Abstract
Background
Prenatal care programs for women with opioid use disorder (OUD) often focus treatment/counseling plans around illicit substances, while concurrent use of alcohol might present an equal or greater risk to the fetus.
Methods
This study evaluated self-reported prevalence of alcohol use in patients participating in a comprehensive prenatal care program for women with substance use disorder (SUD; n=295), of which 95% are treated for OUD, and pregnant women being served through general obstetrical clinics at the University of New Mexico (n=365). During the screening phase of a prospective study, patients were asked to report alcohol use in the periconceptional period, and between the last menstrual period and pregnancy recognition.
Results
The screening interview was conducted at 22.3 (median=22; Q1=16; Q3=29) gestational weeks. Among patients screened at the SUD clinic, 28.8% and 24.1% reported at least one binge drinking episode in the periconceptional period and in early pregnancy, respectively. The prevalence of binge drinking was similar in the general obstetrics population (24.7% and 24.4%, respectively). Among those who reported drinking in early pregnancy, median number of binge drinking episodes was higher among patients screened at the SUD clinic (median=3; Q1=1; Q3=10) compared to the general obstetrics group (median=1; Q1=1; Q3=3; p<0.001).
Conclusions
This study demonstrates a high prevalence of prenatal alcohol use in early pregnancy in both groups, while patients with SUD/OUD consume more alcohol. These findings underscore the need for targeted screening and intervention for alcohol use in all pregnant women, especially those with SUD/OUD.
Keywords: prevalence, alcohol consumption, pregnancy, opioid use disorder
1. Introduction
Expanded use of prescription opioid analgesics and an increasing prevalence of opioid use disorder (OUD) are now widely recognized. Roughly one-third of reproductive-aged women (Ailes et al., 2015) and more than 1 in 5 Medicaid-enrolled pregnant women (Desai et al., 2014) fill a prescription for an opioid analgesic. Results from recent studies using aggregate data collected by the National Survey on Drug Use and Health from 2005–2014 reveal that 0.8% of U.S. pregnant women report past-month non-medical use of opioids (Kozhimannil et al., 2017a), and 5% of pregnant women report non-medical use of opioids during the past year (Kozhimannil et al., 2017b).
Medication-assisted treatment (MAT) with methadone or buprenorphine is the standard of care for pregnant women with OUD (Committee on Obstetric Practice, 2017). MAT during pregnancy improves adherence to prenatal care and substance abuse treatment program requirements, thereby improving overall maternal-fetal wellbeing and outcomes (American Congress of Obstetricians and Gynecologists, 2012). Thus, pregnant women on MAT may represent a unique population of women relative to those who actively use opioids non-medically during pregnancy. In 2009, 5.6 per 1,000 pregnant women who delivered in U.S. hospitals had a diagnosis related to opioid use or dependence, which was a 5-fold increase from the year 2000 (Patrick et al., 2012).
An estimated 30.3% of U.S. pregnancies are affected by prenatal alcohol exposure (PAE) at some point during pregnancy (Ethen et al., 2009), with approximately 1 in 10 pregnant women reporting alcohol use in the past month (Tan et al., 2015). These estimates, based on maternal self-report, are corroborated by our recent estimates in newborns obtained from analyzing ethanol metabolites in dry blood spot cards banked in state repositories, which estimated that on average 8.4% of dry blood spot cards were positive for phosphatidylethanol indicative of PAE within approximately one month before delivery (Bakhireva et al., 2017). A meta-analysis of 24 unique studies estimated that the global prevalence of fetal alcohol spectrum disorders (FASD) is 7.7 per 1000, while some regions and countries have a much higher prevalence, e.g., WHO European region and South Africa (19.8/1000 and 11.1/1000, respectively (Lange et al., 2017). Active case ascertainment studies among school-age children in the U.S. suggest that more than 2% of school-age children might have FASD (May et al., 2014). While FASD is associated with well-documented and lifelong physical, neurodevelopmental, and behavioral disabilities, new estimates of its alarming prevalence have received relatively little attention.
According to the National Survey on Drug Use and Health, 48% of pregnant women who report past-month, non-medical opioid use also report concurrent past-month alcohol use, with a large proportion (32%) of respondents reporting prenatal binge drinking during the same time period (Kozhimannil et al., 2017a). These alarming estimates indicate that co-occurring alcohol misuse is highly prevalent among pregnant women who use opioids prenatally without a prescription. Additionally, unintended pregnancy rates may be nearly 90% in women with SUD, compared to a 45% rate among the general U.S. population (Finer and Zolna, 2016; Heil et al., 2011). Unintended pregnancy is associated with later pregnancy recognition (Ayoola, 2015) posing an additional risk of prolonged PAE during embryogenesis in these women (Parackal et al., 2013). Despite increasing evidence of alcohol-related comorbidities among adults in treatment for SUD (Hartzler et al., 2010), alcohol consumption behaviors among pregnant women in treatment for SUD have not been well-characterized in the U.S. and will not be detected by routine urine drug screening during MAT. The purpose of this study was to compare self-reported prevalence of alcohol use in pregnant women with SUD (n=295), predominantly on MAT for OUD, and the general population of pregnant women recruited from the same medical center (n=365).
2. Material and method
2.1 Study design and population
This study summarizes prevalence estimates obtained during the screening stage of the ENRICH (Ethanol, Neurodevelopment, Infant and Child Health) prospective birth cohort study, which has been described elsewhere (Bakhireva et al., 2015). The University of New Mexico (UNM) Human Research Review Committee (HRRC) approved all study procedures. To determine eligibility for ENRICH, trained study coordinators administered a short screener containing basic demographic characteristics (age, race, ethnicity), reproductive health questions (gravidity, date of the last menstrual period [LMP], singleton/multiple pregnancy), ability to complete a longitudinal study (two-year follow-up), alcohol use in periconceptional period and early pregnancy, and tobacco use history for the past 3 years.
The recruitment procedures were conducted in six prenatal care clinics affiliated with the UNM Departments of Obstetrics and Gynecology or Family Medicine. One of those clinics is a multi-disciplinary specialty program, called Milagro, which provides comprehensive care to pregnant women with an SUD. Non-Milagro pregnant women were screened at UNM midwifery, maternal-fetal medicine, and general outpatient obstetrics clinics. Recruitment procedures were conducted by a study coordinator at the clinics. To minimize participation bias, all pregnant women attending a scheduled prenatal visit on days when a study coordinator was present at the clinic were offered screening for the study.
2.2 Assessment of alcohol consumption at the screening stage of ENRICH
Questions about alcohol use included: 1) the validated AUDIT-C questionnaire which captured alcohol use in the past 12 months (Reinert and Allen, 2007); 2) estimated binge drinking (≥4 drinks/occasion) and average alcohol consumption per week in the periconceptional period (two weeks before and two weeks after LMP); 3) any binge drinking episodes in early pregnancy; and 4) average consumption of at least 3 drinks/week in early pregnancy (yes/no). In this report, alcohol use in ‘early pregnancy’ refers to the time interval between known or estimated LMP (from the expected date of confinement (EDC) and current gestational age provided by the healthcare provider) and the self-reported timing of pregnancy recognition. Local standard clinical practices, following the American College of Obstetrics and Gynecology (ACOG) guidelines (Committee on Obstetric Practice, 2017), determined EDC and gestational age, similar to other studies in the field (Himes et al., 2015).
Between October of 2013 and August of 2017, 666 pregnant women were screened at UNM-affiliated outpatient prenatal care clinics for eligibility to participate in the ENRICH cohort study. This report focuses on 660 subjects (365 from the general obstetrics population and 295 from the Milagro clinic) who completed the alcohol use screening form. Within the two groups, participants who self-reported at least one binge drinking episode or consuming on average ≥3 drinks/week after the LMP were considered ‘binge/chronic moderate users’ in early pregnancy; remaining subjects were considered ‘low/no users.’ Prior reports indicate that women are more likely to disclose risky drinking in the periconceptional period (Jacobson et al., 2002) and there are well-documented risks to the fetus caused by binge or regular alcohol use in early pregnancy (May et al., 2013); thus, these two time intervals were chosen as a focus of our analyses. A subset of patients who went on to enroll in the longitudinal ENRICH cohort were further assessed at multiple time points in pregnancy for PAE using a battery of ethanol biomarkers and repeated prospective Timeline Follow-back interviews – an in-depth assessment method that obtains estimates of daily drinking (Bakhireva et al., 2015). In the current study sample, ninety-seven (26.6%) participants from the general obstetrics group and 74 (25.1%) patients with SUD were classified as binge/chronic moderate alcohol users. We chose to focus this report on the information collected only during the screening stage of the study, regardless of subsequent enrollment into ENRICH to maximize generalizability of the study findings and minimize the effect of ENRICH study-specific eligibility criteria (e.g., level of alcohol consumption, singleton pregnancy, residence in Albuquerque larger metropolitan area, and willingness to be prospectively followed for up to 2 years) on prevalence estimates.
2.3 Statistical analyses
Demographic characteristics and alcohol consumption patterns were compared among the two study groups (general obstetrics and patients with SUD) by t-test or Wilcoxon-Mann-Whitney rank sum test for continuous variables and chi-square or Fisher exact test for categorical variables. Since the intensity of alcohol consumption data (number of drinks per week, number of binge drinking episodes) were not normally distributed, medians and first (Q1) and third (Q3) quartiles were used to describe the data distribution. Asymptotic Wilcoxon rank-sum test was used to assess the difference in distribution between the general obstetrics and patients with SUD groups. In order to visualize these highly skewed distributions containing several extreme outliers, side-by-side box plots were produced on a logarithmic scale where variable value y was graphed at log(y + 0.5) instead of at log(y) on the scale to accommodate values of y = 0. Additionally, a multivariable linear regression was conducted to estimate the effect of the study group and demographic characteristics (ethnicity, maternal age) on the log-transformed intensity of drinking (number of drinks and binge episodes around LMP). Prevalence of tobacco use was included in the summary statistics as one of the most common co-exposures among alcohol-using pregnant women.
3. Results
The mean age of participants at the time of screening was 27.8 (range: 17, 44) years and mean gestational age was 22.3 (range: 5–39; median=22; Q1=16; Q3=29) weeks. Nearly a third (28.8%) of screened patients were primigravida and the majority were Hispanic/Latina (59.4%). As shown in Table 1, there were no differences in maternal age among women screened at the general obstetrics vs. SUD clinics (p = 0.31). The mean gestational age at screening was lower among patients with SUD compared to general obstetrics patients (21.2 vs. 23.1 weeks; p=0.001). The self-reported mean gestational age at pregnancy recognition was 6.2 weeks (5.3 weeks in the general obstetrics and 7.4 weeks in SUD patients; p<0.001). A higher proportion of pregnant women from the general obstetrics clinics were primigravida (34.5% vs. 21.8%; p < 0.001), and a higher proportion of patients with SUD were Hispanic/Latina (48.5% vs. 72.9%; p < 0.001). The prevalence of tobacco use in the last 3 years was much higher among patients with SUD (85.7%) compared to general obstetrics patients (30.7%; p<0.001).
Table 1.
Demographic Characteristics of the Pregnant Patients in the General Obstetrics Clinics and SUD Clinic (n=660).
| Characteristics | General obstetrics clinics (n=365) |
SUD clinic* (n=295) |
p-value |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | ||
| Maternal age at screening (years) | 28.0 (27.4; 28.6) | 27.6 (27.0; 28.2) | 0.31 |
| Gestational age at screening (wks) | 23.1 (22.3; 24.0) | 21.2 (20.3; 22.0) | 0.001 |
| Gestational age at pregnancy recognition (wks) | 5.3 (5.1; 5.5) | 7.4 (6.9; 8.0) | <0.001 |
| n (%) | n (%) | ||
| Primigravida | 126 (34.5) | 64 (21.8) | <0.001 |
| Race/ethnicity: | <0.001 | ||
| Hispanic | 176 (48.5) | 215 (72.9) | |
| Non-Hispanic White | 131 (36.1) | 54 (18.3) | |
| American Indian | 20 (5.5) | 13 (4.4) | |
| Other | 36 (9.9) | 13 (4.4) | |
| Tobacco use in the past 3 years | 112 (30.7) | 253 (85.8) | <0.001 |
SUD, substance use disorder
Screened at the specialty multi-disciplinary clinic for pregnant women with SUD, majority with OUD
In the general obstetrics population, no differences in the demographic characteristics were detected between binge/chronic moderate and low/no users (all p’s > 0.05; data not shown). However, a much higher proportion of binge/chronic moderate users reported having a history of tobacco use (53.6%) compared to low/no users (22.4%; p<0.001) in the general obstetrics population. Among participants obtaining prenatal care in the SUD specialty clinic, no differences in the demographic characteristics or the prevalence of tobacco use were detected between binge/chronic moderate versus low/no users (all p’s > 0.05).
A similar proportion of subjects in both groups had AUDIT-C scores ≥ 3, (41.4% in the general obstetrics sample and 37.6% among patients with SUD; p=0.33) indicative of potential hazardous alcohol use in the past year (Table 2). Approximately one-quarter of respondents (24.7% in the general obstetrics and 28.8% in the patients with SUD samples; p=0.23) reported binge drinking in the periconceptional period. Binge drinking beyond periconceptional period (representing first and second trimesters for most participants) was also prevalent with 24.4% and 24.1% of general obstetrics and patients with SUD, respectively, reporting at least one binge drinking episode (p=0.99). A somewhat smaller proportion of respondents reported chronic use of ≥ 3 drinks/week on average beyond periconceptional period (15.3% and 14.9% in the general obstetrics and patients with SUD groups, respectively; p=0.88).
Table 2.
Alcohol Use among the General Obstetrics Population and Pregnant Women with SUD Screened at the University of New Mexico (n=660).
| Variable | General obstetrics clinics (n=365) |
SUD clinic (n=295) |
p- value |
|---|---|---|---|
| Alcohol use in the past 12 months | |||
| AUDIT-C ≥3 | 151 (41.4%) | 111 (37.6%) | 0.33 |
| Alcohol use in periconceptional perioda | |||
| Any binge drinkingb | 90 (24.7%) | 85 (28.8%) | 0.23 |
| Alcohol in early pregnancyc | |||
| Any binge drinkingb | 89 (24.4%) | 72 (24.1%) | 0.99 |
| ≥3 drinks/ week on average | 56 (15.3%) | 44 (14.9%) | 0.88 |
One month around the last menstrual period
≥4 drinks/occasion
time interval between LMP and pregnancy recognition.
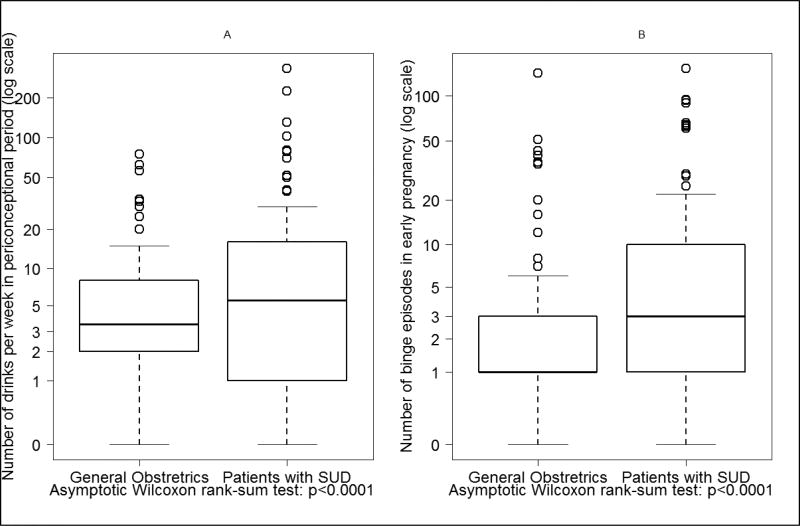
A similar proportion of individuals in the general obstetrics (26.6%) and patients with SUD (25.1%; p=0.66) groups were binge/chronic moderate users in early pregnancy. A subset analysis among these 171 patients demonstrated no difference in the prevalence of binge drinking or chronic use of ≥ 3 drinks/week between general obstetrics and patients with SUD groups (p’s > 0.05; data not shown). A further examination of patterns of alcohol consumption among those who reported alcohol use in early pregnancy, however, revealed differences in intensity of drinking between the two study groups (Figure 1). The median amount of standard drinks consumed per week in the periconceptional period in the general obstetrics group was 3.5 (Q1=2; Q3=8) compared to 5.5 (Q1=1; Q3=16) among patients with SUD (p<0.001). Similarly, median number of binge drinking episodes in early pregnancy in the general obstetrics group was 1 (Q1=1; Q3=3) compared to 3 (Q1=1; Q3=10) in the patients with SUD (p<0.001). Results of multivariable linear regression confirmed that study group (SUD vs. general obstetrics) was a significant predictor of the number of drinks consumed (β=0.5, p=0.02) in the periconceptional period and number of binge episodes (β=0.7, p<0.001) in early pregnancy after adjusting for ethnicity and maternal age.
Figure 1.
Intensity of alcohol consumption among subjects who reported alcohol use in early pregnancy (1.A: Number of Drinks per Week in Periconceptional period; 1.B: Number of Binge Episodes in early pregnancy).
Note: Values transformed to log(x+0.5) to account for x=0
4. Discussion
Results of this large, clinic-based study of 660 pregnant women indicate that alcohol consumption, based on self-report, is prevalent in the periconceptional period and early pregnancy in women attending general obstetrics clinics, as well as a prenatal program dedicated to the care of pregnant women with SUD. While the proportion of pregnant women who consumed alcohol in the periconceptional period or early pregnancy was similar in both groups, alcohol consumption was higher among patients screened at the specialty clinic for pregnant women with SUD. Prevalence estimates obtained in our study are alarming, particularly given the known risk for FASD and vulnerability of the fetus to alcohol exposure beginning in early pregnancy, often prior to pregnancy recognition.
Our data, which indicate that approximately 15% of pregnant women in our sample of general obstetrics and SUD patients consume at least 3 drinks/week on average, are consistent with national estimates reporting 8.5%–30.3% prevalence for any alcohol use (Table 3), either in the past-month or across the duration of pregnancy (Ethen et al., 2009; Slater et al., 2011; Substance Abuse and Mental Health Services Administration, 2013; Tan et al., 2015). However, binge drinking estimates in the periconceptional period (26.7%) and early pregnancy (23.9%) are substantially higher than in the national surveys. This may be at least partly attributed to the fact that drinking before pregnancy recognition was specifically queried and included in our estimates, whereas national surveys do not specifically inquire about drinking after LMP but prior to pregnancy recognition. In this study, pregnant women seen in the SUD clinic had somewhat later pregnancy recognition (by approximately 2 weeks) compared to the general obstetrics population, which is consistent with other reports (Campo et al., 2010; Everett et al., 2016; Heil et al., 2011; Jones et al., 2011; Terplan et al., 2014; Than et al., 2005). Thus, higher alcohol consumption in pregnant women with SUD might, in part, be due to delayed pregnancy recognition.
Table 3.
National Estimates of Self-Reported Alcohol Use in Pregnant Women.
| Source | Alcohol Use (%) | Reported timeframe |
|
|---|---|---|---|
|
| |||
| Any | Binge | ||
| General Population | |||
| Behavioral Risk Factor Surveillance System (BRFSS, CDC) 2011–2013 (Tan et al., 2015) | 10.2 | 3.1* | Past 30 days |
|
| |||
| Substance Abuse and Mental Health Services Administration (NSDUH 2011–2012) (Substance Abuse and Mental Health Services Administration, 2013) | 8.5 | 2.7* | Past 30 days |
| 17.9 | 6.6* | First trimester | |
|
| |||
| National Birth Defects Prevention Study (NBDPS; 1997–2002) (Ethen et al., 2009) | 30.3 | 8.3* | Anytime in pregnancy |
| 22.5 | 7.5* | First month of pregnancy | |
|
| |||
| Across US States (n=324) (Slater et al., 2011) | 21.3 | -- | Anytime in pregnancy |
|
| |||
| Pregnant women who use opioids non-medically | |||
| NSDUH 2005–2014 (Kozhimannil et al., 2017a) | 48.3 | 32.1* | Past 30 days |
Binge drinking is defined by the BRFSS and NBDPS as consuming ≥4 drinks on an occasion and by the NSDUH as consuming ≥5 drinks on occasion
We recognize that the demographics of our study cannot be generalized to the entire U.S. population; the sample included a large number of Hispanic/Latino participants. While historically this group was at low-risk for alcohol abuse, recent reports (Bryant and Kim, 2013), including ours (Bakhireva et al., 2009), indicate that acculturation is a major risk factor for binge drinking in the periconceptional period among pregnant Latina/Hispanic women. Further, we recognize that timing of pregnancy recognition is a subjective measure that might be prone to recall bias. Additionally, we were not able to include measures of socioeconomic status in this report, as this variable was only collected from participants after enrollment in the ENRICH cohort. A prior report of ENRICH cohort participants indicate that annual household income <$20,000 is more than twice as prevalent among OUD/MAT participants compared to those without OUD/MAT (Lowe et al., 2017). Thus, in addition to the increased risks associated with polysubstance use among MAT patients, poverty represents an important consideration when planning early interventions.
To our knowledge, this is the first study using a large U.S. sample of pregnant women to characterize periconceptional and early pregnancy alcohol use behaviors among women in a comprehensive treatment program for SUD, and predominantly OUD. An estimated 38% of U.S. adults seeking treatment for OUD have a clinically significant history of alcohol use problems (Hartzler et al., 2010). Although both alcohol and opioids readily cross the placental barrier, minimal attention has been given to their concurrent use among pregnant women with OUD in the U.S. Limited earlier reports suggested that 21% of pregnant women in a U.S. sample (n=170) attending a comprehensive treatment program for opioid or cocaine use disorder had a concurrent diagnosis of alcohol use disorder (Miles et al., 2001), while only 6.5% of another U.S. sample (n=791) of pregnant women on MAT self-reported a problem with alcohol use prior to pregnancy (Krans et al., 2016); neither of these studies assessed ongoing alcohol use during pregnancy. In the international, multi-site clinical trial, the Maternal Opioid Treatment Human Experimental Research (MOTHER) Study, 1,074 pregnant women with OUD were screened for eligibility across North America and Europe, and 12.2% were determined to be ineligible due to concurrent alcohol use (Stine et al., 2009). Recently, pooled cross-sectional data from the NSDUH, years 2005 to 2014, revealed that among U.S. pregnant women who reported non-medical opioid use (defined as the use of a prescription opioid pain reliever without a prescription) in the past 30 days, 32% also reported binge drinking and 48% reported ‘any’ alcohol use for the same time period (Kozhimannil et al., 2017a). These latter results are notably higher than our own findings, possibly highlighting the positive impact of the Milagro Clinic, which provides a comprehensive treatment program with prescribed MAT throughout pregnancy.
Results of this study should be interpreted in light of its limitations. First, our prevalence estimates, while high, might be an underestimation. One reason for this is potential recall bias, given that alcohol consumption during pregnancy is socially stigmatized, and women who were screened in the second and third trimester could not accurately recall alcohol use several months ago. To minimize reporting biases, patients who screened for this study were asked about their alcohol use in private, non-judgmental settings with the use of recall aids (Muggli et al., 2015). Prior research has found that self-reported alcohol use around conception and before pregnancy recognition is often more accurate and predictive of child outcomes related to PAE than alcohol use later in pregnancy (Coles et al., 2015). Additionally, a validated screening AUDIT-C questionnaire was used, which provides approximately the same accuracy as the full-length AUDIT while minimizing participant burden (Bradley et al., 2007), performs similarly among major racial/ethnic groups, and might be better than other screening questionnaires for identification of risky drinking in pregnancy (Burns et al., 2010). Another reason for potential underestimation of prevalence rates in this study is due to assessment of the majority of patients (88.4%) after the first trimester. Given that binge and/or risky chronic alcohol consumption is known to increase the risk of spontaneous abortion (Avalos et al., 2014), it is possible that highly exposed pregnancies which resulted in termination (either spontaneous or elective) were not captured in our population.
Second, since information was obtained from the screening stage of the ENRICH study, regardless of subsequent enrollment in the prospective cohort, we cannot report changes in alcohol consumption later in pregnancy. While detailed Timeline Follow Back interviews were conducted with alcohol-exposed and unexposed subjects who enrolled in the parent study, we chose to focus on pre-enrollment screening data in order to minimize selection bias on the prevalence estimates reported here.
Third, we cannot report the exact number of subjects with OUD among Milagro patients who participated in the screening since questions about illicit drug use were not asked at the screening stage due to sensitivity. However, among 174 Milagro patients who were subsequently recruited into ENRICH, 165 (94.8%) were treated with MAT for OUD.
Fourth, the general obstetrics group was recruited mostly from ‘low risk’ clinics, e.g., midwifery clinics (90.4%), while 16 subjects (2.4%) were recruited from the Maternal-Fetal Medicine (MFM) clinic, which typically provides care for ‘high risk’ obstetrics patients. Sensitivity analyses revealed, however, that alcohol use estimates were comparable in the MFM group relative to the general obstetrics population (all p-values >0.05); thus, this group was included in the general obstetrics estimates.
5. Conclusions
In summary, to our knowledge this is the first study in a large U.S. sample of pregnant women obtaining prenatal care from a specialty program for women with SUD to characterize periconceptional and early pregnancy alcohol use behaviors and compare them to prevalence estimates in a general obstetrics population. Given the known long-term effects of prenatal alcohol exposure and updated prevalence rates of FASD, continued efforts to decrease drinking during pregnancy are needed to reduce the long-term risk for developmental disorders for all women, including those with OUD. Additional public health efforts should be devoted to preconceptional prevention of alcohol-exposed pregnancies by reducing risky drinking and/or use of effective contraception in women at risk, such as that which has been demonstrated in the CHOICES Program (Hanson et al., 2017;Velasquez et al., 2017). Given high unintended pregnancy rates in women with SUD, additional efforts should be devoted to effective family planning services. Prior research demonstrated that women with SUD use prescription contraception less often than women without SUD, especially long-acting reversible contraception (Terplan et al., 2015). However, women with SUD are open to receiving family planning services in conjunction with substance use treatment (Robinowitz et al., 2016) and will demonstrate similar adherence to prescription contraceptives as women without SUD once prescribed (Griffith et al., 2017). Periconceptional and early pregnancy periods also offer unique opportunities to conduct screening and brief intervention to minimize the risk of FASD. Routine universal screening by validated screening questionnaires for alcohol and tobacco use, followed by brief intervention and referral to treatment is recommended by the ACOG (Committee on Obstetric Practice, 2017) and American Academy of Family Physicians (Wilkes, 2016). ACOG (American Congress of Obstetricians and Gynecologists, 2008), American Academy of Pediatrics (Patrick and Schiff, 2017), and American Nurses Association (American Nurses Association Center for Ethics and Human Rights, 2017) recommend that screening should be applied to all women equally, regardless of age, race, ethnicity, or socioeconomic status, while emphasizing a non-punitive approach towards pregnant women with SUD. Future studies should examine in greater detail the patterns of drinking among pregnant women with OUD and the role of other co-exposures (e.g., amphetamines and tobacco) on alcohol consumption and pregnancy outcomes, as well as examine effectiveness of early screening and brief interventions to minimize alcohol use in women with OUD.
Highlights.
A clinic-based study (n=660) examines prevalence of alcohol use in early pregnancy
Prevalence was similar in the general obstetrics (OB; 24.5%) and substance use disorder (SUD; 24.1%) populations
Intensity of alcohol use (binge episodes, drinks/week) was higher in SUD group
Prenatal programs for women with SUD should screen for alcohol consumption
Acknowledgments
Role of Funding Source
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (NIAAA/NIH), research grant number R01 AA021771. No funding sources had any role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication.
We wish to thank Sandra Cano, Hilda Gutierrez, Sonnie Williams, Alyssa Ortega, Lucinda Flynn, Danielle Kabella, and Dr. Jean Lowe for their assistance with the ENRICH research program. We are also thankful to Dr. Kenneth Lyons Jones for encouraging us to compare prevalence estimates in substance using and general obstetrics populations in our study and to Dr. Ronald Schrader for his review of the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
LB and JS are the Principal Investigators of the parent ENRICH study, which provided the study population and data for this analysis. They secured the funding for the birth cohort and were responsible for the study concept and design with assistance from Co-Investigators (LL and WR). LG and SS were involved in the collection, management, and analysis of the data, as well as literature review. All co-authors were involved in the development of the analytical framework for this analysis and either drafted or critically reviewed the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest
No conflict declared
References
- Ailes EC, Dawson AL, Lind JN, Gilboa SM, Frey MT, Broussard CS, Honein MA. Opioid prescription claims among women of reproductive age--United States, 20082013;2012. MMWR Morb. Mortal. Wkly. Rep. 2015;64:37–41. [PMC free article] [PubMed] [Google Scholar]
- American Congress of Obstetricians and Gynecologists. ACOG Committee Opinion No. 422: at-risk drinking and illicit drug use: ethical issues in obstetric and gynecologic practice. Obstet. Gynecol. 2008;112:1449–60. doi: 10.1097/AOG.0b013e318192499b. [DOI] [PubMed] [Google Scholar]
- American Congress of Obstetricians and Gynecologists. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet. Gynecol. 2012;119:1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- American Nurses Association Center for Ethics and Human Rights. Position Statement: Non-Punitive Treatment for Pregnant and Breast-feeding Women with Substance Use Disorders [Online] Silver Spring, MD: 2017. [Accessed 12/20/2017]. http://www.nursingworld.org/MainMenuCategories/Policy-Advocacy/Positions-and-Resolutions/ANAPositionStatements/Position-Statements-Alphabetically/Non-punitive-Alcohol-and-Drug-Treatment-for-Pregnant-and-Breast-feeding-Women-and-the-Exposed-Childr.pdf. [Google Scholar]
- Avalos LA, Roberts SC, Kaskutas LA, Block G, Li DK. Volume and type of alcohol during early pregnancy and the risk of miscarriage. Subst. Use Misuse. 2014;49:1437–45. doi: 10.3109/10826084.2014.912228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola AB. Late Recognition of unintended pregnancies. Public Health Nurs. 2015;32:462–70. doi: 10.1111/phn.12182. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Lowe JR, Gutierrez HL, Stephen JM. Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) prospective cohort: Study design considerations. Adv. Pediatr. Res. 2015;2:1–13. doi: 10.12715/apr.2015.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC. Prevalence of prenatal alcohol exposure in the State of Texas as assessed by phosphatidylethanol in newborn dried blood spot specimens. Alcohol Clin. Exp. Res. 2017;41:1004–1011. doi: 10.1111/acer.13375. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Young BN, Dalen J, Phelan ST, Rayburn WF. Periconceptional binge drinking and acculturation among pregnant Latinas in New Mexico. Alcohol. 2009;43:475–81. doi: 10.1016/j.alcohol.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Debenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin. Exp. Res. 2007;31:1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Bryant AN, Kim G. The relation between acculturation and alcohol consumption patterns among older Asian and Hispanic immigrants. Aging Ment. Health. 2013;17:147–56. doi: 10.1080/13607863.2012.727382. [DOI] [PubMed] [Google Scholar]
- Burns E, Gray R, Smith LA. Brief screening questionnaires to identify problem drinking during pregnancy: A systematic review. Addiction. 2010;105:601–14. doi: 10.1111/j.1360-0443.2009.02842.x. [DOI] [PubMed] [Google Scholar]
- Campo S, Askelson NM, Spies EL, Losch M. Preventing unintended pregnancies and improving contraceptive use among young adult women in a rural, Midwestern state: Health promotion implications. Women Health. 2010;50:279–96. doi: 10.1080/03630242.2010.480909. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: Developmental effects on 6-month-old infants. Matern Child Health J. 2015;19:2605–14. doi: 10.1007/s10995-015-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet. Gynecol. 2017;130:e81–e94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet. Gynecol. 2014;123:997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA. Alcohol consumption by women before and during pregnancy. Matern. Child Health J. 2009;13:274–85. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett BG, Mccabe KF, Hughes TL. Unintended pregnancy, depression, and hazardous drinking in a community-based sample of sexual minority women. J. Womens Health. 2016;25:904–11. doi: 10.1089/jwh.2015.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N. Engl. J. Med. 2016;374:843–52. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G, Kumaraswami T, Chrysanthopoulou SA, Mattocks KM, Clark RE. Prescription contraception use and adherence by women with substance use disorders. Addiction. 2017;112:1638–1646. doi: 10.1111/add.13840. [DOI] [PubMed] [Google Scholar]
- Hanson JD, Nelson ME, Jensen JL, Willman A, Jacobs-Knight J, Ingersoll K. Impact of the CHOICES Intervention in preventing alcohol-exposed pregnancies in American Indian Women. Alcohol Clin. Exp. Res. 2017;41:828–835. doi: 10.1111/acer.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J. Subst. Abuse Treat. 2010;39:114–23. doi: 10.1016/j.jsat.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Jones HE, Arria A, Kaltenbach K, Coyle M, Fischer G, Stine S, Selby P, Martin PR. Unintended pregnancy in opioid-abusing women. J. Subst. Abuse Treat. 2011;40:199–202. doi: 10.1016/j.jsat.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, Odendaal H, Elliott AJ, Hereld D, Signore C, Willinger M, Huestis MA. Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy. Clin. Chem. 2015;61:523–32. doi: 10.1373/clinchem.2014.233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–25. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jones HE, Berkman ND, Kline TL, Ellerson RM, Browne FA, Poulton W, Wechsberg WM. Initial feasibility of a woman-focused intervention for pregnant african-american women. Int. J. Pediatr. 2011;2011:389285. doi: 10.1155/2011/389285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil KB, Graves AJ, Jarlenski M, Kennedy-Hendricks A, Gollust S, Barry CL. Non-medical opioid use and sources of opioids among pregnant and non-pregnant reproductive-aged women. Drug Alcohol Depend. 2017a;174:201–208. doi: 10.1016/j.drugalcdep.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil KB, Graves AJ, Levy R, Patrick SW. Nonmedical use of prescription opioids among pregnant U.S. women. Womens Health Issues. 2017b;27:308–315. doi: 10.1016/j.whi.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krans EE, Bogen D, Richardson G, Park SY, Dunn SL, Day N. Factors associated with buprenorphine versus methadone use in pregnancy. Subst. Abuse. 2016;37:550–557. doi: 10.1080/08897077.2016.1146649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S. Global prevalence of fetal alcohol spectrum disorder among children and youth: A systematic review and meta-analysis. JAMA Pediatr. 2017;171:948–956. doi: 10.1001/jamapediatrics.2017.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Qeadan F, Leeman L, Shrestha S, Stephen JM, Bakhireva LN. The effect of prenatal substance use and maternal contingent responsiveness on infant affect. Early Hum. Dev. 2017;115:51–59. doi: 10.1016/j.earlhumdev.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–66. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CD, Hoyme HE, Tabachnick B, Seedat S. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013;133:502–12. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DR, Svikis DS, Kulstad Jl, Haug NA. Psychopathology in pregnant drugdependent women with and without comorbid alcohol dependence. Alcohol Clin. Exp. Res. 2001;25:1012–7. [PubMed] [Google Scholar]
- Muggli E, Cook B, O'leary C, Forster D, Halliday J. Increasing accurate self-report in surveys of pregnancy alcohol use. Midwifery. 2015;31:e23–8. doi: 10.1016/j.midw.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Parackal SM, Parackal MK, Harraway JA. Prevalence and correlates of drinking in early pregnancy among women who stopped drinking on pregnancy recognition. Matern. Child Health J. 2013;17:520–9. doi: 10.1007/s10995-012-1026-7. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Schiff DM. A public health response to opioid use in pregnancy. Pediatrics. 2017;139:e20164070. doi: 10.1542/peds.2016-4070. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, Mcallister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307:1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The alcohol use disorders identification test: An update of research findings. Alcohol Clin. Exp. Res. 2007;31:185–99. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Robinowitz N, Muqueeth S, Scheibler J, Salisbury-Afshar E, Terplan M. Family planning in substance use disorder treatment centers: Opportunities and challenges. Subst. Use Misuse. 2016;51:1477–83. doi: 10.1080/10826084.2016.1188944. [DOI] [PubMed] [Google Scholar]
- Slater ME, Linabery AM, Blair CK, Spector LG, Heerema NA, Robison LL, Ross JA. Maternal prenatal cigarette, alcohol and illicit drug use and risk of infant leukaemia: A report from the Children's Oncology Group. Paediatr. Perinat. Epidemiol. 2011;25:559–65. doi: 10.1111/j.1365-3016.2011.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine SM, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Fischer G, Arria AM, Selby P, Jones HE. Characteristics of opioid-using pregnant women who accept or refuse participation in a clinical trial: Screening results from the MOTHER Study. Am. J. Drug Alcohol Abuse. 2009;35:429–33. doi: 10.3109/00952990903374080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) 18 percent of pregnant women drink alcohol during early pregnancy. [Accessed 8/7/17];The NSDUH Report: Data Spotlight. 2013 Available: https://www.samhsa.gov/data/sites/default/files/spot123-pregnancy-alcohol-2013/spot123-pregnancy-alcohol-2013.pdf.
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age - United States, 2011–2013. MMWR Morb. Mortal. Wkly. Rep. 2015;64:1042–6. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Terplan M, Cheng D, Chisolm MS. The relationship between pregnancy intention and alcohol use behavior: An analysis of PRAMS data. J. Subst. Abuse Treat. 2014;46:506–10. doi: 10.1016/j.jsat.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Terplan M, Hand DJ, Hutchinson M, Salisbury-Afshar E, Heil SH. Contraceptive use and method choice among women with opioid and other substance use disorders: A systematic review. Prev. Med. 2015;80:23–31. doi: 10.1016/j.ypmed.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than LC, Honein MA, Watkins ML, Yoon PW, Daniel KL, Correa A. Intent to become pregnant as a predictor of exposures during pregnancy: Is there a relation? J. Reprod. Med. 2005;50:389–96. [PubMed] [Google Scholar]
- Velasquez MM, Von Sternberg KL, Floyd RL, Parrish D, Kowalchuk A, Stephens NS, Ostermeyer B, Green C, Seale JP, Mullen PD. Preventing alcohol and tobacco exposed pregnancies: CHOICES Plus in primary care. Am. J. Prev. Med. 2017;53:85–95. doi: 10.1016/j.amepre.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes J. AAFP releases position paper on preconception care. Am. Fam. Physician. 2016;94:508–10. [PubMed] [Google Scholar]