Abstract
The design of tissue engineered scaffolds based on polymerized high internal phase emulsions (polyHIPEs) has emerged as a promising bone grafting strategy. We previously reported the ability to 3D print emulsion inks to better mimic the structure and mechanical properties of native bone while precisely matching defect geometry. In the current study, redox-initiated hydrogel carriers were investigated for in situ delivery of human mesenchymal stem cells (hMSCs) utilizing the biodegradable macromer, poly(ethylene glycol)-dithiothreitol. Hydrogel carrier properties including network formation time, sol-gel fraction, and swelling ratio were modulated to achieve rapid cure without external stimuli and a target cell-release period of 5–7 days. These in situ carriers enabled improved distribution of hMSCs in 3D printed polyHIPE grafts over standard suspension seeding. Additionally, carrier-loaded polyHIPEs supported sustained cell viability and osteogenic differentiation of hMSCs post-release. In summary, these findings demonstrate the potential of this in situ curing hydrogel carrier to enhance the cell distribution and retention of hMSCs in bone grafts. Although initially focused on improving bone regeneration, the ability to encapsulate cells in a hydrogel carrier without relying on external stimuli that can be attenuated in large grafts or tissues is expected to have a wide range of applications in tissue engineering.
Keywords: MESENCHYMAL STEM CELLS, CELL DELIVERY, POLYHIPES, 3D PRINTING
Graphical Abstract
1. Introduction
Despite the high regenerative potential of bone, treatment of large defects and nonunions remains a significant challenge and often requires surgical intervention. Autologous grafting serves as the current standard of care due to its high regenerative capacity. However, this treatment is unavailable in a large number of patients due to anatomical limitations associated with harvesting.[1] Patients eligible for autologous grafting face elevated risk of donor site morbidity, pain, and infection. Tissue engineering aims to provide a bone replacement that combines the regenerative potential of autologous grafts with the availability and tunability of synthetic materials. Tissue engineered bone grafts are designed to be a porous scaffold that matches defect geometry, degrades at a rate complementary to new tissue formation, and exhibits requisite mechanical properties to withstand physiological loading.[2, 3] It is often challenging to achieve this combination of properties using traditional fabrication techniques. For example, highly porous constructs that facilitate nutrient and waste transport often struggle achieving desired mechanical properties.[4, 5] Expansion of 3D printing technologies into tissue engineering has provided researchers new tools to independently control and optimize these properties. We recently developed a multi-modal printing system to generate tissue engineered scaffolds that mimics the native structure of bone.[6, 7] In this system, fumarate-based emulsion inks with hierarchical porosity were reinforced with a poly(ε-caprolactone) or poly(lactic acid) shell to achieve simultaneous improvements in permeability and compressive properties.
In addition to the design of scaffold properties, success as a tissue engineered bone graft depends on the delivery or recruitment of mesenchymal stem cells (MSCs). These multipotent progenitor cells aid regeneration through a variety of mechanisms including serving as new centers of bone formation and secretion of trophic factors that modulate inflammation, stimulate angiogenesis, and limit fibrosis.[8–11] Despite the therapeutic advantages of MSCs, traditional cell delivery (e.g. direct injection of stem cells via syringe or catheter) are associated with limited cell engraftment, often retaining less than 5% of injected cells.[12–14] Furthermore, the hostile environment of injured or diseased tissue can reduce retention of transplanted cells by depriving them of nutrients or subjecting them to early clearance by surveying inflammatory cells.
To overcome this limitation, researchers have investigated and reviewed numerous hydrogel carriers to improve retention and viability of transplanted cells.[15–18] Encapsulation within an external matrix improves retention by acting as a mechanical barrier to cell wash out and providing an improved substrate for tissue engraftment. Multiple natural and synthetic hydrogel platforms have been investigated for cell encapsulation based on modified gelatin, fibrin, and poly(ethylene glycol)-based systems.[19–24] Although these have improved cell retention, use of hydrogel scaffolds alone are not ideal for bone grafting due to their poor compressive properties. Multiple strategies have been investigated to improve both cell retention and mechanical stability by combining the benefits of mechanically robust scaffolds with hydrogel-mediated cell delivery. One approach has been the incorporation of cell-laden, hydrogel microspheres into injectable graft materials. Specifically, alginate based microspheres have been utilized as cell delivery vehicles in both calcium phosphate and polyurethane scaffolds.[25, 26] Although these systems illustrate the potential of combination delivery methods, these protocols often require significant processing of cell carriers prior to injection. An alternative approach has been the in situ incorporation of cell-laden hydrogels into pre-fabricated scaffolds. Photocurable hydrogel carriers remain one of the most widely investigated systems for use in cell delivery applications due to their tunable nature and cytocompatible properties.[27, 28] Despite this widespread use, photoinitiated systems provide limited potential in composite scaffold fabrication as rapid attenuation and marginal penetration depth of UV sources severely hinders construct size and potential carrier loading.[29] In contrast, gelation of hydrogel cell carriers without external stimuli using either redox-based initiation or Michael-type addition between thiol-derivatives and PEG diacrylates can circumvent this issue and facilitate uniform cell loading.[30, 31] However, carrier degradation and cell release profiles have yet to be adequately established in these systems to maximize therapeutic potential. We propose to use an in situ curing hydrogel as a cell carrier to seed the bone graft with MSCs at the time of surgery, Figure 1, and provide a platform for programmable carrier degradation and temporal control of cell release. In situ MSC seeding of scaffolds has the potential to minimize the costs, treatment delays, and regulatory hurdles of extended pre-culture periods. Furthermore, combination of the cell-releasing hydrogel carriers with advanced 3D manufacturing technologies has the potential to generate a graft with patient specific geometries and improved retention of stem cells.
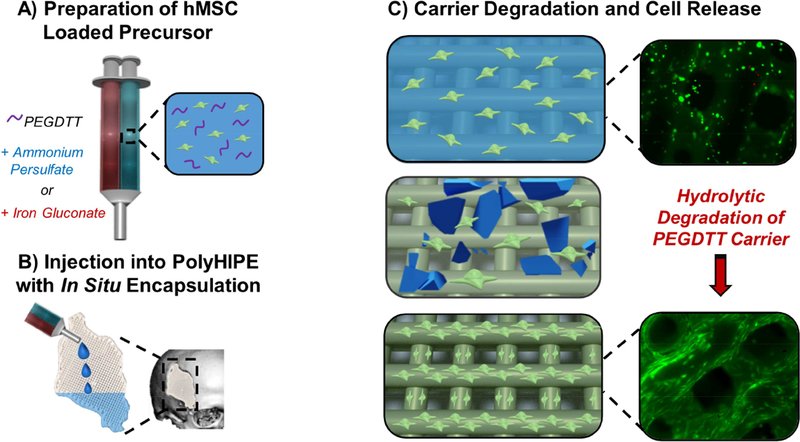
Figure 1.
Schematic illustrating hMSC loading in hydrogel precursor solutions (A), injection and encapsulation in 3D printed polyHIPE scaffold (B), and protection and release during early stages of implantation (C).
In this study, 3D printed polyHIPE scaffolds were seeded with human MSCs using a cell-releasing hydrogel carrier that cures in situ using redox initiation. The hydrolytically degradable macromer, poly(ethylene glycol)-dithiothreitol, was investigated as a cell carrier and the effect of the oxidant-to-reductant ratio on network formation time, sol-gel fraction, and swelling ratio was investigated to identify candidate cell carriers. The viability and release profiles of MSCs encapsulated in these in situ cured hydrogels was then characterized. To confirm the benefits of hydrogel delivery in 3D printed polymerized high internal phase emulsions (polyHIPEs), MSCloaded macromer solutions were injected into multi-layered constructs and cell distribution compared to a traditional suspension seeding method. Mesenchymal stem cell activity on 3D printed polyHIPEs was monitored using established alkaline phosphatase and mineralization assays to ensure delivered cells retained the ability to undergo osteoblastic differentiation. We previously reported that unmodified scaffolds based on propylene fumarate dimethacrylate promoted osteoblastic differentiation under standard culture conditions, demonstrating the inherent osteoinductive character of these grafts.[32, 33] In the current study, we aimed to better understand the mechanism behind this osteoinductive character by isolating the effects of scaffold chemistry and surface area on osteoblastic differentiation. Collectively, this work aims to highlight the potential of cell-laden 3D printed scaffolds to serve as rigid cell carriers and improve the regenerative capacity of tissue engineered bone grafts.
2. Materials and Methods
Materials
Polyglycerol polyricinoleate (PGPR 4125) was donated by Palsgaard. Human mesenchymal stem cells (hMSCs) were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White. All other chemicals were purchased and used as received from Sigma–Aldrich, unless otherwise noted.
hMSC Culture
Bone marrow-derived hMSCs were obtained as passage 1 from the Center for the Preparation and Distribution of Adult Stem Cells at Texas A&M Health Science Center College of Medicine, Institute for Regenerative Medicine at Scott & White through NIH Grant # P40RR017447. Cells were cultured to 80% confluency on tissue-culture polystyrene flasks in standard growth media containing Minimum Essential Media α (MEM α, Life Technologies) supplemented with 16.5% fetal bovine serum (FBS, Atlanta Biologicals) and 1% L-glutamine (Life Technologies) prior to passaging. All experiments were performed with cells at passage 3.
PEGDTT Synthesis
Poly(ethylene glycol)-dithiothreitol (PEGDTT) was synthesized by adding a solution of d,ldithiothreitol (DTT), triethylamine (TEA), and dichloromethane (DCM) dropwise to a solution of poly(ethylene glycol)-diacrylate (PEGDA) 2kDa in DCM. The molar ratios of DTT, PEGDA and TEA were 2:3:0.9. After the addition of the DTT and TEA solution in DCM, the reaction was stirred for 24 hours at room temperature. The resulting solution was then precipitated in cold diethyl ether, washed, filtered, and dried under ambient conditions for 24 hours. The resulting macromer was placed under vacuum to remove excess solvent until the weight was stabilized.
Fabrication of Redox-Initiated Hydrogels
The effects of redox initiator concentration on hydrogel properties were investigated to identify carrier formulations suitable for subsequent cell encapsulation. Briefly, hydrogel precursor solutions were prepared by dissolving PEGDTT (10 wt %) in phosphate-buffered saline (PBS) containing either ammonium persulfate (APS) as initiator or iron gluconate dihydrate (IG) as reducing agent at a concentration of 1.25, 2.5, 5, 10 or 25 mM and APS:IG molar ratio of 1.0:0.5, 1.0:1.0 and 1.0:2.0. Initiator and reducing agent precursor solutions were then loaded into double-barrel syringes and injected through a mixing head to facilitate crosslinking. Post-injection conditions are detailed below.
Rheological Characterization
Cure profiles of hydrogel carriers were characterized by determining gelation onset and complete network formation using an Anton Paar MCR 301 rheometer. Hydrogel precursor solutions were prepared at an initiator concentration of 1.25, 2.5, 5, 10 or 25 mM with an APS:IG molar ratio of 1.0:0.5, 1.0:1.0 and 1.0:2.0, loaded into double-barrel syringes, and injected through a mixing head onto a parallel-plate configuration heated to 37 °C. Storage, loss, and complex moduli were measured every 3 seconds with a 1 mm gap and 0.5% strain. Gelation onset was determined as the crossing of loss and storage modulus. Complete network formation was determined as the fourth point after which there was a less than 1% change in complex viscosity. Values were reported as the average of three specimens.
Characterization of Network Formation
Network formation of hydrogel carriers was determined by monitoring sol-gel fraction and swelling ratio as a function of redox initiator concentration. Precursor solutions were prepared with an initiator concentration of 1.25, 2.5, 5, 10 or 25 mM with an APS:IG molar ratio of 1.0:0.5, 1.0:1.0 and 1.0:2.0, loaded into double-barrel syringes, injected through a mixing head into 8 mm cylindrical tubes, and allowed to cure for 1 hour at 37 °C. Specimens were then sectioned (8 mm diameter × 2 mm height) and vacuum-dried for 24 hours. The dry polymer mass was obtained and specimens placed in DCM at a ratio of 1 mL of DCM to 10 mg of specimen to extract the soluble fraction. After extraction for 24 hours, DCM was decanted and specimens were vacuum-dried for 24 hours at ambient temperature. Final polymer mass was obtained and gel fraction calculated as the final mass divided by the original mass. To measure swelling ratio, specimens were prepared and sectioned (8 mm diameter × 2 mm height) as described above and allowed to swell in reverse osmosis water for 3 hours to reach equilibrium swelling mass. Specimens were then dried under vacuum for 24 hours and dry polymer mass obtained. The equilibrium volumetric swelling ratio, Q, was calculated as equilibrium swelling mass divided by dry polymer mass.
In Vitro Hydrolytic Degradation
The hydrolytic degradation profile of the selected cell carrier formulation was determined by monitoring swelling ratio over the degradation period. Briefly, precursor solutions were prepared with an initiator or reducing agent concentration of 10 mM, injected through a double barrel mixing syringe into 8 mm cylindrical tubes, and allowed to cure for 1 hour at 37 °C. Specimens were then sectioned (8 mm diameter, 2 mm thick) and vacuum-dried for 24 hours. Dry polymer mass was obtained followed by incubation in PBS at 37°C (pH = 7.4). Equilibrium swelling masses were recorded every 2 days, beginning at day 1 and continuing until complete dissolution occurred. The equilibrium volumetric swelling ratio, Q, was calculated as equilibrium swelling mass divided by dry polymer mass and used as a measure of hydrolytic degradation.
Preparation of Emulsion Inks and 3D Printed PolyHIPEs
Propylene fumarate dimethacrylate (PFDMA) was synthesized in a two-step process detailed previously.[34] Briefly, propylene oxide was added dropwise to a solution of fumaric acid and pyridine in 2-butanone and refluxed at 75°C to yield the diester, bis(1,2 hydroxypropyl). Following purification, the diester was end-capped with methacrylate groups using methacryloyl chloride in the presence of trimethylamine and purified to yield the final product. To prepare emulsion inks, propylene fumarate dimethacrylate was combined with 10 wt% surfactant (polyglycerol polyricinoleate), 1 wt% initiator (phenylbis (2,4, 6-trimethylbenzoyl)- phosphine oxide)(BAPO), and mixed in a FlacTek Speedmixer. An aqueous solution of calcium chloride (1 wt%) was then added to the organic phase (w:o 75:25) and mixed. Emulsion inks were extruded layer-by-layer through respective syringes and motor actuated plungers as described previously.[7] Each deposited layer was actively polymerized with an ultraviolet cure-ondispense technique via a modified UV source consisting of four LEDs (365 nm, 700 mW radiant flux) positioned at a 5 cm vertical distance and 2 cm radial distance from the printing nozzle, Figure 2A. Simple models were created in OpenSCAD by creating a cylinder (h = 1 mm, r = 4 mm). G-code was created with slic3r version 1.2.9 using the following key settings: printing speeds of 10 mm/s, nonprinting speed of 25 mm/s, layer thickness of 200 μm, infill of 70% (rectilinear grid), extrusion width of 0.6 mm, one perimeter, and no top or bottom solid layers.
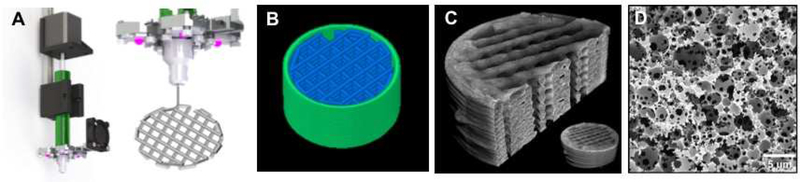
Figure 2.
Custom setup with modified paste extruder based on HYREL EMO-25 with UV LED and heat sink for printing photocurable emulsion inks with Cure-on-Dispense (CoD) (A), g-code rendering of the model (B), representative μCT images (C), and SEM micrograph of the emulsion templated microporosity (D).
All specimens were dried under vacuum for 24 hours to remove water prior to imaging the macroscopic architecture microcomputed tomography (μCT) and microscale porosity with scanning electron microscopy (SEM). Following previously established protocols, specimens were scanned using a SkyScan 1272 μCT imaging system (SkyScan, Aartselaar, Belgium) at a current of 250 mA and a voltage of 40 kV without a filter.[35] Specimens were scanned at a resolution of 2.2 μm/pixel to create high resolution (4096 × 2160 pixel) images. NReconn and CTVox software (SkyScan, Aartselaar, Belgium) were used to reconstruct the serial tomograms and generate representative 3D models. To image the microscale porosity, specimens were coated with gold and examined under SEM (Phenom Pro, Nanoscience Instruments). Representative images of the 3D printed macroscopic architecture and the emulsion-templated microscale porosity are displayed in
Effect of Redox Initiator Concentration on hMSC Viability
A standard LIVE/DEAD assay kit (Molecular Probes) was used to determine the effect of redox initiator concentration on hMSC viability and to confirm hydrogel precursor solution cytocompatibility prior to encapsulation. Briefly, hMSC monolayers were cultured in 48-well tissue-culture plates and exposed for 10 minutes to 1.25, 2.5, 5, 10 or 25 mM concentration of either APS or IG in standard growth media. Viability analysis was performed immediately following exposure. Additionally, hMSC monolayers were exposed to indicated initiator concentrations for 10 minutes, followed by culture at a 10-fold initiator dilution for an additional 24 hours. Viability analysis was performed immediately following this 24-hour period. For LIVE/DEAD analysis, cells were washed with PBS and stained with 2 μM calcein-AM (live) and 2 μM ethidium homodimer-1 (dead) for 30 minutes at 37°C. Cells were then washed again with PBS prior to imaging. Imaging (3 images per specimen) was conducted on four specimens (n = 12) with a fluorescence microscope (Nikon Eclipse TE2000-S).
Fabrication and Cytocompatibility of hMSC-releasing Hydrogels
Cell-releasing hydrogels were prepared by dissolving PEGDTT (10 wt %) in phosphate-buffered saline (PBS) containing either APS as initiator or IG as reducing agent. Precursor solutions were used to resuspend hMSCs at a density of 1 million cells/mL, loaded into double barrel syringes, and injected through a mixing head to facilitate crosslinking. Hydrogels were injected into neat hydrogel constructs (8 mm diameter × 1 mm height) or into pre-fabricated polyHIPE prints (8 mm diameter × 1 mm height) and allowed to cure for ten minutes. Following cure, cell-laden constructs were moved to 48-well tissue-culture plates and incubated in standard growth media containing Minimum Essential Media α (MEM α, Life Technologies) supplemented with 16.5% fetal bovine serum (FBS, Atlanta Biologicals) and 1% L-glutamine (Life Technologies).
Hydrogel formulations with requisite cure times (2.5, 5, 10 or 25 mM APS concentration at 1:1 APS:IG ratio) were selected to investigate the effects of mixing, radical formation, and resultant encapsulation on hMSC viability. hMSC viability and density in neat hydrogel scaffolds was characterized 24 hours post-injection utilizing the LIVE/DEAD analysis procedure outlined above. Additionally, the hydrogel formulation with 10 mM APS and 1:1 APS:IG ratio was utilized to investigate the effects of carrier degradation and cell release on viability and attachment of hMSCs onto tissue-culture polystyrene and polyHIPE scaffolds. Hydrogel cell carriers cured with 10 mM APS concentration and 1:1 APS:IG ratio were allowed to degrade for 7 days to facilitate cell release followed by an additional 7-day culture period and LIVE/DEAD characterization. The hydrogel formulation cured with 10 mM APS concentration and 1:1 APS:IG ratio was selected for all further characterization of cell activity.
Distribution of hMSCs in 3D Printed PolyHIPE Scaffolds
A Quant-iT™ PicoGreen® dsDNA Assay Kit (Molecular Probes) was utilized to quantify cell distribution throughout multi-layer polyHIPE scaffolds. The hMSC-PEGDTT precursor solutions were prepared utilizing 10 mM APS (1:1 APS:IG ratio) and injected into 3D printed constructs consisting of 3 layers of stacked polyHIPE prints (8 mm diameter × 3 mm height). Scaffolds were allowed to cure for ten minutes and transferred to 48-well tissue-culture plates with standard growth media. Cell density was characterized at 1-day post release from the hydrogel carrier to confirm improved distribution of hMSCs throughout the bulk of the 3D printed scaffold. Cell distribution was quantified at three discrete depths of the scaffold (bottom: 0–1 mm, middle: 1–2 mm, top: 2–3 mm). At the specified time point, polyHIPEs were removed from the culture wells and placed in unused wells for thermal shock lysis procedure to ensure only DNA from cells adhered to the scaffolds was measured. The assay was performed according to manufacturer instructions and fluorescence intensity was assessed using a plate reader (Tecan Infinite M200Pro) with excitation/emission wavelengths of 480/520 nm, respectively. Average cell number was approximated by converting dsDNA values to individual cell number using 6.9 pg DNA/cell.[36] Specimens were analyzed in triplicate.
Alkaline Phosphatase Activity of hMSCs Released into 3D Printed PolyHIPE Scaffolds
Alkaline phosphatase activity (ALP) of cells encapsulated and released into 3D printed polyHIPE scaffolds or onto tissue-culture polystyrene from neat hydrogel scaffolds (8 mm diameter × 1 mm height) was determined by monitoring the conversion of p-nitrophenyl phosphate (PNPP, Thermo Scientific) to p-nitrophenol. Standard growth media or osteogenic media (growth media supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone) was added and changed every 2 days for 7 or 14 days post release from carrier, followed by measurement of ALP activity. Scaffold cultures were lysed using thermal shock treatment and incubated with pNPP for 30 min. ALP activity was determined as the rate of PNPP conversion to p-nitrophenyl by measuring the absorbance at 405 nm (Tecan Infinite M200Pro) and normalized to cell number obtained from the PicoGreen assay. Specimens were analyzed in triplicate.
Mineralization Activity of hMSCs Released into 3D Printed PolyHIPE Scaffolds
Alizarin red staining (ARS) was performed to detect calcium phosphate mineral deposition of hMSCs encapsulated and released into 3D printed polyHIPE scaffolds or onto tissue-culture polystyrene from neat hydrogel scaffolds (8 mm diameter × 1 mm height). Standard growth media or osteogenic media (growth media supplemented with 50 μg/mL ascorbic acid, 10 mM βglycerophosphate, and 10 nM dexamethasone) was added and changed every 2 days for 28 days post release following staining of mineralized nodules. hMSCs were fixed in 3.7% glutaraldehyde and incubated for 5 minutes in 2% Alizarin Red S. Scaffolds were then washed with PBS to remove excess stain and photographed under optical microscopy. A semi-quantitate procedure was then performed by destaining scaffolds in 10% acetic acid solution and monitoring absorbance at 405 nm. Specimens were analyzed in triplicate.
Mechanism of PolyHIPE Osteoinductivity
To investigate the underlying mechanism of polyHIPE osteoinductivity, 2-week ALP activity of seeded hMSCs, 25,000 cells/cm2, was monitored on three differing substrates, PFDMA polyHIPEs, PFDMA films, and poly(lactic acid) (PLA) films. Chroma-Line brand PLA (NatureWorks 4042D resin, CAS# 26100–51-6, Mn = 127 ± 2 kDa) filament was selected for high purity and absence of pigments or additives and used as received. A HYREL MK1 thermoplastic extruder was modified with an E3Dv6 hot end to allow for printing of the PLA filament into films. Briefly, poly(lactic acid) filament was heated to 200°C, extruded into films with 100% infill (8 mm diameter × 1 mm height), and allowed to cool under ambient temperatures. PFDMA films were fabricated by mixing the pre-polymer with 1 wt% BAPO, adding dropwise into round molds (8 mm diameter × 1 mm height), and cured via 6-minute exposure to long-wave UV light (UVP High Performance Transilluminator 365 nm). Finally, PFDMA polyHIPE scaffolds were fabricated as described previously with the exception of utilizing a 100% infill density. Scaffolds were sterilized using ethylene oxide sterilization prior to cell culture.
Statistical Analysis
The data are displayed as mean ± standard deviation for each composition. An analysis of variance (ANOVA) comparison was used for multiple composition comparisons with a Tukey’s multiple comparison to analyze the significance of the data. A Student’s t-test was performed to determine any statistically significant differences if only two compositions were present. All tests were carried out at a 95% confidence interval (p<0.05).
3. Results and Discussion
Rheological Characterization of Redox Initiated PEGDTT Hydrogels
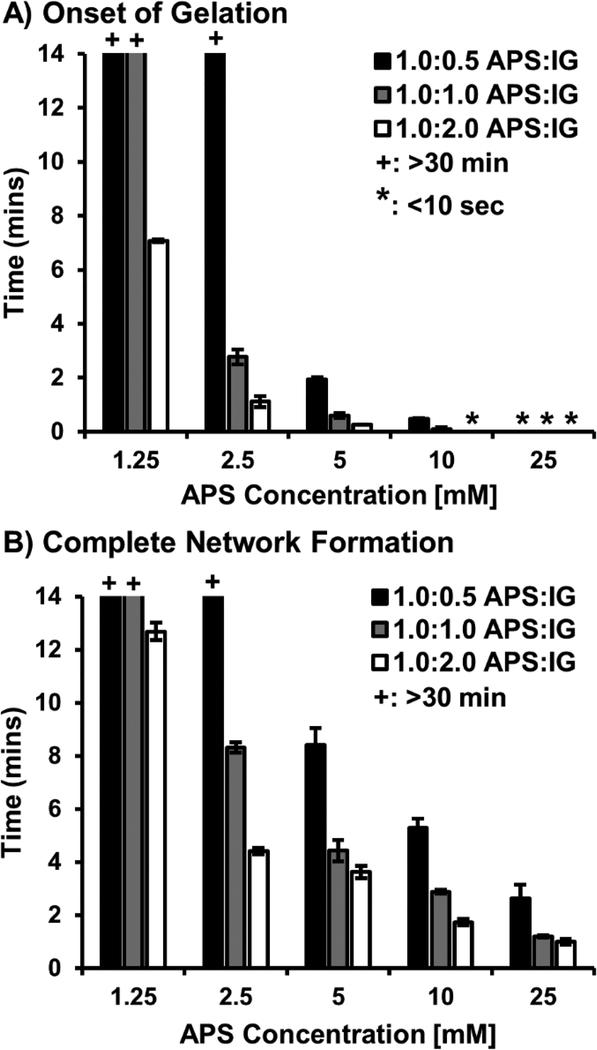
Photopolymerization of acrylated poly(ethylene glycol) is a widely studied delivery platform that allows for cell encapsulation in mild conditions with high cell survival.[37–39] Despite these advantages, reliance on an external UV source to initiate polymerization often results in depth dependent properties that can limit translation of these systems.[40] Increased depths and irregular geometries of bone injuries make sufficient filling and curing within these defects difficult with photopolymerization alone. In contrast, this study aimed to develop and characterize an in situ curing hydrogel based on redox initiation of the biodegradable macromer, poly(ethylene glycol)-dithiothreitol. An in situ curing cell carrier would allow for facile incorporation of marrow derived cells in the surgical setting and improve spatial distribution of cells within large, 3D tissue engineered scaffolds. To assess the feasibility of the proposed carrier, polymerization times were characterized as a function of initiator concentration to ensure encapsulation occurred in a 5–10 minute time scale suitable for clinical deployment.[41] First, onset of gelation was determined by monitoring the rheological transition between the liquid and gelled state to ensure appropriate time for injection and filling of the scaffold was allowed, Figure 3A. Next, complete network formation was determined by identifying the plateau of complex viscosity to ensure uniform property formation and retention of cells at the defect site, Figure 3B. Multiple researchers have reported use of the water-soluble initiator, ammonium persulfate in cell encapsulation platforms.[42, 43] Temeneoff et al. demonstrated rapid encapsulation of rat marrow stromal cells in a thermal initiated, oligo(poly(ethylene glycol) fumarate) hydrogel with gelation onset occurring in less than 5 minutes at an initiator concentration of 25 mM. Traditional redox carriers such as these often utilize N, N, N, Ntetramethylethylendiamine (TMED) to accelerate radical formation. As an alternative, ferrous based reducing agents facilitate radical formation at an elevated rate, introducing the potential to retain rapid polymerization rates with reduced initiator concentrations.[44, 45] In this study, ammonium persulfate was added at concentrations of 1.25, 2.5, 5, 10 and 25 mM with equal molar iron gluconate dihydrate added as reducing agent. As expected, increasing initiator concentration resulted in more rapid gelation onset ranging from approximately 10 minutes to less than 10 seconds. Uniquely, the use of a ferrous reducing agent allowed for gelation to occur at rates comparable to other APS systems with the benefit of a 10-fold reduction in required concentration. Furthermore, complete network formation time could be tuned from approximately 15 minutes to less than 5 minutes. To further develop tools that could be utilized to modulate cure rate independent of initiator concentration, the effect of initiator: reductant ratio on cure rate was explored by adding IG concentration at relative molar concentrations of 1:0.5, 1.0:1.0, 1.0:2.0 (APS:IG). Thoughtful selection of iron based reducing agent concentration is required as excessive concentrations of ferrous ions are known to inhibit polymerization through oxidation of propagating radicals.[46] In this work, no inhibitory effects of increasing IG concentration was observed and it is hypothesized that a more rapid production of the APS-IG complex led to a tunable range of cure rates with a single initiator concentration. A minimum of 2-fold range in polymerization times were observed with increasing ratio of reductant to initiator. Studies investigating enzyme-mediated redox initiation of hydrogel cell carriers have also demonstrated no inhibitory effects in similar ranges of ferrous ion concentration.[46] However, the potential impact on final double bond conversion has been noted, and as such, it is critical to ensure requisite network formation in this system.
Figure 3.
Effect of initiator concentration and reducing agent ratio on gelation onset (A) and complete network formation (B) of hydrogel carrier. The + represents a gelation and network formation time of greater than 30 minutes. The * represents a gelation onset time of less than 10 seconds. All data represents average ± standard deviation for n = 3. Statistical analysis provided in Supplemental Figure S1.
Network Formation of Redox Initiated PEGDTT Hydrogels
Complete and consistent network formation is critical to controlling carrier properties and degradation profiles.[47, 48] To this end, sol-gel fraction and swelling ratio were characterized to assess the effect of oxidant and reductant concentration on hydrogel network formation, Supplemental Table S1 Sol-gel fraction increased with elevated initiator, ranging from greater than ~95% for 25 mM concentration to ~70% for 1.25 mM concentration. For comparison, photopolymerized PEGDTT hydrogels were cured with 5mM concentration of Irgacure 2959 and exhibited a sol-gel fraction of 98%. Differences in sol-gel fraction for redox systems versus UV systems is attributed to dissociation of the photoinitiator resulting in a pair of free radicals, versus only a single free radical site being generated with the formation of each redox complex site.[45] Furthermore, due to double barrel mixing, initiator concentration is diluted in half upon mixing of the two phases. Therefore, when matched by radical generation, sol-gel fractions were more closely aligned at greater than 95%. Hydrogel swelling ratios further confirmed efficient network formation with a less than 15% change in swelling for all compositions when compared to photoinitiated control. After completing physical characterization of the hydrogel carrier, selection criteria were implemented to identify potential carriers to investigate in cytocompatibility studies. Only initiator concentrations of 2.5 mM or greater were selected for subsequent testing based on the requirements of complete network formation times of less than 10 minutes, sol-gel fraction greater than 80%, and less than 10% change in swelling ratio as compared to the photoinitated control.
Effect of Redox Initiation on Stem Cell-Loaded Hydrogel Viability
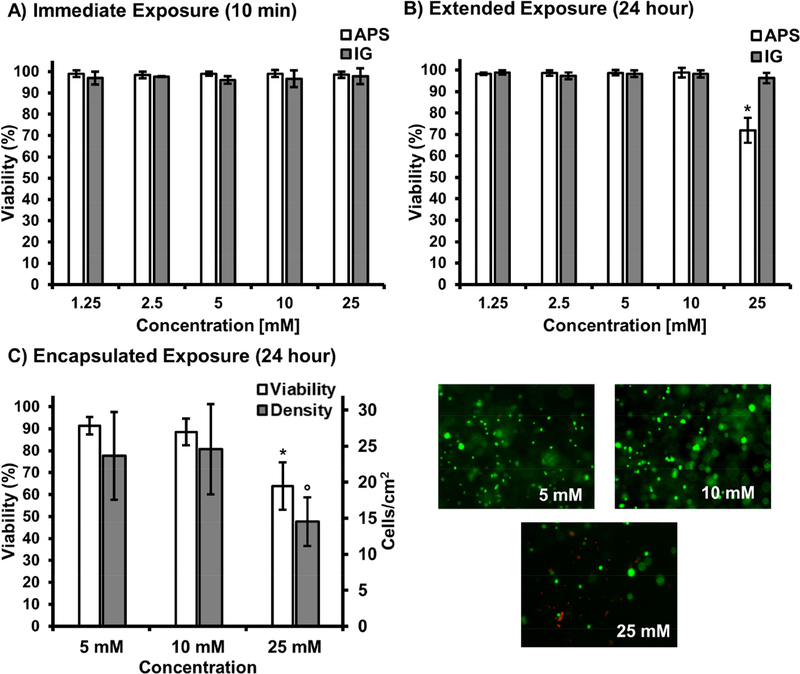
In situ delivery of marrow derived cells within a biodegradable carrier has the potential to improve translation by eliminating timely pre-culture and reducing external equipment required for the surgical procedure. However, these benefits must be achieved without compromising cytocompatibility observed in photoinitiated systems.[49, 50] As PEGDTT has been demonstrated cytocompatible in previous cell encapsulation studies, the only anticipated source of potential cytotoxicity was due to the redox initiators.[51, 52] Cell delivery in this in situ system occurs through mixing of two distinct hydrogel suspensions containing the cell payload and either the initiator or reducing agent. As a result, it was critical to identify the exposure limits of these agents to ensure a formulation was selected that optimizes cell survival during encapsulation. Previously, it has been reported that extended exposure to elevated concentrations of redox initiators (100 mM and greater) can result in poor cytocompatibility resulting from significant changes to pH.[53] However, cell carrier studies that utilize redox initiator concentrations at the maximum end of our experimental design demonstrate marginal pH change in buffered environments, with a return to neutral conditions after mixing.[20, 42] These studies also confirmed cells encapsulated in redox carriers supported markers of osteoblast and chondrocyte differentiation. Cytocompatibility studies were developed to model the in vitro exposure conditions cells would encounter during the encapsulation process to determine an appropriate concentration range for the initiator system. First, viability of non-encapsulated hMSC monolayers was assessed following ten minutes of exposure to either the initiator or reducing agent, mimicking anticipated conditions during early stages of carrier polymerization. As shown in Figure 4A, all concentrations of APS and IG supported viability greater than 95%. Next, to ensure encapsulated cells did not experience long term effects due to precursor exposure, cells were exposed to full initiator concentration for ten minutes and cultured for an additional 24h at a 10-fold dilution to mimic hydrogel swelling and dilution of excess initiator following cure and transfer to culture conditions, Figure 4B. It is anticipated that in vivo application will result in a similar dilution of residual initiator. All APS concentrations lower than 25 mM supported high viability of greater than 95%. The small reduction in viability, ~70%, for the 25 mM APS is attributed to the reduction in media pH resulting from initiator addition and demonstrates cytocompatibility constraints of the system.[42, 53] After confirming hMSCs would survive exposure to initiating agents, the effect of initiator mixing, radical formation, and resultant encapsulation on viability was assessed, Figure 4C. Two critical observations were made during the encapsulation procedure. First, all compositions below 25 mM experienced excellent viability and retained high cell densities, which was consistent with the direct exposure initiator studies. The reduction in viability at elevated initiator concentration was attributed to an increased generation of reactive oxygen species that has previously been shown to result in increased cell damage, apoptosis, and inhibit attachment of mesenchymal stem cells.[54–56] Second, a cell-dependent effect was observed on the lowest, 2.5 mM APS concentration, resulting in the composition failing to cure within the allotted ten minute period allowed for cell encapsulation. This was attributed to the radical scavenging effect of the encapsulated cells that caused the gel to cure slower than observed during rheological characterization. Based on this finding, this combination was deemed not suitable for the in situ encapsulation procedure and not selected for subsequent studies. The hydrogel formulation cured with 10 mM APS concentration and 1:1 ratio of APS:IG was selected for subsequent cell release and osteogenic activity studies.
Figure 4.
Percent hMSC viability following 10 min exposure to redox agents, ammonium persulfate and iron gluconate (A); 24h additional culture (B). Percent hMSC viability and cell density following carrier encapsulation with micrographs illustrating live (green) and dead (red) cells in hydrogel formulations at varying initiator concentrations of 5, 10, 25 mM at 1:1 APS:IG ratio (C). All data represents average ± standard deviation for n = 12. The * represents a significant difference (p<0.05) for the 25 mM concentration of APS compared to all other APS concentrations. The ° represents a significant difference (p<0.05) for the 25 mM concentration of iron gluconate compared to all other iron gluconate concentrations.
Improved Stem Cell Loading of 3D Printed PolyHIPE Scaffolds
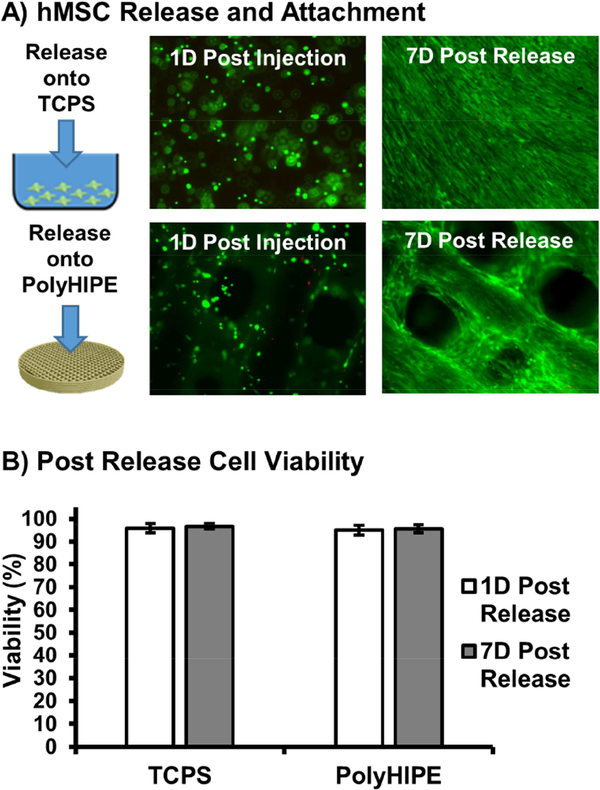
The primary aim of this work was to develop a transient, hydrogel carrier that could improve cell retention during early stages of delivery, then undergo biodegradation and provide targeted stem cell release into 3D printed polyHIPE scaffolds under more hospitable conditions. Degradable hydrogels formed by the Michael-type addition reaction of dithiothreitol and poly(ethylene glycol) diacrylate have been successfully explored for cell encapsulation and delivery applications.[51, 52, 57] Biodegradation is achieved by introducing hydrolytically labile DTT linkages into the macromer backbone. Resultant thioether linkages change the atomic charge of adjacent acrylate ester bonds, increasing susceptibility to nucleophilic attack and hydrolytic degradation.[58] In vitro hydrolytic degradation was first assessed for the selected hydrogel composition prior to cell encapsulation to gain preliminary insight into potential cell release profiles. Supplemental Figure S2 illustrates increases in swelling ratio over the degradation period with complete degradation following one week incubation in buffered saline conditions. Next, cell-loaded PEGDTT hydrogels were cultured for 2 weeks to assess hMSC loaded carrier degradation and viability of released cells. As high encapsulation viability was observed in PEG based systems under this target degradation time frame, any viability effects would arise from exposure to carrier degradation products.[59] Partial release of the hMSC loaded carrier was observed after 4 days in culture, with significant cell numbers remaining entrapped in the hydrogel network. After 7 days in culture, hMSCs were released from the hydrogel carrier and successfully adhered to tissue culture polystyrene substrate and polyHIPE substrates, Figure 5A. Viability was monitored for an additional 7 days to ensure cells remained viable and persisted on the target substrate, Figure 5B. hMSCs released onto 3D printed polyHIPE scaffolds demonstrated viability of greater than 90% with morphology matching that of a direct-seeded polyHIPE scaffold, indicating no effects of degradation products on cell survival.[6] In this study, a single hydrogel degradation profile was studied; however, use of the PEGDTT platform allows for facile tuning of degradation through modulation of the number of DTT linkages or combination with PEGDA. Previously, we demonstrated that varying the compositional ratios of PEGDA and PEGDTT of similar molecular weight can be used to tune degradation rate without affecting other physical properties.[52, 60] It is expected that the in vivo degradation rate will be different, likely faster, than that of the buffered saline experiment; however, the system can be readily tuned to achieve target cell release profiles by adjusting PEGDTT:PEGDA ratio. The ability to modulate carrier degradation profile has been shown to play a major role in cell retention. Qui et al. demonstrated that a range of carrier degradation profiles could be engineered utilizing the PEGDTT macromer and that increasing carrier degradation rate resulted in a greater than 2-fold increase in cell retention in a tendon tissue explant model.[51]
Figure 5.
Micrographs illustrating live (green) and dead (red) cells 1 day post encapsulation and 7 days post release onto TCPS and polyHIPE substrates (A). Percent hMSC viability 1 day and 7 days following release and attachment onto TCPS and polyHIPE substrates (B). All data represents average ± standard deviation for n = 12.
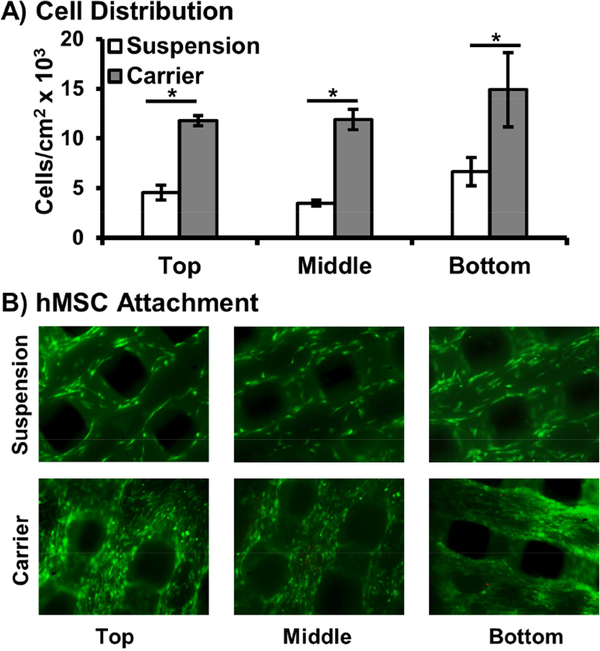
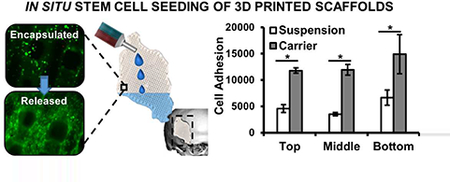
A major hurdle to achieving the full potential of stem cell therapies is retaining cells at the target site after transplantation. Direct injection often results in rapid dispersal of cells away from the injury site, resulting in subtherapeutic retention levels.[13, 61] Rapid curing hydrogels act as a physical barrier to cell dispersal and 3D substrate to improve cell engraftment. A primary hypothesis of this work was that an in situ curing hydrogel carrier would improve spatial distribution and retention of hMSCs in our 3D printed polyHIPE grafts. To this end, cell density was monitored after encapsulation and release onto multilayered polyHIPE scaffolds and compared to a standard suspension seeding method, Figure 6. Cell distribution was quantified at three uniform depths of a multilayered polyHIPE scaffold 3 mm tall. In contrast to standard suspension seeding, carrier loading resulted in uniform cell distribution across the three scaffold layers. Suspension seeding demonstrated irregular distribution with a significantly reduced number of cells observed on the top two layers and high settling at the bottom most layer. Furthermore, a 3-fold increase in cell density was observed on the top two layers for carrier seeding while retaining a 2-fold increase over the bottom layer. This improved control over seeding density across scaffold depth is a significant advantage over standard seeding techniques as seeding density has been shown to influence osteoblastic differentiation of transplanted marrow stromal cells on poly(propylene fumarate) scaffolds.[62, 63] Kim et al. investigated the role of cell-cell paracrine signaling distance, demonstrating that control of cell seeding density could be used to better direct early or late stage osteogenic markers of marrow derived cells and control temporal gene expression profiles of endogenous growth factors.[63] As such, it is hypothesized that improved control over spatial distribution will allow for targeted control over cell activity using these previously reported relationships. Furthermore, it is believed that in vivo application of this system will result in greater improvements in cell retention as rapid wash out effects are often observed during the early stages following surgery and improve spatial distribution.[64] Current studies are investigating improved loading of printed constructs with sizes greater than 2 cm in the aim of developing a platform to explore healing in critical sized defects of more clinically relevant large animal models.[65–67] Additionally, pilot studies are underway to investigate the hypothesis that hydrogel encapsulation of mesenchymal stem cells will improve retention in vivo using a subcutaneous rat model and protect transplanted cells from rapid clearance.
Figure 6.
Distribution of hMSCs using carrier seeding onto multilayer polyHIPE scaffolds determined by dsDNA quantification and compared to suspension seeding control (A). Representative micrographs of top, middle and bottom layers of scaffold (B). All data represents average ± standard deviation for n = 3. The * represents significant difference (p<0.05) between suspension and carrier seeding at specified layer.
Osteoblastic Activity of hMSCs Released into 3D Printed PolyHIPE Scaffolds
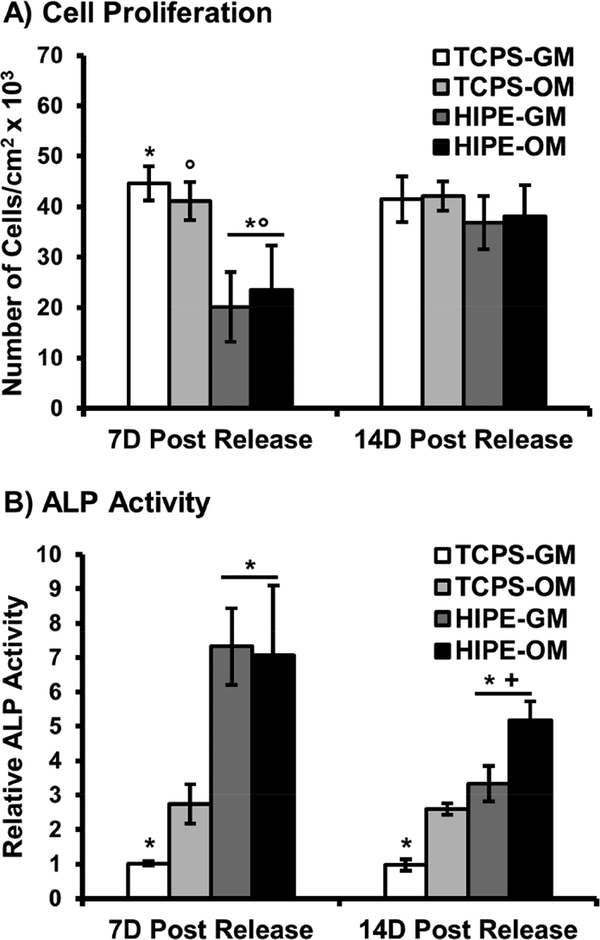
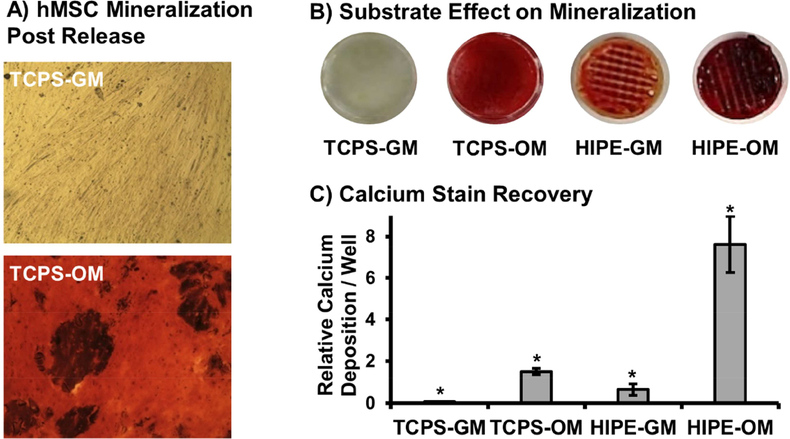
To fully assess the potential of this in situ cell carrier to improve the regenerative capacity of our 3D printed bone graft, it was essential to confirm encapsulated stem cells retained their potency and ability to undergo osteoblastic differentiation post release. To this end, alkaline phosphatase activity was investigated as an early marker of differentiation at 7 and 14 days post release onto tissue culture polystyrene and polyHIPE substrates, Figure 7. ALP activity of hMSCs released onto TCPS increased ~3-fold when cultured in osteogenic conditions compared to control cells cultured in standard growth conditions. A 7-fold increase in ALP activity was observed at 7D post release on polyHIPE substrates, with no statistical effect of culture in osteogenic media observed. This peak was followed by a 5-fold increase in activity at 14D for polyHIPE substrates in osteogenic conditions. These expression profiles support conclusion of osteoblastic differentiation as observed in other systems investigating MSC differentiation.[68, 69] It is noted that cells released onto polyHIPE scaffolds exhibited reduced proliferation rates during the first week of culture that is consistent with activity profiles during early stages of differentiation.[70] Confluency was achieved as confirmed by cell density quantification after two week culture. Next, mineralization was characterized as a late stage marker of differentiation. Alizarin red staining indicated encapsulated hMSCs retained the ability to facilitate mineralization 4 weeks following release onto TCPS substrates, Figure 8A. Calcium deposition was further characterized as a function of TCPS or polyHIPE substrate, Figure 8B-C. A synergistic effect was observed for hMSCs cultured on polyHIPE scaffolds in osteogenic conditions as a 5-fold increase in calcium deposition was observed over hMSCs cultured on TCPS in same conditions. Alizarin red staining was also performed on polyHIPE scaffolds seeded with human dermal fibroblasts to confirm calcium deposition observed in growth conditions over the 4 week period was due to the effects of polyHIPE substrate on hMSC activity, Supplemental Figure S3. Overall, these profiles support retention of stem cell potency after encapsulation and promotion of osteoblastic activity after release onto 3D printed polyHIPEs, demonstrating the strong potential of stem cell seeded polyHIPE grafts.
Figure 7.
Adhesion of hMSCs released onto TCPS and polyHIPE substrates determined by dsDNA quantification (A). Alkaline phosphatase activity of hMSCs 7D and 14D post carrier release. Cells were cultured in growth media (GM) and osteogenic media (OM) as positive control. All data represents average ± standard deviation for n = 3. The * represents significant difference (p<0.05) for TCPS-GM substrates compared to HIPE-GM and HIPE-OM for cell density at 7D and ALP activity at 7D and 14D. The ° represents significant difference (p<0.05) for TCPS-OM substrates compared to HIPE-GM and HIPE-OM for cell density at 7D. The + represents significant difference (p<0.05) for 14D ALP activity on HIPE-GM and HIPE-OM substrates.
Figure 8.
Alizarin red staining of hMSC cultures 4 weeks following carrier release. hMSC mineralization post release onto TCPS substrates in growth vs osteogenic media (A). Effect of release substrate (TCPS vs polyHIPE) and media conditions (growth vs osteogenic) on hMSC mineralization (B). Semi-quantitative analysis of mineralization determined by alizarin red stain recovery (C). All data represents average ± standard deviation for n = 4. The * represents significant difference (p<0.05) between all compositions.
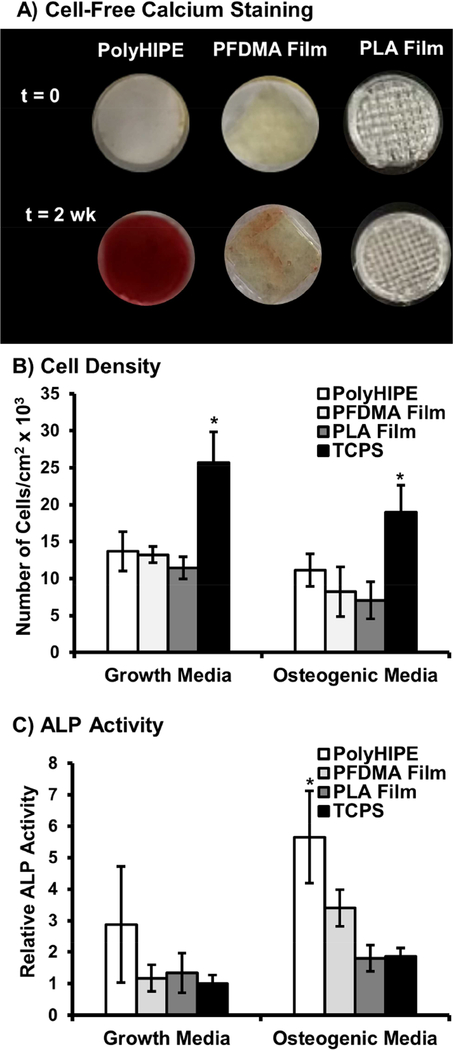
We previously demonstrated the ability of fumarate-based polyHIPEs to promote osteoblastic activity in the absence of dexamethasone supplements through increased deposition of media derived calcium ions onto the polyHIPE surface.[32] In addition to confirming osteogenic potential, this study aimed to elucidate a potential mechanism behind this increased calcium deposition and assess its impact on osteoblastic differentiation. Extracellular calcium is known to play a key role in bone regeneration via direct activation of Ca-sensing receptors that result in increased osteoblast proliferation, expression of osteoinductive factors, and matrix mineralization.[71–73] To this end, three substrates were tested to isolate and explore the effects of surface area and scaffold chemistry on calcium deposition and osteoblastic activity, Figure 9. First, PFDMA polyHIPES were compared to PFDMA films to investigate effects of substrate surface area. Additionally, activity on PFDMA films was compared to PLA films, a commonly selected bone grafting material, to elucidate effects of PFDMA scaffold chemistry in comparison to a clinically relevant material. PLA was selected based on its extensive use as a fixation device in orthopedic repair and has been demonstrated suitable for fabrication of tissue engineered scaffolds.[74, 75] Alizarin red staining was performed on neat, cell-free scaffolds that had been soaked in growth media for 2 weeks for a qualitative assessment of calcium deposition. Fumarate-based chemistries have been shown to support surface mineralization when soaked in concentrated solutions of simulated body fluid.[76] In this study, calcium concentrations were limited to those present in basal media, ~1.6 mM, plus calcium found in the fetal bovine serum supplement. Despite a buffered environment conducive to mineral deposition, minimal calcium was observed on neat PFDMA and PLA films. In contrast, PFDMA polyHIPEs displayed significant levels of staining. Surface roughening has been used previously in titanium implants to provide an improved substrate for apatite precipitation.[77, 78] Specifically, Chen et al. identified surface grooves approximately 3 microns wide to be ideal for surface mineralization.[77] It is hypothesized that the increased surface area of polyHIPE scaffolds over neat films, combined with a pore size on the scale of several microns had a similar effect, providing an ideal substrate for surface deposition. Initial investigation of hMSC activity supports this hypothesis as polyHIPE scaffolds promoted increased levels of ALP activity in standard growth media. Elevated activity was also observed when cultured in osteogenic conditions as the polyHIPE substrate most likely provided an ideal surface for nucleation and mineral growth. Similarly, benefits of increased surface area on osteoblastic activity have been reported previously in osteoinductive grafts such as porous ceramics and have been shown to improve bone regeneration.[73, 79]
Figure 9.
Effect of scaffold chemistry (PFDMA vs PLA films) and scaffold porosity (PFDMA polyHIPE vs PFDMA film) on calcium deposition in cell free conditions (A). Quantification of hMSC adhesion on varied substrate determined by dsDNA quantification (B). Effect of substrate on hMSC alkaline phosphatase activity after 14 days (C). Cells were cultured in growth media (GM) and osteogenic media (OM) as positive control. All data represents average ± standard deviation for n = 4. The * represents significant difference (p<0.05) for indicated composition compared to all others in respective media.
4. Conclusions
This study developed a biodegradable poly(ethylene glycol)-dithiothreitol hydrogel system to serve as injectable stem cell carrier to improve in situ seeding of 3D printed polyHIPE grafts. Oxidant and reductant concentration were modulated to achieve desired cure rates while maintaining high cell viability during cure and after release. This redox-based initiator system demonstrated the ability to encapsulate stem cells without relying on external stimuli (e.g. UV) that can be attenuated in large constructs or tissues. Rapid, in situ seeding of tissue engineered grafts has the potential to reduce the added costs and regulatory delays of lengthy pre-culture periods. As expected, the cell carrier improved cell distribution in 3D printed polyHIPE scaffolds over standard suspension cell seeding. Finally, this work demonstrated that polyHIPEs support long term viability and osteoblastic differentiation of carrier-delivered hMSCs in vitro and provided preliminary investigation into potential osteoinductive mechanisms of these scaffolds. Overall, this approach has strong potential to improve bone regeneration but requires validation in animal models to confirm improved cell retention and the corollary impact on bone regeneration. It is expected that the highly tunable cure rates without external stimuli will facilitate implementation needs of different applications and the programmable degradation rate permits the investigation of the temporal effect of cell release on the regenerative potential of hMSC delivery.
Supplementary Material
ACKNOWLEDGEMENTS
Funding was provided by NIH R21 AR057531 and NIH P41 EB023833. The authors acknowledge Palsgaard USA for providing PGPR 4125. Human mesenchymal stem cells were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40RR017447.
Footnotes
DATA AVAILABILITY STATEMENT
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
SUPPORTING INFORMATION
Quantification of PEGDTT gel fraction and swelling ratio, statistical analysis of cure rate, in vitro hydrolytic degradation profile of encapsulation formulation, and effect of cell type on mineralization is provided as supplementary material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D, et al. Bone autografting of the calvaria and craniofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. World Neurosurgery. 2003;60:71–9. [DOI] [PubMed] [Google Scholar]
- [2].Lanza R, Langer R, Vacanti JP. Principles of tissue engineering: Academic press; 2011. [Google Scholar]
- [3].Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. The lancet. 1999;354:S32–S4. [DOI] [PubMed] [Google Scholar]
- [4].Kim CW, Talac R, Lu L, Moore MJ, Currier BL, Yaszemski MJ. Characterization of porous injectable poly‐(propylene fumarate)‐based bone graft substitute. Journal of Biomedical Materials Research Part A. 2008;85:1114–9. [DOI] [PubMed] [Google Scholar]
- [5].Fisher J P, Holland TA, Dean D, Engel PS, Mikos AG. Synthesis and properties of photocross-linked poly (propylene fumarate) scaffolds. Journal of Biomaterials Science, Polymer Edition. 2001;12:673–87. [DOI] [PubMed] [Google Scholar]
- [6].Sears N, Dhavalikar P, Whitely M, Cosgriff-Hernandez E. Fabrication of biomimetic bone grafts with multi-material 3D printing. Biofabrication. 2017;9:025020. [DOI] [PubMed] [Google Scholar]
- [7].Sears NA, Dhavalikar PS, Cosgriff‐Hernandez EM. Emulsion inks for 3D printing of high porosity materials. Macromolecular rapid communications. 2016;37:1369–74. [DOI] [PubMed] [Google Scholar]
- [8].Caplan A Why are MSCs therapeutic? New data: new insight. Journal of Pathology. 2009;217:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue engineering. 2005;11:1198–211. [DOI] [PubMed] [Google Scholar]
- [10].Caplan Arnold I, Correa D. The msc: an injury drugstore. Cell Stem Cell. 2011;9:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry. 2006;98:1076–84. [DOI] [PubMed] [Google Scholar]
- [12].Burdick JA, Mauck RL, Gerecht S. To serve and protect: hydrogels to improve stem cellbased therapies. Cell stem cell. 2016;18:13–5. [DOI] [PubMed] [Google Scholar]
- [13].Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nature medicine. 1999;5:309. [DOI] [PubMed] [Google Scholar]
- [14].Templin C, Lüscher TF, Landmesser U. Cell-based cardiovascular repair and regeneration in acute myocardial infarction and chronic ischemic cardiomyopathy–current status and future developments. International journal of developmental biology. 2011;55:407–17. [DOI] [PubMed] [Google Scholar]
- [15].Marquardt LM, Heilshorn SC. Design of injectable materials to improve stem cell transplantation. Current stem cell reports. 2016;2:207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gómez‐Barrena E, Rosset P, Müller I, Giordano R, Bunu C, Layrolle P, et al. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. Journal of cellular and molecular medicine. 2011;15:1266–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Loebel C, Burdick JA. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell stem cell. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering Part B: Reviews. 2008;14:149–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hoffman MD, Van Hove AH, Benoit DS. Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta biomaterialia. 2014;10:3431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou H, Xu HH. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 2011;32:7503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vo T, Shah S, Lu S, Tatara A, Lee E, Roh T, et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016;83:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Man Y, Wang P, Guo Y, Xiang L, Yang Y, Qu Y, et al. Angiogenic and osteogenic potential of platelet-rich plasma and adipose-derived stem cell laden alginate microspheres. Biomaterials. 2012;33:8802–11. [DOI] [PubMed] [Google Scholar]
- [24].Yao R, Zhang R, Luan J, Lin F. Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication. 2012;4:025007. [DOI] [PubMed] [Google Scholar]
- [25].Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guo R, Ward CL, Davidson JM, Duvall CL, Wenke JC, Guelcher SA. A transient cellshielding method for viable MSC delivery within hydrophobic scaffolds polymerized in situ. Biomaterials. 2015;54:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tan H, Marra KG. Injectable, biodegradable hydrogels for tissue engineering applications. Materials. 2010;3:1746–67. [Google Scholar]
- [28].Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone research. 2017;5:17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burdick JA, Peterson AJ, Anseth KS. Conversion and temperature profiles during the photoinitiated polymerization of thick orthopaedic biomaterials. Biomaterials. 2001;22:1779–86. [DOI] [PubMed] [Google Scholar]
- [30].Visser J, Melchels FP, Jeon JE, Van Bussel EM, Kimpton LS, Byrne HM, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nature communications. 2015;6:6933. [DOI] [PubMed] [Google Scholar]
- [31].Kim M, Hong B, Lee J, Kim SE, Kang SS, Kim YH, et al. Composite system of PLCL scaffold and heparin-based hydrogel for regeneration of partial-thickness cartilage defects. Biomacromolecules. 2012;13:2287–98. [DOI] [PubMed] [Google Scholar]
- [32].Robinson JL, McEnery MA, Pearce H, Whitely ME, Munoz-Pinto DJ, Hahn MS, et al. Osteoinductive PolyHIPE Foams as Injectable Bone Grafts. Tissue Engineering Part A. 2016;22:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Whitely ME, Robinson JL, Stuebben MC, Pearce HA, McEnery MA, Cosgriff-Hernandez E. Prevention of Oxygen Inhibition of PolyHIPE Radical Polymerization Using a Thiol-Based Cross-Linker. ACS biomaterials science & engineering. 2017;3:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moglia RS, Holm JL, Sears NA, Wilson CJ, Harrison DM, Cosgriff-Hernandez E. Injectable PolyHIPEs as High-Porosity Bone Grafts. Biomacromolecules. 2011;12:3621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Trachtenberg JE, Placone JK, Smith BT, Fisher JP, Mikos AG. Extrusion-based 3D printing of poly (propylene fumarate) scaffolds with hydroxyapatite gradients. Journal of Biomaterials science, Polymer edition. 2017;28:532–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gregory TR. Nucleotypic effects without nuclei: genome size and erythrocyte size in mammals. Genome. 2000;43:895–901. [DOI] [PubMed] [Google Scholar]
- [37].Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature materials. 2008;7:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–23. [DOI] [PubMed] [Google Scholar]
- [39].Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue engineering. 2003;9:679–88. [DOI] [PubMed] [Google Scholar]
- [40].Bowman CN, Kloxin CJ. Toward an enhanced understanding and implementation of photopolymerization reactions. AIChE Journal. 2008;54:2775–95. [Google Scholar]
- [41].Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. Journal of Biomedical Materials Research. 1998;43:422–7. [DOI] [PubMed] [Google Scholar]
- [42].Temenoff JS, Park H, Jabbari E, Conway DE, Sheffield TL, Ambrose CG, et al. Thermally cross-linked oligo (poly (ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules. 2004;5:5–10. [DOI] [PubMed] [Google Scholar]
- [43].Griffin DR, Kasko AM. Photodegradable macromers and hydrogels for live cell encapsulation and release. Journal of the American Chemical Society. 2012;134:13103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mawad D, Martens PJ, Odell RA, Poole-Warren LA. The effect of redox polymerisation on degradation and cell responses to poly (vinyl alcohol) hydrogels. Biomaterials. 2007;28:947–55. [DOI] [PubMed] [Google Scholar]
- [45].Sarac A Redox polymerization. Progress in Polymer Science. 1999;24:1149–204. [Google Scholar]
- [46].Johnson LM, Fairbanks BD, Anseth KS, Bowman CN. Enzyme-mediated redox initiation for hydrogel generation and cellular encapsulation. Biomacromolecules. 2009;10:3114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. Journal of controlled release. 2002;78:199–209. [DOI] [PubMed] [Google Scholar]
- [48].Metters A, Anseth K, Bowman C. Fundamental studies of a novel, biodegradable PEG-bPLA hydrogel. Polymer. 2000;41:3993–4004. [Google Scholar]
- [49].Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–8. [DOI] [PubMed] [Google Scholar]
- [50].Fedorovich NE, Oudshoorn MH, van Geemen D, Hennink WE, Alblas J, Dhert WJ. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:34453. [DOI] [PubMed] [Google Scholar]
- [51].Qiu Y, Lim JJ, Scott L Jr, Adams RC, Bui HT, Temenoff JS. PEG-based hydrogels with tunable degradation characteristics to control delivery of marrow stromal cells for tendon overuse injuries. Acta biomaterialia. 2011;7:959–66. [DOI] [PubMed] [Google Scholar]
- [52].Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly (ethylene glycol) networks. Biomacromolecules. 2008;9:842–9. [DOI] [PubMed] [Google Scholar]
- [53].Temenoff JS, Shin H, Conway DE, Engel PS, Mikos AG. In vitro cytotoxicity of redox radical initiators for cross-linking of oligo (poly (ethylene glycol) fumarate) macromers. Biomacromolecules. 2003;4:1605–13. [DOI] [PubMed] [Google Scholar]
- [54].Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–63. [DOI] [PubMed] [Google Scholar]
- [55].Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem cells and development. 2015;24:1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Roberts JJ, Bryant SJ. Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials. 2013;34:9969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jongpaiboonkit L, King WJ, Lyons GE, Paguirigan AL, Warrick JW, Beebe DJ, et al. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29:3346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Van De Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly (ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. Journal of Controlled Release. 2005;102:619–27. [DOI] [PubMed] [Google Scholar]
- [59].Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. Journal of Biomedical Materials Research Part A. 2004;68:773–82. [DOI] [PubMed] [Google Scholar]
- [60].Cereceres S, Touchet T, Browning MB, Smith C, Rivera J, Hoeoek M, et al. Chronic wound dressings based on collagen-mimetic proteins. Advances in wound care. 2015;4:444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clinical Pharmacology & Therapeutics. 2011;90:532–41. [DOI] [PubMed] [Google Scholar]
- [62].Kim K, Dean D, Lu A, Mikos AG, Fisher JP. Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta biomaterialia. 2011;7:1249–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly (propylene fumarate) disks. Biomacromolecules. 2009;10:1810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Xu HH, Takagi S, Quinn JB, Chow LC. Fast‐setting calcium phosphate scaffolds with tailored macropore formation rates for bone regeneration. Journal of Biomedical Materials Research Part A. 2004;68:725–34. [DOI] [PubMed] [Google Scholar]
- [65].Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. JBJS. 1998;80:985–96. [DOI] [PubMed] [Google Scholar]
- [66].Arinzeh TL, Peter SJ, Archambault MP, Van Den Bos C, Gordon S, Kraus K, et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. JBJS. 2003;85:1927–35. [DOI] [PubMed] [Google Scholar]
- [67].Ball AN, Donahue SW, Wojda SJ, McIlwraith CW, Kawcak CE, Ehrhart N, et al. The Challenges of Promoting Osteogenesis in Segmental Bone Defects and Osteoporosis. Journal of Orthopaedic Research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:103647. [DOI] [PubMed] [Google Scholar]
- [69].Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture‐expanded human mesenchymal stem cells in vitro. Journal of cellular biochemistry. 1997;64:295–312. [PubMed] [Google Scholar]
- [70].Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Critical Reviews in Oral Biology & Medicine. 1992;3:269–305. [DOI] [PubMed] [Google Scholar]
- [71].Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26:4847–55. [DOI] [PubMed] [Google Scholar]
- [72].Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone. 2010;46:571–6. [DOI] [PubMed] [Google Scholar]
- [73].Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. [DOI] [PubMed] [Google Scholar]
- [74].Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Advanced drug delivery reviews. 2016;107:247–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. [DOI] [PubMed] [Google Scholar]
- [76].Lan PX, Lee JW, Seol Y-J, Cho D-W. Development of 3D PPF/DEF scaffolds using microstereolithography and surface modification. Journal of Materials Science: Materials in Medicine. 2009;20:271–9. [DOI] [PubMed] [Google Scholar]
- [77].Chen X, Nouri A, Li Y, Lin J, Hodgson PD, Wen Ce. Effect of surface roughness of Ti, Zr, and TiZr on apatite precipitation from simulated body fluid. Biotechnology and bioengineering. 2008;101:378–87. [DOI] [PubMed] [Google Scholar]
- [78].Chen X-B, Li Y-C, Du Plessis J, Hodgson PD, Wen Ce. Influence of calcium ion deposition on apatite-inducing ability of porous titanium for biomedical applications. Acta biomaterialia. 2009;5:1808–20. [DOI] [PubMed] [Google Scholar]
- [79].Jones JR, Hench LL. Regeneration of trabecular bone using porous ceramics. Current Opinion in Solid State and Materials Science. 2003;7:301–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.