), infection (2) and aGvHD (1). The median survival in patients with DSAs was 112 days and not reached in patients without DSAs. Patients with DSAs had significantly worse OS (HR: 4.65, CI: 1.98 – 10.90) (Figure 1A). A model including HCT-CI score ≥ 3, age, prior transplant and DRI demonstrated a worse survival in patients with DSAs (aHR: 4.27, CI: 1.63 – 11.14). Other significant factors included prior transplant (aHR: 3.32, CI: 1.10 – 9.99) and “Very High Risk” DRI (aHR: 2.91 CI: 1.12 – 7.56).
Fig. 1A.
Overall Survival by DSA status. The solid line represents no DSAs, and the dashed line represents patients without DSAs (no HLA-antibody or no donor specific anti-HLA antibody).
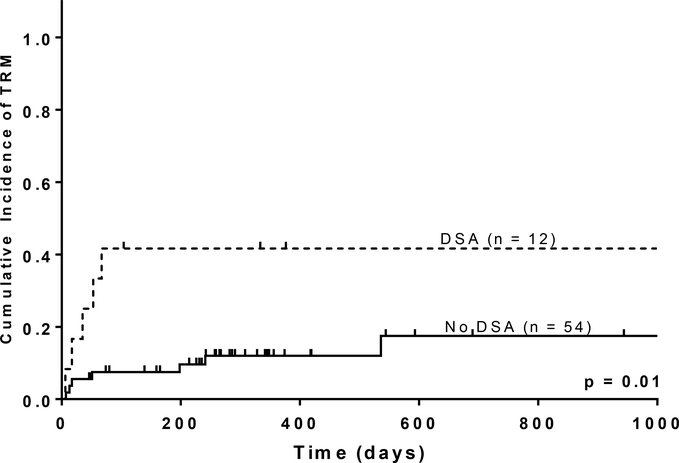
No difference was observed in the cumulative incidence of aGVHD or cGVHD in patients with DSA. Similarly, relapse was not significantly different between cohorts (p = 0.56). However, patients with DSAs had significantly higher TRM (Figure 1B). Due to the small sample size, cumulative incidence estimates of relapse and TRM for patients with DSAs were too imprecise to be meaningful.
Fig. 1B.
Treatment-Related Mortality.
Patients with DSAs were significantly more likely to experience engraftment delay (40% vs. 13%, RR: 2.91, CI: 1.04 – 8.12) and engraftment failure (30% vs. 6%, RR: 5.10, CI: 1.20 – 21.74). Patients with DSAs were also significantly more likely to have mixed or absent donor chimerism at day +30 (40% versus 12%, RR: 3.47, CI: 1.19 – 10.10) but not to die without engrafting (33% versus 11%, RR: 3.00, CI: 0.99 – 9.01). However, cumulative NE and PE were not significantly in patients with DSAs.
Eleven of 12 (92%) patients with DSAs were tested for complement activation with the C1q test. Eight patients were C1q positive and 3 were negative. C1q status was not associated with any difference in OS (Figure 1C). All cases of engraftment failure (43%, 3/7) or delay (57%, 4/7) occurred in C1q positive patients. Similarly, 57% (4/7) of C1q positive patients had mixed or absent donor chimerism on day +30, compared to 0% (0/2) C1q negative patients. However, none of these comparisons reached statistical significance.
Fig. 1C.
Survival by C1q Status. The solid line represents C1q negative, and the dashed line represents C1q positive.
Most of our results are consistent with previous reports concerning DSAs in haplo-HCT with BM graft. Female sex and parity are known risk factors (10,11). Since most women in our study were parous, we were unable to disentangle the effect of parity from gender. DSAs have also been consistently associated with graft failure in haplo-HCT (6,8,9,11). One study reported a relative risk of 7.80 (CI: 2.50 – 24.33) in haplo-HCT with PTCy, regardless half of the patient with DSA undergoing desensitization (11). Our relative risk of engraftment failure is lower than previous reports, despite the lack of desensitization. However, our 95% confidence interval includes the point estimates from previous studies and we consequently cannot draw conclusions from this difference. The role of complement fixation in this setting is still unclear. The observed effect was smaller in our cohort than previously reported (3/7 versus 5/5 patients) (11).
Less data are available regarding the impact of DSA on survival following HCT with peripheral stem cell grafts. Two previous studies have reported inferior OS in patients experiencing graft failure (8,11). Chang et al observed significantly worse OS in patients with DSA with MFI > 10,000 in patients undergoing haplo-HCT with the GIAC protocol (8). Our study is, to the best of our knowledge, the first to associate DSAs (MFI > 2000) with significantly poor OS. Of note, only one death was attributable directly to non-engraftment. Most patients died of relapse (56%), suggesting that patients with DSAs may have inferior immune reconstitution and a blunted graft-versus-leukemia effect.
Our study has several limitations. First, we were limited by the relatively small number of patients with DSAs. Consequently, our ability to make strong inferences regarding the impact of DSA on survival was reduced and we could not assess the impact of DSA-specific variables such as complement fixation, antibody intensity or antibody class. Second, only a small number of patients had banked serum, leading to the exclusion of a significant percentage of patients. Third, follow-up time in our cohort was limited. However, we expected DSAs to have the strongest impact in the early post-transplant period.
In conclusion, we found that the presence of untreated DSA is associated with significantly worse outcomes in PBSC haplo-HCT including poor OS and engraftment failure. Consequently, pre-transplant screening is prudent and, in cases of positive screening, a different donor should be sought or antibody-directed therapy should be performed. Our study also offers a valuable baseline for assessing these therapies, including desensitization, and studies are ongoing at our center.
Acknowledgements
Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449, from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. We would like to acknowledge Kathryn Trinkaus PhD for her work on the statistical analysis for this manuscript.
References
- 1.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008/05/21 ed. 2008;14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciurea SO, Zhang M-J, Bacigalupo A a, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with post-transplant cyclophosphamide versus matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):blood – 2015–04 – 639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashey A, Zhang X, Sizemore C a., Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–6. [DOI] [PubMed] [Google Scholar]
- 4.Rashidi A, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Cashen AF, et al. Comparison of outcomes following peripheral blood haploidentical vs. matched unrelated donor allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia: a retrospective single-center review. Biol Blood Marrow Transplant. 2016. May; [DOI] [PubMed] [Google Scholar]
- 5.Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. United States; 2009. October;88(8):1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciurea SO, Thall PF, Wang X, Wang S a., Hu Y, Cano P, et al. Donor-specific anti-HLAAbs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118(22):5957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y-J, Zhao X-Y, Xu L-P, Zhang X-H, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. Journal of Hematology & Oncology; 2015;8(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant . Nature Publishing Group; 2012;47(4):508–15. [DOI] [PubMed] [Google Scholar]
- 10.Gladstone DE, Zachary A a., Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially Mismatched Transplantation and Human Leukocyte Antigen Donor-Specific Antibodies. Biol Blood Marrow Transplant. Elsevier Ltd; 2013;19(4):647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-Binding Donor-Specific Anti-HLA Antibodies and Risk of Primary Graft Failure in Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. Elsevier Inc; 2015;21(June):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-Blood Stem Cells versus Bone Marrow from Unrelated Donors. N Engl J Med. 2012;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor P a., Ehrhardt MJ, Roforth MM, Swedin JM, Panoskaltsis-Mortari A, Serody JS, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109(3):1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Kim H, Logan B. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation ( HCT )– specific comorbidity index : a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]