Atherosclerosis is a chronic inflammatory disease of large and medium-sized arteries that causes ischemic heart disease, strokes, and peripheral vascular disease, collectively called cardiovascular disease (CVD). Atherosclerosis requires elevated low-density lipoprotein (LDL) cholesterol, which can be controlled by statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. Both effectively control LDL cholesterol and reduce major adverse cardiovascular events (MACE) by about 50% 1.
When LDL cholesterol is under control, the remaining risk for MACE is believed to be inflammatory in nature. The recent CANTOS trial showed that canakinumab, an antibody blocking IL-1β, reduces MACE 2. However, canakinumab treatment impacted host defense, leading to a significant increase of lethal infections 2.
Atherosclerosis is accompanied by an autoimmune response to LDL and other antigens that can exacerbate or ameliorate the course of the disease. Recently, various approaches to immunotherapy and vaccination have shown promise in curbing atherosclerosis in animal models 3. Unlike antibodies to cytokines, immunotherapies and vaccinations are antigen-specific and thus spare host defense. This viewpoint focuses on the modulation of atherosclerosis by adaptive immune responses, especially CD4 T cells recognizing self-antigens, which forms the basis for the development of a new type of therapy targeting adaptive immune responses.
Immune response in atherosclerosis
Atherosclerotic plaques can become unstable, rupture or erode, which leads to MACE. Plaque stability is related to the level of inflammatory cells and the thickness of the cap: plaques with thin caps and full of immune cells are called “soft” or vulnerable plaques. Immune cell infiltration is initiated by chemokines and adhesion molecules.
Initially, the innate immune response regulates the adaptive immune response 4. Antigen-presenting cells provide major histocompatibility complex (MHC) molecules, costimulatory molecules and cytokines in response to molecules derived from pathogens, microbes and altered self 5 and thereby determine the polarization of the adaptive immune response. Macrophages and dendritic cells are found in the arterial adventitia and neointima and are activated by Toll-like receptor ligands and scavenger receptors 6. Inflammatory cytokines exacerbate and perpetuate atherosclerosis and attract more immune cells. The inflammatory cytokine IL-1β is a proven therapeutic target to treat atherosclerosis 2. In mature atherosclerotic plaque, atherosclerosis antigen-specific T cells secrete cytokines, perpetuate inflammation and shape the immune cell infiltrate 7 (Figure).
Figure. Adaptive immune responses in atherosclerosis.
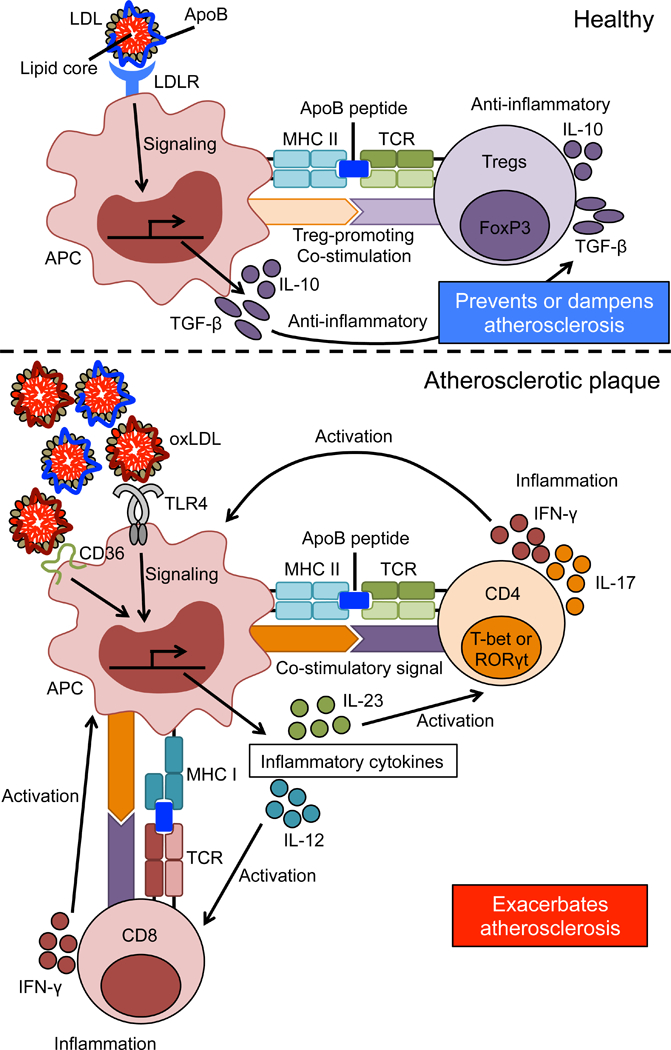
Top: In healthy individuals, LDLR on antigen presenting cells (APCs) recognizes and takes up LDL, inducing production of anti-inflammatory cytokines. Self-reactive Tregs recognize ApoB peptides presented by MHC-II on APCs in the context of co-stimulatory molecules that promote Tregs, which also produce anti-inflammatory cytokines production, thereby preventing or dampening atherosclerosis.
Bottom: Modified and oxidized (ox)LDL accumulates in atherosclerotic lesions and is recognized by receptors like TLR14 and CD36 to induce transcription of inflammatory cytokines like IL-12 and IL-23. CD4 T cells recognize ApoB peptides in the context of pro-inflammatory cytokines and strong costimulation, which induces Th1 (T-bet+) and Th17 (RORγt+) T cells. Their production of IFN-γ and IL-17 exacerbates atherosclerosis. Now, CD8 T cells also recognize ApoB epitopes presented by MHC-I and exacerbate inflammation by producing IFN-γ. TCR: T cell receptor, TLR4: toll-like receptor 4.
Atherosclerosis is always accompanied by an autoimmune response. Antibodies against oxidized (oxLDL) are produced by B-cell-derived plasma cells and are detectable in the serum of humans or animals with atherosclerosis. T cells against atherosclerosis antigens are also found. Almost all the mechanistic insight into the role of immune cells in atherosclerosis comes from two mouse models, Apoe−/− mice and Ldlr−/− mice. Regulatory CD4 T cells (Tregs) have long known to be atheroprotective in these mouse models 8.
Recently, we showed that CD4 T cells specific for an epitope in apolipoprotein B, the core lipoprotein of LDL, VLDL, and chylomicron, are mostly Tregs in people without CVD, but assume mixed and effector phenotypes in people with CVD 9. The exact mechanism of atheroprotection may involve other T and B cell subsets and is an area of active investigation.
Chronic inflammatory diseases with a secondary autoimmune component
Autoimmune diseases are defined by autoantibodies, autoreactive cells, identifiable autoantigen(s), and transmission by immunization. Atherosclerosis is not a classic autoimmune disease. In atherosclerosis, autoantibodies, T cells and B cells have all been shown to exacerbate or ameliorate10 the disease. Similarly, Parkinson’s disease and emphysema are not considered autoimmune diseases, but Parkinson’s and emphysema patients harbor epitope-specific T and B cells. Thus, we propose that these three diseases form a new class of chronic inflammatory diseases with a secondary autoimmune component.
Atherosclerosis and autoreactive CD4 T cells
Patients with atherosclerosis have anti-oxLDL and anti-heat-shock protein (hsp) 65 antibodies. However, anti-oxLDL antibodies are also found in healthy individuals and might protect against atherosclerosis and CVD. The majority of CD4+ T cells in atherosclerotic lesions are memory T cells (CD45RO+) and respond to oxLDL to produce inflammatory cytokines. Many immunogenic epitopes derived from mouse ApoB have been identified and used as vaccine antigens against atherosclerosis in animal models.
Until recently, there was no direct evidence that ApoB epitope-specific CD4 T cells exist. To address this question, we developed MHC-II tetramers to detect such cells in both humans and mice. A tetramer consists of four molecules of recombinant MHC-II loaded with the antigenic peptide and held together by fluorochrome-conjugated streptavidin. We focused on the ApoB epitope P18, which is sequence-identical in mouse ApoB and human APOB. P18 binds the mouse MHC-II allele I-Ab and DRB1*07:01 expressed by about 8% of humans. To detect APOB-specific CD4+ T cells, we created human APOB-peptide P18:DRB1*07:01 tetramers and found that P18-recognizing CD4+ T cells exist in human PBMCs. These P18-specific CD4+ T cells were found in subjects with and without subclinical CVD. Interestingly, the majority of P18-specific CD4+ T cells from individuals without CVD expressed FoxP3, the defining transcription factor of Tregs. On the other hand, P18-specific CD4+ T cells from subclinical CVD patients expressed the Th17-defining transcription factor RORγτ or the Th1-defining transcription factor T-bet alone or together with FoxP3 9. This is the first evidence that self-peptide recognizing CD4+ T cells exist in human PBMCs. The phenotype of these cells appears to change during atherosclerosis progression.
Future atherosclerosis therapy and prevention
Vaccination is the most successful intervention in medicine. At least 27 infectious diseases and several cancers are preventable by vaccination. Now, the vaccine development field is moving from vaccines against infectious diseases to vaccines for non-communicable diseases such as cancer, atherosclerosis, hypertension, Alzheimer’s disease, and diabetes.
To develop an atherosclerosis vaccine, vaccine antigen(s) must be identified. Possible atherosclerosis vaccine antigens include PCSK9, HSP65, and ApoB. The concept of a vaccine against PCSK9 is to induce a neutralizing antibody response11. This is similar to the use of monoclonal antibodies (passive immunization). Antibodies to PCSK9 are already in clinical use. Targeting PCSK9 is known to be safe, because humans with null mutations in PCSK9 are asymptomatic except for being resistant to atherosclerosis 12.
Immunization with ApoB-derived MHC-II-restricted peptides strongly induced peptide-specific antibody responses in Apoe- or Ldlr-deficient mice, associated with reduced atherosclerotic lesions 13. This observation resulted in a clinical trial (GLACIER), in which a monoclonal antibody to oxLDL was administered 14. Atherosclerosis was assessed by PET imaging after injection of FDG glucose, a marker for myeloid cell accumulation. This trial showed no evidence of efficacy of antibody treatment. In mouse models, it is known that vaccine-induced antibody responses are dispensable for atheroprotection 15.
Several vaccines against ApoB induce anti-inflammatory responses such as IL-10 production or Tregs. Immunization with the APOB-derived peptide P210 elicited CD4 T cell responses with IL-10 production. Immunization with other ApoB-derived peptides also induced IL-10+ and FoxP3+CD4+ T cells. However, these studies did not demonstrate induction of vaccine antigen-specific CD4 T cells9. Interestingly, P18 immunization increased the number of P18-specific CD4 T cells compared to the adjuvant only group, and many vaccine-induced P18-specific CD4 T cells were Tregs. We also found that P18-specific CD4 T cells produced IL-10 and P18 immunization significantly reduced atherosclerotic lesions in Apoe−/− mice 9. Thus, vaccines inducing antigen-specific Tregs might be a novel and viable approach for atherosclerosis therapy. Important for clinical translation, Addavax, a squalene oil similar to the clinically approved MF59, may be useful as an adjuvant for a future atherosclerosis vaccine for humans 15.
Conclusions
In spite of the success of statins and PCSK9 antibodies, atherosclerosis-triggered diseases are still the #1 killer in the world. Atherosclerosis is a chronic inflammatory disease with a secondary autoimmune component. Self antigen-specific adaptive immune responses are found in both humans with atherosclerosis and in animal models. These immune responses are strongly involved in the progression of atherosclerosis and atheroprotection. The recent discovery of APOB-specific CD4 T cells in both humans and mice and the induction of Tregs by vaccination with an ApoB peptide suggests that vaccination to self antigens could be a viable approach to prevent atherosclerosis. It is currently unknown how long the vaccination-induced atheroprotection lasts, how often the vaccine would have to be administered, and what the best dose, formulation and route of administration would be.
Acknowledgments
Sources of Funding
This work was supported by grant from the National Institutes of Health R01 HL121697, P01 HL088093, and P01 HL136275 to K.L.
Footnotes
Disclosures
None.
References
- 1.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ Jr, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, Group JS . Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. The New England journal of medicine. 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory therapy with canakinumab for atherosclerotic disease. The New England journal of medicine. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 3.Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity. 2015;48:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills CD, Ley K, Buchmann K, Canton J. Sequential immune responses: The weapons of immunity. Journal of innate immunity. 2015;7:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley K, Pramod AB, Croft M, Ravichandran KS, Ting JP. How mouse macrophages sense what is going on. Frontiers in immunology. 2016;7:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clinical immunology. 2010;134:5–24 [DOI] [PubMed] [Google Scholar]
- 7.Wolf D, Zirlik A, Ley K. Beyond vascular inflammation--recent advances in understanding atherosclerosis. Cellular and molecular life sciences : CMLS. 2015;72:3853–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nature medicine. 2006;12:178–180 [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, Jenkins MK, James EA, Kwok WW, Hanna DB, Kaplan RC, Strickler HD, Durkin HG, Kassaye SG, Karim R, Tien PC, Landay AL, Gange SJ, Sidney J, Sette A, Ley K. Regulatory cd4(+) t cells recognize mhc-ii-restricted peptide epitopes of apolipoprotein b. Circulation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley K 2015 russell ross memorial lecture in vascular biology: Protective autoimmunity in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chackerian B, Remaley A. Vaccine strategies for lowering ldl by immunization against proprotein convertase subtilisin/kexin type 9. Current opinion in lipidology. 2016;27:345–350 [DOI] [PubMed] [Google Scholar]
- 12.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH Jr. Sequence variations in pcsk9, low ldl, and protection against coronary heart disease. The New England journal of medicine. 2006;354:1264–1272 [DOI] [PubMed] [Google Scholar]
- 13.Gistera A, Klement ML, Polyzos KA, Mailer RK, Duhlin A, Karlsson MCI, Ketelhuth DFJ, Hansson GK. Ldl-reactive t cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehrer-Graiwer J, Singh P, Abdelbaky A, Vucic E, Korsgren M, Baruch A, Fredrickson J, van Bruggen N, Tang MT, Frendeus B, Rudd JH, Hsieh F, Ballantyne CM, Ghoshhajra B, Rosenson RS, Koren M, Roth EM, Duprez DA, Fayad ZA, Tawakol AA. Fdg-pet imaging for oxidized ldl in stable atherosclerotic disease: A phase ii study of safety, tolerability, and anti-inflammatory activity. JACC. Cardiovascular imaging. 2015;8:493–494 [DOI] [PubMed] [Google Scholar]
- 15.Kobiyama K, Vassallo M, Mitzi J, Winkels H, Pei H, Kimura T, Miller J, Wolf D, Ley K. A clinically applicable adjuvant for an atherosclerosis vaccine in mice. European journal of immunology. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


