To the Editor:
Asthma is characterized by strong time-of-day rhythms: symptoms worsen around 04:00 (1) along with increased airway narrowing, as reflected by a reduced peak expiratory flow or FEV1 (2). Clinically useful biomarkers in asthma include sputum and blood eosinophils (3), and results from several small studies looking at circadian variations in airway inflammation in asthma are conflicting (4–9). The sputum eosinophil percentage is used to guide management decisions in severe-asthma clinics (3), and therefore any diurnal variation in sputum eosinophilia could influence the management of patients.
We studied circadian variations in blood and sputum eosinophils in a cohort of patients with mild/moderate, atopic asthma compared with healthy control subjects. We then retrospectively compared sputum eosinophil counts from a severe-asthma clinic cohort in relation to the time of day of collection (morning vs. afternoon).
Methods
Ten healthy volunteers and 10 adults with mild/moderate, atopic asthma (Asthma Control Questionnaire 7, 0.9 [0.8–1.1]) who were on regular inhaled corticosteroids (equivalent to beclomethasone dipropionate 400 μg [400–650 μg]) were recruited for this study. The study protocol was approved by the Health Research Authority, National Research Ethics Service Committee of North West–Greater Manchester Central (REC 14/NW/1352). Written informed consent was obtained from all participants. Spirometry, blood eosinophils, induced sputum, and serum were collected during four visits, each 1 week apart, avoiding any potentiating effect of sputum induction on a subsequent sample. Visit 1 (V1) occurred at 16:00, V3 was at 10:00, and V4 was at 22:00. V2 involved an overnight stay, with sampling occurring at 16:00, 22:00, 04:00, and 10:00 the following morning.
Next, a circadian analysis was performed retrospectively on sputum eosinophil counts obtained from patients with severe asthma attending either a morning or afternoon clinic.
The median (interquartile range) is reported. Wilcoxon’s test, the Mann-Whitney U test, and two-way ANOVA were used to analyze the data. A post hoc power analysis performed on the sputum eosinophil data resulted in a power of 81.86% for our observed effect size (1.057), and the minimum detectable effect size was 1.037 given 80% power. Power calculations were based on a Wilcoxon signed-rank test to detect the time-of-day difference in asthma patients. General linear modeling was used to analyze the severe-asthma cohort data and potential confounders. Chi-squared Fisher’s exact test and the Pearson correlation coefficient were also used for analyses. P ≤ 0.05 was considered statistically significant.
Results
Mild/moderate asthma
Healthy and asthma groups were matched for age (P = 0.63) and body mass index (P = 0.9). FEV1% predicted was lower in the asthma group (82.3% [73.0–89.0%]) than in the healthy group (97.7% [91.7–105.3%]; P < 0.001), with more reversibility in the asthma group (255 ml [172.5–355 ml]) than in the healthy group (35 ml [−15 to 122.5 ml]; P < 0.01). There was a nocturnal dip in FEV1 in the asthma group (P < 0.0001) at 04:00.
For patients with mild/moderate asthma and control subjects, the number of blood eosinophils showed a time-of-day difference, peaking at 04:00 (P < 0.01), with no difference between groups. Serum eotaxin did not vary by time of day or between groups.
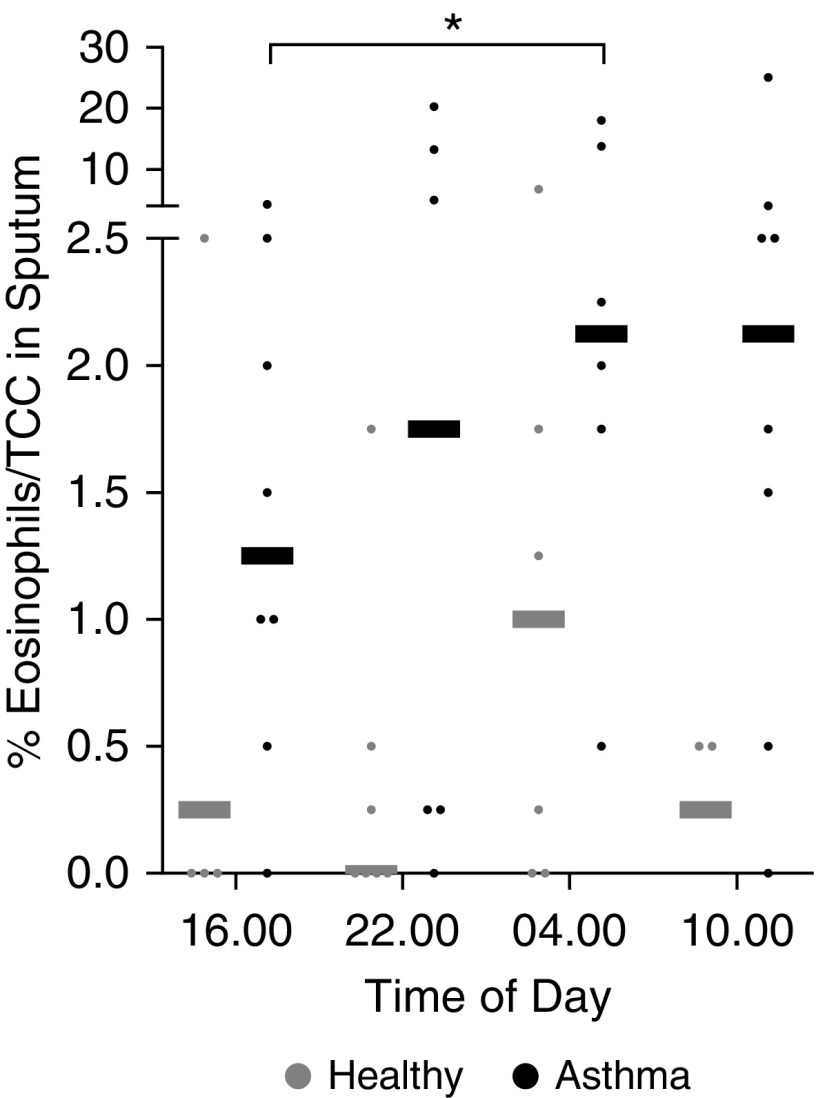
Among patients with mild/moderate asthma, the sputum eosinophil percentages were significantly higher at 04:00 than at 16:00 (P < 0.05; Figure 1). There was no significant time-of-day variation in sputum eosinophils in the healthy group (P = 0.63). Sputum eosinophil percentages were significantly increased in the asthma group compared with the healthy group (P < 0.01). Sputum eotaxin was significantly increased at 04:00 compared with 16:00 in the asthma group (62.9 [28.2–120.4] vs. 33.0 [14.5–73] pg/ml; P < 0.05), but not in the healthy group (29.7 [16.2–74.2] vs. 37.0 [25.5–54.5] pg/ml).
Figure 1.
Diurnal variation in blood and sputum eosinophils. Sputum eosinophil percentages increased significantly at 04:00 compared with 16:00 in patients with asthma (*P < 0.05, Mann-Whitney U test). This was not seen in the healthy group (P = 0.63). Sputum eosinophil percentages were significantly higher in patients with asthma compared with healthy control subjects (P < 0.01, two-way ANOVA). Shown are individual sputum eosinophil percentages (dots) and medians (bars) for healthy control subjects (gray) and patients with asthma (black). TCC = total cell count.
Severe asthma
A total of 131 patients attended the morning clinic and 193 attended the afternoon clinic. Groups were well matched for age (P = 0.11), body mass index (P = 0.25), FEV1% predicted (P = 0.85), smoking status (P = 0.3), serum total IgE (P = 0.23), fractional exhaled nitric oxide (P = 0.58), blood eosinophil count (P = 0.58), and treatment (intramuscular triamcinolone [P = 0.71], oral prednisolone [P = 0.31], or daily inhaled corticosteroids [beclomethasone dipropionate equivalent]; P = 0.31). Significantly more subjects in the morning group produced sputum spontaneously than by induction (77.1% vs. 62.1%; P < 0.005).
An analysis of the severe-asthma clinic cohort data revealed a significant time-of-day effect: sputum produced in the morning clinic contained a significantly higher percentage of sputum eosinophils than that produced in the afternoon clinic (morning sputum eosinophil percentage 1.25% [0.00–8.75%] vs. afternoon 0.5% [0.00–2.25%]; P = 0.008). A general linear modeling analysis showed that the time-of-day effect persisted even if patients were on high-dose steroids (P = 0.41) and was not affected by the type of sputum (spontaneous vs. induced; P = 0.54).
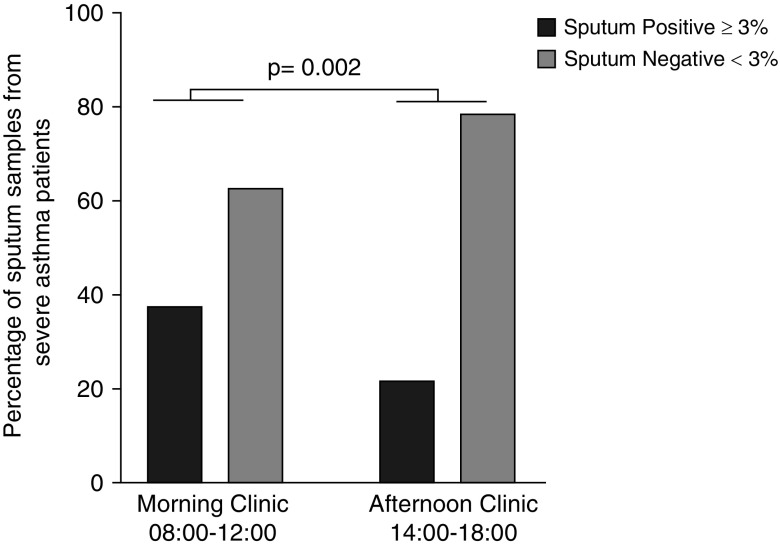
A significantly higher proportion of patients with severe asthma attending the morning clinic had positive sputum eosinophil counts (≥3%) compared with those attending the afternoon clinic (37.4% vs. 21.6%; P = 0.002; Figure 2).
Figure 2.
Analysis of sputum from patients attending morning and afternoon severe-asthma clinics. The figure shows the percentage of positive (black) and negative (gray) sputum samples from patients with severe asthma attending a morning or afternoon clinic: 37.4% of patients with severe asthma had positive sputum eosinophil counts (≥3%) in the morning clinic, compared with 21.6% in the afternoon clinic (P = 0.002, chi-squared, Fisher’s exact test).
We found no difference in the proportion of cell counts that were classed as eosinophilic (≥3% eosinophils) between spontaneously produced sputum and induced sputum (morning P = 0.6, afternoon P = 1).
Discussion
This is the most comprehensive circadian study of biomarkers in asthma to date. We report, for the first time, a circadian variation in sputum eotaxin, peaking at 04:00. We confirmed that airway eosinophils showed a significant circadian rhythm in induced sputum from patients with mild/moderate asthma, with a peak influx at 04:00 coinciding with peak sputum eotaxin concentrations suggesting a chemotactic mechanism. We demonstrated that this was in antiphase with FEV1. In contrast to airway eosinophils, blood eosinophils oscillated diurnally in both healthy subjects and patients with asthma, suggesting that the physiological mechanism that controls the circadian variation in blood eosinophils is not upregulated in asthma. In support of this, there was no difference in serum eotaxin levels between the groups.
We noticed a nadir in sputum eosinophil levels at 16:00, and although the difference was not statistically significant, sputum eosinophil levels appeared to be higher at 10:00, hinting at a possible time-of-day effect that would be relevant within a clinical working day. We postulated that a patient might produce a sputum sample with higher eosinophil counts while attending a morning clinic versus an afternoon clinic. In our retrospective evaluation, we identified that patients with severe asthma attending a morning clinic were almost twice as likely (37.4% vs. 21.6%) to have sputum eosinophilia (≥3%) than those attending an afternoon clinic. These findings require confirmation in a prospective study; however, the implications are clinically important. In patients with severe asthma, increased sputum eosinophil counts are an indicator for treatment escalation (3). Based on our results, we propose that different clinical decisions could be made based on whether the patient is allocated a morning or afternoon appointment.
Footnotes
H.J.D. is supported by an Asthma UK Senior Clinical Academic Development Award (AUK-SCAD-2013-229), the J P Moulton Charitable Foundation, a North West Lung Centre Charity project grant, and the University of Manchester Dean’s Prize for Clinicians. R.J.M. is funded by grants from the Wellcome Trust (107849/Z/15/Z) and the Medical Research Council (MR/P023576/1). A.S.I.L. is a Wellcome Trust Investigator (107849/Z/15/Z). D.W.R. is a Wellcome Trust Investigator (107849/Z/15/Z) and received a Medical Research Council Program grant (MR/P023576/1). J.F.B. is funded by a Medical Research Council grant (MR/L006499/1). D.S. received research grants from Apellis, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Johnson and Johnson, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Skypharma, Teva, Therevance, and Verona. A.S. was supported by grants from the Medical Research Council, National Institute for Health Research, EU FP7, and the NIHR Manchester Biomedical Research Centre.
Author Contributions: H.J.D. conceived of the study, secured the funding, ran the study, analyzed the results, and prepared the manuscript. S.J.F. analyzed the results and prepared the manuscript. G.O.G.-T. analyzed the severe-asthma cohort data and prepared the manuscript. R.J.M. ran the statistical analysis, advised on the statistics used, and prepared the manuscript. K.K. performed eotaxin cytokine assays on serum and sputum samples, and analyzed the data. A.S.I.L. and J.F.B. prepared the manuscript. D.S. conceived the study, provided support to run the study, and prepared the manuscript. D.W.R. and A.S. conceived the study, analyzed the results, and prepared the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201807-1289LE on August 29, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Turner-Warwick M. Nocturnal asthma: a study in general practice. J R Coll Gen Pract. 1989;39:239–243. [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland ER. Nocturnal asthma. J Allergy Clin Immunol. 2005;116:1179–1186, quiz 1187. doi: 10.1016/j.jaci.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 4.Popov TA, Shenkada MS, Tzoncheva AV, Pravtchanska MP, Mustakov TB, Dimitrov VD. Circadian changes in the sputum of asthmatic subjects and healthy controls. World Allergy Organ J. 2008;1:74–78. doi: 10.1097/WOX.0b013e3181752d02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson WJ, Wong LE, The S, Leigh R. The impact of diurnal variation on induced sputum cell counts in healthy adults. Clin Transl Allergy. 2013;3:8. doi: 10.1186/2045-7022-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panzer SE, Dodge AM, Kelly EA, Jarjour NN. Circadian variation of sputum inflammatory cells in mild asthma. J Allergy Clin Immunol. 2003;111:308–312. doi: 10.1067/mai.2003.65. [DOI] [PubMed] [Google Scholar]
- 7.Oosterhoff Y, Kauffman HF, Rutgers B, Zijlstra FJ, Koëter GH, Postma DS. Inflammatory cell number and mediators in bronchoalveolar lavage fluid and peripheral blood in subjects with asthma with increased nocturnal airways narrowing. J Allergy Clin Immunol. 1995;96:219–229. doi: 10.1016/s0091-6749(95)70011-0. [DOI] [PubMed] [Google Scholar]
- 8.Jarjour NN, Busse WW, Calhoun WJ. Enhanced production of oxygen radicals in nocturnal asthma. Am Rev Respir Dis. 1992;146:905–911. doi: 10.1164/ajrccm/146.4.905. [DOI] [PubMed] [Google Scholar]
- 9.Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154:1505–1510. doi: 10.1164/ajrccm.154.5.8912772. [DOI] [PubMed] [Google Scholar]