Abstract
The use of new psychoactive substituted 2,5-dimethoxy-N-benzylphenethylamines is associated with abuse and toxicity in the United States and elsewhere and their pharmacology is not well known. This study compares the mechanisms of action of 2(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe), 2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25E-NBOMe), 2-(2,5dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25H-NBOMe), 2-(((4-iodo-2,5dimethoxyphenethyl)amino)methyl)phenol (25I-NBOH); and 2-(2,5-dimethoxy-4-nitrophenyl)-N(2-methoxybenzyl)ethanamine) (25N-NBOMe) with hallucinogens and stimulants. Mammalian cells heterologously expressing 5-HT1A, 5-HT2A, 5-HT2B or 5-HT2C receptors, or dopamine, serotonin or norepinephrine transporters (DAT, SERT and NET, respectively) were used to assess drug affinities at radioligand binding sites. Potencies and efficacies were determined using [35S]GTPγS binding assays (5-HT1A), inositol-phosphate accumulation assays (5-HT2A, 5-HT2B and 5-HT2C), and uptake and release assays (transporters). The substituted phenethylamines were very low potency and low efficacy agonists at the 5-HT1A receptor. 25D-NBOMe, 25E-NBOMe, 25HNBOMe, 25I-NBOH and 25N-NBOMe had very high affinity for, and full efficacy at, 5HT2A and 5-HT2C receptors. In the 5-HT2A receptor functional assay, 25D-NBOMe, 25ENBOMe, 25I-NBOH and 25N-NBOMe had subnanomolar to low nanomolar potencies similar to (+)lysergic acid diethylamide (LSD) while 25H-NBOMe had lower potency, similar to serotonin. At the 5-HT2C receptor, four had very high potencies, similar to LSD and serotonin, while 25H-NBOMe had lower potency. At the 5-HT2B receptor, the compounds had lower affinity, potency and efficacy compared to 5-HT2A or 5-HT2C. The phenethylamines had low to mid micromolar affinities and potencies at the transporters.
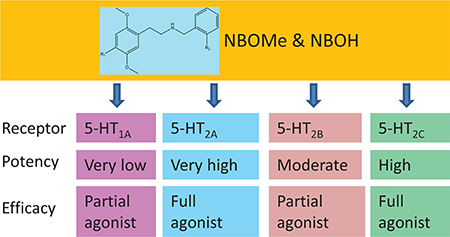
These results demonstrate that these –NBOMe and –NBOH substituted phenethylamines have a biochemical pharmacology consistent with hallucinogenic activity, with little psychostimulant activity.
Keywords: Substituted phenethylamine, Serotonin receptor, Lysergic acid diethylamide (LSD), NBOMe, Drug abuse
Graphical Abstract
1. Introduction
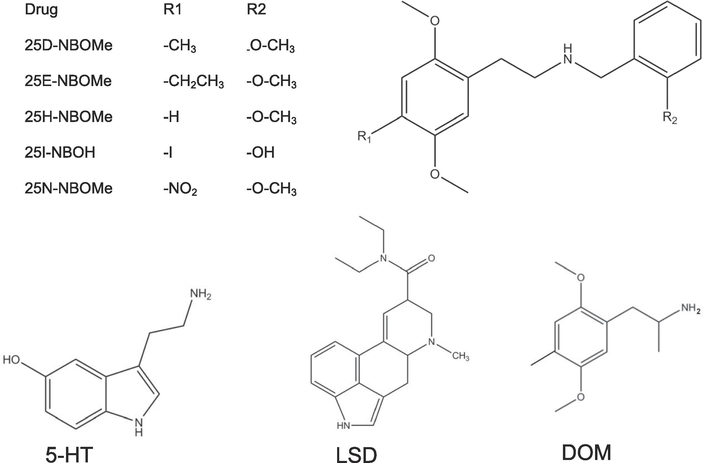
New psychoactive substances, including psychedelic substituted phenethylamines, are public health and regulatory challenges [1]. Substituted phenethylamines, similar in structure to mescaline, were found in drug seizures in the European Union [2]. The United States Drug Enforcement Administration (DEA) has categorized some hallucinogenic compounds, including the substituted phenethylamines 2,5-dimethoxy-4- methylphenethylamine (2C-D), and 2,5-dimethoxy-4-ethylphenethylamine (2C-E) as Schedule 1 substances, i.e., having no therapeutic use and having high potential for abuse and adverse health effects [3]. Recently developed N-benzylmethoxy (NBOMe) compounds are derivatives of the 2C-X family of phenethylamine hallucinogens (see Figure 1) [4;5]. The drugs are administered orally or sublingually/buccally, available via the internet and known as “n-bomb” [4]. There are several case reports of abuse of 25BNBOMe, 25C-NBOMe, and 25I-NBOMe and their harmful effects include prolonged agitation, hallucinations, seizures, rhabdomyolysis, acute kidney injury and death [6–10]. In animal studies, these three NBOMe compounds substituted for the discriminative stimulus effects of the hallucinogen DOM [11] and were recently categorized as Schedule 1 compounds [12]. There is much less information available regarding other NBOMe compounds although anectodal evidence indicates that many are psychoactive and hallucinogenic. 25D-NBOMe, 25E-NBOMe and 25H-NBOMe (Fig 1) have been detected on commercially available blotter paper [13;14] and the latter was detected in postmortem blood and urine [15]. 25I-NBOH (Fig 1) has been found on blotter paper [16] and is an N-hydroxybenzyl derivative of the hallucinogen 2C-I, the demethylated analog of 25I-NBOMe.
Figure 1.
Chemical structures of 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, 25N-NBOMe, 5-HT, LSD and DOM.
Agonist activation of 5-HT2A receptors is essential for hallucinogenic activity of serotonergic compounds such as LSD and (-)2,5-dimethoxy-4-methylamphetamine (DOM) [17–19]. Conformational changes of 5-HT2A receptors induced by the binding of LSD are critical for its time course [20]. For the substituted phenethylamine 2,5dimethoxy-4-propylthiophenethylamine (2C-T-7), antagonists of 5-HT2A receptors can decrease drug-induced head twitch behavior in mice and its ability to substitute for the discriminative stimulus properties of LSD in rats [21]. The psychoactive dimethoxyphenethylamine series of compounds, including 2C-D, 2C-E, 2C-I and 2C-T-2 [22] and others, have high affinity and potency at the 5-HT2A receptor and many of these compounds also bind and have high potency agonist activity at additional 5-HT receptors [23;24]. Addition of the NBOMe substituent increases the affinity for the 5HT2A receptor [5;24–26]. The potential interaction of these drugs with the 5-HT2B receptor is of concern, as prolonged activation by agonists at this receptor can cause cardiac valvulopathy [27;28]. The role of the 5-HT2C receptor activation in the psychoactivity of hallucinogens is less well understood (Reviewed in [19]).
The hallucinogenic activity of the NBOMe and NBOH series is indicated by animal studies. A structurally similar compound, 2-([2-(r-cyano-2,5dimethoxyphenyl)ethylamine]methyl)phenyl (25CN-NBOH) is a 5-HT2A receptor agonist, causes head twitches in mice that are inhibited by a 5-HT2A antagonist and substitutes partially for the discriminative stimulus of 2,5-dimethoxy-4-iodoamphetamine (DOI) [5;29]. 25I-NBOMe is more potent at inducing head twitches than the parent compound 2C-I [30]. Derivatives of 25I-NBOMe have high affinity for 5-HT2A and 5-HT2c receptors as determined using [125I]DOI, and 5-HT2B receptor using [3H]LSD and have very low affinity for other 5-HT receptors [31]. 25D-NBOMe, 25E-NBOMe, 25H-NBOMe and 25N-NBOMe have micromolar affinity for 5-HT1A receptors, rank order of affinity of 5-HT2A >5HT2C >5-HT2B receptors, mid-nanomolar potency but low efficacy at the 5-HT2A receptor as measured by Ca2+ mobilization assays and have low-mid micromolar affinities for the dopamine, serotonin and norepinephrine transporters (DAT, SERT and NET, respectively)[24]. However, the latter report involved the use of antagonist ligands at the 5-HT2A and 5-HT2C receptors and an agonist ligand at the 5-HT1A receptor.
The goal of this research was to characterize the pharmacological activity of 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH and 25N-NBOMe at relevant receptors and transporters to aid in the determination of abuse potential and DEA scheduling decisions. We now report results of experiments characterizing the interactions of NBOMe analogues with agonist ligand binding sites and signal transduction across four 5-HT receptors. The specific aims were to 1) determine the affinities of four NBOMe compounds and 25I-NBOH for the 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors using agonist radioligands, as well as affinities for the DAT, SERT and NET, 2) determine the potencies and efficacies of the compounds as agonists in functional assays for the 5HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors, and 3) determine the potencies and efficacies of the compounds in uptake and release assays with DAT, SERT and NET.
2. Materials and Methods
2.1. Drugs
25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH and 25N-NBOMe were purchased from Cayman Chemicals (Ann Arbor, MI). (+)LSD(-)tartrate, (-)DOM, (-)cocaine, and S(+)METH were provided by the National Institute on Drug Abuse Drug Supply Program (Rockville, MD). [3H]8-OH-DPAT, [125I]2,5-dimethoxy-4-iodoamphetamine (DOI), [125I]RTI-55, [3H]DA, [3H]5-HT, [3H]NE and [35S]GTPγS were purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA). The IP-1 Elisa kit was purchased from Cisbio (Bedford, MA). Other reagents were purchased from Sigma (St. Louis, MO).
2.2. 5-HT1A receptor: Radioligand binding
Human embryonic kidney cells expressing the human 5-HT1A receptor (HEK-5-HT1A, passage numbers 10, 14, 15 and 20) were used. The methods for transfection of HEK cells, cell membrane preparation, and [3H]8-OH-DPAT agonist binding have been described previously [32]. The density and affinity of [3H]8-OH-DPAT binding sites was 1670 fmol/mg protein and 5.0 nM. Briefly, the binding reaction mixture contained test compound, cell homogenate (0.05 mg of protein) and [3H]8-OH-DPAT (0.5 nM final concentration) in a final volume of 1 ml (assay buffer: 25 mM Tris-HCl, pH 7.4, containing 1 mM ascorbic acid and 10 μM pargyline) and was incubated for 1h. Nonspecific binding was determined with 1 μM dihydroergotamine. The reaction was terminated by filtration through polyethylenimine-soaked “A” filtermats on a Tomtec 96well cell harvester (Tomtec, Hamden, CT) and radioactivity was counted on a Perkin Elmer (Boston, MA) microbeta scintillation counter.
2.3. 5-HT1A receptor: [35S]GTPγS binding
The method for [35S]GTPγS binding has been described [32]. In brief, cell membranes (40–75 μg protein) were preincubated (10 min, room temperature) with test compound in duplicate in assay buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2, 100 mM NaCl, and 0.2 mM dithiothreitol). The reaction was initiated by addition of GDP (3 μM) and [35S]GTPγS (~150,000 cpm, 1350 Ci/mmol) in a final volume of 1 ml. The reaction was incubated for 1h at 25oC and terminated as described above. Agonist efficacy is expressed relative to that of 100 nM 5-HT, which was determined for each experiment.
2.4. 5-HT2A and 5-HT2C Receptors: [125I]DOI binding
[125I]DOI binding to 5-HT2A and 5-HT2C receptors was tested in HEK-293 cells expressing either the human 5-HT2A receptor (HEK-5-HT2A cells, passage numbers 1718) or the human 5-HT2C receptor (HEK-5-HT2C cells, passage number 11) adapting methods described earlier [23;32]. The density and affinity of [125I]DOI binding sites were 612 and 900 fmol/mg protein and 3.62 and 4.18 nM for h5-HT2A and h5-HT2C receptors, respectively. Briefly, the binding reaction mixture contained test compound, cell homogenate and [125I]DOI (0.05 nM final concentration) in a final volume of 250 μl (assay buffer: 50 mM Tris-HCl, pH 7.4, containing 5 mM ascorbic acid, 5 mM CaCl2, 10 μM pargyline). The assay was incubated for 1h at 37oC and terminated as described above. Nonspecific binding was determined with 10 μM serotonin.
2.5. 5-HT2B Receptor: [3H]5-HT binding
[3H]5-HT binding to 5-HT2B receptors was tested in HEK-293 cells stably expressing the human 5-HT2B receptor (HEK-5-HT2B cells, passage numbers 7–11) adapting methods described earlier for [125I]DOI binding to 5-HT2A and 5-HT2C receptors [23;32]. The cDNA, subcloned into the mammalian expression vector pCMV6-AC, was purchased from Origene (Rockville, MD). The density and affinity of [3H]5-HT binding sites were 1,910 ± 240 fmol/mg protein and 3.56 ± 0.19 nM. Briefly, the binding reaction mixture contained test compound, cell homogenate and [3H]5-HT (3–4 nM final concentration) in a final volume of 250 μl (assay buffer: 50 mM Tris-HCl, pH 7.4, containing 5 mM ascorbic acid, 5 mM CaCl2, 10 μM pargyline). The assay was incubated for 45 min at 37oC and terminated as described above. Nonspecific binding was determined with 10 μM serotonin.
2.6. 5-HT2A, 5-HT2B and 5-HT2C Receptors: Inositol monophosphate (IP-1) formation
Activation of 5-HT2A (passage numbers 9–17), 5-HT2B (passage numbers 4–8), and 5HT2C receptors (passage numbers 7–10) was tested by measuring the accumulation of inositol monophosphate using the Cisbio IP-1 Elisa kit as described previously [23;32]. Briefly, cells were plated at a density of 400,000 cells per well in 24 well plates. The next day, cells were starved with DMEM for 1 h, medium was removed, and stimulation buffer was added. After 10 min incubation, agonists were added and plates were incubated for 60 min. Cells were lysed, and 50 μl aliquots of the lysates were added to the IP-1 plate. The assay was conducted according to kit instructions. Stimulated IP-1 formation was normalized to the maximal effect of 5-HT, which was determined in each assay.
2.6. Biogenic amine transporters: Inhibition of [125I]RTI-55 binding to, and [3H]neurotransmitter uptake by, hDAT, hSERT or hNET in Clonal Cells
The methods for characterizing radioligand binding and functional uptake assays have been described previously [33]. Human embryonic kidney (HEK-293) cells expressing the recombinant hDAT (HEK-hDAT, passage numbers 7,9,10,17,18,28), hSERT (HEKhSERT, passage numbers 11, 15,16,18,20,26) or hNET (HEK-hNET, generous gift from Dr. Randy Blakely, Florida Atlantic University, passage numbers 9,19–23) were used. The density and affinity of [125I]RTI-55 binding sites was 7.9, 0.85, and 3.6 pmol/mg protein and 1.83, 0.98, and 12.1 nM for DAT, SERT and NET, respectively [33]. Binding assays were conducted with a total particulate membrane preparation. The uptake assay was conducted in duplicate and initiated by the addition of [3H]DA, [3H]5-HT, or [3H]NE (20 nM final concentration) to intact detached cells.
2.7. Biogenic amine transporters: [3H]Neurotransmitter release
The methods for characterizing drug-induced release of pre-loaded [3H]neurotransmitter from HEK-hDAT (passage numbers 24,26,27), HEK-hSERT (passage numbers 13–19) and HEK-hNET (passage numbers 18,19,22–25) cells have been described previously [32]. In brief, cells were loaded with [3H]neurotransmitter, centrifuged, resuspended in Krebs HEPES buffer (pH 7.4; 122 mM NaCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 10 μM pargyline, 100 μM tropolone, 0.2% glucose and 0.02% ascorbic acid, buffered with 25 mM HEPES), and added to the superfusion device (Brandel, Gaithersburg, MD). Buffer was perfused for 12–15 min, and the last 6 min (3 fractions) were collected for baseline. Drug was added, and 11 × 2 min fractions of effluent were collected. SDS (1%) was then perfused, and 4 × 2.5 min fractions were collected. Data were normalized to the maximal effects of the positive control METH. Radioactivity in the samples was determined using conventional liquid scintillation spectrometry. Fractional release was the amount of radioactivity in a fraction divided by the total radioactivity remaining in the sample.
2.8. Data analysis
For competition binding assay results, data were normalized to the specific binding in the absence of drug. Three or more independent competition experiments were conducted with duplicate determinations. GraphPAD Prism (La Jolla, CA) was used to analyze the ensuing data, with IC50 values converted to Ki values using the Cheng-Prusoff equation [34]. For signal transduction assays, GraphPAD Prism was used to calculate EC50 values using data expressed as % 5-HT-stimulation for 5-HT1A-stimulated [35S]GTPγS binding and 5-HT2A-, 5-HT2B- and 5-HT2C-receptor-mediated IP-1 formation and for % total specific [3H]neurotransmitter uptake for transporters. For [3H]neurotransmitter release assays, area under the curve (AUC) for fractional release in the absence or presence of test compound over time was calculated using GraphPad Prism, and EC50 values were determined using logarithms of drug concentrations and sigmoidal dose-response nonlinear regression. Differences in affinities, potencies or efficacies were assessed by one way ANOVA using the logarithms of the Ki or EC50 values for test compounds and standards. Statistical significance was set at p<0.05. Dunnett’s multiple comparison test was used to compare NBOMe and NBOH compounds to a drug standard. GraphPad Prism was used to calculate the Spearman correlation coefficient for the affinities and potencies at each 5-HT receptor using the logarithms of the Ki and EC50 values.
3. Results
3.1. 5-HT1A receptors
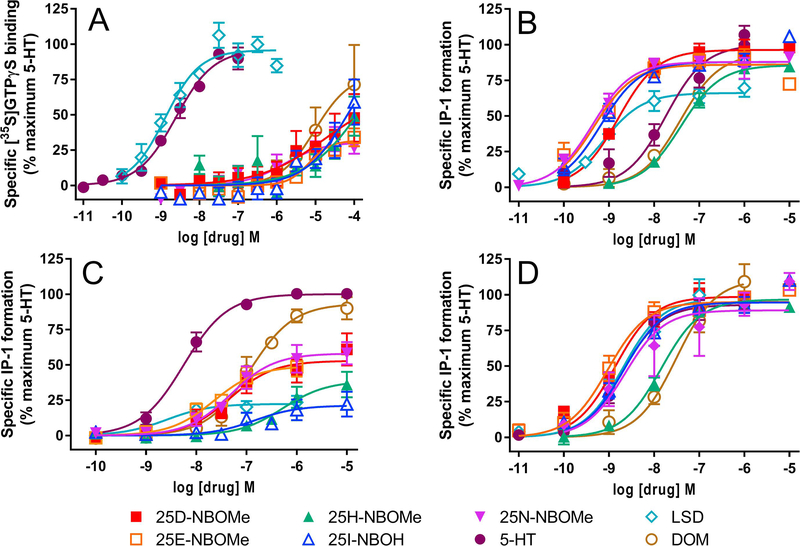
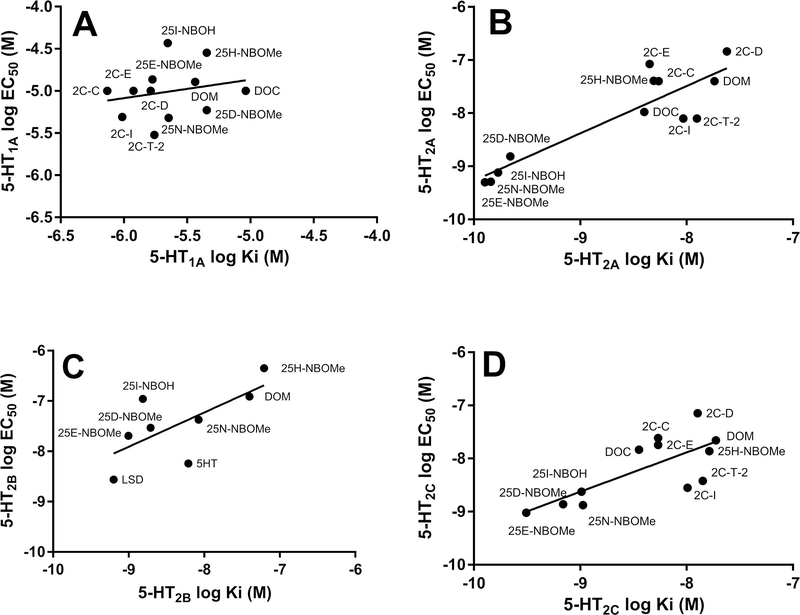
At the recombinant 5-HT1A receptor, 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25INBOH, 25N-NBOMe and DOM had lower affinities compared to 5-HT (ps<0.0001, one way ANOVA followed by Dunnett’s multiple comparison test) and LSD had a similar affinity to that of 5-HT (Table 1). The Ki values for the NBOMes and NBOH compounds were all in the micromolar range. Similar results were obtained in the 5-HT1A functional assay, with EC50 values for stimulating [35S]GTPγS binding in the micromolar range for 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, 25N-NBOMe and DOM, and with significantly lower potencies than 5-HT (ps<0.0001) while LSD had potency similar to that of 5-HT (Table 2, Fig 2A). 25D-NBOMe, 25E-NBOMe, and 25N-NBOMe were partial agonists with lower efficacies for stimulation of [35S]GTPγS binding compared to 5-HT (p<0.05–0.01), while the efficacies of 25H-NBOMe, 25I-NBOH, DOM and LSD were not significantly different from 5-HT (Table 2, Fig 2A). In addition, there was no correlation between affinities for the [3H]8-OH-DPAT binding site and potencies for stimulating [35S]GTPγS binding, except that both values were low (Fig 3A).
Table 1.
Affinity of substituted phenethylamines and other compounds for recombinant 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors.
| Inhibition of radioligand binding Ki mean ± sem (nM) (n) | Selectivity (binding ratios) | ||||||
|---|---|---|---|---|---|---|---|
| Drug | 5-HT1A [3H]8OH-DPAT | 5-HT2A [125I]DOI | 5-HT2B [3H]5-HT | 5-HT2C [125I]DOI | 5-HT2A : 5-HT1A | 5-HT2A : 5-HT2B | 5-HT2A : 5-HT2C |
| 25D-NBOMe | 4510 ± 730 (3) | 0.22 ± 0.026 (5) | 2.05 ± 0.49 (3) | 0.69 ± 0.02 (4) | 20500 | 9.3 | 3.1 |
| 25E-NBOMe | 1680 ± 350 (3) | 0.127 ± 0.019 (5) | 1.11 ± 0.32 (3) | 0.311 ± 0.071 (4) | 13200 | 8.7 | 2.4 |
| 25H-NBOMe | 4520 ± 3+0 (3) | 4.9 ± 1.3 (5) | 62.9 ± 7.5 (3) | 16.4 ± 5.0 (6) | 920 | 13 | 3.3 |
| 25I-NBOH | 2220 ± 590 (3) | 0.169 ± 0.013 (5) | 1.91 ± 0.48 (6) | 1.03 ± 0.24 (4) | 13100 | 11 | 6.1 |
| 25N-NBOMe | 2260 ± 230 (3) | 0.144 ± 0.042 (5) | 8.7 ± 1.6 (3) | 1.06 ± 0.35 (4) | 15700 | 60 | 7.4 |
| 5-HT | 1.71 ± 0.24 (4) | 16.8 ± 2.2 (3) | 6.31 ± 0.94 (4) | 2.52 ± 0.45 (7) | 0.10 | 0.38 | 0.15 |
| LSD | 1.22 ± 0.23 (4) | 0.091 ± 0.044 (3) | 0.57 ± 0.14 (3) | 1.60 ± 0.27 (4) | 13.4 | 6.3 | 17.6 |
| DOM | 3660 ± 470 (4) | 18.3 ± 1.7 (4) | 41.0 ± 5.9 (3) | 18.9 ± 2.4 (4) | 200 | 2.2 | 1.0 |
(n) Number of independent experiments conducted in duplicate.
Binding Hill slopes for h5-HT1A [3H]8-OH-DPAT ranged from -0.58 to -0.90, for h5-HT2A [125I]DOI ranged from -0.32 to -1.78, for h5-HT2B [3H]5-HT ranged from -0.87 to -1.17 and for h5-HT2C [125I]DOI ranged from -0.58 to -0.96.
Table 2.
Potency and efficacy of substituted phenethylamines and other compounds at recombinant 5-HT1A, 5-HT2A and 5-HT2C receptors.
| Drug | EC50 mean ± sem (nM) (n) % maximum stimulation ± sem* | Selectivity (functional ratios) | |||||
|---|---|---|---|---|---|---|---|
| 5-HT1A Stimulation of [35S]GTPγS binding | 5-HT2A Stimulation of IP-1 formation | 5-HT2B Stimulation of IP-1 formation | 5-HT2C Stimulation of IP-1 formation | 5-HT2A: 5-HT1A | 5-HT2A: 5-HT2B | 5-HT2A: 5-HT2C | |
| 25D-NBOMe | 5900 ± 1900 (4) 55 ± 20% | 1.53 ± 0.10 (3) 95.1 ± 6.8% | 32.3 ± 6.8 (4) 47.7 ± 8.1% | 1.37 ± 0.36 (3) 96.1 ± 2.5% | 3900 | 21 | 0.90 |
| 25E-NBOMe | 13700 ± 5600 (6) 38.0 ± 6.2% | 0.50 ± 0.14 (4) 87.1 ± 6.6% | 23.5 ± 6.0 (5) 49.4 ± 6.8% | 0.95 ± 0.18 (3) 91.8 ± 4.4% | 27,000 | 47 | 1.9 |
| 25H-NBOMe | 28400 ± 8600 (4) 52 ± 14% | 40.7 ± 8.1 (4) 85.9 ± 1.7% | 463 ± 71 (4) 37.7 ± 9.0% | 13.8 ± 2.6 (3) 95.5 ± 3.0% | 700 | 11 | 0.34 |
| 25I-NBOH | 37000 ± 13000 (3) 74 ± 18% | 0.76 ± 0.26 (4) 87.5 ± 4.1% | 111 ± 13 (4) 21.3 ± 8.3% | 2.38 ± 0.72 (3) 94.0 ± 4.0% | 49,000 | 146 | 3.1 |
| 25N-NBOMe | 4800 ± 1800 (5) 35.6 ± 6.6% | 0.51 ± 0.19 (3) 87.9 ± 3.4% | 47 ± 11 (4) 57.6 ± 8.6% | 1.32 ± 0.27 (3) 99.4 ± 0.4% | 9400 | 92 | 2.6 |
| 5-HT | 2.88 ± 0.61 (6) 94.7 ± 3.6% | 19.4 ± 3.7 (8) 98.7 ± 2.7% | 6.3 ± 1.2 (5) 100.2 ± 2.8% | 2.8 ± 1.0 (6) 98.9 ± 3.3% | 0.15 | 0.32 | 0.14 |
| LSD | 1.31 ± 0.46 (3) 93.2 ± 6.4% | 0.706 ± 0.065 (3) 64.5 ± 6.3% | 3.07 ± 0.65 (6) 23.3 ± 4.9% | 1.74 ± 0.29 (3) 92.5 ± 4.8% | 1.9 | 4.3 | 2.5 |
| DOM | 12800 ± 4700 (3) 74 ± 25% | 39.9 ± 5.4 (3) 94.6 ± 4.1% | 145 ± 47 (4) 96.2 ± 3.2% | 21.9 ± 5.9 (3) 96.0 ± 4.0% | 320 | 3.6 | 0.55 |
(n) Number of independent experiments conducted in duplicate.
Drug-induced stimulation is normalized to the maximal stimulation by 5-HT.
Figure 2.
Agonist activity of -NBOMe phenethylamines at recombinant 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors. All data were normalized to the maximal effect of 5-HT, which was measured on each experimental day. A. 5-HT1A [35S]GTPγS binding. N=3–7 independent experiments conducted with duplicate determinations. B. 5-HT2A agonist IP-1 assay. N=3–4 independent experiments conducted with duplicate determinations. C. 5-HT2B IP-1 assay. N=4–6 independent experiments conducted with duplicate determinations. D. 5-HT2C IP-1 assay. N=3–4 independent experiments conducted with duplicate determinations. Data shown are mean ± sem.
Figure 3.
Correlation of affinities and agonist potencies of substituted phenethylamines at 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors. The linear regression for the data in each graph is shown. A. 5-HT1A affinities as measured with [3H]8-OH-DPAT binding vs 5-HT1A potencies as measured using [35S]GTPγS binding. Spearman r=0.19, p>0.05. B. 5-HT2A affinities as measured with [125I]DOI binding vs 5-HT2A potencies as measured using the IP-1 assay. Spearman r=0.72, p=0.01. C. 5-HT2B affinities as measured with [3H]5-HT binding vs 5-HT2B potencies as measured using the IP-1 assay. Spearman r=0.76, p<0.05. D. 5-HT2C affinities as measured with [125I]DOI binding vs 5-HT2C potencies as measured with IP-1 assay. Spearman r=0.67, p<0.05. Values for 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2 and DOC are from [23]
3.2. 5-HT2A receptors
Results were very different with the recombinant 5-HT2A compared to the 5-HT1A receptor. Using the agonist [125I]DOI as the radioligand in the binding assay, affinities of 25D-NBOMe, 25E-NBOMe, 25I-NBOH, 25N-NBOMe and LSD were all higher than that of 5-HT, with subnanomolar Ki values (ps<0.0001, one way ANOVA Dunnett’s multiple comparison test, Table 1). 25H-NBOMe and DOM had Ki values that were similar to 5HT Ki values, which were in the low nanomolar range. In the IP-1 functional assay, 25D-NBOMe, 25E-NBOMe, 25I-NBOH, 25N-NBOMe and LSD had higher potencies than 5-HT (ps<0.01–0.0001) at the 5-HT2A receptor, with EC50 values ranging from 0.511.5 nM (Table 2, Fig 2B). 25H-NBOMe and DOM had potencies similar to 5-HT, with EC50 values of about 40 nM. All compounds except LSD (64.5%) were full agonists at the 5-HT2A receptor, with efficacies ranging from 85.9–95.1% of the maximal stimulation by 5-HT. There was an excellent correlation between affinities for the 5-HT2A [125I]DOI binding site and potencies in the IP-1 signal transduction assay (Fig 3B).
3.3. 5-HT2B receptors
Using the agonist [3H]5-HT as the radioligand in binding assays, 25D-NBOMe, 25E-NBOMe, 25I-NBOH and LSD had higher affinities than 5-HT with Ki values of 2.05, 1.11, 1.91 and 0.57 respectively (ps<0.05–0.001). 25N-NBOMe had similar affinity to that of 5-HT. 25H-NBOMe and DOM had lower affinities than 5-HT (ps<0.001).
However, in the 5-HT2B receptor IP-1 functional assay, 25D-NBOMe, 25E-NBOMe, 25I-NBOH, 25N-NBOMe and DOM had lower potencies than 5-HT (ps<0.01–0.0001), with EC50 values ranging from 23.5–463 nM (Table 2, Fig 2C). LSD and 5-HT had similar, low nanomolar potencies. In addition, only 5-HT and DOM were full agonists at the 5-HT2B receptor. The rest of the compounds had efficacies ranging from 21.3–57.6% of the maximal stimulation by 5-HT (Table 2). There was good correlation between affinities for the 5-HT2B [3H]5-HT binding site and potencies in the IP-1 functional assay (Fig 3C).
3.4. 5-HT2C receptors
At the recombinant 5-HT2C receptor, using the agonist [125I]DOI as the radioligand in the binding assay, the affinities of 25D-NBOMe and 25E-NBOMe were higher than that of 5HT, with subnanomolar Ki values (ps<0.05–0.001, one way ANOVA Dunnett’s multiple comparison test, Table 1). 25I-NBOH, 25N-NBOMe and LSD had affinities similar to the affinity of 5-HT, with Ki values in the low nanomolar range. 25H-NBOMe and DOM had lower affinities than 5-HT, with Ki values of 16–19 nM (ps<0.001). In the IP-1 functional assay, 25H-NBOMe and DOM had lower potencies than 5-HT (ps<0.001) with EC50 values of 13.8 and 21.9 nM (Table 2). 25D-NBOMe, 25E-NBOMe, 25INBOH, 25N-NBOMe and LSD had similar potencies for stimulation of IP-1 formation compared to 5-HT, with EC50 values ranging from 0.95–2.38 nM (Table 2, Fig 2D). All compounds were full agonists at the 5-HT2C receptor. There was good correlation between affinities for the 5-HT2C [125I]DOI binding site and the potencies of drugs in the IP-1 functional assay (Fig 3D).
3.5. DAT, SERT and NET: inhibition of [125I]RTI-55 binding and [3H]neurotransmitter uptake
3.5.1. DAT
25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, and 25N-NBOMe had very low affinities for the radioligand binding site on the DAT, with values ranging from 8.5–81.4 μM (Table 3). LSD and DOM had no measurable affinity, and the uptake blockers, cocaine and mazindol, had mid- and low-nanomolar Ki values. Similar results were seen for inhibition of [3H]DA uptake, with IC50 values for the five compounds being higher than the Ki values. Again there was no measurable effect of LSD and DOM on uptake, while cocaine and mazindol had mid- and low-nanomolar potencies, respectively.
Table 3.
Affinity and potency of substituted phenethylamines and other compounds at recombinant hDAT, hSERT and hNET.
| Inhibition of [125I]RTI-55 binding Ki mean ± sem (μM) (n) | Inhibition of [3H]neurotransmitter uptake IC50 mean ± sem (μM) (n) | |||||
|---|---|---|---|---|---|---|
| Drug | hDAT | hSERT | hNET | hDAT [3H]DA | hSERT [3H]5-HT | hNET [3H]NE |
| 25D-NBOMe | 34.5 ± 4.2 (3) | 1.78 ± 0.28 (3) | 6.7 ± 1.3 (4) | >85 μM (3) | 1.024 ± 0.078 (3) | 1.17 ± 0.14 (3) |
| 25E-NBOMe | 19.6 ± 1.2 (3) | 1.59 ± 0.12 (4) | 5.4 ± 1.0 (3) | 34 ± 10 (5) | 1.44 ± 0.14 (3) | 2.31 ± 0.70 (3) |
| 25H-NBOMe | 81.4 ± 8.9 (4) | 2.22 ± 0.34 (3) | 16.3 ± 2.6 (4) | >100 μM (2) | 2.08 ± 0.31 (3) | 3.65 ± 0.88 (4) |
| 25I-NBOH | 8.50 ± 0.38 (3) | 1.22 ± 0.21 (3) | 4.06 ± 0.65 (3) | 30.7 ± 5.0 (3) | 1.72 ± 0.25 (3) | 0.629 ± 0.093 (4) |
| 25N-NBOMe | 37.9 ± 4.6 (3) | 5.81 ± 0.90 (3) | 11.3 ± 3.5 (3) | >100 μM (2) | 5.79 ± 0.53 (3) | 15.0 ± 3.8 (4) |
| 5-HT | 375 ± 83 (3) | 2.33 ± 0.52 (4) | 392 ± 10 (4) | 3.36 ± 0.32 (4) | 0.192 ± 0.049 (3) | 7.2 ± 2.5 (6) |
| LSD | >100 μM (2) | 62.9 ± 5.0 (4) | 76.9 ± 8.5 (4) | >100 μM (2) | >90 μM (3) | >100 μM (3) |
| DOM | >100 μM (2) | >100 μM (4) | >100 μM (2) | >100 μM (2) | 64 ± 12 (4) | >70 μM (2) |
| Cocaine | 0.595 ± 0.059 (5) | 0.468 ± 0.049 (6) | 1.88 ± 0.42 (4) | 0.37 ± 0.11 (4) | 0.361 ± 0.077 (6) | 0.212 ± .013 (4) |
| Mazindol | 0.0289 ± 0.0026 (4) | 0.127 ± 0.039 (4) | 0.0116 ± 0.0035 (5) | 0.0154 ± 0.0021 (5) | 0.069 ± 0.012 (4) | 0.0011 ± 0.0003 (5) |
(n) Number of independent experiments conducted in duplicate.
Data are normalized to specific binding or specific uptake in the absence of drugs. Drugs were tested in binding assays at concentrations ranging from 1 nM to either 10 μM or 100 μM. Hill slopes for binding ranged from -0.93 to -2.39.
3.5.2. NET
25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, and 25N-NBOMe had low affinities for the [125I]RTI-55 binding site on the recombinant NET, with values ranging from 4.06–16.3 μM (Table 3). All had higher affinities for NET than for DAT. LSD and DOM had no measurable affinity, and cocaine and mazindol had low micromolar and low nanomolar Ki values, respectively. For all except 25N-NBOMe, IC50 values for inhibition of [3H]NE uptake were lower than the Ki values. 25N-NBOMe had similar values in both assays. All five compounds had higher potencies at NET compared to their potencies at DAT. Again there was no measurable effect of LSD and DOM, while cocaine and mazindol had mid- and low-nanomolar potencies, respectively.
3.5.3. SERT
25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, and 25N-NBOMe had low affinities for the [125I]RTI-55 binding site on the SERT, with values ranging from 1.22 to 5.81 μM (Table 3). All had higher affinities for SERT than for DAT or NET. LSD had mid-micromolar affinity and DOM had no measurable affinity, and cocaine and mazindol had mid-nanomolar Ki values. The –NBOMe compounds had IC50 values for inhibition of [3H]5-HT uptake that were similar to their Ki values. 25D-NBOMe, 25E-NBOMe, 25INBOH, and 25N-NBOMe had higher potencies at the recombinant SERT compared to their potencies at DAT or NET. 25H-NBOMe had a rank order of potency of NET > SERT >DAT. There was no measurable effect of LSD, while DOM had mid-micromolar and cocaine and mazindol had mid- and low-nanomolar potencies, respectively.
3.6. [3H]Neurotransmitter Release via DAT, SERT or NET
Assays measuring release of preloaded [3H]neurotransmitter from cells or other tissue preparations have been used to determine if a compound is a transporter substrate [35]. 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, 25N-NBOMe, LSD and DOM had little to no efficacy at inducing release of preloaded [3H]neurotransmitter from recombinant DAT, SERT or NET, tested at concentrations from 10 nM – 100 μM (Table 4). Thus, even though the compounds were efficacious at inhibition of uptake at 1–5 μM at SERT (Table 3), they did not induce release, and thus are blockers, not substrates, at SERT. METH was efficacious at all three transporters, with potencies that agree with previous reports [35].
Table 4.
Potency and efficacy of substituted phenethylamines and other compounds to release preloaded [3H]neurotransmitter from HEK-hDAT, HEK-hSERT and HEK-hNET cells.
| Drug | Drug-induced release of [3H]neurotransmitter | ||
|---|---|---|---|
| EC50 mean ± sem (μM) (n) | |||
| % maximum release ± sem* | |||
| hDAT [3H]DA | hSERT [3H]5-HT | hNET [3H]NE | |
| 25D-NBOMe | >100 μM (2) | >100 μM (2) | >33 μM (3) |
| 0.75 ± 0.48% | 0.59 ± 0.24% | 14.3 ± 8.5% | |
| 25E-NBOMe | >85 μM (3) | >100 μM (2) | >66 μM (3) |
| 5.8 ± 6.4% | 0.21 ± 0.77% | 17.1 ± 5.9% | |
| 25H-NBOMe | >100 μM (2) | >100 μM (2) | >66 μM (3) |
| −0.18 ± 0.26% | −0.27 ± 0.49% | 21 ± 12% | |
| 25I-NBOH | >100 μM (2) | >100 μM (2) | >100 μM (2) |
| −0.56 ± 0.35% | −0.27 ± 0.40% | 16 ± 16% | |
| 25N-NBOMe | >100 μM (2) | >100 μM (2) | >38 μM (3) |
| −0.89 ± 0.91% | −0.25 ± 0.54% | 25 ± 15% | |
| LSD | >54 μM (4) | >100 μM (2) | >91 μM (3) |
| 7.4 ± 4.7% | −0.59 ± 0.55% | 35.7 ± 4.2% | |
| DOM | >100 μM (2) | >100 μM (2) | >42 μM (4) |
| −0.20 ± 0.16% | 2.9 ± 3.4% | 25.5 ± 2.0% | |
| METH | 0.43 ± 0.15 (5) | 27.5 ± 7.0 (4) | 0.152 ± 0.056 (3) |
| 104.1 ± 1.3 | 104.9 ± 6.8% | 106.7 ± 3.3% | |
(n) Number of independent experiments.
Maximum release is defined as the maximum release (maximal AUC) induced by METH (1–10 μM, hDAT; 0.3–1 mM, hSERT; 0.3–1 μM, hNET) for each experiment.
4. Discussion
25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, and 25N-NBOMe had very high affinities for the 5-HT2A receptor, in the subnanomolar range, except for 25H-NBOMe, which had low nanomolar affinity. These affinities are higher (3–5 fold) than those reported [24] using an antagonist radioligand. This difference is consistent with [26], in that many substituted phenethylamines in the 25H and 25I families show lower affinity for the 5-HT2A receptor when displacing an antagonist compared to displacement of agonist radioligand. In 5-HT2A functional assays, these compounds had very high potency at inducing the accumulation of inositol phosphates (IP-1 assay). These EC50 values were 12–320 times lower (higher potency) than those reported by [24], which were measured using a Ca2+ mobilization assay. For 25H-NBOMe and 25I-NBOH, Braden et al. [26] reports EC50 values in a radiolabeled inositol phosphate accumulation assay similar to or about three fold lower than the values reported herein. In addition, we observed full functional efficacy for the five compounds, ranging from 86–95% of maximal serotonin effect, in agreement with [26] and much higher efficacy than in the Ca2+ mobilization assay [24]. Thus these compounds may be of high concern when considering the possibility of overdose and adverse effects resulting from high potency and full efficacy at the 5-HT2A receptor.
25D-NBOMe, 25E-NBOMe had sub-nanomolar, 25H-NBOMe had low nanomolar, and 25I-NBOH and 25N-NBOMe had about 1 nM affinity for the 5-HT2C receptor. These affinities are higher (4–23 fold) than those reported by [24] who used an antagonist radioligand. This is the first report that, in 5-HT2C functional assays, these compounds had very high potency for inducing the accumulation of inositol phosphates (IP-1 assay). In addition, we observed full functional efficacy for the five compounds, ranging from 91.8–99.4% of maximal 5-HT effect. The role of the 5-HT2C receptor in the psychoactive properties of the substituted phenethylamines is still debatable. A 5-HT2C antagonist does not, but a 5-HT2A antagonist does, modify head twitch behavior by 25CN-NBOH [29]. The affinities of 5-HT2A receptor antagonists correlate with their IC50 values for blockade of LSD and DOM as stimuli in drug discrimination studies, while the drugs’ affinities for the 5-HT2C receptor do not correlate with the behavioral data [17]. However, activation of 5-HT2C receptors can activate differentially distinct signal transduction pathways, dependent on agonist characteristics, which may contribute to psychoactive properties of these compounds [36]. For example, Canal and Murnane recently hypothesized that the non-addictive nature of many hallucinogens is due to 5-HT2C receptor activation inhibiting potassium Kv1.c channels on nucleus accumbens medium spiny neurons [37].
Hallucinogenic indoleamines depress raphe cell firing by binding with high affinity to somatodendritic 5-HT1A autoreceptors (reviewed in [19]). However, the phenethylamines have very low affinity for 5-HT1A receptors, and thus do not directly mediate this effect. In addition, although these compounds had high affinity for the 5HT2B receptor, they had 11–146 times lower potencies at activating 5-HT2B compared to 5-HT2A receptors, and only partial efficacy, which suggests that they could cause cardiac valvulopathy only with chronic and high dose use [28].
Forensic evidence indicates that relevant brain concentrations for 5-HT2A activation are reached following drug ingestion. Human blood concentrations, taken hours after ingestion, of 0.29 ng/ml 25H-NBOMe and 2.80 ng/ml 25C-NBOMe have been measured [15]. The ~0.9 nM 25H-NBOMe blood concentration is lower than its EC50 for 5-HT2A IP-1 hydrolysis (40.7 nM) but the ~8 nM 25I-NBOMe concentration is higher than its EC50 [26] and similar concentrations have been reported for 25B-NBOMe [38]. Postmortem analysis of peripheral blood and brain tissue yielded 25I-NBOMe concentrations of 0.405 ng/ml and 2.54ng/g, respectively, suggesting preferential distribution to, and accumulation in, brain tissue [39]. Thus brain concentrations sufficient to activate the 5HT2A and 5-HT2C receptors may have been attained. Serum concentrations for other compounds tested herein were not found in the literature.
Consistent with other reports [26;30;40], affinity for some 5-HT receptors increased with the N-benzyl additions to the phenethylamines. For parent compounds of 25D-NBOMe, 25E-NBOMe and 25I-NBOH at 5-HT1A, 5-HT2A and 5-HT2C receptors, using the same assay conditions, 2C-D had affinities of 1630, 23.9 and 12.7 nM; 2C-E had affinities of 1190, 4.5, and 5.4 nM; and 2C-I had affinities of 970, 9.3, 10.2 nM, respectively [23]. Comparison of these values with Ki values for the corresponding – NBOMe and –NBOH analogs (Table 1) indicates that affinities for 5-HT1A were decreased while 5-HT2A affinities were increased 35–100 times by addition of –NBOMe and –NBOH moieties, and 5-HT2C affinities were increased 10–18 times, similar to affinity shifts of other –NBOMe [41]. Thus the affinity selectivity for the 5-HT2A receptor was increased with the N-benzyl additions. Modelling of -NBOMe compounds indicates that the increase in 5-HT2A affinities is due to stabilization of the N-benzyl moiety with Phe339 in transmembrane 6 [26]. In Fig 3, the –NBOMe series is grouped to the left with high affinity and potency for both 5-HT2A and 5-HT2C receptors. Selectivity for binding affinity for 5-HT2A over 5-HT1A ranged from 920–20,500 fold across –NBOMe drugs, and between 5-HT2A and 5-HT2C ranged from 2.4–7.4 fold (Table 1). There was also a high selectivity of functional activity for the –NBOMe and –NBOH analogs for 5HT2A over 5-HT1A receptors (Table 2), while the potencies at 5-HT2A and 5-HT2C were similar.
The current results indicate that the –NBOMe and –NBOH substituted phenethylamines examined here are full, very high potency agonists at the 5-HT2A and 5-HT2C receptors. The biochemical pharmacology of these compounds is consistent with psychoactive hallucinogenic activity with minimal stimulant activity, as indicated by lower affinities and potencies at neurotransmitter (DA and NE) transporters. At high, toxic doses, these compounds may cause symptoms, including tachycardia and hypertension, that are elicited by activation of additional pathways [6].
Acknowledgements
Support
Funding for this study was provided by the Department of Justice Drug Enforcement Administration [Interagency agreement D-15-OD-0002], Veterans Affairs Merit Review and Career Scientist programs (AJ), the Methamphetamine Abuse Research Center Grant P50 DA018165 (A.J), and National Institutes of Health National Institute on Drug Abuse [Interagency agreement ADA12013 (AJ, AJE)]. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Role of funding source
DEA project officers contributed to study design and had no further role in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Abbreviations
- (25D-NBOMe)
2-(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine
- (25E-NBOMe)
2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine
- (25H-NBOMe)
2-(2,5dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine
- (25I-NBOH)
2-(((4-iodo-2,5dimethoxyphenethyl)amino)methyl)phenol
- (25N-NBOMe
2-(2,5-dimethoxy-4-nitrophenyl)-N-(2methoxybenzyl)ethanamine)
- (LSD)
(+)Lysergic acid diethylamide
- ((DOM) -)
2,5dimethoxy-4-methylamphetamine
- (DOI)
2,5-dimethoxy-4-iodoamphetamine
- (METH)
(S+)methamphetamine
- (5-HT)
Serotonin hydrochloride
- (DA)
Dopamine hydrochloride
- (NE)
Norepinephrine bistartrate
- (DAT)
Dopamine transporter
- (NET)
Norepinephrine transporter
- (SERT)
Serotonin transporter
- (2C-B)
2,5-dimethoxy-4-bromophenethylamine
- (2C-C)
2,5dimethoxy-4-chlorophenethylamine
- (2C-E)
2,5-dimethoxy-4-ethylphenethylamine
- (2C-I)
2,5dimethoxy-4-iodophenethylamine
- (2C-T-2)
2,5-dimethoxy-4-ethylthiophenethylamine
- (DOC)
2,5-dimethoxy-4-chloroamphetamine
- ([125I]RTI-55)
[125I]methyl (1R,2S,3S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- ([3H]8-OH-DPAT)
[3H]8-hydroxy-N,N-dipropyl-2aminotetralin
- ([35S]GTPγS)
[35S]guanosine triphosphate
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
Chemical compounds studied in this article
2-(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe); (PubChem CID: 118536027);
2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25E-NBOMe); (PubChem CID: 121230757);
2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25H-NBOMe); (PubChem CID: 121230760);
2-(((4-iodo-2,5-dimethoxyphenethyl)amino)methyl)phenol (25I-NBOH) (PubChem CID: 10001761);
2-(2,5-dimethoxy-4-nitrophenyl)-N-(2-methoxybenzyl)ethanamine) (25N-NBOMe) (PubChem CID: 118536028)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Griffiths P, Evans-Brown M, Sedefov R, Getting up to speed with the public health and regulatory challenges posed by new psychoactive substances in the information age. Addiction 2013; 108:1700–1703. [DOI] [PubMed] [Google Scholar]
- 2.EMCDDA. European Drug Report. European Monitoring Centre for Drugs and Drug Addiction, 2015.
- 3.DEA. Title 21 United States Code Controlled Substances Act, Part B, Section 812, Schedule 1(c) hallucinogenic substances, 2017.
- 4.Lawn W, Barratt M, Williams M, Horne A, Winstock A, The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J Psychopharmacology 2014; 28:780–788. [DOI] [PubMed] [Google Scholar]
- 5.Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Brauner-Osborne H, Kristensen JL, Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci 2014; 5:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki J, Dekker MA, Valenti ES, Arbelo Cruz FA, Correa AM, Poklis JL, Poklis A, Toxicities associated with NBOMe ingestion-a novel class of potent hallucinogens: a review of the literature. Psychosomatics 2015; 56:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isbister GK, Poklis A, Poklis JL, Grice J, Beware of blotting paper hallucinogens: severe toxicity with NBOMes. Med J Aust 2015; 203:266–267e. [DOI] [PubMed] [Google Scholar]
- 8.Laskowski LK, Elbakoush F, Calvo J, Exantus-Bernard G, Fong J, Poklis JL, Poklis A, Nelson LS, Evolution of the NBOMes: 25C- and 25B- Sold as 25I-NBOMe. J Med Toxicol 2015; 11:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood DM, Sedefov R, Cunningham A, Dargan PI, Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin Toxicol (Phila) 2015; 53:85–92. [DOI] [PubMed] [Google Scholar]
- 10.Forrester MB, NBOMe designer drug exposures reported to Texas poison centers. J Addict Dis 2014; 33:196–201. [DOI] [PubMed] [Google Scholar]
- 11.Gatch MB, Dolan SB, Forster MJ, Locomotor and discriminative stimulus effects of fournovel hallucinogens in rodents. Behav Pharmacol 2017; 28:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DEA, Schedules of Controlled Substances: Placement of Three Synthetic Phenethylamines Into Schedule I. Final rule. Fed Regist 2016; 81:66181–4. [PubMed] [Google Scholar]
- 13.Poklis JL, Raso SA, Alford KN, Poklis A, Peace MR, Analysis of 25I-NBOMe, 25B-NBOMe,25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper. J Anal Toxicol 2015; 39:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuba D and Sekula K, Analytical characterization of three hallucinogenic N-(2methoxy)benzyl derivatives of the 2C-series of phenethylamine drugs. Drug Test Anal 2013; 5:634–645. [DOI] [PubMed] [Google Scholar]
- 15.Morini L, Bernini M, Vezzoli S, Restori M, Moretti M, Crenna S, Papa P, Locatelli C, Osculati AMM, Vignali C, Groppi A, Death after 25C-NBOMe and 25H-NBOMe consumption. Forensic Sci Int 2017; 279:e1–e6. [DOI] [PubMed] [Google Scholar]
- 16.Arantes LC, Junior EF, de Souza LF, Cardoso AC, Alcantara TLF, Liao LM, Machado Y,Lordeiro RA, Neto JC, Andrade AFB, 25I-NBOH: a new potent serotonin 5-HT2A receptor agonist identified in blotter paper seizures in Brazil. Forensic Toxicol 2017; 35:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorella D, Rabin RA, Winter JC, The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl) 1995; 121:347–356. [DOI] [PubMed] [Google Scholar]
- 18.Glennon RA, Titeler M, McKenney JD, Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 1984; 35:2505–2511. [DOI] [PubMed] [Google Scholar]
- 19.Halberstadt AL and Geyer MA, Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011; 61:364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL, Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017; 168:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH, Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 2005; 181:496–503. [DOI] [PubMed] [Google Scholar]
- 22.Shulgin AT. Pihkal: a chemical love story. Berkeley, CA: Transform Press, 1991. [Google Scholar]
- 23.Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB, Behavioraland neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology (Berl) 2014;231: 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME, Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015; 99:546–553. [DOI] [PubMed] [Google Scholar]
- 25. Halberstadt AL, Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens.Curr Top Behav Neurosci 2017; 32:283–311. [DOI] [PubMed] [Google Scholar]
- 26.Braden MR, Parrish JC, Naylor JC, Nichols DE, Molecular interaction of serotonin 5-HT2Areceptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol 2006; 70:1956–1964. [DOI] [PubMed] [Google Scholar]
- 27.Elangbam CS, Drug-induced valvulopathy: an update. Toxicol Pathol 2010; 38:837–848. [DOI] [PubMed] [Google Scholar]
- 28.Roth BL, Drugs and valvular heart disease. N Engl J Med 2007; 356:6–9. [DOI] [PubMed] [Google Scholar]
- 29.Fantegrossi WE, Gray BW, Bailey JM, Smith DA, Hansen M, Kristensen JL, Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CNNBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT(2)A receptors, in mice. Psychopharmacology (Berl) 2015; 232:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halberstadt AL and Geyer MA, Effects of the hallucinogen 2,5-dimethoxy-4iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 2014; 77:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ, NBenzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues . ACS Chem Neurosci 2015; 6:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatch MB, Forster MJ, Janowsky A, Eshleman AJ, Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther 2011; 338:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A, Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther 1999; 289:877–885. [PubMed] [Google Scholar]
- 34.Cheng Y and Prusoff WH, Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction.Biochem Pharmacol 1973; 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- 35.Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A, Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 2013; 85:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chagraoui A, Thibaut F, Skiba M, Thuillez C, Bourin M, 5-HT2C receptors in psychiatric disorders: A review. Prog Neuropsychopharmacol Biol Psychiatry 2016; 66:120–135. [DOI] [PubMed] [Google Scholar]
- 37.Canal CE and Murnane KS, The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens. J Psychopharmacol 2017; 31:127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poklis JL, Nanco CR, Troendle MM, Wolf CE, Poklis A, Determination of 4-bromo-2,5dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe) in serum and urine by high performance liquid chromatography with tandem mass spectrometry in a case of severe intoxication. Drug Test Anal 2014; 6:764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poklis JL, Devers KG, Arbefeville EF, Pearson JM, Houston E, Poklis A, Postmortemdetection of 25I-NBOMe [2-(4-iodo-2,5-dimethoxyphenyl-N-[(2methoxyphenyl)methyl]ethanamide] in fluids and tissues determined by high performance liquid chromatography with tandem mass spectrometry from a traumatic death. Forensic Sci Int 2014; 234:e14–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blaazer AR, Smid P, Kruse CG, Structure-activity relationships of phenylalkylamines as agonist ligands for 5-HT(2A) receptors. ChemMedChem 2008; 3:1299–1309. [DOI] [PubMed] [Google Scholar]
- 41.Elmore JS, Decker AM, Sulima A, Rice KC, Partilla JS, Blough BE, Baumann MH, Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology; doi 10.1016/j.neuropharm.2018.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]