Abstract
Rationale: Puberty may influence lung function, but the precise role of pubertal height growth in lung development is unclear.
Objectives: To examine associations of timing of puberty and peak velocity of pubertal height growth with lung function in adolescence and early adulthood.
Methods: Longitudinal analyses of repeat height measurements from age 5 to 20 years for a British birth cohort with 4,772 males and 4,849 females were conducted to characterize height growth trajectories and to derive pubertal age and peak height velocity using the validated SITAR (SuperImposition by Translation and Rotation) model. Association of these estimates with prebronchodilator and post-bronchodilator spirometry measures: FEV1; FVC; FEV1/FVC; FEF25–75% at age 15 and 24 years were investigated using multivariable regression models adjusted for lung function at age 8 years, height and age at time of outcome measurements, and potential confounders.
Measurements and Main Results: Later pubertal age and greater peak velocity were associated with higher FEV1 and FVC at 24 years in both sexes. A 1-year increase in pubertal age was associated with a 263-ml higher FVC (95% confidence interval [CI], 167–360 ml) for males (n = 567) and 100-ml (95% CI, 50–150 ml) higher FVC for females (n = 990). A 1-cm/yr increase in peak velocity was associated with 145-ml (95% CI, 56–234 ml) and 50-ml (95% CI, 2–99 ml) increases in FVC for males and females, respectively. No associations were found with FEV1/FVC.
Conclusions: Later onset and greater peak velocity of height growth in puberty are associated with increased FEV1 and FVC in young adults but there was no evidence of dysanapsis of pubertal lung growth.
Keywords: Avon Longitudinal Study of Parents and Children, SuperImposition by Translation and Rotation, pubertal age, velocity of pubertal height growth, maximal lung function
At a Glance Commentary
Scientific Knowledge on the Subject
Low lung function at the physiological plateau in early adulthood is associated with chronic obstructive pulmonary disease in later life. Lung development during childhood and adolescence influences maximally attained lung function in early adulthood. Puberty is a crucial phase in this process, during which a series of programmed biological changes occur that may influence lung function. Height, a key developmental factor during puberty, is strongly correlated with lung function, but little is known about the influence of the characteristics of the pubertal height growth on subsequent lung function.
What This Study Adds to the Field
This population-based, birth cohort study with repeat measurements of anthropometry and lung function from childhood to early adulthood shows that later onset and higher velocity of pubertal growth are associated with higher maximally attained lung function at age 24 years in both sexes, with a greater magnitude in males than females.
Pubertal growth is a pivotal phase in the life course whose characteristics can influence adulthood health outcomes including type 2 diabetes (1), cardiovascular mortality (2), and ovarian (3) and testicular (4) cancers. Understanding the role of pubertal growth in subsequent health conditions may provide insight into the heterogeneous pathophysiology of related diseases and explain growth-related differences in their associated risks (5–7). Timing of pubertal growth has shown a secular trend toward earlier ages over the years (8), which is related to childhood life-style and social factors, for example, diet, obesity, and social deprivation and psychological stress, as well as environmental exposures including endocrine disruptors found in several household products (9, 10).
Previous studies investigating the association of puberty with later health outcomes have largely relied on deriving pubertal age using recalled sexual development such as age at first menstrual bleeding (11–14), self-reported attainment of breast (15) and pubic hair (16) Tanner stages for females; and self-reported attainment of testicular and pubic hair Tanner stages (17) and voice deepening (18) for males. Although these methods have some validity, their reliability may depend on characteristics of the study population, including age at recall and socioeconomic factors, such as educational attainment and social class (19). Moreover, methods of tracking pubertal growth using Tanner stages may not reveal precise pubertal timing because sexual characteristics are assessed at specific time points and the attained Tanner stage is reported, with no information on exact age at attainment. In addition, subjective assessments, particularly around the transition to a higher Tanner stage, may lead to misclassification problems. As a result, these methods may bias estimates of the effects of pubertal age. The velocity (speed) of pubertal growth is another parameter whose role in relevant health outcomes, for example, adult lung function, remains incompletely understood.
Age at first menstrual bleeding has been associated with respiratory health conditions in women (14, 20), including low lung function, a major predictor of disability and mortality in adults (21). Impairments of lung function, either obstructive, defined as a low ratio of FEV1 to FVC, or restrictive, defined as low FVC, are major causes of disability and death worldwide (22–24). There has been a growing interest in identifying childhood risk factors associated with impaired lung function in adulthood, particularly those related to pubertal development. For instance, earlier age at menarche has been associated with reduced FVC in women with no effect found on FEV1/FVC (14). These findings were confirmed by a Mendelian randomization study of 46,944 adult women and 3,025 adolescent girls, investigating the causal effect of age at menarche on lung function, using 122 genetic variants as instrumental variables (20). Of note, consistent findings were shown in men in whom the same genetic variants were used as instrumental variables for sexual development (20). This may suggest a role for pubertal age in general rather than for menarche specifically.
Pubertal height growth has been used as an effective marker for pubertal development (25). Height is an objective measure that can be easily obtained, and of particular importance when respiratory health is considered because it is related to lung volume (26). Through identification of the individual height growth trajectories, we can examine association of age as well as velocity of pubertal height growth with lung function in both males and females; and reveal sex- and growth-related differences in lung development.
In this study we used a validated SITAR (SuperImposition by Translation and Rotation) model (27) to characterize individual height growth from age 5 to 20 years and to investigate the associations of pubertal age and height velocity with lung function in adolescent and young adult males and females.
Methods
Study Design, Setting, and Population
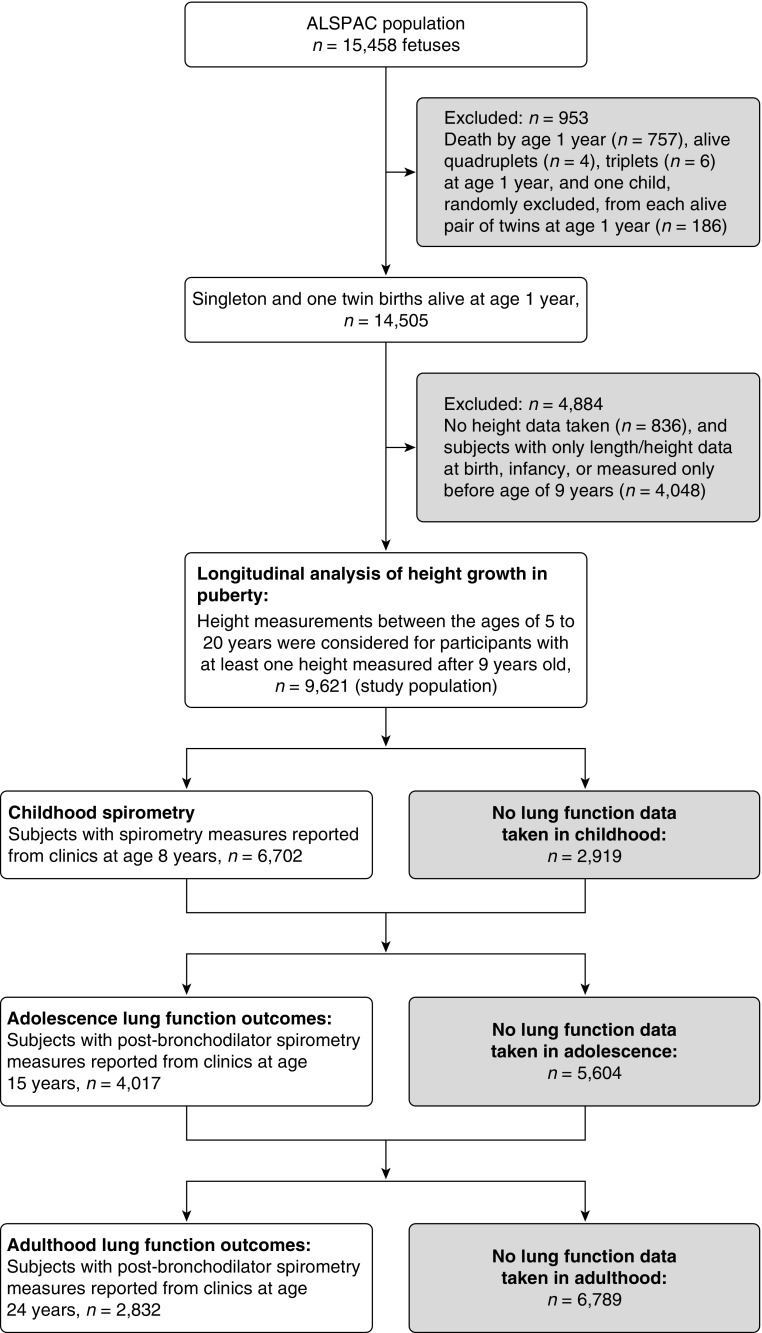
We studied participants in ALSPAC (Avon Longitudinal Study of Parents and Children), a population-based birth cohort. The study protocol was described previously (28), and a detailed description is reported in the online supplement. Briefly, 15,247 pregnant women resident in Avon, UK, with expected delivery dates between April 1, 1991, and December 31, 1992, were recruited, and their live-born children were monitored prospectively. There were 15,458 fetuses, resulting in 14,775 live births and 14,701 children who were alive at 1 year of age. Participant flow is shown in Figure 1. The study was approved by the ALSPAC Ethics and Law Committee and local research ethics committees.
Figure 1.
Flow chart of study subjects included in the analyses. ALSPAC = Avon Longitudinal Study of Parents and Children.
Height Growth in Puberty
Longitudinal measurements of height were obtained mainly by direct measurement in annual research clinics from age 7 to 13 years and at 15, 17, and 24 years. These were supplemented with maternal and self-reported measures throughout the duration of the study. Standing height was measured to the last complete millimeter by trained clinic staff. For characterizing individual height growth trajectories, we considered measurements between the ages of 5 and 20 years for those participants who had at least one height measured after reaching 9 years of age (n = 9,621, excluding quadruples, triples, and one random child from each alive twin).
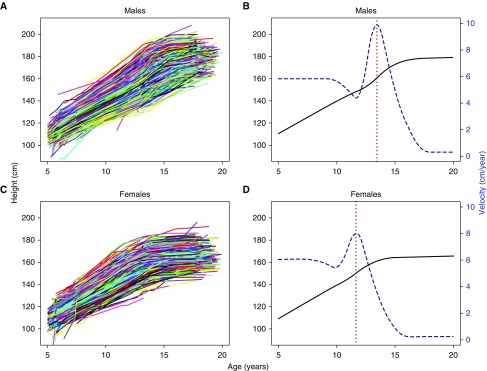
Height growth curves by sex were fitted using the SITAR model, a validated nonlinear mixed effects model (27). The SITAR model explains most of the population heterogeneity in pubertal height growth through characterizing variability in pubertal age, measured by age at peak height velocity (APV), and magnitude of peak height velocity (PV) (see Figure 2). Additional detail on the SITAR model is provided in the online supplement. Median, interquartile range (IQR), correlation of APV and PV, and standard deviations and correlations of the SITAR model’s random effect parameters were obtained to describe the height growth in puberty.
Figure 2.
Individual height growth curves for (A) 4,772 males, with 33,367 measurements, and (C) 4,849 females, with 33,783 measurements, from age 5 to 20 years. Mean curves, fitted by the SITAR models, are illustrated for height growth (in solid black) and height velocity (in dashed blue) for (B) males and (D) females, with vertical lines (in dotted red) indicating mean pubertal age (13.5 and 11.7 yr for males and females, respectively). The right vertical axis in B and D represents the height velocity (cm/yr). SITAR = SuperImposition by Translation and Rotation.
Lung Function
We performed spirometry according to standards of the American Thoracic Society/European Respiratory Society criteria (29, 30) in research clinics at ages 8, 15, and 24 years. Lung function at 15 and 24 years was reported before and 15 minutes after receiving 400 μg of salbutamol administered by metered aerosol through a spacer (31, 32). The highest measurement for each lung function variable (FEV1, FVC, FEV1/FVC, and FEF25–75% [forced expiratory flow, midexpiratory phase]) among the best three technically acceptable flow–volume curves was used for analyses. Post-bronchodilator (post-BD) lung function variables at age 15 and 24 years were used as the primary outcomes, which were summarized using the median and IQR. Missing lung function data were examined to assess whether they were plausibly missing at random; details are reported in the online supplement.
Statistical Analysis
The associations of APV and PV with lung function outcomes in adolescence and early adulthood were examined by using multivariable linear regression models adjusted for confounders, lung function at age 8 years, and age and height at clinic visits for spirometry. We estimated differences in lung functions at age 15 and 24 years associated with a 1-year increase in APV, and a 1-cm/yr increase in PV, mutually adjusted for each other. We estimated odds of risk for asthma symptoms and wheezing at ages 16 and 23 years associated with a 1-year increase in APV, and a 1-cm/yr increase in PV by using logistic regression models adjusted for confounders. A detailed description of statistical analyses is reported in the online supplement.
Sex differences among characteristics of interest were examined by χ2 and Mann-Whitney tests for categorical and continuous factors, respectively. Associations of potential confounders with lung function outcomes were examined using multiple linear regression models. We identified the following variables as being associated with at least one of the lung function measures and adjusted for them in subsequent analyses: parity (≥1 sibling); maternal history of asthma or allergy; maternal smoking during pregnancy; birth weight; ever doctor-diagnosed asthma by age 14 years; exposure to tobacco smoke from birth to 8 years of age; and smoking status at 14 years.
Several secondary analyses were performed to address the robustness of our findings: 1) Prebronchodilator (pre-BD) measurements were used as lung function outcomes. 2) Secondary sexual characteristics such as age at menarche for females and pubic hair development for males were used as measures of pubertal development. 3) Post-BD lung function measurements, excluding the lowest and highest 1% of measurements, were used to examine the sensitivity to extreme spirometry values. 4) We performed k-means cluster analysis to identify groups of subjects with similar pubertal growth in terms of APV and PV. The associations of clusters of identified pubertal patterns with lung function were then examined to assess pattern-specific differential risks. 5) A sensitivity analysis was performed to investigate the association between lung function at 8 years old and pubertal growth to assess potential reverse causation (i.e., that lung function in childhood was affecting puberty). 6) An analysis restricted to subjects with complete lung function data at ages 8, 15, and 24 years was performed to examine sensitivity to incomplete lung function cases. Details of the secondary analyses are provided in the online supplement. All analyses were adjusted for lung function at age 8 years, and for height and age at time of lung function measurements. The analyses were conducted with R software (R Foundation) (33).
Results
Characteristics of the Study Population
Among 9,621 subjects with at least one observation of height measured after age 9 years (the study population), 4,849 (50.4%) were female. Table 1 reports characteristics of the study population by sex. A similar proportion of male and female subjects had an asthmatic or allergic mother (47.4% in males vs. 46.5% in females), were exposed to maternal smoking during pregnancy (23.3% vs. 22.3%), had at least one sibling (53.8% vs. 54.5%), and low birth weight (4.3% vs. 4.7%). More male subjects were born preterm (5.9% vs. 4.7%; P = 0.01) and reported positive doctor-diagnosed asthma ever by 14 years of age (36.4% vs. 30.2%; P = 2 × 10−7) compared with female subjects. Female subjects, on the other hand, were more exposed to maternal anxiety during pregnancy (32.6% vs. 29.8%; P = 0.009) and had a higher proportion of smokers by age 14 years (31% vs. 18.6%; P = 2 × 10−16) compared with male subjects. Male subjects had higher baseline FEV1 and FVC measurements at 8 years of age (P = 2 × 10−16), were taller and attended earlier, in relation to their birth date, at the 15-year visit clinic (largest P = 7 × 10−4), and were taller and attended later, in relation to their birth date, at the 24-year visit clinic (largest P = 0.001) compared with female subjects.
Table 1.
Characteristics of Subjects by Sex
| Characteristic | Male (n = 4,772) |
Female (n = 4,849) |
P Value* | ||
|---|---|---|---|---|---|
| n | Percent or Median (IQR) | n | Percent or Median (IQR) | ||
| Potential confounders | |||||
| Lower maternal education† | 2,553 | 59.5 | 2,567 | 59.8 | 0.778 |
| Having ≥1 sibling (parity) | 2,331 | 53.8 | 2,359 | 54.5 | 0.538 |
| Maternal history of asthma or allergy | 1,998 | 47.4 | 1,971 | 46.5 | 0.398 |
| Maternal smoking during pregnancy | 985 | 23.3 | 957 | 22.3 | 0.294 |
| Maternal anxiety during pregnancy‡ | 1,171 | 29.8 | 1,268 | 32.6 | 0.009 |
| Low birth weight (<2.5 kg) | 193 | 4.3 | 208 | 4.7 | 0.456 |
| Preterm delivery (<37 wk) | 267 | 5.9 | 213 | 4.7 | 0.010 |
| White ethnic group | 4,015 | 95.6 | 4,036 | 95.8 | 0.684 |
| Ever doctor-diagnosed asthma by age 14 yr | 1,164 | 36.4 | 931 | 30.2 | 2 × 10−7 |
| Day care attendance within first year | 272 | 6.7 | 253 | 6.3 | 0.438 |
| Exposure to smoke from birth to age 8 yr | 2,452 | 64.7 | 2,505 | 66.7 | 0.063 |
| Smoking by 14 yr | 454 | 18.6 | 966 | 31.0 | 2 × 10−16 |
| Smoking by 23 yr | 1,753 | 79.4 | 2,724 | 79.6 | 0.816 |
| Control variables | |||||
| Childhood spirometry | |||||
| FEV1 at age 8 yr, L | 3,325 | 1.73 (1.56–1.92) | 3,377 | 1.64 (1.49–1.80) | 2 × 10−16 |
| FVC at age 8 yr, L | 3,325 | 1.98 (1.78–2.20) | 3,377 | 1.83 (1.65–2.03) | 2 × 10−16 |
| FEV1/FVC at age 8 yr, % | 3,325 | 88.0 (83.2–92.0) | 3,377 | 89.8 (85.8–93.4) | 2 × 10−16 |
| FEF25–75% at age 8 yr, L/s | 3,325 | 2.04 (1.67–2.39) | 3,377 | 2.07 (1.74–2.43) | 0.001 |
| Variables at 15 yr | |||||
| Age, yr | 2,556 | 15.3 (15.3–15.5) | 2,856 | 15.4 (15.2–15.6) | 7 × 10−4 |
| Height, m | 2,535 | 1.75 (1.70–1.8) | 2,807 | 1.65 (1.61–1.70) | 2 × 10−16 |
| Variables at 24 yr | |||||
| Age, yr | 1,358 | 24.5 (24.0–25.1) | 2,169 | 24.4 (23.8–25.0) | 0.001 |
| Height, m | 1,355 | 1.80 (1.75–1.84) | 2,151 | 1.66 (1.62–1.70) | 2 × 10−16 |
Definition of abbreviations: FEF25–75% = forced expiratory flow, midexpiratory phase; IQR = interquartile range.
P value from the χ2 or Mann-Whitney test.
Educated to the General Certificate of Education level (school-leaving certificate) or lower.
Anxious mothers were defined as being in the fourth quartile of the Crown-Crisp Experiential Index (40).
Longitudinal Analysis of Pubertal Height Growth
A total of 33,367 and 33,783 measurements were included in the analysis for 4,772 males (median, 8 measurements; IQR, 4–9; and range, 1–20) and 4,849 females (median, 8; IQR, 5–9; range, 1–18), respectively. The SITAR model explained 96.2% and 96.6% of the height growth variation in males and females with residual SD of 13 and 12 mm, respectively (see Table E2 in the online supplement). The median (IQR) APV was 13.5 (13.0–13.9) years for males and 11.7 (11.2–12.1) years for females, and the median (IQR) PV was 9.9 (9.3–10.5) and 8.0 (7.5–8.5) cm/yr, respectively (Figure 2 and Table E2). The correlations between APV and self-reported pubertal staging, that is, age at menarche and age at advanced pubic hair Tanner stage (>2), were 0.71 and 0.26 for females and males, respectively. In both sexes, growth curves were steeper for those with an early puberty and shallower for individuals who attained puberty later (correlation [APV, PV], −0.70 and −0.62 for males and females, respectively). The estimated subject-specific parameters confirmed these relationships (Table E3). There were positive correlations (0.29 and 0.20 for males and females, respectively) between velocity and size (magnitude of height). These suggest that children with relatively higher PV tend to remain on their height trajectory, achieving above-average adulthood height (Table E3).
Lung Function Outcomes
Structures of missingness of lung function data at ages 8, 15, and 24 years are presented in Figure E1. Table E4 shows that parity, maternal smoking during pregnancy, exposure to smoke from birth to age 8 years, ever doctor-diagnosed asthma by age 14 years, and smoking by age 23 years were related to chance of missing the next lung function data, but the current lung function measurements were not. This implies that data are likely to be missing at random and that our analyses, considering all variables related to missingness, should be unbiased.
Table 2 summarizes the distribution of lung function outcomes at age 15 and 24 years in the study population and restricted to subjects with complete data on confounding and control variables by sex. At age 24 years, the median FVC was 5.50 L (IQR, 5.00–6.06 L) and 3.89 L (IQR, 3.53–4.24 L) and the median FEV1 was 4.61 L (IQR, 4.19–5.04 L) and 3.36 L (IQR, 3.06–3.66 L) for male and female subjects, respectively. Male subjects had higher spirometry measurements (FEV1, FVC, and FEF25–75%) at both ages compared with female subjects. These measurements were similar when data was restricted to subjects with complete data on confounders and control variables.
Table 2.
Descriptive Statistics of Lung Function Outcomes in the Study Population and Restricted to Subjects with Complete Data on Confounders and Control Variables by Sex
| Lung Function Outcome | All Subjects in Study Population |
Restricted to Subjects with Complete Data on Confounders* and Control Variables† |
||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Adolescence (age, 15 yr) | n = 1,909 | n = 2,108 | n = 1,177 | n = 1,235 |
| FEV1, L | 3.86 (3.32–4.37) | 3.10 (2.71–3.46) | 3.86 (3.32–4.36) | 3.09 (2.69–3.46) |
| FVC, L | 4.23 (3.63–4.78) | 3.30 (2.88–3.69) | 4.20 (3.64–4.76) | 3.28 (2.90–3.68) |
| FEV1/FVC, % | 92.0 (87.2–96.7) | 94.4 (89.7–98.1) | 92.2 (87.5–96.8) | 94.2 (89.7–97.9) |
| FEF25–75%, L/s | 4.62 (3.85–5.45) | 4.04 (3.37–4.71) | 4.65 (3.88–5.46) | 3.99 (3.36–4.72) |
| Early adulthood (age, 24 yr) | n = 1,062 | n = 1,770 | n = 567 | n = 990 |
| FEV1, L | 4.61 (4.19–5.04) | 3.36 (3.06–3.66) | 4.61 (4.17–5.04) | 3.37 (3.10–3.66) |
| FVC, L | 5.50 (5.00–6.06) | 3.89 (3.53–4.24) | 5.49 (4.97–6.09) | 3.89 (3.55–4.25) |
| FEV1/FVC, % | 84.2 (80.3–87.7) | 86.9 (83.5–90.3) | 84.4 (80.6–87.9) | 86.7 (83.5–90.3) |
| FEF25–75%, L/s | 4.80 (4.04–5.55) | 3.82 (3.25–4.39) | 4.84 (4.06–5.59) | 3.84 (3.29–4.37) |
Definition of abbreviation: FEF25–75% = forced expiratory flow, midexpiratory phase.
Data are shown as median (interquartile range).
Confounders: parity; maternal history of asthma or allergy; maternal smoking during pregnancy; birth weight; ever doctor-diagnosed asthma by age 14 years; exposure to smoke from birth to age of 8 years; and smoking status.
Control variables: baseline lung function at 8 years old; and age and height at visit clinics in which outcomes, reported in the first column, were measured.
Associations of Pubertal Height Growth with Lung Function
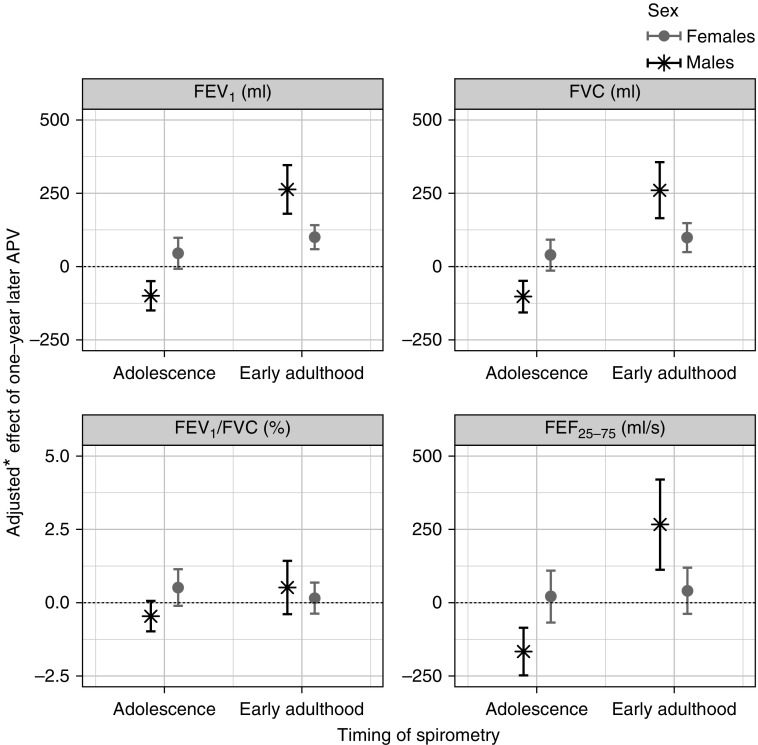
At age 24 years, a 1-year increase in APV was associated with a 263-ml (95% CI, 180–346 ml; P = 1 × 10−9) increase in FEV1 in males and a 100-ml (95% CI, 59–141 ml; P = 1 × 10−6) increase in females. Similar findings were obtained for FVC (263 ml; 95% CI, 167–360; P = 1 × 10−7 in males; 100 ml; 95% CI, 50–150; P = 8 × 10−5 in females) and FEF25–75% in males (270 ml/s; 95% CI, 114–425; P = 7 × 10−4); see Figure 3 and Table E6. In contrast, at age 15 years the APV in males was inversely associated with lung function measurements: FEV1 (−100 ml; 95% CI, −150 to −50 ml; P = 8 × 10−5), FVC (−103 ml; 95% CI, −158 to −49 ml; P = 2 × 10−4), and FEF25–75% (−168 ml/s; 95% CI, −250 to −86 ml/s; P = 6 × 10−5). There was no evidence of association between pubertal age and lung function at 15 years in females.
Figure 3.
Adjusted differences (with 95% confidence intervals) in lung function measurements in adolescence (age, 15 yr) and early adulthood (age, 24 yr) associated with 1-year later pubertal age. *Adjusted for: lung function at age 8 years; age and height at visit clinics in which lung function outcomes were measured (at ages 15 and 24 yr); parity; maternal history of asthma or allergy; maternal smoking during pregnancy; birth weight; ever doctor-diagnosed asthma by age 14 years; exposure to smoke from birth to 8 years of age; and smoking status. APV = age at peak height velocity; FEF25–75% = forced expiratory flow, midexpiratory phase.
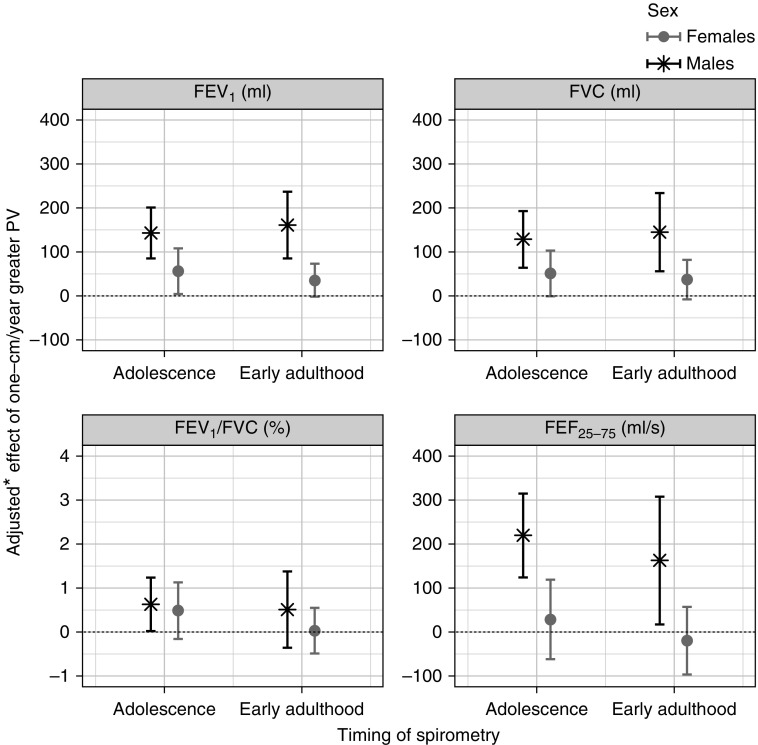
At age 24 years, a 1-cm/yr increase in PV was associated with a 161-ml (95% CI, 85–237 ml; P = 4 × 10−5) increase in FEV1 in males and a 46-ml (95% CI, 6–86 ml; P = 0.025) increase in females (Figure 4 and Table E7). Similarly for FVC, a 1-cm/yr increase in PV was associated with a 145-ml (95% CI, 56–234 ml; P = 0.001) increase in males and a 50-ml (95% CI, 2–99 ml; P = 0.043) increase in females. Evidence of association with FEF25–75% was found only in males (163 ml/s per 1-cm/yr increase in PV; 95% CI, 17–308 ml/s; P = 0.029). At age 15 years, lung function measurements, that is, FEV1, FVC, and FEF25–75%, showed the same direction of association with PV as at age 24 years in males (largest P = 9 × 10−5), with no or little evidence of association in females (lowest P = 0.034). For both sexes, results show that PV is more important than APV as a predictor for adolescence lung function, whereas for adulthood lung function the APV plays the dominant role; see Tables E6 and E7 and the section Statistical Analyses in the online supplement for more details.
Figure 4.
Adjusted differences (with 95% confidence intervals) in lung function measurements in adolescence (age, 15 yr) and early adulthood (age, 24 yr) associated with 1-cm/yr greater peak height velocity. *Adjusted for: lung function at age 8 years; age and height at visit clinics in which lung function outcomes were measured (at ages 15 and 24 yr); parity; maternal history of asthma or allergy; maternal smoking during pregnancy; birth weight; ever doctor-diagnosed asthma by age 14 years; exposure to smoke from birth to 8 years of age; and smoking status. PV = peak height velocity.
Results obtained from secondary analyses confirmed the findings of the study (Tables 3 and E8–E11 and Figures E5 and E6).
Table 3.
Adjusted* Associations of Age at Menarche and Pubic Hair Development for Females and Males, Respectively, with Lung Function Measurements at 15 and 24 Years
| Lung Function Outcome | Adolescence: Age, 15 Years |
Early Adulthood: Age, 24 Years |
||||||
|---|---|---|---|---|---|---|---|---|
| Females: Menarche† (n = 1,181) |
Males: Pubic Hair‡ (n = 1,123) |
Females: Menarche† (n = 943) |
Males: Pubic Hair‡ (n = 529) |
|||||
| β (95% CI) | P Value§ | β (95% CI) | P Value§ | β (95% CI) | P Value§ | β (95% CI) | P Value§ | |
| FEV1, ml | −13 (−40 to 14) | 0.337 | −254 (−421 to −87) | 0.003 | 28 (7 to 48) | 0.009 | 227 (177 to 603) | 4 × 10−4 |
| FVC, ml | −8 (−35 to 19) | 0.558 | −335 (−516 to −154) | 3 × 10−4 | 27 (2 to 52) | 0.036 | 283 (26 to 540) | 0.031 |
| FEV1/FVC, % | −0.1 (−0.4 to 0.3) | 0.606 | 0.4 (−1.3 to 2.1) | 0.620 | 0 (−0.3 to 0.3) | 0.827 | 2.4 (−0.1 to 4.8) | 0.055 |
| FEF25–75%, ml/s | −23 (−71 to 24) | 0.331 | −234 (−502 to 34) | 0.869 | 23 (−18 to 65) | 0.266 | 557 (195 to 919) | 0.003 |
Definition of abbreviations: β = estimate of effect of 1-year increase in age at first menstrual bleeding for females or age at attainment of advanced pubic hair Tanner stage (>2) for males on lung function; CI = confidence interval; FEF25–75% = forced expiratory flow, midexpiratory phase.
Adjusted for: lung function at age 8 years; age and height at clinic visits for spirometry; parity; maternal history of asthma or allergy; maternal smoking during pregnancy; birth weight; ever doctor-diagnosed asthma by age 14 years; exposure to smoke from birth to 8 years of age; and smoking status.
Age at menarche: age at the first day of the first menstrual bleeding.
Age at attainment of pubic hair Tanner stage >2, estimated by parametric survival models (13) from interval-censored maternal and self-reported data collected annually on pubic hair Tanner staging.
P values from Wald test.
Associations of Pubertal Height Growth with Asthma Symptoms and Wheezing
Increased APV and PV were associated with 28% (95% CI, 13–41%) and 21% (95% CI, 6–34%) lower risk of asthma symptoms, respectively, in adolescence, and with 21% (95% CI, 6–34%) and 22% (95% CI, 7–34%) lower risk of wheezing in early adulthood for females. For males, there was some evidence that higher PV is associated with 19% (95% CI, 2–34%) lower risk of asthma symptoms in adolescence; see Table 4.
Table 4.
Adjusted* Associations of Pubertal Age and Magnitude of Peak Height Velocity with Risk of Asthma Symptoms and Wheezing at Ages 16 and 23 Years by Sex
| Pubertal Height Growth | Adolescence: Age, 16 Years |
Early Adulthood: Age, 23 Years |
||||||
|---|---|---|---|---|---|---|---|---|
| Females |
Males |
Females |
Males |
|||||
| Adjusted OR (95% CI)* | P Value† | Adjusted OR (95% CI)* | P Value† | Adjusted OR (95% CI)* | P Value† | Adjusted OR (95% CI)* | P Value† | |
| Asthma symptoms |
n = 1,703 |
n = 1,278 |
n = 1,758 |
n = 924 |
||||
| APV | 0.72 (0.59–0.87) | 7 × 10−4 | 0.85 (0.68–1.07) | 0.171 | 0.84 (0.67–1.07) | 0.156 | 0.85 (0.56–1.28) | 0.433 |
| PV | 0.79 (0.66–0.94) | 0.010 | 0.81 (0.66–0.98) | 0.033 | 0.77 (0.61–0.96) | 0.022 | 0.83 (0.59–1.17) | 0.277 |
| |
||||||||
| Wheezing |
n = 2,120 |
n = 1,564 |
n = 2,041 |
n = 1,128 |
||||
| APV | 0.89 (0.75–1.05) | 0.152 | 0.97 (0.79–1.20) | 0.799 | 0.79 (0.66–0.94) | 0.009 | 0.88 (0.68–1.15) | 0.357 |
| PV | 0.88 (0.76–1.03) | 0.101 | 0.99 (0.82–1.18) | 0.892 | 0.78 (0.66–0.93) | 0.006 | 0.87 (0.69–1.10) | 0.247 |
Definition of abbreviations: APV = age at peak height velocity; CI = confidence interval; OR = odds ratio; PV = peak height velocity.
Adjusted for: parity; maternal history of asthma or allergy; maternal smoking during pregnancy; birth weight; exposure to smoke from birth to 8 years of age; and smoking status.
P values from Wald test.
Discussion
Main Findings
This large, population-based birth cohort study shows that both APV and PV, estimated from individual-level longitudinal height growth curves, are associated with lung function in early adult life, around the time of maximal lung function attainment. In both sexes, we found an association of later pubertal age and rapid pubertal growth with increased FVC, with no evidence of an effect on large airway obstruction (FEV1/FVC), suggesting that the effect of pubertal growth is manifested by an increase in both FEV1 and FVC with no evidence of dysanaptic growth occurring during pubertal development. In contrast, in adolescent males, we showed that later pubertal age was associated with decreased lung function, which may indicate a lag between height growth and lung function maturation during puberty. In addition, we showed that rapid pubertal growth was associated with increased FVC, FEV1, and FEF25–75% in males in adolescence and early adulthood, with less evidence of an effect in females.
Findings in the Context of the Literature
The findings of this study support previous evidence suggesting an increase in FVC of 123 ml (P = 0.01) in women associated with 1-year-later age at menarche, but no association with FEV1/FVC (14). Our finding in adult males, showing a similar association with FVC, confirms the Mendelian randomization findings suggesting effects of pubertal age in general rather than menarche specifically (20).
The inverse association of APV with FVC at 15 years in males is intriguing and contrasts with the relationships in females. Lung volume increases during childhood are generally linear up until puberty and related largely to height growth in both sexes (34). However, during the pubertal growth spurt, there is uncoupling of growth in chest wall dimensions with height increases, such that thoracic growth lags behind leg and height growth (35). Lung volumes continue to increase after height growth ceases, but there is evidence that the pattern differs between males and females; the latter have a shorter duration of lung development, and lung volume increases have completed by the occurrence of menarche (36). In contrast, lung volume increases in males continue throughout puberty and beyond the point at which final adult height is reached. We interpret the differential relationship seen at 15 years in this study in the context of most females having completed menarche by this age but a sizeable proportion of males being in midpuberty. Coupled with the lag between height and thoracic development growth, we believe this observation is most likely explained by the timing of lung function measurement in relation to pubertal status.
Although pubertal growth clearly has an important influence on lung function, the plateau phase of lung function development usually occurs in the early to mid-twenties (21). The association of greater magnitude of PV with increased lung function at 15 and 24 years could possibly be explained by rapid pubertal growth influencing lung function directly as well as leaving a longer interval for postpubertal lung development before achieving plateau values. It is conceivable that our findings reflect the inverse relationship between APV and the PV for both sexes. This confirms the clinical observation that pubertal timing and velocity are inversely correlated (37).
Strengths and Limitations
This study offers insights into the roles of pubertal height growth in maximal lung function, which can shape respiratory health in late adulthood. Our study used repeated height measurements, covering the span from childhood through early adulthood, to which we applied a well-validated mixed-effects model (SITAR) for characterizing growth in puberty. As a result, APV and PV were derived and explained more than 96% of variability in pubertal height growth among subjects. This approach avoided potential problems of most traditional methods used for investigating growth in puberty such as the Tanner stages, for example, misclassifications of pubertal stages. Our findings highlight potential implications of the secular trend shifting to earlier attainment of puberty over the years (8). We addressed the associations of height growth with various lung function parameters including FEV1, FVC, and FEF25–75%. We minimized potential reverse causation by adjusting results for lung function at age 8 years. Another strength is that the study accounts for a wide range of potential confounders with detailed information provided by the ALSPAC cohort.
There are some limitations to our study design and findings. We had no information on hormone levels, the “gold standard” by which to measure puberty. Therefore, the influences of hormones could not be addressed. However, our secondary analyses showed consistency in results using other proxy measures, including timing of appearance of secondary sexual characteristics. We supplemented height measurements taken at the research visit clinics with some self-reported heights. The complex physiological changes that occur in puberty challenge the identification of precise mechanisms underlying our findings on APV and PV, suggesting a need for further research to investigate them. Our study population is liable to loss to follow-up, related to socioeconomic factors. However, there is no evidence that its potential bias might affect the association of puberty with lung function.
Conclusions
Our study provides evidence for associations of later onset and steeper pubertal growth with greater maximally attained lung volumes by analyzing longitudinal heights around the time of puberty, using a validated approach for both males and females. We found that pubertal growth patterns associated with increased lung volumes were associated with reduced reporting of asthma-like symptoms in this population of young adults despite the absence of evidence of specific effects on airway obstruction. We have shown previously that low FEV1 tracks through childhood and may be associated with chronic obstructive pulmonary disease in adults (38, 39), but the long-term implications on adult lung disease of lung volume changes we observed during puberty without evidence of dysanaptic growth have yet to be fully determined.
Our findings, together with evidence of a secular trend toward earlier puberty (8), may have population-level consequences on lifetime lung function and respiratory symptoms and raise a public health implication for targeting modifiable childhood factors, such as obesity and overweight, that may contribute to pubertal growth patterns.
Acknowledgments
Acknowledgment
The authors are extremely grateful to all the families who took part in this study, the midwives for help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Footnotes
This project was conducted within the Ageing Lungs in European Cohorts study and received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement 633212. The UK Medical Research Council and Wellcome Trust (grant reference: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and O.M. and J.H. will serve as guarantors for the contents of this paper.
Author Contributions: O.M. and J.H. conceived and designed the study. O.M. planned, designed, and conducted the statistical analyses, and drafted the manuscript. O.M., R.G., K.T., C.M., J.G.-A, J.W.H., A.C., D.J., J.S., and J.H. contributed to the interpretation of the results, critically reviewed the manuscript, and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201802-0274OC on July 11, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171:334–344. doi: 10.1093/aje/kwp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai CM, Gunnarsdottir I, Gudnason V, Aspelund T, Birgisdottir BE, Thorsdottir I, et al. Early peak height velocity and cardiovascular disease mortality among Icelandic women. Ann Med. 2013;45:545–550. doi: 10.3109/07853890.2013.852347. [DOI] [PubMed] [Google Scholar]
- 3.Gong TT, Wu QJ, Vogtmann E, Lin B, Wang YL. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer. 2013;132:2894–2900. doi: 10.1002/ijc.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richiardi L, Askling J, Granath F, Akre O. Body size at birth and adulthood and the risk for germ-cell testicular cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:669–673. [PubMed] [Google Scholar]
- 5.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuh D, Muthuri SG, Moore A, Cole TJ, Adams JE, Cooper C, et al. Pubertal timing and bone phenotype in early old age: findings from a British birth cohort study. Int J Epidemiol. 2016;45:1113–1124. doi: 10.1093/ije/dyw131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. A possible link between the pubertal growth of girls and prostate cancer in their sons. Am J Hum Biol. 2012;24:406–410. doi: 10.1002/ajhb.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams Hillard PJ. Menstruation in adolescents: what’s normal, what’s not. Ann N Y Acad Sci. 2008;1135:29–35. doi: 10.1196/annals.1429.022. [DOI] [PubMed] [Google Scholar]
- 9.Fisher MM, Eugster EA. What is in our environment that effects puberty? Reprod Toxicol. 2014;44:7–14. doi: 10.1016/j.reprotox.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisonet M, Christensen KY, Rubin C, Holmes A, Flanders WD, Heron J, et al. Role of prenatal characteristics and early growth on pubertal attainment of British girls. Pediatrics. 2010;126:e591–e600. doi: 10.1542/peds.2009-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen KY, Maisonet M, Rubin C, Flanders WD, Drews-Botsch C, Dominguez C, et al. Characterization of the correlation between ages at entry into breast and pubic hair development. Ann Epidemiol. 2010;20:405–408. doi: 10.1016/j.annepidem.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin C, Maisonet M, Kieszak S, Monteilh C, Holmes A, Flanders D, et al. Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2009;23:492–504. doi: 10.1111/j.1365-3016.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 13.Monteilh C, Kieszak S, Flanders WD, Maisonet M, Rubin C, Holmes AK, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25:75–87. doi: 10.1111/j.1365-3016.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 14.Macsali F, Real FG, Plana E, Sunyer J, Anto J, Dratva J, et al. Early age at menarche, lung function, and adult asthma. Am J Respir Crit Care Med. 2011;183:8–14. doi: 10.1164/rccm.200912-1886OC. [DOI] [PubMed] [Google Scholar]
- 15.Christensen KY, Maisonet M, Rubin C, Holmes A, Flanders WD, Heron J, et al. Progression through puberty in girls enrolled in a contemporary British cohort. J Adolesc Health. 2010;47:282–289. doi: 10.1016/j.jadohealth.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen KY, Maisonet M, Rubin C, Holmes A, Flanders WD, Heron J, et al. Pubertal pathways in girls enrolled in a contemporary British cohort. Int J Pediatr. 2010;2010:329261. doi: 10.1155/2010/329261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole TJ, Pan H, Butler GE. A mixed effects model to estimate timing and intensity of pubertal growth from height and secondary sexual characteristics. Ann Hum Biol. 2014;41:76–83. doi: 10.3109/03014460.2013.856472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousefi M, Karmaus W, Zhang H, Roberts G, Matthews S, Clayton B, et al. Relationships between age of puberty onset and height at age 18 years in girls and boys. World J Pediatr. 2013;9:230–238. doi: 10.1007/s12519-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 19.Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth MEJ, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health. 2006;60:993–997. doi: 10.1136/jech.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill D, Sheehan NA, Wielscher M, Shrine N, Amaral AFS, Thompson JR, et al. Age at menarche and lung function: a Mendelian randomization study. Eur J Epidemiol. 2017;32:701–710. doi: 10.1007/s10654-017-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low lung function in young adult life is associated with early mortality. Am J Respir Crit Care Med. 2017;195:1399–1401. doi: 10.1164/rccm.201608-1561LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66:49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- 23.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agustí A. Chronic obstructive pulmonary disease and cardiac diseases: an urgent need for integrated care. Am J Respir Crit Care Med. 2016;194:1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 24.Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Lung function in young adult life and mortality risk [abstract] Am J Respir Crit Care Med. 2015;191:A2315. [Google Scholar]
- 25.Aksglaede L, Olsen LW, Sørensen TI, Juul A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS One. 2008;3:e2728. doi: 10.1371/journal.pone.0002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow–volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 27.Cole TJ, Donaldson MD, Ben-Shlomo Y. SITAR: a useful instrument for growth curve analysis. Int J Epidemiol. 2010;39:1558–1566. doi: 10.1093/ije/dyq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: the “children of the 90s”—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 30.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. American Thoracic Society/European Respiratory Society Working Group on Infant and Young Children Pulmonary Function Testing. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 31.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing: 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrino R, Decramer M, van Schayck CP, Dekhuijzen PN, Troosters T, van Herwaarden C, et al. Quality control of spirometry: a lesson from the BRONCUS trial. Eur Respir J. 2005;26:1104–1109. doi: 10.1183/09031936.05.00026705. [DOI] [PubMed] [Google Scholar]
- 33. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017.
- 34.Lebowitz MD, Sherrill DL. The assessment and interpretation of spirometry during the transition from childhood to adulthood. Pediatr Pulmonol. 1995;19:143–149. doi: 10.1002/ppul.1950190210. [DOI] [PubMed] [Google Scholar]
- 35.DeGroodt EG, van Pelt W, Borsboom GJ, Quanjer PH, van Zomeren BC. Growth of lung and thorax dimensions during the pubertal growth spurt. Eur Respir J. 1988;1:102–108. [PubMed] [Google Scholar]
- 36.Nève V, Girard F, Flahault A, Boulé M. Lung and thorax development during adolescence: relationship with pubertal status. Eur Respir J. 2002;20:1292–1298. doi: 10.1183/09031936.02.00208102. [DOI] [PubMed] [Google Scholar]
- 37.Tanner JM. Growth at adolescence; with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Oxford: Blackwell Scientific; 1962.
- 38.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6:526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 39.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 40.Birtchnell J, Evans C, Kennard J. The total score of the Crown-Crisp Experiential Index: a useful and valid measure of psychoneurotic pathology. Br J Med Psychol. 1988;61:255–266. doi: 10.1111/j.2044-8341.1988.tb02787.x. [DOI] [PubMed] [Google Scholar]