To the Editor:
Reduced lung BMPR2 (bone morphogenetic protein receptor type 2) expression and female predominance are two major features of most pulmonary arterial hypertension (PAH) subtypes (1). In addition, germline mutations in BMPR2 are present in more than 75% of patients with heritable PAH, and about 20% of patients with idiopathic PAH (2). However, only 14% of males, compared with 42% of females, who harbor BMPR2 mutations develop PAH (3).
There is a growing body of molecular and in vivo work supporting the concept that sex and BMPR2 are intimately related to each other, to PAH pathogenesis, and perhaps to right ventricular adaptation. For example, estrogen receptor α binds to the BMPR2 promoter in Cos-7 cells, leading to decreased expression and signaling of BMPR-II, whereas female human pulmonary artery smooth muscle cells exhibit estrogen-driven suppression of BMPR-II signaling (4, 5); however, estrogen signaling appears to support right ventricular response to stress (6). Although likely relevant, a focus only on sex hormones ignores an obvious difference between females and males: the sex chromosomes (XX vs. XY). Importantly, recently in the Journal, Umar and colleagues demonstrated a protective effect of the Y chromosome in murine hypoxia-induced pulmonary hypertension (7). We explored the hypothesis that the higher female incidence in PAH is driven in part by factors specific to the Y chromosome that enhance BMPR2 expression, with a focus on the transcription factor SRY (sex-determining region Y).
To start, we analyzed BMPR2 proximal regulator sequences using the transcription element search system and transcription factor database (http://www.gene-regulation.com). These analyses predicted BMPR2 upstream regulatory regions had at least five SRY binding sites. This suggested to us that SRY may regulate BMPR2 mRNA expression.
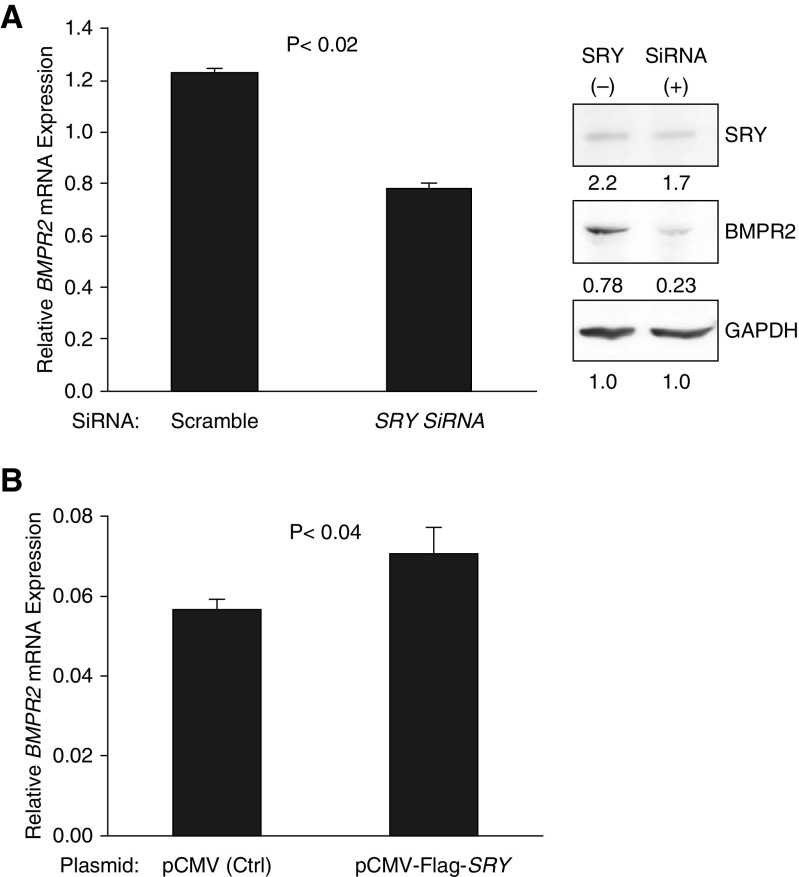
We next evaluated SRY expression in different cell types and found that SRY expression was low in multiple different male lung vascular cell lines but high in dermal fibroblast cell lines from multiple control patients and patients with PAH (data not shown). We then sought to determine whether SRY regulates native BMPR2 expression. We used RNA interference to knockdown SRY expression in fibroblasts from a male patient with PAH. We found that reducing SRY expression resulted in decreased BMPR2 mRNA and protein expression (Figure 1A). Although Smad 1/5/8 protein expression was not demonstratively reduced (data not shown), the breadth of canonical and noncanonical BMPR2 signaling was not assessed. We then used a SRY expression construct to overexpress SRY in a female HEK293 cell line (which does not express SRY). We found that SRY overexpression resulted in ∼20% increased BMPR2 expression compared with control (Figure 1B).
Figure 1.
SRY (sex-determining region Y) positively regulates BMPR2 (bone morphogenetic protein receptor type 2) expression in human cells. (A) SRY knockdown resulted in lower BMPR2 mRNA and protein expression in male fibroblasts. The dermal fibroblasts, derived from a male patient with pulmonary arterial hypertension, were transfected with scrambled siRNA or SRY siRNA. BMPR2 mRNA levels were quantified by real-time PCR, and protein levels by Western blot. (B) SRY overexpression resulted in increased BMPR2 mRNA expression in female HEK293 cells (which do not express native SRY). HEK293 cells were transfected with plasmids: control pCMV or pCMV-FLAG-SRY (which expressed SRY). BMPR2 mRNA levels were quantified by real-time PCR.
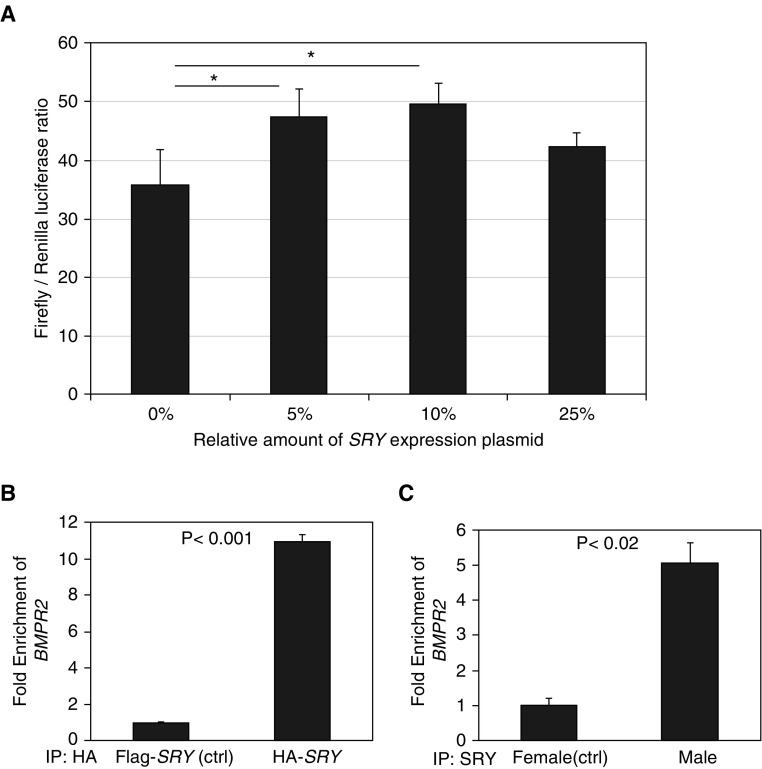
We next investigated whether BMPR2 is regulated by SRY in a dose-dependent manner. A BMPR2 promoter expression construct pGL3-BMPR2-Luciferase (containing the predicted SRY binding sites) was used in a cotransfection assay with a varying amount of SRY expression construct. We found that an increased quantity of SRY resulted in an increased expression of luciferase, suggesting that SRY can positively regulate the BMPR2 promoter in a dose-dependent manner (Figure 2A).
Figure 2.
SRY (sex-determining region Y) directly binds to the BMPR2 (bone morphogenetic protein receptor type 2) promoter. (A) SRY induction of a BMPR2 promoter−driven luciferase. Dual luciferase assay was performed in human microvascular endothelial cells (HMECs). HMECs were transfected with a pGL3-luc-BMPR2 firefly vector, an internal control herpes simplex virus thymidine kinase promoter Renilla vector, and a variable amount of SRY expression plasmids. The transfected cells were lysed, and luminescence was measured on a luminometer. The relative luminescent ratios of luciferase firefly/Renilla were plotted to indicate BMPR2 expression (*P < 0.05). (B) SRY binds to the BMPR2 promoter in HEK293 cells. Chromatin immunoprecipitation (ChIP) assay was performed in HEK293 cells (female) overexpressing a control plasmid (Flag-SRY) or plasmid HA-SRY. Anti-HA antibody was used to immunoprecipitate HA-tagged SRY. PCR was performed to detect the BMPR2 promoter sequence, using the EpiTect ChIP quantitative PCR assay designed for human BMPR2. Results were calculated as fold enrichment of BMPR2 and represent the mean ± SEM. (C) Endogenous SRY binds to the BMPR2 promoter in cells of a male patient with pulmonary arterial hypertension (PAH). ChIP assay was performed in dermal fibroblasts derived from one female patient with PAH and one male patient with PAH. Anti-SRY antibody was used to immunoprecipitate endogenous SRY. PCR was performed to detect the BMPR2 promoter sequence, using EpiTect ChIP quantitative PCR assay designed for human BMPR2. Results were calculated as fold enrichment of BMPR2 and represent the mean ± SEM. ctrl = control; HA = human influenza hemagglutinin; IP = immunoprecipitation.
We then used a chromatin immunoprecipitation assay to determine whether SRY directly bound to human BMPR2 promoter sequences, using the female cell line HEK293 overexpressing human influenza hemagglutinin–tagged SRY. We found that SRY bound to BMPR2 promoter sequences (Figure 2B). Next, we repeated the chromatin immunoprecipitation assay in fibroblast cell lines from a female and a male to determine whether endogenous SRY can also bind to BMPR2 promoter sequences. Although the female cells (lacking SRY) showed no binding, the SRY in the male fibroblasts bound to BMPR2 promoter sequences (Figure 2C). Taken together, our chromatin immunoprecipitation data show that SRY binds to BMPR2 promoter sequences.
Our data thus demonstrate that SRY binds to and positively regulates the BMPR2 promoter. These findings add to our understanding of the contribution of sex to PAH and its relationship to BMPR2. Given the crucial role of reduced BMPR2 in PAH, including data that reduced BMPR-II signaling (regardless of the presence of a BMPR2 mutation) is detrimental for the pulmonary vasculature, factors that regulate BMPR2 are likely to modify overall PAH risk and resilience. SRY appears to be one of these factors.
SRY is a member of the SOX (SRY-like box) family of transcription factors, and is known to both positively and negatively regulate gene expression (8). The SRY gene is localized on chromosome Yp11.2, and as such is expressed exclusively in males. It is a critical gene for male sex determination, as well as other components of development (9). However, SRY also appears to modulate several other pathways through cis and trans effects across the genome, including pathways highly relevant to PAH pathogenesis, such as WNT signaling (8, 10, 11).
One of the findings in our study was that the positive effect of SRY on BMPR2 expression had an upper limit, above which adding more SRY did not result in a corresponding increase in BMPR2 expression. This is not a surprising finding, as most transcription factors have a limited range of function. Furthermore, BMPR2 expression is likely controlled by a coordinated action of multiple different transcriptional regulators, and thus one transcription factor would not be expected to modify its expression to an unlimited extent. For example, previous studies have identified other factors that regulate BMPR2 expression, such as estrogen receptor α (4, 5). Thus, the integration of multiple factors may explain why SRY has a limited, but important, capacity to increase BMPR2 expression. Each factor may play a role in PAH susceptibility or resilience. It is important to acknowledge that in some assays, the effect sizes were modest; this may reflect the fact that multiple factors likely contribute to BMPR2 expression, not simply SRY activity. Finally, much of this work was conducted using PAH fibroblasts because of the low expression of SRY in typical lung vascular cells. Although the amount of data suggesting that fibroblasts may contribute to PAH pathogenesis is growing, future work will determine whether SRY contributes to variations in lung vascular cell health and function.
In conclusion, SRY binds to and positively regulates BMPR2 expression. This builds on recent novel work by Umar and colleagues, which demonstrated the relevance of the Y chromosome to pulmonary hypertension (7). Our findings advance the concept that protective factors on the Y chromosome contribute to pulmonary hypertension, with a focus on the reduced male incidence in PAH via sex-specific BMPR2 regulation.
Footnotes
Funding was provided by NIH P01 HL 108800 (E.D.A.), NIH R01 HL134802 (E.D.A.), and NIH R01 HL 102020 (R.H.) awards.
Author Contribution: L.Y. conducted experiments, analyzed data, and wrote the manuscript; J.D.C. analyzed data; L.K.H. and B.N. conducted experiments; and L.Y., R.H., and E.D.A. conceived the study; designed the experiments; acquired, analyzed, and interpreted the data; and wrote and edited the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201802-0308LE on September 25, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 2.Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D13–D21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Larkin EK, Newman JH, Austin ED, Hemnes AR, Wheeler L, Robbins IM, et al. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:892–896. doi: 10.1164/rccm.201205-0886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3:6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mair KM, Yang XD, Long L, White K, Wallace E, Ewart MA, et al. Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2015;191:693–703. doi: 10.1164/rccm.201410-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahm T, Frump AL, Albrecht ME, Fisher AJ, Cook TG, Jones TJ, et al. 17β-Estradiol mediates superior adaptation of right ventricular function to acute strenuous exercise in female rats with severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;311:L375–L388. doi: 10.1152/ajplung.00132.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, et al. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2018;197:952–955. doi: 10.1164/rccm.201707-1345LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau YF, Li Y. The human and mouse sex-determining SRY genes repress the Rspol/beta-catenin signaling. J Genet Genomics. 2009;36:193–202. doi: 10.1016/S1673-8527(08)60107-1. [DOI] [PubMed] [Google Scholar]
- 9.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 10.Awad KS, West JD, de Jesus Perez V, MacLean M. Novel signaling pathways in pulmonary arterial hypertension (2015 Grover Conference Series) Pulm Circ. 2016;6:285–294. doi: 10.1086/688034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wissmüller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006;34:1735–1744. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]