Abstract
Background:
Transcranial direct current stimulation (tDCS) has been used to enhance endurance performance but its precise mechanisms and effects remain unknown.
Objective:
To investigate the effect of bilateral tDCS on neuromuscular function and cycling time to task failure (TTF) test.
Methods:
Twelve participants in randomized order received a placebo tDCS (SHAM) or real tDCS with two cathodes (CATHODAL) or two anodes (ANODAL) over bilateral motor cortices and the opposite electrode pair over the ipsilateral shoulders. Each session lasted 10 min and current was set at 2mA. Neuromuscular assessment was performed before and after tDCS and was followed by a cycling time to task failure (TTF) test. Heart rate (HR), ratings of perceived exertion (RPE), leg muscle pain (PAIN) and blood lactate accumulation B[La−] in response to the cycling TTF test were measured.
Results:
Corticospinal excitability increased in the ANODAL condition (P < 0.001) while none of the other neuromuscular parameters showed any change. Neuromuscular parameters did not change in the SHAM and CATHODAL conditions. TTF was significantly longer in the ANODAL (P = 0.003) compared to CATHODAL and SHAM condition (12.61 ± 4.65 min; 10.61 ± 4.34 min; 10.21 ± 3.47 min respectively), with significantly lower RPE and higher B[La−] accumulation (P < 0.001). No differences were found for HR (P = 0.803) and PAIN between conditions during the cycling TTF test (P = 0.305).
Conclusion:
Our findings demonstrate that tDCS with the anode over both motor cortices using a bilateral extracephalic reference improved endurance performance.
Keywords: endurance performance, fatigue, perception of effort, tDCS
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that delivers a constant, weak electrical current flow to the brain by placing two or more electrodes over the scalp [1]. The neuromodulatory effect of tDCS is polarity specific with an excitatory effect under the anodal electrode and an inhibitory effect under the cathodal electrode [1]. When applied to the primary motor cortex (M1), cortical excitability has been shown to increase after anodal stimulation and to be reduced after cathodal stimulation [2], as demonstrated by changes in the motor evoked potential (MEP) elicited via transcranial magnetic stimulation (TMS). This neuromodulatory effect is probably achieved by a shift of the resting membrane potential of the targeted neural cells [1]. tDCS has been widely used in cognitive neuroscience to understand brain function [3,4], and in the treatment of various neurological disorders [5], and psychiatric disorders [6].
More recently, there has been great interest in the use of tDCS to enhance sport performance [7,8], and to facilitate neuroplasticity and training adaptations [9]. With specific reference to the enhancement of endurance performance, the acute administration of anodal stimulation over the contralateral primary motor cortex (M1) prior to or during isometric time to task failure (TTF) tests of isolated muscle groups has induced either an improvement [10–13], or no effect [14,15]. A similar inconsistency in endurance performance outcomes has been also reported in cycling studies [16–19]. The inconsistent effects of tDCS on endurance performance found in previous experimental studies might be partly caused by the different electrode montages adopted [20]. For example, Angius et al., [21] did not find any improvement in TTF during cycling exercise when anodal tDCS was delivered over M1 with the cathode over the right dorsolateral prefrontal cortex [21]. Such a cephalic montage may induce effects under the cathode [11] that may modulate or even nullify the effect of the anode over M1. In a follow-up study, Angius et al. [11] compared cephalic and extracephalic tDCS montages by targeting M1 with the anodal electrode and they found that isometric TTF of the knee extensor muscles was significantly longer when the extracephalic montage was used. Therefore, it seems that an extracephalic montage may be preferable when tDCS is applied to enhance endurance performance. Furthermore, various studies have demonstrated the efficacy and safety of extracephalic montages [11,22] and monopolar montages [23] in other experimental and clinical settings [24].
The primary aim of the present study was to verify that the positive effect of an anodal electrode over M1 with an extracephalic montage on endurance performance during an isometric TTF test with the knee extensor muscles can be replicated in an exercise mode (cycling) more relevant to real endurance competitions, involving continuous, dynamic, whole-body exercise lasting more than 75 s [25]. As cycling exercise involves both lower limbs, the extracephalic montage proposed by Angius et al., [11] might be more targeted than the unilateral one used in a previous cycling study [21]. Overall, this montage simultaneously stimulates both primary motor cortices while also potentially avoiding the effects of the cathode over other brain areas. The secondary aim of the study was to investigate some of the potential physiological and psychological mechanisms for the hypothesised positive effect on endurance performance. Specifically, we tested the hypotheses that an extracephalic bilateral tDCS montage with anodal electrodes over M1 and cathodes placed over the shoulders increases corticospinal excitability during submaximal contractions of the knee extensor muscles and reduces perception of effort during cycling exercise. Therefore, because the neural signals processed by the brain to generate perception of effort seem to be corollary discharges from SMA and other cortical areas upstream of M1 [26–29], an increase in cortical excitability should lead to a lower perception of effort during cycling exercise at the same power output.
Methods
Participants
Twelve recreationally active participants (4 women and 8 men; mean ± SD, age: 24 ± 5 yr, height: 175 ± 12 cm, weight: 74 ± 17 kg) volunteered for this study. Eligibility criteria were age between 18 and 44 yr old and performing regular aerobic training (at least three hours per week). Participants were not included in the study if they had any somatic or mental disorder or were taking any medication at the time of the study. Prior to providing written informed consent, all participants were given instructions about the experimental procedures. Approval for the experiment was obtained from the local Research Ethics Committee (approval number: Prop 98_2014_2015), in accordance with the Declaration of Helsinki. After completion of all their experimental visits, participants were fully debriefed on the actual study aims, and provided with their individual results. To minimize the subject-expectancy effect, participants were told that the aim of the experiment was to study the effect of tDCS on the cardiovascular response during exhaustive exercise. After debriefing, participants were asked not to discuss the real purpose of the study purpose with any other participants until the entire data collection had been completed.
Experimental protocol
Participants visited the laboratory on four different occasions that included one preliminary visit and three experimental visits. During the three experimental visits, participants were randomly assigned in a double-blind, randomised, counterbalanced order to a sham (SHAM), anodal (ANODAL) and cathodal (CATHODAL) stimulation conditions (see Transcranial direct current stimulation procedures for more details). Participants were given instructions to avoid caffeine, alcohol, stimulants or depressants, and strenuous exercise for 48 h prior to each visit. All experimental visits were completed within 14 days and were interspaced by at least 48 h. Each visit was performed at the same time of the day in a temperature-controlled room (20°C, relative humidity between 40–50%).
During the first visit participants were familiarized with the laboratory equipment and all the experimental procedures. In addition, they performed an incremental cycling test on a cycle ergometer (Excalibur Spot, Lode, Groningen, Netherlands) to establish individual peak power output (Wpeak). In this test, participants performed a 5 min warm up at 100 W, followed by an incremental protocol in which the power increased by 5 W every 15 s−1 until task failure (i.e. operationally defined as a pedal frequency of less than 60 revolutions/min (RPM) for more than 5 s despite strong verbal encouragement). The cycle ergometer rider position was recorded for each participant so that it could be reproduced for all the following visits.
In visits 2–4, participants performed a neuromuscular assessment before (pre) and after (post) tDCS administration (see Neuromuscular assessment for more details), as well as a time to task failure (TTF) test (see Time to task failure test for more details). A schematic summary of all procedures performed and timing during each experimental visit is illustrated in Fig 1, panel B.
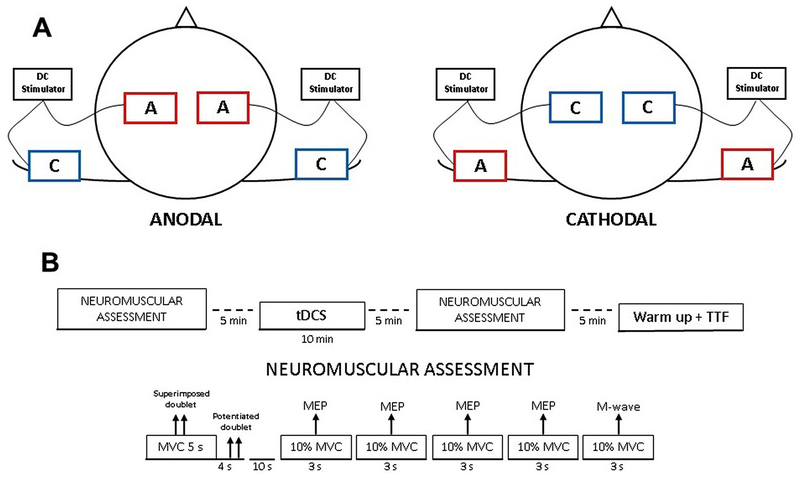
Fig. 1.
Overall view of transcranial direct current stimulation (tDCS) montages and phases of the experimental protocol. Panel A. Schematic illustration showing placement of electrodes. The montage for ANODAL condition and for CATHODAL condition are respectively illustrated on the left and right side of the panel. Anodal electrode (A) and cathodal electrode (C). Panel B. Maximal muscular wave (M-wave); motor evoked potential (MEP); maximal voluntary contraction (MVC); transcranial direct current stimulation (tDCS); cycling time to task failure (TTF) test.
Transcranial direct current stimulation procedures
For the present experiment, an extracephalic tDCS montage similar to the one used by by Angius et al. [11] was adopted. For the ANODAL condition both anodal electrodes were placed over bilateral M1 (C3 and C4 according to the 10–20 EEG system) in correspondence with the TMS stimulation point, while the cathodal electrodes were placed respectively above the ipsilateral shoulders (see Fig.1, panel A). For the CATHODAL condition, the position of the electrodes was simply reversed with respect to the ANODAL condition (see Fig.1, panel B). For the SHAM condition, the same set up of ANODAL condition was used. tDCS was administered by two direct current stimulators (TCT Research Limited, Hong Kong) using two rubber target electrodes (size: 7×5 cm) and two rubber return electrodes (size 5×5 cm) and water-soaked synthetic sponge. Stimulation intensity was set at 2.0 mA for 10 min, whereas during the SHAM condition lasted only 30 s. For all three conditions, the current was ramped up and down for 10 s. To ensure good conductance, electrode sponges were soaked with standard saline solution (NaCl 9%) and elasticated straps were used to maintain all the electrodes on the scalp and both shoulders. The electrical resistance was constantly monitored on the stimulator’s display within a range between 4 to 5 kΩ.
Neuromuscular assessment
After a standardised warm up consisting of 10 brief (5 s) submaximal voluntary isometric contractions at 50% of the estimated MVC torque, the neuromuscular assessment was performed before and after tDCS stimulation (see Fig 1). All participants performed a 5 s isometric maximal voluntary contraction (MVC) of the right knee extensor muscles with superimposed doublet followed (3 s post MVC) by a resting potentiated doublet (Doublet). Ten seconds after the MVC, participants were asked to perform four brief submaximal isometric contractions (3 s) at 10% of MVC with superimposed TMS, followed by one submaximal contraction (3 s) at 10% of MVC with superimposed nerve stimulation. Each contraction was interspaced by 3 s. Submaximal muscle contraction has been shown to provide a facilitation of the corticospinal tract, thus requiring less than 100% of the maximum stimulator output to elicit the minimum measurable MEP response of the targeted muscle [30,31] and also reduces the unpleasantness caused by the high stimulator intensity for the participants.
Transcranial magnetic stimulation (TMS).
Excitability of the left M1 was measured by means of TMS. Stimulation was delivered with a 110 mm diameter concave coil over the left M1 by a magnetic stimulator (Magstim 2002, The Magstim Company Ltd, Whitland, UK). The precise site of the stimulation was determined at the beginning of each visit and then marked on the scalp to maintain the same coil position during the visit. The coil position was determined in order to elicit the largest MEP response of the right vastus lateralis (VL) and a small MEP response (<10% of right VL MEP amplitude) in the antagonist muscle (biceps femoris, BF). After determination of the exact coil position, the stimulus intensity was set to elicit the largest MEP response during a brief (3 s) submaximal isometric contraction at the 10% of MVC of the knee extensor muscles. The stimulation intensity was determined during each experimental visit before commencing with the neuromuscular assessment. The mean stimulation intensity was 65 ± 4% of the maximum stimulator output.
Femoral nerve stimulation.
Transcutaneous electrical stimulation of the right femoral nerve was delivered by a high-voltage constant-current stimulator (model DS7 modified, Digitimer, Hertfordshire, UK). The femoral nerve was stimulated by a cathode electrode (2 × 2 cm, Swaromed, Nessler Medizintechnik, Innsbruck, Austria) positioned over the right femoral triangle with the anodal electrode (10 × 5 cm; Phoenix Healthcare Products Ltd., Nottingham, UK) placed in the right gluteal fold. Stimulation intensity was increased by 20 mA until the electrical compound action potential response (M-wave) did not further increase both at rest and during a submaximal 10% MVC contraction. The stimulation intensity was determined during each experimental visit before commencing the neuromuscular assessment. The optimal intensity of stimulation (Mmax) was then set at 130% of the intensity required to elicit the highest M-wave. The stimulus duration was 200 μs, with an interval between stimuli in the doublet of 10 ms (100 Hz frequency). The mean stimulation intensity was 290 ± 71 mA.
Mechanical recordings.
The neuromuscular assessment was performed on an isokinetic dynamometer (Cybex NORM, CMSi, Computer Sports Medicine Inc., Stoughton, USA). All participants performed the neuromuscular assessment in isometric conditions with the right leg at a knee flexion of 90° (0° = full extension) and a hip angle of 90°.
The dynamometer set-up was recorded and kept constant over all visits for each participant to maintain the same position. Mechanical signals were recorded at a sampling frequency of 1 kHz and analysed with commercially available software (Acqknowledge 4.2 for MP Systems, Biopac Systems Inc., Goleta, USA).
Electromyographic recordings.
Surface electromyography (EMG) of the VL and BF were acquired with two square surface electrodes (Swaromed, Nessler Medizintechnik, Innsbruck, Austria). The recording site was circular (10 mm diameter) in the centre of the electrode (center-to-center distance of 20 mm). Electrodes were placed according to the SENIAM guidelines [32]. More specifically electrodes for VL were placed on the muscle belly at 2/3 of the line from the anterior spina iliaca superior and the lateral side of the patella while for the BF electrodes were placed on the muscle belly at 50% on the line between the ischial tuberosity and the lateral epicondyle of the tibia with the reference electrode placed over the patella. Before starting the neuromuscular assessment, the skin was shaved and cleaned with alcohol swabs. The electrical signal was then amplified with a bandwidth frequency ranging from 10 Hz to 500 Hz (gain = 500) with commercially available software (Acqknowledge 4.2 for MP Systems, Biopac Systems Inc., Goleta, USA).
Cycling time to task failure test
After the final neuromuscular assessment, participants performed the TTF test on the cycle ergometer at 70% of their Wpeak. The cycling TTF test terminated when the participants were not able to maintain a pedal frequency above 60 revolution/min for more than 5 s despite strong verbal encouragement. The cycling TTF test was preceded by a 5 min warm up at 100 W. Participants were verbally encouraged throughout the cycling TTF test by a researcher blinded to the condition allocation.
Ratings of perceived exertion (RPE) and leg muscle pain (PAIN) were measured respectively using the 15-point RPE scale [33] and a 10-point numerical scale for PAIN [34] administered 30 s after the start of the cycling TTF test, at the end of each min, and at exhaustion. Heart rate (HR) was continuously monitored using a HR monitor (Polar RS400; Polar Electro Oy, Kempele, Finland) and averaged to provide data points to coincide with RPE and PAIN ratings. Blood lactate concentration (B[La−]) was measured at rest before the first neuromuscular assessment (baseline) and immediately after the cycling TTF test (at exhaustion). A 10μl sample of capillary blood was collected from the thumb of the right hand and immediately analysed for B[La−] (Biosen; EFK Diagnostics, London, UK).
Data analysis
Peak torque obtained during the MVC was used as a measure of the force-generating capacity of the locomotor muscles. Voluntary activation level (VAL) during the MVC was obtained according to the following formula:
The EMG amplitude obtained during the MVC was quantified with the root mean square (RMS) for a 0.5 s interval during the peak torque (250 ms either side at the peak torque). The RMS of EMG was automatically calculated with the software (Acqknowledge 4.2 for MP Systems, Biopac Systems Inc., Goleta, USA). The MEP area (MEParea) was manually calculated and then averaged for the four brief submaximal contractions performed at 10% MVC, and was normalized for the M-wave area (MEParea/M-wave ratio) obtained during the 10% MVC contraction. This procedure was performed both for VL muscle (VLMEParea/M-wave ratio) and BF muscle (BFMEParea/M-wave ratio). The following MEP parameters were also calculated: MEP peak to peak amplitude (MEPamp), MEP peak to peak duration (MEPdur). The MEP cortical silent period (CSP) was measured from the onset of the MEP to the return of EMG signal. The isotime data of RPE, PAIN and HR were measured at the selected time points to allow the within-subjects comparison of temporal changes during the cycling TTF test. The shortest TTF was identified for each individual over the three visits and considered as 100% isotime. The values for each variable obtained at the final minute of the shortest cycling TTF test was compared to the value obtained at the equivalent minute in the other two visits. The minute identified as 100% isotime was divided by four and rounded up to obtain the necessary value corresponding to 25, 50 and 75% isotime. Isotime values for 0% were attained by taking into account data collected at 30 s of each cycling TTF test [35,36].
Statistical analysis
Unless specified, data are presented as mean ± SD. Assumptions of statistical tests such as normal distribution was checked by using the Shapiro-Wilk and sphericity of data was checked by using the Mauchly’s test. The Greenhouse-Geisser correction to the degrees of freedom was applied when violations to sphericity were present. A one-way measure analysis of variance (ANOVA) with repeated measures was used to compare TTF across the tDCS conditions and to check whether there was a statistical significance at baseline for MVC, VAL, Doublet, CSP, MEPdur, MEPamp and VLMEParea/M-wave ratio and BFMEParea/M-wave ratio. Furthermore, intraclass correlation coefficient (ICC) was also calculated according to Hopkins et al., [37]
A 3×5 fully repeated measures (condition × time) ANOVA were performed to test the effects of tDCS condition on RPE, PAIN and HR during the cycling TTF test. The effects of tDCS condition on RPE, PAIN, HR and accumulation of B[La−] were analysed by using the Friedman test because the normal distribution assumption was violated. A 3×2 fully repeated measures (condition × test) ANOVA was performed to verify the effect of condition on MVC, VAL, Doublet, MEPamp, MEPdur, VLMEParea/M-wave ratio, BFMEParea/M-wave ratio and CSP measured before and after tDCS. When a significant simple main effect of time or condition was found, a Holm-Bonferroni follow-up test was performed. Correlation coefficients (r) were determined by using Pearson’s r. Post-hoc analysis for Friedman test was performed by means of multiple comparison with the Wilcoxon Signed-Rank test. Pearson correlation was computed to observe the relationships between MEP change and change in TTF. Statistical significance was set at P < 0.05. Statistics analysis was performed by using SPSS version 20.
Results
Effects of tDCS on neuromuscular function and cortical responses
All participants completed the three experimental visits. Participants reported an itching sensation on the scalp in all the tDCS conditions and none reported any side effects during, or after tDCS administration.
There were no statistical differences at baseline between each experimental condition for MVC (P = 0.822), VAL (P = 0.348), Doublet (P = 0.671), MEPamp (P = 0.176), MEPdur (P = 0.340), VLMEParea/M-wave ratio (P = 0.108) and BFMEParea/M-wave ratio (P = 0.885), CSP (P = 0.466). ICC of reliability data with upper and lower 95% confidence intervals for MVC, VAL, Doublet, MEPamp, MEPdur, VLMEParea/M-wave ratio, BFMEParea/M-wave ratio and CSP were reported in table 1.
Table 1.
Intraclass correlation coefficient (ICC) with lower and upper 95% confidence intervals for key variables measured.
| Coefficient of variation | Intraclass Correlation Coefficient | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower bound | Upper Bound | |||
| MVC | 9.37 | 0.915 | 0.794 | 0.972 |
| Doublet | 6.67 | 0.955 | 0.886 | 0.986 |
| VAL | 4.12 | 0.404 | 0.047 | 0.744 |
| CSP | 5.96 | 0.946 | 0.866 | 0.983 |
| MEPdur | 5.83 | 0.756 | 0.490 | 0.914 |
| MEPamp | 5.59 | 0.982 | 0.952 | 0.994 |
| M-wave 10% MVC | 5.75 | 0.856 | 0.671 | 0.952 |
| VLMEParea/M-wave ratio | 6.70 | 0.959 | 0.896 | 0.987 |
| BFMEParea/M-wave ratio | 17.84 | 0.123 | −0.183 | 0.541 |
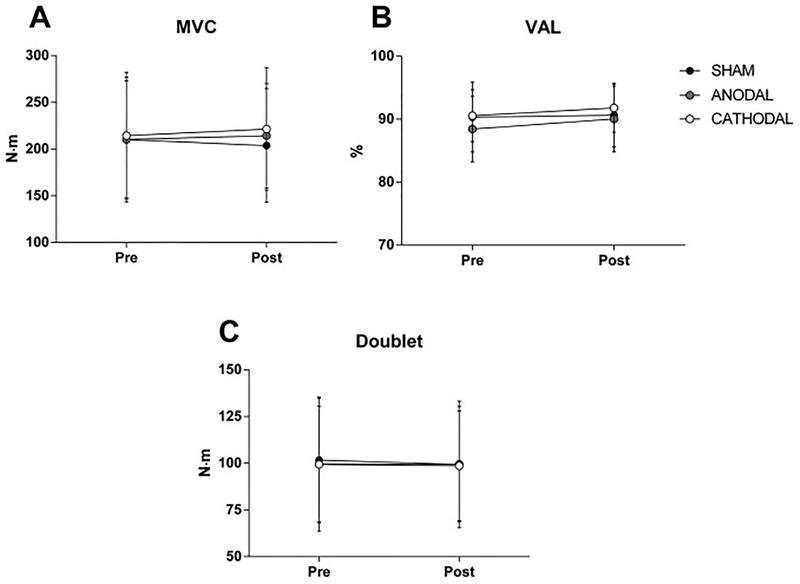
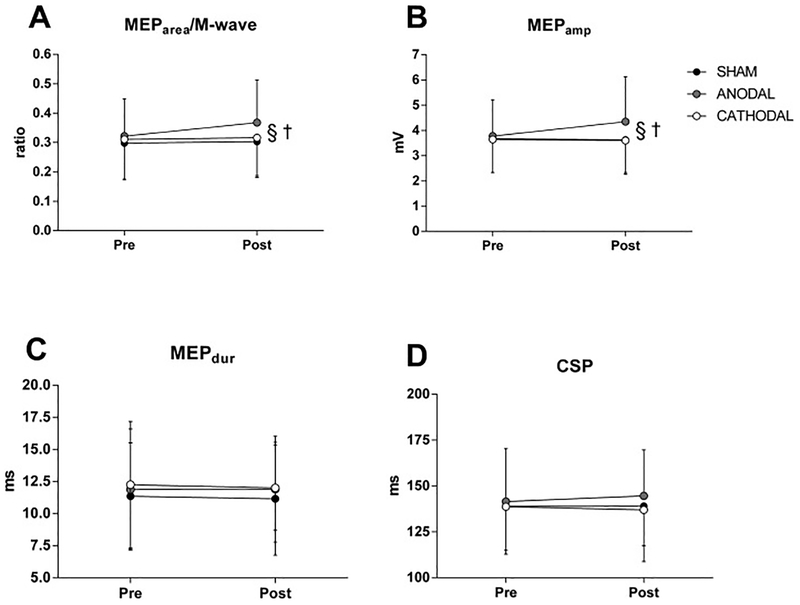
There were no significant interactions and no significant main effects of condition or time on MVC, VAL, Doublet, MEPdur, BFMEParea/M-wave ratio and CSP (all Ps > 0.208) (see Fig 3 and 4). There were, however, significant condition by time interactions for MEPamp (P = 0.004) and VLMEParea/M-wave ratio (P < 0.005). Follow-up tests revealed a significant increase in MEPamp (P = 0.001) and VLMEParea/M-wave ratio (P < 0.001) in the ANODAL condition, but not in the SHAM AND CATHODAL conditions (see Fig 4).
Fig. 3.
Neuromuscular function before and after transcranial direct current stimulation (tDCS). Panel A shows maximal voluntary contraction (MVC) torque; Panel B shows voluntary activation level (VAL); Panel C shows peak torque of the doublet (Doublet). Data are presented as mean ± SD (n = 12).
Fig. 4.
Corticospinal response before and after transcranial direct current stimulation (tDCS). Panel A shows motor evoked potential area (MEParea) and muscular wave (Mwave) MEParea/M-wave ratio. Panel B shows MEP peak to peak amplitude (MEPamp). Panel C shows MEP peak to peak duration (MEPdur); Panel D shows MEP cortical silent period (CSP); §Denotes significant difference from CATHODAL and SHAM (P < 0.05); †Denotes significant condition × time interaction (P < 0.05). Data are presented as mean ± SD (n = 12).
Effects of tDCS on performance and physiological/perceptual responses during the cycling TTF test
Wpeak obtained during the incremental cycling test was 257 ± 58 W with a power output during the cycling TTE test corresponding to 180 ± 40 W.
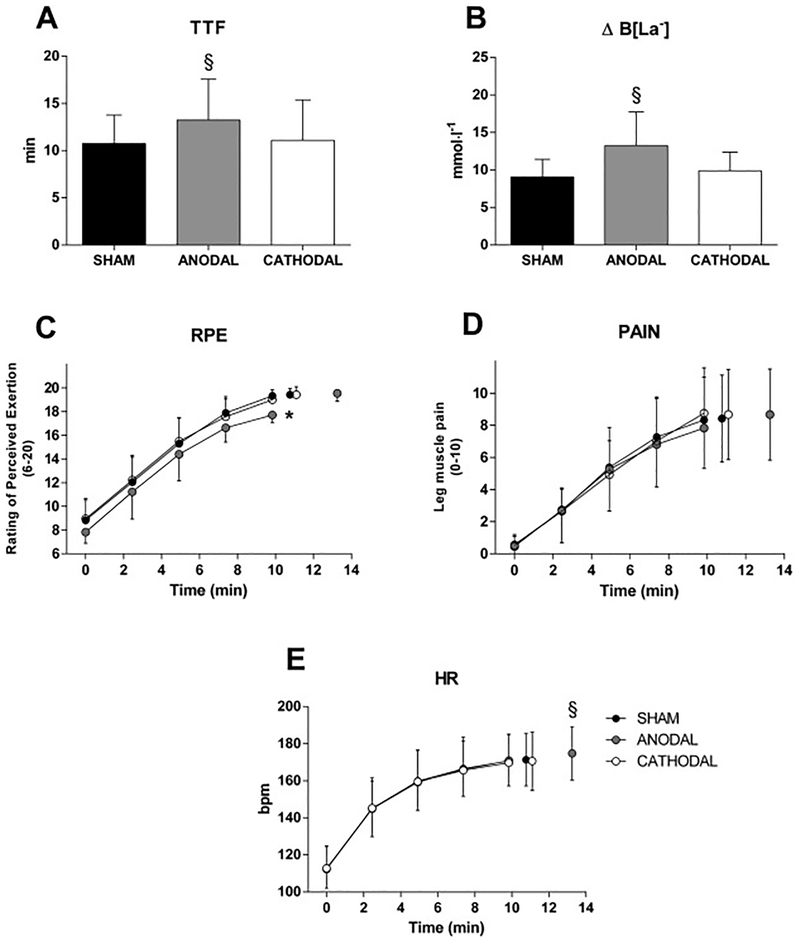
There was a significant main effect of condition on TTF (P = 0.003). Follow-up tests showed a significantly longer TTF in the ANODAL condition (13.25 ± 4.34 min) compared to CATHODAL (11.10 ± 4.28 min, P = 0.004) and SHAM condition (10.76 ± 3.03 min, P = 0.024). No significant difference between CATHODAL and SHAM conditions was found (P = 0.1) (see Fig 2).
Fig. 2.
Effects of transcranial direct current stimulation (tDCS) on performance and perceptual/physiological responses during the cycling TTF test. Panel A shows time to task failure (TTF) in different conditions; Panel B shows blood lactate accumulation (ΔB[La−]) in different conditions; Panel C, D and E show respectively time courses of rating of perceived exertion(RPE), leg muscle pain (PAIN) and heart rate (HR) during the TTF test. *Denotes significant main effect of condition (P < 0.05); §Denotes significant difference from CATHODAL and SHAM (P < 0.05); Data are presented as mean ± SD (n = 12).
Analyses of isotime data revealed significant main effects of time for RPE, PAIN and HR (all Ps < 0.001), but no condition × time interactions were found (all Ps > 0.305). A simple main effect of condition for RPE was found (P = 0.001). Specifically, participants rated perceived exertion lower in the ANODAL condition compared to CATHODAL condition (P = 0.023) and SHAM condition (P = 0.008). No significant main effects of condition were found for PAIN (P = 0.305) or HR (P = 0.803).
A main effect of condition for both HR at task failure (P = 0.004) and B[La−] (P < 0.001) was found. Follow up tests revealed a higher HR in the ANODAL condition (174 ± 14 bpm) compared to CATHODAL condition (170 ± 15, P = 0.023) and SHAM condition (171 ± 14, P = 0.003). Follow up tests revealed a higher B[La−] in the ANODAL condition (13.26 ± 4.47 mmol·l−1) compared to CATHODAL condition (9.90 ± 2.51 mmol·l−1, P = 0.011) and SHAM condition (9.09 ± 2.33 mmol·l−1, P = 0.006). RPE and PAIN were not significantly different at task failure (P = 0.779 and P = 0.326 respectively) (see Fig 2). There was no correlation between MEP change and TTF (P = 0.377, r = −0.281).
Discussion
The present study demonstrates that extracephalic anodal stimulation over bilateral M1 significantly improves TTF during cycling exercise by 23%. As hypothesised, this positive effect on endurance performance occurred alongside a lower perception of effort during cycling exercise. Cathodal stimulation over bilateral M1 using the same montage did not have any significant effect on these variables.
Effect of anodal tDCS on endurance performance
To the best of our knowledge only four studies have previously investigated the effects of tDCS on various measures of endurance performance [17–19,21]. In a previous study from our laboratory, we found no significant changes in TTF during cycling exercise when a cephalic montage was administered with a single anodal electrode over one M1 and with the cathode over contralateral prefrontal cortex [21]. The lack of improvement in endurance performance in that study may be explained by the isolated effect of the anodal electrode over the left M1 while cycling exercise requires both legs. The absence of a significant effect on TTF may also be due to the tDCS montage used as any benefit from the anodal electrode over M1 could have been negated by the cathodal electrode over the right dorsolateral prefrontal cortex (F4). These speculations are supported by the results of a recent study showing that extracephalic montage with anode over M1 and cathode over the ipsilateral shoulder elicits a significant 17% improvement in TTF during single leg isometric exercise [11]. Contrarily, in the same study, the cephalic montage with the anode over M1 and cathode over the opposite dorsolateral prefrontal cortex did not have any significant effect on this kind of endurance performance.
On the basis of previous studies implicating the SMA in the generation of perception of effort during physical tasks [27,28,38], Vitor-Costa et al. [19] placed the centre of one electrode (9 × 4 cm) over Cz region (both SMA), thus each side (4.5 cm) were also placed over both M1, and found a significant improvement in TTF during cycling exercise following anodal stimulation. This finding suggests that anodal stimulation of cortical areas upstream of M1 may also improve endurance cycling performance. However, the mechanisms for this ergogenic effect are not clear as the hypothesised reduction in perception of effort was only a trend (P = 0.07) and no electrophysiological or neuromuscular parameters were measured.
Three other studies investigated the effects of an electrode montage aimed at reducing the perception of effort via the stimulation of the insular cortex (tDCS with anodal electrode over T3 and cathodal over the contralateral supraorbital area, Fp2) [17,18]. The results, however, are contrasting. Okano et al. [18], reported a reduction in perception of effort and ~4% improvement in peak power output during an incremental cycling test, whereas Barwood et al., [17] did not find any perceptual or performance improvement during a cycling TTF and time trial test in the heat. Although testing and environmental differences may explain these contrasting results, further replications are needed to establish the ergogenic effect of this specific tDCS procedure.
Effects of tDCS on neuromuscular function and cortical responses
Neuromuscular assessment of the knee extensors was performed as a manipulation check (corticospinal excitability) and to investigate the effects of tDCS on the force-generating capacity of the locomotor muscles. The latter can significantly affect perception of effort and endurance performance during cycling exercise [39,40]. Similar to previous findings [11], acute anodal stimulation over M1 did not change MVC torque, VAL and doublet torque of the knee extensor muscles. In healthy participants an increase in MVC force after anodal stimulation over M1 has been reported only for the pinching muscle in the foot [41].
There is a limited number of studies in healthy participants investigating the effects of tDCS administration on lower limb motor cortex [11,42,43,41,44]. In line with previous experiments, the findings of the current study demonstrated an increased corticospinal excitability of the VL after anodal stimulation over M1 [42,43], as demonstrated by the increase MEParea/M-wave ratio and MEPamp without any significant change in the MEP size of the antagonist muscle (BF). In support of this, Krishan et al [45] reported an increase in activation of the bicep brachii (agonist muscle) with no effect on the tricep brachii (antagonist muscle). However, it is important to note that our protocol was designed to evaluate the response of the knee extensor muscles, and therefore was not optimised to detect possible changes in corticospinal excitability of BF muscle. Therefore, further studies should be performed to clarify the potential effect of tDCS on selective muscle recruitment.
Similarly to our findings, cathodal stimulation failed to induce suppression of corticospinal excitability [42]. The lack of diminished corticospinal excitability in the CATHODAL condition in this study is in contrast to previous studies which have investigated tDCS effects on the upper limb [2]. These conflicting findings might be caused by fewer inhibitory circuits available to suppress leg excitability compared to the hand, or due to a different neuroanatomical structure and orientation of the leg motor cortex, which make cathodal stimulation less effective [42]. Further, the different cortical organization and projection to the spinal cord between upper and lower limbs might explain the lack of effect [46,47].
Effects of tDCS on physiological and perceptual responses during cycling exercise
In the current study, HR increased over time and similarly to B[La−] was significantly higher at exhaustion after ANODAL condition compared to SHAM and CATHODAL conditions, most likely because of the longer exercise duration [48]. Our results are in line with previous findings where anodal stimulation over the M1 did not induce significant changes in HR response [11,21] or other cardiovascular and autonomic parameters at a given time point during exercise [22,23]. A reduction in HR has been previously reported during the initial phases of a maximal incremental cycling test [18] albeit in this study anodal stimulation was administered over T3.
Contrarily to previous studies where anodal stimulation over M1 induced changes in pain perception during various types of experimentally induced pain [49,50], our data did not show any significant effect on exercise-induced muscle pain. Previous studies did not elicit any analgesic effect of tDCS on PAIN during cycling [21] or isometric exercise [11,14]. As discussed by Angius et al. [21] many factors could explain why exercise-induced muscle pain seems to be insensitive to the analgesic effects of tDCS with anode over M1. These factors include the type of nociceptive stimulus, attentional focus, release of endogenous opioids or catecholamines and supraspinal nociceptive inhibitory mechanisms.
As hypothesised, RPE during cycling exercise was significantly lower in the ANODAL condition compared to CATHODAL and SHAM conditions. Because RPE at task failure was not significantly affected by anodal tDCS, participants reached similar levels of RPE at task failure later than in the other two experimental conditions. According to the psychobiological model of endurance performance proposed by Marcora [39,51], this perceptual effect of anodal tDCS is sufficient to explain its effect on performance during TTF tests. Indeed, according to this model, which is based on motivational intensity theory [52], task failure occurs because people voluntary stop exercising when their perception of effort coincides with the maximum effort they are willing to exert in order to succeed in the task (potential motivation).
It is likely that M1 excitability was higher during cycling exercise performed after anodal stimulation compared to both cathodal stimulation and sham tDCS. Such effect provides a plausible neurophysiological explanation for the observed effect on RPE during cycling exercise. Perception of effort seems to originate from processing of corollary discharges from cortical areas upstream of M1 [27,28,38,53]. These cortical areas include the supplementary motor area (SMA) and provide excitatory inputs into M1 that eventually lead to its discharge and recruitment of the locomotor muscles. Because locomotor muscle function (as measured by doublet torque) was not affected by anodal tDCS, we can safely assume that recruitment of the locomotor muscles whilst cycling at the same power output was the same after the three tDCS conditions. However, because M1 excitability was increased by anodal stimulation, less excitatory input into M1 was required to produce the same level of locomotor muscle recruitment. Therefore, the activity of SMA and other motor and premotor areas providing excitatory inputs into M1 and producing the corollary discharges processed by the brain to generate perception of effort should be lower after anodal stimulation compared to cathodal stimulation and sham tDCS. Accordingly, further studies involving neuroimaging techniques such as fMRI or PET would be required to verify this hypothesis and clarify the neurophysiological mechanisms for the reduction in perception of effort.
Technical considerations and limitations
A possible limitation is that the exact propagation of the electrical current in the brain for the montage used in this study is unknown. tDCS has been demonstrated to have a widespread distribution on an area larger than the targeted one [54]. In light of this, an accurate evaluation of the current field distribution could optimize this montage to specifically target the brain areas of interest. Another limitation is that we did not measure cortical activity during cycling exercise. Therefore, our hypothesised neurophysiological mechanisms for the reduction in perception of effort during cycling exercise observed after anodal stimulation remain hypothetical. Further studies using motor-related cortical potentials [27,28], single-photon-emission computed tomography [55], or functional magnetic resonance imaging [56] should be carried out to test the hypotheses that i) anodal stimulation over M1 reduces the activity of the SMA and other cortical areas upstream of M1 during subsequent voluntary submaximal muscle contractions, and ii) that this cortical effect is associated with a reduction in perception of effort.
In this experiment, knee extensor muscles were used to monitor the neuromuscular response given their role in cycling exercise. However, it is important to recognise that other muscles contribute to power production during cycling (e.g. calf muscle, hip muscles, tibialis anterior) and therefore their contribution is likely to change over time during prolonged and fatiguing cycling exercise.
A possible explanation for the lack of correlation between MEP change and TTE duration might be due to the small sample size for this experiment. Further, it should be taken into account that the precise neurophysiological mechanism between excitability of M1 and perception of effort is still not clear and therefore the relationship between these two variables might not be direct. Further experiments would be required to elucidate the link between these two variables.
Conclusion
In conclusion, this study demonstrates that by applying anodal stimulation over both M1 via an extracephalic montage improves TTF and reduces RPE during cycling exercise in a group of healthy participants. Our data suggest that the increase in endurance performance might be the result of higher excitability of the motor cortex leading to a reduction in perception of effort.
HIGHLIGHTS:
Anodal stimulation increased corticospinal excitability of knee extensor muscles;
Perception of effort was reduced following anodal stimulation;
Anodal stimulation over bilateral motor cortices improved endurance performance;
Acknowledgements
The authors would like to thank all participants who took part in the study and for their efforts. Dr. Pascual-Leone and Dr. Santarnecchi are partially supported by Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014-13121700007. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the ODNI, IARPA, or the U.S. Government. Dr. Pascual-Leone is further supported by the Berenson-Allen Foundation, the Sidney R. Baer Jr. Foundation, grants from the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, the Sidney R. Baer Jr. Foundation.
Footnotes
Conflict of interests
Dr. Santarnecchi serves as a consultant for EBNeuro Ltd, a manufacturer of TMS and tDCS devices. None of the devices used in the present experiments were provided by EBNeuro. Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
References
- [1].Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- [2].Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 Pt 3:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci 2014;37:742–53. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- [4].Santarnecchi E, Brem A-K, Levenbaum E, Thompson T, Kadosh RC, Pascual-Leone A. Enhancing cognition using transcranial electrical stimulation. Curr Opin Behav Sci 2015;4:171–8. doi: 10.1016/j.cobeha.2015.06.003. [DOI] [Google Scholar]
- [5].Kuo M-F, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage 2014;85 Pt 3:948–60. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- [6].Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): A Review. Exp Neurol 2009;219:14–9. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- [7].Davis NJ. Neurodoping: Brain Stimulation as a Performance-Enhancing Measure. Sports Med 2013;43:649–53. doi: 10.1007/s40279-013-0027-z. [DOI] [PubMed] [Google Scholar]
- [8].Reardon S. “Brain doping” may improve athletes’ performance. Nature 2016;531:283–4. doi: 10.1038/nature.2016.19534. [DOI] [PubMed] [Google Scholar]
- [9].Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J NeuroEngineering Rehabil 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abdelmoula A, Baudry S, Duchateau J. Anodal transcranial direct current stimulation enhances time to task failure of a submaximal contraction of elbow flexors without changing corticospinal excitability. Neuroscience 2016;322:94–103. doi: 10.1016/j.neuroscience.2016.02.025. [DOI] [PubMed] [Google Scholar]
- [11].Angius L, Pageaux B, Hopker J, Marcora SM, Mauger AR. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience 2016. doi: 10.1016/j.neuroscience.2016.10.028. [DOI] [PubMed] [Google Scholar]
- [12].Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci 2007;26:242–9. doi: 10.1111/j.1460-9568.2007.05633.x. [DOI] [PubMed] [Google Scholar]
- [13].Williams PS, Hoffman RL, Clark BC. Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PloS One 2013;8:e81418. doi: 10.1371/journal.pone.0081418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kan B, Dundas JE, Nosaka K. Effect of transcranial direct current stimulation on elbow flexor maximal voluntary isometric strength and endurance. Appl Physiol Nutr Metab Physiol Appliquée Nutr Métabolisme 2013;38:734–9. doi: 10.1139/apnm-2012-0412. [DOI] [PubMed] [Google Scholar]
- [15].Muthalib M, Kan B, Nosaka K, Perrey S. Effects of transcranial direct current stimulation of the motor cortex on prefrontal cortex activation during a neuromuscular fatigue task: an fNIRS study. Adv Exp Med Biol 2013;789:73–9. doi: 10.1007/978-1-4614-7411-1_11. [DOI] [PubMed] [Google Scholar]
- [16].Angius L, Hopker JG, Marcora SM, Mauger AR. The effect of transcranial direct current stimulation of the motor cortex on exercise-induced pain. Eur J Appl Physiol 2015;115:2311–9. doi: 10.1007/s00421-015-3212-y. [DOI] [PubMed] [Google Scholar]
- [17].Barwood MJ, Butterworth J, Goodall S, House JR, Laws R, Nowicky A, et al. The Effects of Direct Current Stimulation on Exercise Performance, Pacing and Perception in Temperate and Hot Environments. Brain Stimul Basic Transl Clin Res Neuromodulation 2016;0. doi: 10.1016/j.brs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- [18].Okano AH, Fontes EB, Montenegro RA, Farinatti P de TV, Cyrino ES, Li LM, et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med 2015;49:1213–8. doi: 10.1136/bjsports-2012-091658. [DOI] [PubMed] [Google Scholar]
- [19].Vitor-Costa M, Okuno NM, Bortolotti H, Bertollo M, Boggio PS, Fregni F, et al. Improving Cycling Performance: Transcranial Direct Current Stimulation Increases Time to Exhaustion in Cycling. PloS One 2015;10:e0144916. doi: 10.1371/journal.pone.0144916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Angius L, Hopker J, Mauger AR. The Ergogenic Effects of Transcranial Direct Current Stimulation on Exercise Performance. Front Physiol 2017;8:90. doi: 10.3389/fphys.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Angius L, Hopker JG, Marcora SM, Mauger AR. The effect of transcranial direct current stimulation of the motor cortex on exercise-induced pain. Eur J Appl Physiol 2015;115:2311–9. doi: 10.1007/s00421-015-3212-y. [DOI] [PubMed] [Google Scholar]
- [22].Vandermeeren Y, Jamart J, Ossemann M. Effect of tDCS with an extracephalic reference electrode on cardio-respiratory and autonomic functions. BMC Neurosci 2010;11:38. doi: 10.1186/1471-2202-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Santarnecchi E, Feurra M, Barneschi F, Acampa M, Bianco G, Cioncoloni D, et al. Time Course of Corticospinal Excitability and Autonomic Function Interplay during and Following Monopolar tDCS. Front Psychiatry 2014;5:86. doi: 10.3389/fpsyt.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liew S-L, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci 2014;8. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McCormick A, Meijen C, Marcora S. Psychological Determinants of Whole-Body Endurance Performance. Sports Med Auckl NZ 2015;45:997–1015. doi: 10.1007/s40279-015-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Marcora S. Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J Appl Physiol Bethesda Md 1985 2009;106:2060–2. doi: 10.1152/japplphysiol.90378.2008. [DOI] [PubMed] [Google Scholar]
- [27].de Morree HM, Klein C, Marcora SM. Perception of effort reflects central motor command during movement execution. Psychophysiology 2012;49:1242–53. doi: 10.1111/j.1469-8986.2012.01399.x. [DOI] [PubMed] [Google Scholar]
- [28].de Morree HM, Klein C, Marcora SM. Cortical substrates of the effects of caffeine and time-on-task on perception of effort. J Appl Physiol Bethesda Md 1985 2014;117:1514–23. doi: 10.1152/japplphysiol.00898.2013. [DOI] [PubMed] [Google Scholar]
- [29].Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 2012;92:1651–97. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- [30].Han TR, Kim JH, Lim JY. Optimization of facilitation related to threshold in transcranial magnetic stimulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2001;112:593–9. [DOI] [PubMed] [Google Scholar]
- [31].Lim CL, Yiannikas C. Motor evoked potentials: a new method of controlled facilitation using quantitative surface EMG. Electroencephalogr Clin Neurophysiol 1992;85:38–41. [DOI] [PubMed] [Google Scholar]
- [32].Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol 2000;10:361–74. [DOI] [PubMed] [Google Scholar]
- [33].Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998. [Google Scholar]
- [34].O’Connor PJ, Cook DB. Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc Sport Sci Rev 1999;27:119–66. [PubMed] [Google Scholar]
- [35].Blanchfield A, Hardy J, Marcora S. Non-conscious visual cues related to affect and action alter perception of effort and endurance performance. Front Hum Neurosci 2014;8:967. doi: 10.3389/fnhum.2014.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Blanchfield AW, Hardy J, De Morree HM, Staiano W, Marcora SM. Talking yourself out of exhaustion: the effects of self-talk on endurance performance. Med Sci Sports Exerc 2014;46:998–1007. doi: 10.1249/MSS.0000000000000184. [DOI] [PubMed] [Google Scholar]
- [37].Hopkins WG, Schabort EJ, Hawley JA. Reliability of power in physical performance tests. Sports Med Auckl NZ 2001;31:211–34. [DOI] [PubMed] [Google Scholar]
- [38].Zénon A, Sidibé M, Olivier E. Disrupting the supplementary motor area makes physical effort appear less effortful. J Neurosci Off J Soc Neurosci 2015;35:8737–44. doi: 10.1523/JNEUROSCI.3789-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marcora SM, Bosio A, de Morree HM. Locomotor muscle fatigue increases cardiorespiratory responses and reduces performance during intense cycling exercise independently from metabolic stress. Am J Physiol Regul Integr Comp Physiol 2008;294:R874–83. doi: 10.1152/ajpregu.00678.2007. [DOI] [PubMed] [Google Scholar]
- [40].Rønnestad BR, Hansen EA, Raastad T. Effect of heavy strength training on thigh muscle cross-sectional area, performance determinants, and performance in well-trained cyclists. Eur J Appl Physiol 2010;108:965–75. doi: 10.1007/s00421-009-1307-z. [DOI] [PubMed] [Google Scholar]
- [41].Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res Exp Hirnforsch Exp Cerebrale 2009;196:459–65. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 2007;182:281–7. doi: 10.1007/s00221-007-1093-y. [DOI] [PubMed] [Google Scholar]
- [43].Madhavan S, Stinear JW. Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimulat 2010;3:42. doi: 10.1016/j.brs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tatemoto T, Yamaguchi T, Otaka Y, Kondo K, Tanaka S. Anodal Transcranial Direct Current Stimulation over the Lower Limb Motor Cortex Increases the Cortical Excitability with Extracephalic Reference Electrodes In: Pons JL, Torricelli D, Pajaro M, editors. Converging Clin. Eng. Res. Neurorehabilitation, Springer Berlin Heidelberg; 2013, p. 829–34. [Google Scholar]
- [45].Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimulat 2014;7:443–50. doi: 10.1016/j.brs.2014.01.057. [DOI] [PubMed] [Google Scholar]
- [46].Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- [47].Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol 1992;448:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Coyle EF, González-Alonso J. Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev 2001;29:88–92. [DOI] [PubMed] [Google Scholar]
- [49].Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol Off J Eur Fed Neurol Soc 2008;15:1124–30. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- [50].Zandieh A, Parhizgar SE, Fakhri M, Taghvaei M, Miri S, Shahbabaie A, et al. Modulation of cold pain perception by transcranial direct current stimulation in healthy individuals. Neuromodulation J Int Neuromodulation Soc 2013;16:345–8; 10.1111/ner.12009. [DOI] [PubMed] [Google Scholar]
- [51].Marcora SM, Staiano W. The limit to exercise tolerance in humans: mind over muscle? Eur J Appl Physiol 2010;109:763–70. doi: 10.1007/s00421-010-1418-6. [DOI] [PubMed] [Google Scholar]
- [52].Brehm JW, Self EA. The intensity of motivation. Annu Rev Psychol 1989;40:109–31. doi: 10.1146/annurev.ps.40.020189.000545. [DOI] [PubMed] [Google Scholar]
- [53].McCloskey DI. Corollary Discharges: Motor Commands and Perception Compr. Physiol, John Wiley & Sons, Inc.; 2011. [Google Scholar]
- [54].Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. NeuroImage 2013;70:48–58. doi: 10.1016/j.neuroimage.2012.12.034. [DOI] [PubMed] [Google Scholar]
- [55].Williamson JW, McColl R, Mathews D, Ginsburg M, Mitchell JH. Activation of the insular cortex is affected by the intensity of exercise. J Appl Physiol Bethesda Md 1985 1999;87:1213–9. [DOI] [PubMed] [Google Scholar]
- [56].Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human Brain Activation During Sustained and Intermittent Submaximal Fatigue Muscle Contractions: An fMRI Study. J Neurophysiol 2003;90:300–12. doi: 10.1152/jn.00821.2002. [DOI] [PubMed] [Google Scholar]