Abstract
As the number of older adults in the U.S. increases, so too will the incidence of cancer and cancer-related cognitive impairment (CRCI). However, the exact underlying biological mechanism for CRCI is not yet well understood. We utilized susceptibility-weighted imaging with quantitative susceptibility mapping, a non-invasive MRI-based technique, to assess longitudinal iron deposition in subcortical gray matter structures and evaluate its association with cognitive performance in women age 60+ with breast cancer receiving adjuvant chemotherapy and age-matched women without breast cancer as controls. Brain MRI scans and neurocognitive scores from the NIH Toolbox for Cognition were obtained before chemotherapy (time point 1) and within one month after the last infusion of chemotherapy for the patients and at matched intervals for the controls (time point 2). There were 14 patients age 60+ with breast cancer (mean age 66.3 ± 5.3 years) and 13 controls (mean age 68.2 ± 6.1 years) included in this study. Brain iron increased as age increased. There were no significant between- or within-group differences in neurocognitive scores or iron deposition at time point 1 or between time points 1 and 2 (p > 0.01). However, there was a negative correlation between iron in the globus pallidus and the fluid cognition composite scores in the control group at time point 1 (r= −0.71; p < 0.01), but not in the chemotherapy group. Baseline iron in the putamen was negatively associated with changes in the oral reading recognition scores in the control group (r=0.74, p < 0.01), but not in the chemotherapy group. Brain iron assessment did not indicate cancer or chemotherapy related short-term differences, yet some associations with cognition were observed. Studies with larger samples and longer follow-up intervals are warranted.
Keywords: Brain iron, quantitative susceptibility mapping (QSM), gray matter nucleus, cancer-related cognitive impairment (CRCI), breast cancer, chemotherapy
1. Introduction
Cancer is a disease of aging [1] and the number of older adults in the U.S. is growing.[2] Because the number of older adults with cancer is increasing, understanding cancer-related cognitive impairment (CRCI) in older adults has been identified as a research priority. [3] A subset of patients report cognitive changes during and after cancer [4] and systemic chemotherapy has been implicated as a potential cause.[5, 6] The cognitive side effects of chemotherapy may disrupt an individual’s ability to concentrate and plan [7], and thus hinder independence. As a result, older adults have expressed that they are less likely to accept a treatment that has cognitive side effects.[8] In addition, prior research has provided evidence that the underlying biological mechanisms of CRCI may overlap with those of aging, thus contributing to accelerated aging.[9] Therefore, a clearer understanding of the mechanisms underlying CRCI could improve diagnosis and treatment, thereby improving quality of life for cancer survivors and other aging individuals.
Candidate mechanisms for CRCI include the following: direct neurotoxic effects of chemotherapy drugs crossing the blood brain barrier, secondary effects of neuroinflammation, DNA damage, and host factors, such as genetic predisposition.[10–12] However, the exact underlying biological mechanism is not yet well understood. There is increased inflammation in the brain after exposure to chemotherapy, with increased levels of protein oxidation and lipid peroxidation.[13] New non-invasive neuroimaging techniques that measure brain structural changes such as iron deposition could provide valuable novel insight into CRCI and aging.
Brain iron deposition measured by MRI methods is considered a proxy indicator and biomarker of brain function and cognition in both healthy aging and neurodegenerative disorders [14]. Further, increased iron content in the caudate nucleus, a subcortical gray matter structure, was associated with reduced improvement in working memory after repeated testing in healthy adults.[15, 16] Iron deposition in subcortical gray matter nuclei has been implicated in brain aging and cognition.[14] However, the relationship between brain iron deposition and CRCI in older adults with cancer is not known.
The primary objective of this study was to assess short-term changes in brain iron deposition in women age 60+ with breast cancer receiving adjuvant chemotherapy and compared to similarly aged women without breast cancer using susceptibility-weighted imaging (SWI) with quantitative susceptibility mapping, a non-invasive brain MRI technique.
2. Materials and methods
2.1. Study design
The present study is a prospective longitudinal study of older women with breast cancer scheduled to receive adjuvant chemotherapy and age- & sex-matched healthy controls. The eligibility criteria for women with cancer included the following: stage I-III breast cancer, age ≥ 60 years, and scheduled to receive adjuvant chemotherapy. The exclusion criteria for the women with cancer included the following: metastatic disease, history of neurological or psychiatric disorders or stroke, and MRI exclusion criteria such as claustrophobia, cardiac pacemaker, and orbital metal implants. Age-matched female controls with no history of cancer or chemotherapy exposure were recruited from the community with similar inclusion and exclusion criteria, except the control participants did not have a cancer diagnosis. The study design and this study cohort have been reported previously.[17] This study was carried out in accordance with the recommendations of the Institutional Review Board at City of Hope National Medical Center. The protocol was approved by the Institutional Review Board at City of Hope National Medical Center. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
A pre-chemotherapy assessment was performed after surgery and before the start of adjuvant chemotherapy (time point 1, baseline). This assessment included a brain MRI scan and neuropsychological testing using the NIH Toolbox for Cognition.[18, 19] The follow-up assessment with the same components for the women with breast cancer was conducted within one month after the last infusion of chemotherapy (time point 2). The chemotherapy treatment lasted approximately 60 to 120 days in duration. So, the follow-up intervals between time point 1 at baseline and time point 2 were 3 to 5 months counting in the duration of chemotherapy treatment. The healthy control group underwent the same assessments at matched intervals as the breast cancer patient group receiving adjuvant chemotherapy.
2.2. Iron quantification
All brain MRI scans were performed on the same in-house Siemens 3T Verio magnet (Siemens, Erlangen, Germany). A 3D flow-compensated SWI sequence was acquired as part of the brain MRI protocol. The SWI scan parameters included the following: TR/TE=27/18 ms, receiver bandwidth=120 Hz/pixel, Flip angle=15o; number of slices=128, voxel size=0.5×0.5×1.0 mm3 with flow compensation. The 3D T2-weighted sagittal FLAIR and T1-weighted 3D MPRAGE sequences were used to rule out brain structural abnormalities.
SWI phase images on the brain MRI scans were used to generate images for quantitative susceptibility mapping to measure brain iron levels. SWI phase images were processed to generate susceptibility maps using the MATLAB-based software SMART v2.0 (Susceptibility Mapping and phase Artifacts Removal Toolbox, MRI Institute for Biomedical Research, Detroit, MI). This post-processing method was published previously.[20] We have used threshold-based k-space division (TKD) as the method of choice for susceptibility measurements in this study.[21, 22] Susceptibility maps were generated using the following steps: BET for brain tissue extraction [23] using the magnitude images, 3D-SRNCP for phase unwrapping [24], SHARP for background field removal [25], and finally an iterative TKD algorithm for QSM reconstruction [21, 26]. The iterative QSM reconstruction algorithm reduces streaking artifact and is a very fast algorithm [21, 27, 28]. It has also been demonstrated that the iron content quantified using this technique results in similar values when compared to other susceptibility mapping techniques in different brain structures [22, 29]. Moreover, TKD has shown consistency with the literature when it is utilized to compare ferritin levels to actual iron concentration in various brain structures.[29]To address the issue of normalization for iron measurements, we chose 10 random cases from this study and performed two ways of measuring mean susceptibility values for the caudate nucleus (CN), putamen (PUT) and globus pallidus (GP): 1) Mean susceptibility of the structures without normalization, and 2) mean susceptibility of a homogenous area in the CSF subtracted from the mean susceptibility of the structures. For all three structures, there were no significant differences observed between the means of the susceptibilities measured using these two methods. This is not surprising since the offset in susceptibility mapping is arbitrary and it is only differences in susceptibility that matter. Furthermore, since this study was a longitudinal study with a relative comparison of brain iron deposition before and after chemotherapy, normalization of the values would not be critical as the reference susceptibilities of the corresponding measurements would have cancelled each other out in estimating changes in susceptibility.
Manual segmentation of the subcortical gray matter structures, including the caudate nucleus, globus pallidus, putamen, and thalamus, was performed according to the anatomical features in the susceptibility maps. We exercised caution to ensure accurate drawing of the boundaries for each subcortical structure, especially for the thalamus which had less clear borders. The boundaries for the thalamus were first drawn on the QSM maps and then were copied to the corresponding T1 image. If necessary, the boundaries of thalamus were slightly modified on T1 data and then were copied back to the QSM maps for final measurement. Contiguous scan slices were drawn manually to cover the subcortical gray matter nuclei for evaluation of their magnetic susceptibility in 3D (Fig 1). The manual segmentation of MRI images was performed by a co-author (KB) with more than five years of experience under direct supervision of a senior researcher and co-author (EMH). For the whole-region analysis, the 3D regions of interest (ROI) were analyzed for iron deposition quantitatively using the software SPIN (Signal Processing in NMR, MR Innovations, Detroit, MI).[29] An age-related threshold was applied to split each ROI into a global low-iron-content region and a high-iron-content region. For each structure, the thresholds used were the upper 95% prediction interval values for the whole-region analysis linear regressions. Any pixels with iron content above this threshold were allocated to the high iron content region, using a previously published methodology for assessing iron content.[29] The brain iron deposition value for each nucleus was the mean value for both hemispheres.
Fig 1. Representative images for brain iron measurement in a 66-year-old female control subject.
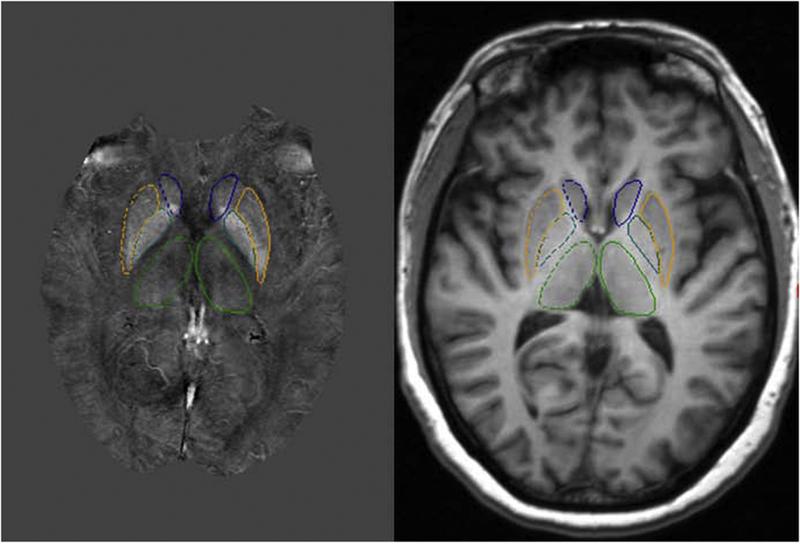
Left panel: showing regions of interest manually traced on quantitative susceptibility mapping of the subcortical gray matter nuclei. Right panel: showing corresponding 3D axial T1- weighted image at the same brain level as the left panel, indicating the anatomical reference of the subcortical gray matter nuclei. The subcortical gray matter nuclei included the following: caudate nucleus (blue), globus pallidus (green blue), putamen (orange) and thalamus (green).
2.3. Neuropsychological testing
All study participants completed neuropsychological testing using the NIH Toolbox for Cognition.[19] The NIH Toolbox cognition batteries yielded three summary composite scores and seven individual scores for cognitive subdomains, including executive function and attention, episodic memory, working memory, and language processing. All the neuropsychological scores were standardized and reported using a mean of 100 with a standard deviation of 15.[31] The performance for each individual was compared to a nationally representative sample and adjusted for key demographic variables such as age, gender, race, and education. Higher scores indicated higher levels of cognitive functioning. The neuropsychological batteries were administered outside of the MRI scanner. The cognitive assessment was overseen by the study neuropsychologist (SKP) who has close to 20 years of clinical andresearch experience in neuropsychological assessment of cancer patients.
2.4. Demographic and disease characteristics
The participants’ demographic characteristics, including age, education, race, and ethnicity, were obtained through a self-report questionnaire. Disease stage and treatment information (chemotherapy regimen) were obtained through medical record abstraction. Treatment duration was calculated as the days between the first and last infusion of chemotherapy.
2.5. Statistical analysis
The controls were frequency matched to the patients with breast cancer for age distribution. Unconditional logistic regression was used to compare the patients with breast cancer receiving chemotherapy and the controls for ethnicity and education. All controls were white; thus, Fisher’s exact test was used to compare the race and ethnicity distribution between the patients and the controls.
Linear mixed modeling, considering the correlation of repeated measurements within subjects, was used for longitudinal analysis.[32] Within-subject correlations were accounted for by using a compound symmetry covariance structure. Time point (1 vs. 2) and group (chemotherapy group vs. control group) were both considered categorical fixed effects in the model. The group-by-time interaction term was included in the model to examine whether the changes in brain iron in the chemotherapy group differed significantly from those of the control group. Using this linear mixed effects model with a compound symmetry covariance structure to account for correlation between repeated measurements, we examined: 1) whether there were any differences in brain iron deposition between the chemotherapy group and the control group at time point 1 and time point 2; 2) whether there were any significant changes from time point 1 to time point 2 within the chemotherapy group and within the control group; and 3) whether the iron deposition changes differed by group (group-by-time interaction).
Correlative analyses were conducted between baseline brain iron deposition and age, and the neuropsychological testing scores in the control group and the chemotherapy group. Pearson correlation coefficients and p-values were computed. All statistical tests were two-sided. This study measured the iron deposition in the subcortical nuclei including the caudate nucleus, globus pallidus, putamen, and thalamus. A conservative Bonferroni method was used to correct for multiple testing; thus, p <0.01 were considered significant. The data were analyzed using SAS 9.3 (SAS Institute, Cary, NC).
3. Results
3.1. Demographic data
The demographic data for all study participants are summarized in Table 1. A total of 16 patients with breast cancer and 15 age-matched controls without cancer were enrolled into the study. Two patients were excluded: one patient had poor quality SWI scan images from cusp artifacts in the original phase images, and the second patient did not complete the SWI sequence for time point 2. Two controls were also excluded: one control had noisy SWI post-processing data with poor quality, and the second control had an extreme sinus artifact at time point 2. Therefore, there were 14 patients with breast cancer (mean age 66.3 ± 5.3 years, range 60–82 years) and 13 healthy controls (mean age 68.2 ± 6.1 years, range 60–78 years) in this analysis. There were no significant differences between the chemotherapy group and the control group for age or overall education (p=0.25). All study participants were female and right-handed. The chemotherapy group included 10 (71.4%) white and 4 (28.6%) African-American patients, while all controls (n=13) were white (p=0.04). The difference in ethnicity (non-Hispanic versus Hispanic) between the groups was not significant. There were 6 (42.9%) patients with stage I, 5 (35.7%) patients with stage II, and 3 (21.4%) patients with stage III breast cancer. Out of the 14 patients, seven received docetaxel and cyclophosphamide (TC) and seven received a chemotherapy regimen other than TC: two received paclitaxel and trastuzumab, two received a regimen with trastuzumab, docetaxel, carboplatin (TCH) plus pertuzumab, and the remaining 3 patients received different chemotherapy regimens, as noted in Table 1. The median treatment duration was 63 days.
Table 1.
Demographic and disease characteristics for study participants.
| Variables | Control Group (n=13) |
Chemotherapy Group (n=14) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 68.2 (6.1) | 66.3 (5.3) |
| Median (range) | 67.0 (60–78) | 65.5 (60–82) |
| Race | ||
| White | 13 (100%) | 10 (71.4%) |
| African-American | 0 | 4 (28.6%) |
| Ethnicity | ||
| Non-Hispanic | 11 (84.6%) | 12 (85.7%) |
| Hispanic | 2 (15.4%) | 2 (14.3%) |
| Education | ||
| High school | 1 (8%) | 4 (29%) |
| College | 9 (69%) | 9 (64%) |
| Advanced degree | 3 (23%) | 1 (7%) |
| Treatment Duration (days) | ||
| Mean (SD) | N/A | 75.4 (21.2) |
| Median (range) | N/A | 63.0 (42–112) |
| Stage | ||
| I | N/A | 6 (42.9%) |
| II | N/A | 5 (35.7%) |
| III | N/A | 3 (21.4%) |
| Regimen | ||
| TC | N/A | 7 (50.1%) |
| TCH + pertuzumab | N/A | 2 (14.3%) |
| Paclitaxel/trastuzumab | N/A | 2 (14.3%) |
| TAC | N/A | 1 (7.1%) |
| Carboplatin/paclitaxel | N/A | 1 (7.1%) |
| ddAC followed by paclitaxel | N/A | 1 (7.1%) |
Abbreviations for chemotherapy regimen: TC: docetaxel and cyclophosphamide; TCH + pertuzumab: trastuzumab, pertuzumab, docetaxel, carboplatin; paclitaxel/trastuzumab: paclitaxel, trastuzumab; TAC: docetaxel, doxorubicin, and cyclophosphamide; carboplatin/paclitaxel: carboplatin, paclitaxel; ddAC followed by paclitaxel: dose-dense Adriamycin and cyclophosphamide followed by paclitaxel.
3.2. Brain iron deposition
Brain iron deposition at time point 1:
Table 2 summarizes the baseline brain iron deposition for the control group and the chemotherapy group. No significant differences were observed between the groups. The iron deposition in the globus pallidus showed a trend toward increase in the chemotherapy group (242.1 ppb) compared to the control group (228.3 ppb) with a p- value of 0.04, which was not considered statistically significant after correction for multiple comparisons.
Table 2.
Baseline comparison of brain iron deposition (ppb [STD]) in subcortical gray matter structures between the control group and chemotherapy group.
| Region | Overall N=27 |
Control Group N=13 |
Chemotherapy Group N=14 |
Difference | p-value |
|---|---|---|---|---|---|
| Caudate nucleus | 105.3 (12.9) | 106.8 (14.4) | 103.8 (11.8) | −3.0 | 0.56 |
| Globus pallidus | 235.5 (20.4) | 228.3 (10.9) | 242.1 (25.1) | 13.8 | 0.04 |
| Putamen | 159.4 (16.9) | 162.9 (16.5) | 156.2 (17.2) | −6.8 | 0.34 |
| Thalamus | 30.9 (6.9) | 29.4 (4.0) | 32.4 (8.7) | 3.0 | 0.27 |
Note: The brain iron deposition value for each nucleus was the mean value for both hemispheres in the unit of ppb. The brain iron deposition values presented here were the high iron values (RII) in the subcortical gray matter nuclei.
Overall relationship between brain iron deposition and age:
The mean susceptibility for each of the measured subcortical gray matter structures in the control and the chemotherapy groups, for both time points, increased as a function of age (Fig 2). The mean susceptibility values from this study followed a linear upward trend with increasing age and were fitted into the upper right extension of the previously published data from a normal population-based study.[29] In addition, the mean brain iron deposition values in the high-iron regions for both groups and for both time points fell within the 95% prediction intervals of the normal population for all four subcortical gray matter structures, based on data from the same published study.[29]
Fig 2. Brain iron deposition indicated by the mean susceptibility for the high iron regions as a function of age in the caudate nucleus.
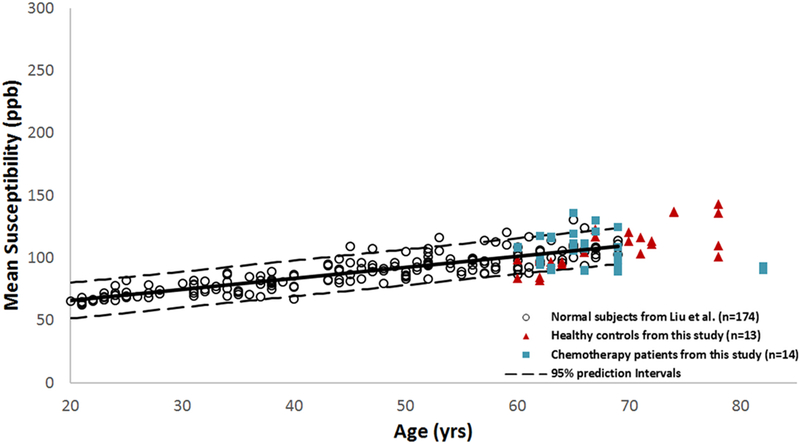
Brain iron mean susceptibility values for both controls and chemotherapy patients for both time point 1 and time point 2 followed the linear upward trend and were fitted into the upper right extension of the previously published data from a normal population-based study.[29]
Correlation between brain iron deposition and age at time point 1:
There was a positive correlation between brain iron deposition and age in the control group at time point 1, especially in the caudate nucleus (r=0.75, p=0.003) (Fig 3). In the chemotherapy group at time point 1, we observed a non-significant negative correlation (r=-0.17, p=0.55) initially. However, after excluding one outlier patient (age 82), the negative correlation in the chemotherapy group did not persist. Nevertheless, we did not observe similar positive correlation between iron and age in the chemotherapy group as noted in the control group at time point 1. There was no significant correlation between caudate nucleus and age in the chemotherapy group after excluding the 82-year-old outlier patient at time point 1(r=0.26, p=0.39) (Fig 3).
Fig 3. Correlations between brain iron mean susceptibility values in the high iron regions and age at baseline.
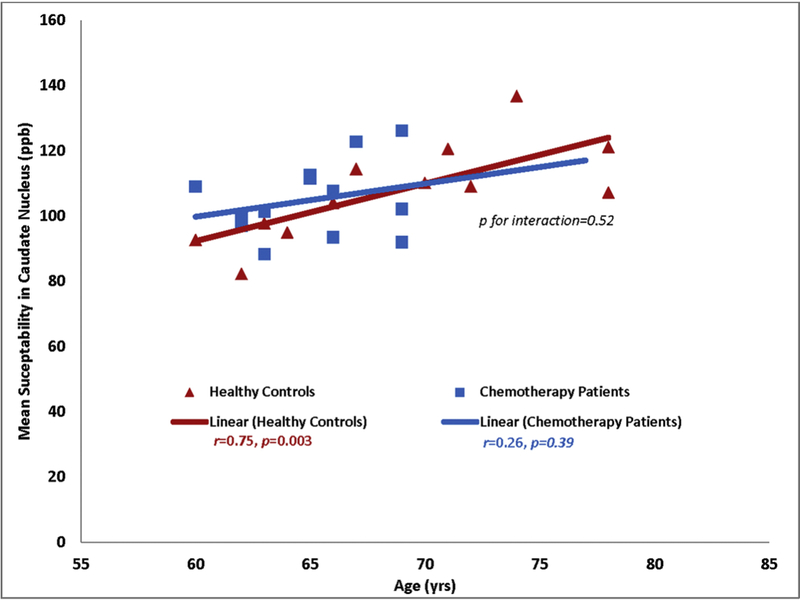
Age-associated increases in brain iron mean susceptibility values were noted in the control group but not in the chemotherapy group. This figure showed the iron data for the caudate nucleus after excluding an 82-year-old outlier patient.
Longitudinal changes in brain iron deposition:
Within each group, there were no statistically significant changes in brain iron deposition values between time points 1 and 2. All p-values were greater than 0.01 (Table 3). No significant between-group difference was noted at time point 2. There were also no significant group-by-time interactions when comparing the brain iron deposition changes between the groups.
Table 3.
Longitudinal changes in brain iron deposition in the control group and chemotherapy group.
| Control Group | Chemotherapy Group | |||
|---|---|---|---|---|
| Region | Iron changes (STD) | p-value | Iron changes (STD) | p-value |
| Caudate nucleus | −0.6 (10.1) | 0.85 | −2.9 (10.8) | 0.31 |
| Globus pallidus | 0.5 (6.6) | 0.92 | −5.9 (23.4) | 0.22 |
| Putamen | 0.1 (5.5) | 0.94 | 2.2 (5.3) | 0.14 |
| Thalamus | −0.6 (2.8) | 0.40 | −0.1 (2.5) | 0.88 |
Note: The brain iron deposition value for each nucleus was the mean value for both hemispheres in the unit of ppb.
3.3. Neuropsychological data
Neuropsychological testing scores at time point 1:
Baseline comparison of neuropsychological testing scores between the control group and chemotherapy group are presented in Table 4. There were no significant differences between groups.
Table 4.
Baseline comparison of neuropsychological testing scores (STD) between the control group and the chemotherapy group.
| Neuropsychological scores | Overall scores | Control Group | Chemotherapy Group | Difference scores | p-value |
|---|---|---|---|---|---|
| Crystallized Cognition Composite | 111.2 (16.0) | 110.2 (14.8) | 112.2 (17.6) | 2.05 | 0.74 |
| Fluid Cognition Composite | 99.7 (12.9) | 99.5 (10.1) | 99.9 (15.5) | 0.32 | 0.95 |
| Total Cognition Composite | 104.8 (16.9) | 103.4 (13.2) | 106.0 (2.03) | 2.60 | 0.70 |
| Dimensional Change Card Sort | 102.2 (10.7) | 102.6 (12.1) | 101.7 (9.7) | −0.92 | 0.81 |
| Picture Sequence Memory Test | 109.4 (18.1) | 104.5 (15.2) | 114.0 (19.9) | 9.47 | 0.23 |
| Flanker Inhibitory Control and Attention | 93.2 (12.5) | 97.6 (7.9) | 89.1 (14.7) | −8.41 | 0.04 |
| Oral Reading Recognition | 109.1 (12.8) | 105.2 (12.2) | 112.7 (12.8) | 7.52 | 0.14 |
| Pattern Comparison Processing Speed | 94.2 (14.4) | 96.0 (14.7) | 92.6 (14.5) | −3.32 | 0.56 |
| Picture Vocabulary | 109.7 (14.6) | 112.1 (13.1) | 107.5 (16.1) | −4.52 | 0.42 |
| List Sorting Working Memory | 100.8 (16.7) | 100.4 (16.6) | 101.1 (17.5) | 0.74 | 0.90 |
Correlation between brain iron and neuropsychological testing scores at time point 1:
There was a significant negative correlation between brain iron deposition in the globus pallidus and the fluid composite score in the control group (Pearson correlation coefficient = -0.71; p < 0.01), but not in the chemotherapy group.
Relationship between iron deposition at time point 1 and changes in neuropsychological testing scores:
Baseline brain iron deposition in the putamen was negatively associated with changes in oral reading recognition testing scores in the control group (Pearson correlation coefficient = -0.74; p < 0.01). This indicated that higher baseline iron levels in the putamen were associated with less change in oral reading recognition testing scores in the control group. No significant correlations between brain iron deposition and changes in neuropsychological testing scores were noted for the chemotherapy group.
Longitudinal changes in neuropsychological scores:
Table 5 summarizes the changes in testing scores between time points 1 and 2 for the control and the chemotherapy groups. There were no significant changes over time within groups, nor were there significant group-by-time interactions.
Table 5.
Longitudinal change in neuropsychological testing scores (STD) in the control group and chemotherapy group.
| Score changes | CC | FC | TC | DCCS | PSMT | FIC | ORR | PS | PV | WM |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | −0.9 | 4.3 | 2.0 | 3.0 | 2.7 | 3.1 | −2.3 | 0.7 | 0.8 | 3.8 |
| (6.8) | (11.0) | (8.9) | (10.0) | (18.5) | (12.0) | (6.4) | (14.3) | (9.1) | (12.2) | |
| Control Group | −0.9 | 5.3 | 2.9 | 5.8 | 8.2 | 2.1 | −0.3 | −3.1 | −2.2 | 2.8 |
| (6.6) | (12.5) | (9.2) | (12.5) | (21.7) | (5.8) | (6.8) | (14.9) | (6.1) | (13.8) | |
| p-value | 0.66 | 0.10 | 0.25 | 0.05 | 0.12 | 0.54 | 0.87 | 0.41 | 0.35 | 0.42 |
| Chemotherapy Group | −0.9 | 3.3 | 1.2 | 0.5 | −2.3 | 4.0 | −4.3 | 4.3 | 3.5 | 4.6 |
| (7.2) | (9.7) | (8.9) | (6.5) | (13.8) | (15.9) | (5.6) | (13.3) | (10.6) | (10.9) | |
| p-value | 0.64 | 0.28 | 0.63 | 0.86 | 0.64 | 0.23 | 0.02 | 0.27 | 0.15 | 0.18 |
Abbreviations for neuropsychological testing scores: CC: Crystallized Cognition Composite; FC: Fluid Cognition Composite; TC: Total Cognition Composite; DCCS: Dimensional Change Card Sort; PSMT: Picture Sequence Memory Test; FIC: Flanker Inhibitory Control and Attention; ORR: Oral Reading Recognition; PS: Pattern Comparison Processing Speed; PV: Picture Vocabulary; WM: List Sorting Working Memory. Note: Corrected for multiple comparisons of 10 neuropsychological testing scores, the significant p value threshold was at p < 0.005. The p-value of 0.02 for the ORR score in the chemotherapy group was not considered statistically significant.
4. Discussion
To the best of our knowledge, this is the first prospective longitudinal study to assess subcortical brain iron deposition and cognitive performance in older patients with breast cancer receiving adjuvant chemotherapy. Our study confirmed the previously reported age-associated increase in brain iron deposition for older women without cancer. We observed brain iron trending upward with increasing age in older women with or without cancer. We also observed an association between higher iron and lower fluid composite scores in the older women without cancer, but not in the older women with breast cancer. In addition, the baseline iron levels in the putamen were negatively associated with changes in oral reading recognition scores in the control group, but not in the breast cancer group receiving adjuvant chemotherapy. However, we found no short-term, longitudinal alteration of brain iron deposition in either group.
The current study furthers our knowledge regarding brain iron deposition in older adults with or without cancer. The association between brain iron and age is consistent with the published literature. Subcortical brain iron content has been reported to increase with age in healthy adults between 20 and 69 years of age.[29] Our study, with a focus on older study participants, provides evidence that brain iron continues to increase into even older age, from 60 to 82 years of age.
The association between brain iron deposition and cognitive changes in older individuals without cancer is also consistent with the published literature.[34] An exploratory study on a small sample of 10 healthy older adults ranging from 65 to 79 years identified that higher iron deposition in the caudate nucleus and putamen was correlated with lower Dementia Rating Scale scores.[34] A longitudinal study of healthy human adults (age 19–77 years at baseline) over a 2-year period showed that higher baseline iron content in the caudate nucleus predicted reduced improvement in Working Memory testing scores.[15] Furthermore, age-related increases in iron deposition in subcortical gray matter nuclei correlated with poor performance in various cognitive tasks.[14] These studies have implicated brain iron deposition as a risk factor for cognitive aging in adults without cancer. We observed similar findings: higher iron in the globus pallidus associated with a lower fluid composite score in older adults without cancer at baseline.
Neuroimaging studies have provided evidence for potential association between chemotherapy and cancer-related cognitive impairment.[10] We directly estimated brain iron content from quantitative susceptibility mapping data generated from processing SWI sequences in brain MRI scans. This differed from prior studies, which estimated oxidative stress, inflammation, and DNA damage via peripheral blood testing and correlated these values with brain structural and metabolic changes on neuroimaging.[35–37] Conroy et al., showed increased oxidative DNA damage measured in peripheral lymphocytes in breast cancer survivor group compared with healthy control group, which was related to brain alteration and cognitive function in the breast cancer survivor group.[37] In addition, a study by Ganz et al. showed that the levels of proinflammatory cytokines, such as soluble tumor necrosis factor-alpha receptor II, were associated with memory complaints and diminished metabolism in the inferior frontal cortex on positron emission tomography scan imaging in chemotherapy-exposed cancer patients.[35] Other studies indicated that cytokine levels and left hippocampal volumes were associated with memory performance.[36] Although we did not find significant short-term changes in iron deposition in our study, our MRI-focused brain iron measurement offered a new approach to studying cancer-related cognitive impairment.
There are several limitations of the study. This was a pilot study with a modest number of participants. Further, the follow-up interval was short: only 3 to 5 months. Given the higher brain iron content and larger variation in iron deposition in older adults [29], it was not surprising that we did not identify changes in brain iron deposition within such a short period of time. Previous longitudinal studies of brain iron levels were performed over a much longer period of 2–7 years.[14, 38] In addition, the chemotherapy regimen was heterogeneous for the older women enrolled in this study, and different chemotherapy regimens may produce different neurotoxicity profiles.[39] However, our study was not powered to assess chemotherapy regimen-specific effects on brain iron levels due to a modest sample size. These limitations reduced our ability to detect cancer-associated changes in brain iron levels. Lastly, our study did not include patients with breast cancer who did not receive chemotherapy. Future studies will benefit from the inclusion of breast cancer patients not receiving chemotherapy, as addition of this group will provide the ability to differentiate the effect of chemotherapy from the cancer diagnosis itself.
Despite these limitations, this study also has strengths. Our MRI-based non-invasive methodology for brain iron measurement is promising and could potentially be useful in studies of CRCI in older healthy adults and older patients with cancer. In addition, it provides much needed longitudinal prospective information on brain iron deposition and its association with cognitive performance in older women without cancer and in older women with cancer undergoing adjuvant chemotherapy.
In summary, we observed an age-related increase in brain iron levels in older women without breast cancer. We also observed that higher baseline iron levels correlated with lower cognitive performance in the older women without cancer. However, we did not find any short-term changes in subcortical brain iron deposition in the older women without cancer or those with cancer undergoing chemotherapy for breast cancer. Future studies with larger sample sizes over a longer follow-up duration are needed to examine the trajectory of brain iron deposition and its association with cognition in older adults with or without cancer.
Acknowledgments
The preliminary data was presented as an electronic scientific poster at the American Society of Neuroradiology Annual Meeting April 22–27, 2017, in Long Beach, CA, USA. Nancy Linford, PhD, provided editing assistance.
Funding
This study was funded by NIH/NIA grants R03 AG045090–02 (BTC), R01 AG037037–01A1 (AH) and K24 AG055693–01 (AH).
Summary declaration of interest statement
AH reports research funding from Celgene, Novartis, and GSK, and has served as a consultant for MJH Healthcare Holdings, LLC, Pierian Biosciences, Sanofi, Boehringer Ingelheim Pharmaceuticals, and Carevive, outside the submitted work. KG and EMH are employed by Magnetic Resonance Innovations, Inc.
Footnotes
Trial registration: ClinicalTrials.gov, NCT01992432.
All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report. Atlanta: U.S: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017. [Google Scholar]
- [2].Ortman JM, Velkoff VA, Hogan H. An aging nation: The older population in the United States Current Population Reports, P25–1140. Washington, DC: U.S. Census Bureau; 2014. [Google Scholar]
- [3].Dale W, Mohile SG, Eldadah BA, Trimble EL, Schilsky RL, Cohen HJ, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst 2012;104(8):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 2014;26(1):102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 2011;12(7):703–8. [DOI] [PubMed] [Google Scholar]
- [6].Hurria A, Somlo G, Ahles T. Renaming "chemobrain". Cancer Invest 2007;25(6):373–7. [DOI] [PubMed] [Google Scholar]
- [7].Dutta V Chemotherapy, neurotoxicity, and cognitive changes in breast cancer. J Cancer Res Ther 2011;7(3):264–9. [DOI] [PubMed] [Google Scholar]
- [8].Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002;346(14):1061–6. [DOI] [PubMed] [Google Scholar]
- [9].Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol 2013;40(6):709–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev 2013;37(8):1311–21. [DOI] [PubMed] [Google Scholar]
- [11].Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex 2014;54(Supplement C):33–50. [DOI] [PubMed] [Google Scholar]
- [12].Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 2007;7(3):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, et al. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radic Res 2005;39(11):1147–54. [DOI] [PubMed] [Google Scholar]
- [14].Daugherty AM, Raz N. Appraising the Role of Iron in Brain Aging and Cognition: Promises and Limitations of MRI Methods. Neuropsychol Rev 2015;25(3):272–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Daugherty AM, Haacke EM, Raz N. Striatal iron content predicts its shrinkage and changes in verbal working memory after two years in healthy adults. J Neurosci 2015;35(17):6731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rodrigue KM, Daugherty AM, Haacke EM, Raz N. The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb Cortex 2013;23(7):1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen BT, Sethi SK, Jin T, Patel SK, Ye N, Sun CL, et al. Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res 2018;20(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH Toolbox for Assessment of Neurological and Behavioral Function. Neurology 2013;80(11 Supplement 3):S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80(11 Supplement 3):S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haacke EM, Tang J, Neelavalli J, Cheng YC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging 2010;32(3):663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang J, Liu S, Neelavalli J, Cheng YC, Buch S, Haacke EM. Improving susceptibility mapping using a threshold-based K-space/image domain iterative reconstruction approach. Magn Reson Med 2013;69(5):1396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghassaban K, Liu S, Jiang C, Haacke EM. Quantifying iron content in magnetic resonance imaging. Neuroimage 2018. [DOI] [PubMed] [Google Scholar]
- [23].Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17(3):143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abdul-Rahman HS, Gdeisat MA, Burton DR, Lalor MJ, Lilley F, Moore CJ. Fast and robust three-dimensional best path phase unwrapping algorithm. Appl Opt 2007;46(26):6623–35. [DOI] [PubMed] [Google Scholar]
- [25].Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 2011;54(4):2789–807. [DOI] [PubMed] [Google Scholar]
- [26].Haacke EM, Tang J, Neelavalli J, Cheng YC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. Journal of magnetic resonance imaging : JMRI 2010;32(3):663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deistung A, Schweser F, Reichenbach JR. Overview of quantitative susceptibility mapping. NMR Biomed 2017;30(4). [DOI] [PubMed] [Google Scholar]
- [28].Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med 2009;62(6):1510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu M, Liu S, Ghassaban K, Zheng W, Dicicco D, Miao Y, et al. Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. Journal of magnetic resonance imaging : JMRI 2016;44(1):59–71. [DOI] [PubMed] [Google Scholar]
- [30].Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3(1):41–51. [DOI] [PubMed] [Google Scholar]
- [31].Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Slotkin J, et al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc 2014;20(6):567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- [33].Zheng W, Nichol H, Liu S, Cheng YC, Haacke EM. Measuring iron in the brain using quantitative susceptibility mapping and X-ray fluorescence imaging. Neuroimage 2013;78:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sullivan EV, Adalsteinsson E, Rohlfing T, Pfefferbaum A. Relevance of Iron Deposition in Deep Gray Matter Brain Structures to Cognitive and Motor Performance in Healthy Elderly Men and Women: Exploratory Findings. Brain Imaging Behav 2009;3(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun 2013;30 Suppl:S99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun 2013;30 Suppl:S109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, et al. Alterations in brain structure and function in breast cancer survivors: effect of postchemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat 2013;137(2):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daugherty AM, Raz N. Accumulation of iron in the putamen predicts its shrinkage in healthy older adults: A multi-occasion longitudinal study. Neuroimage 2016;128:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kesler SR, Blayney DW. Neurotoxic Effects of Anthracycline- vs Nonanthracycline- Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol 2016;2(2):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


