Abstract
BACKGROUND
Heart transplant allocation in the United States is made on the basis of coarse tiers, defined by mechanical circulatory devices and therapy for advanced heart failure, updated infrequently as a patient’s condition deteriorates. Thus, many patients die awaiting heart transplantation. What is needed is a tool that continuously updates risk of mortality as a patient’s condition changes to inform clinical decision making.
OBJECTIVES
This study sought to develop a decision aid that aggregates adverse events and measures of end-organ function into a continuously updated waitlist mortality estimate.
METHODS
From 2008 to 2013, 414 patients were listed for heart transplantation at Cleveland Clinic, Cleveland, Ohio. The endpoint was waitlist death. Pre-listing patient characteristics and events and laboratory results during listing were analyzed. At each event or measurement change, mortality was recomputed from the resulting model.
RESULTS
There were 77 waitlist deaths, with 1- and 4-year survival of 85% and 57%, respectively. When time-varying events and measurements were incorporated into a mortality model, pre-listing patient characteristics became nonsignificant. Neurological events (hazard ratio [HR]: 13.5; 95% confidence interval [CI]: 7.63 to 23.8), new requirement for dialysis (HR: 3.67; 95% CI: 1.88 to 7.14), more respiratory complications (HR: 1.79 per episode; 95% CI: 1.23 to 2.59), and higher serum bilirubin (p < 0.0001) and creatinine (p < 0.0001) yielded continuously updated estimates of patient-specific mortality across the waitlist period.
CONCLUSIONS
Mortality risk for patients with advanced heart failure who are listed for transplantation is related to adverse events and end-organ dysfunction that change over time. A continuously updated mortality estimate, combined with clinical evaluation, may inform status changes that could reduce mortality on the heart transplant waiting list.
Keywords: heart failure, mathematical modeling, mechanical circulatory support, risk score
A heart allocation score largely calculated on the basis of quantitative data on survival and risk factors for patients with advanced heart disease remains a long-term goal of the United Network for Organ Sharing (UNOS) and the transplantation community (1,2). Given the generally low mortality after heart transplantation in the United States(3), the focus of present efforts to refine heart allocation is directed primarily at minimizing mortality in patients on the heart transplant waiting list while maintaining post-transplantation survival (1,4). However, despite the dynamically changing clinical condition of patients with end-stage heart failure who are listed for transplantation, the UNOS database is updated infrequently (time of listing, change in priority status, and time of transplant), and heart allocation is made on the basis of coarse tiers mainly defined by use of mechanical circulatory support (MCS) devices and inotropes, rather than objective evidence of hemodynamic compromise or adverse events (5,6). This is particularly troublesome in an era of widespread use of temporary and durable MCS devices (7), with their accompanying benefits and sometimes devastating complications (8–12). We hypothesized that reducing waitlist mortality requires a continuously updated quantitative decision aid that aggregates adverse events and changing measures of end-organ function while patients are on the waitlist into a nearly real-time mortality estimate to alert the heart transplantation team to changes that may signal a need to upgrade or lower a patient’s transplantation urgency level.
To test the feasibility of developing and implementing such a patient-specific precision medicine tool, we developed a model that continuously updates risk of mortality with occurrence of adverse events and changes in end-organ function among patients awaiting heart transplants at a single academic medical center.
PATIENTS AND METHODS
PATIENTS.
From January 2008 to June 2013, 532 patients were listed for heart transplantation or underwent implantation of a durable MCS as a bridge to transplantation at Cleveland Clinic, Cleveland, Ohio. Of these patients, we excluded 118 who were listed for multiorgan transplantation or who were younger than 18 years of age at listing, thus resulting in a study cohort of 414 patients. No patient who had an MCS device at our center was considered as “bridge to candidacy,” and patients receiving MCS as destination therapy were not considered for this study.
Mean age at baseline was 54 years, the cohort predominately consisted of white men, and nearly one-half had nonischemic cardiomyopathy (Table 1). About one-third of these patients were status 1A and one-half were status 2. MCS was provided for 43%, inotropes for hemodynamic support for 33%, and mechanical ventilation for 8%. About one-third were diabetic, nearly one-half had coronary artery disease, and three-fourths had an implantable cardioverterdefibrillator. Dialysis was rare, and mean estimated glomerular filtration rate was 42 ml·min −1·1.73 m−2.
TABLE 1.
Patient Characteristics at Baseline
| n* | ||
|---|---|---|
| Demographics | ||
| Age, yrs | 414 | 54 ± 13 |
| Body mass index, kg/m2 | 414 | 27 ± 5.2 |
| Male | 306 (74) | |
| Race | 414 | |
| Black | 65 (16) | |
| White | 336 (81) | |
| Other than black or white | 13 (3.1) | |
| Waitlist status | 414 | |
| 1A | 130 (31) | |
| IB | 80 (19) | |
| 2 | 204 (49) | |
| Diagnosis | 414 | |
| Cardiomyopathy | ||
| Nonischemic | 177 (43) | |
| Ischemic | 150 (36) | |
| Restrictive | 55 (13) | |
| Valvular heart disease | 18 (4.3) | |
| Congenital heart disease | 14 (3.4) | |
| Medical condition | ||
| Medical condition | 414 | |
| Not hospitalized | 185 (45) | |
| Hospitalized, not in intensive care unit | 93 (22) | |
| Intensive care unit | 136 (33) | |
| Life support† | 414 | 179 (43) |
| Inotropes | 137 (33) | |
| Intra-aortic balloon pump | 48 (12) | |
| Mechanical circulatory support at time zero | 104 (25) | |
| Extracorporeal membrane oxygenation | 24 (5.8) | |
| Mechanical ventilation | 34 (8.2) | |
| Nitric oxide | 5 (1.2) | |
| Cardiac comorbidities | ||
| Ejection fraction, % | 370 | 20 ± 11 |
| Cardiac index, l·min−1·m−2 | 402 | 3.0 ± 2.0 |
| Right ventricular systolic pressure, mm Hg | 308 | 47 ± 15 |
| Pulmonary artery mean pressure, mm Hg | 395 | 32 ± 10 |
| Coronary artery disease | 414 | 196 (47) |
| History of ventricular tachycardia | 392 | 169 (43) |
| History of ventricular fibrillation | 392 | 133 (34) |
| Implantable cardioverter-defibrillator | 414 | 312 (75) |
| Noncardiac comorbidities | ||
| Hypertension | 414 | 194 (47) |
| Diabetes | 414 | 115 (28) |
| Dialysis | 414 | 27 (6.5) |
| Cerebrovascular disease or stroke | 414 | 42 (10) |
| Bilirubin, mg/dl | 411 | 0.5/0.9/1.9‡ |
| Creatinine, mg/dl | 411 | 1.3 ± 0.48 |
| Glomerular filtration rate, ml·min−1·1.73 m−2 | 411 | 42/66/99‡ |
| Hemoglobin, mg/dl | 411 | 12 ± 2.05 |
Values are mean ± SD or n (%), unless otherwise indicated.
Patients with data available.
Not mutually exclusive.
15th/50th/85th percentiles.
TIME ZERO (BASELINE).
Time zero-and baseline-for this study was active UNOS listing or date of implantation of an MCS device as bridge to transplantation, thereby excluding destination therapy patients. Thus, for the 104 patients who had a durable MCS device implanted as bridge to transplantation before listing (25% of the 414), the date of device implantation was considered time zero. For patients with end-stage heart failure without durable MCS, time zero was the initial date of active UNOS listing for heart transplantation.
ENDPOINT.
The primary endpoint was all-cause mortality before heart transplantation, with follow-up until November 2013. Patients with MCS were followed according to Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) criteria of 1 week, 1 month, 3 months, 6 months, and every 6 months thereafter (13). Status 1B outpatients were followed at a minimum every month at Cleveland Clinic, and every 1 to 2 weeks by their local physician, on the basis of clinical condition. Status 2 outpatients were followed at a minimum every 3 to 4 months at Cleveland Clinic.
Patients were censored at the time of transplantation. Patients alive at the end of the study and who had not received a heart transplant were censored at that time. Patients lost to follow-up after time zero were censored at the last documented encounter within our health system according to electronic medical record documentation (transferred to another center [n = 3], noncompliant with appointment or treatment [n = 4], treated at another center by patient’s choice [n = 1]). Delisting was not considered a censoring event, in keeping with the principle of intent to treat, and patients were not excluded or censored if their condition worsened.
Median follow-up time for survivors before transplantation was 0.71 years. Among these survivors, 25% were followed for more than 1.8 years and 10% for more than 3.8 years. A total of 507 person-years of vital status data were available for analysis.
CLINICAL DATA.
Baseline data.
Baseline characteristics were the most recent values recorded before time zero. Clinical data, including events while on the waitlist, were extracted from our Electronic Data Interface for Transplant (EDIT) database, which transplant coordinators update during the course of clinical care, and the Cardiovascular Information Registry (CVIR), a prospective registry of all cardiovascular procedures. These data were supplemented with queries of the electronic medical record to resolve inconsistencies and fill in incomplete data. Baseline variables at listing included the patient’s demographics, patient’s size (height, weight, body mass index, body surface area, height-to-weight ratio), symptoms and medical condition at listing, echocardiographic findings, heart catheterization hemodynamics, comorbidities, laboratory findings, and year of listing (Online Appendix). These data were approved for use in research by the Cleveland Clinic Institutional Review Board, with patient consent waived.
Events, complications, and end-organ function on the waitlist.
Of particular importance for this study were data reflecting major complications while awaiting transplantation and end-organ statusneurological, respiratory, cardiac, hepatic, and renal. These data were abstracted from all outpatient and inpatient encounters, as well as from surgical intervention reports, as were variables related to MCS, including device insertion and removal and device infections, thrombosis, and other device-related complications (Online Appendix). Events and complications associated with fewer than 10 deaths or fewer than 25 patients were considered insufficient data for separate analysis, but they were incorporated into composite events (14). This resulted in 377 occurrences of events and complications while patients awaited heart transplantation: 102 MCS implants after time zero (including 91 devices in 88 patients who did not have an MCS device before listing and 11 device exchanges among the 104 patients who had an MCS device before listing); 60 respiratory complications (mechanical ventilation with or without tracheostomy); 51 MCS-related infections (driveline and pump pocket); 45 neurological complications (stroke and intracranial hemorrhage); 42 episodes of gastrointestinal bleeding; 28 instances of new dialysis; 25 thrombotic complications; and 24 cardiac surgical events other than MCS implantation (Table 2).
TABLE 2.
Events on the Waitlist
| Total | N | |
|---|---|---|
| MCS device implant | 102 | 99 |
| Infection (driveline, pump pocket) | 51 | 49 |
| Thrombosis (confirmed, suspected) | 25 | 24 |
| Respiratory (tracheostomy, mechanical ventilation) | 60 | 47 |
| Neurological (stroke, intracranial hemorrhage) | 45 | 39 |
| Gastrointestinal bleed | 42 | 38 |
| New requirement for dialysis | 28 | 28 |
| Cardiac surgical procedure other than MCS implant | 24 | 23 |
| Delayed chest closure | 1 | 1 |
| Reoperation for bleeding/tamponade | 7 | 7 |
| Mediastinal reoperation for tamponade and wound debridement | 1 | 1 |
| Cardiac ablation | 3 | 3 |
| Intubation or reintubation, pulmonary embolus | 1 | 1 |
| IVC filter | 1 | 1 |
| MCS controller change | 3 | 3 |
| Left atrial thrombus removal | 1 | 1 |
| Wound debridement | 4 | 3 |
| Implant relocation | 1 | 1 |
| ICD implantation | 1 | 1 |
ICD = implantable cardioverter-defibrillator; IVC = inferior vena cava; MCS = mechanical circulatory support.
Serum creatinine and bilirubin measurements obtained while these patients were on the waitlist were downloaded from the electronic medical record. Thus, kidney and liver functions were represented by 19,983 laboratory measurements of creatinine in 395 patients (Online Figure 1A) and 14,412 laboratory measurements of bilirubin in 387 patients (Online Figure 1B). Among these, 56 patients had creatinine values >3 mg/dl while on the waitlist: 27 died; 4 were delisted alive; 13 underwent transplantation; and 12 remained on the waitlist at the end of follow-up. A total of 61 patients had bilirubin values >5 mg/dl while on the waitlist: 23 died; 3 were delisted alive; 21 underwent transplantation; and 14 remained on the waitlist at the end of follow-up.
We refrained from incorporating variables strictly related to the probability of transplantation and its timing rather than waitlist mortality. These included variables such as baseline blood type, longitudinal assessment of panel reactive antibodies, UNOS status at transplantation, and status changes.
DATA ANALYSIS.
All analyses were performed using SAS statistical software version 9.2 (SAS, Inc., Cary, North Carolina) and R software version 3.0.2 (R Foundation, Vienna, Austria). Uncertainty is expressed by confidence limits (CL) equivalent to ±1 standard error (68%). Continuous variables are summarized as mean ± SD or as equivalent 15th, 50th (median), and 85th percentiles when their distribution was skewed. Categorical data are summarized using frequencies and percentages.
Mortality.
Mortality was assessed nonparametrically by the Kaplan-Meier method and parametrically by a multiphase nonproportional hazards model (15). This nonproportional completely parametric model was chosen because instantaneous risk of mortality (hazard) was time varying, with an early peaking risk, signifying that different risk factors may be driving it and other factors were driving later risk. A parametric model also facilitates calculating patient-specific changing risk of mortality. The model resolved 2 phases of instantaneous risk of death (16). An extended version of the Kaplan-Meier estimator was used to assess the effect of time-varying covariates nonparametrically (17).
Model development.
Our strategy was first to develop a model for time-related mortality on the basis of only baseline variables and then to add time-varying covariables. Bagging (bootstrap aggregation) was used for model variable selection from baseline variables listed in the Online Appendix (18–20). For this, 500 bootstrap samples were analyzed by unsupervised forward stepwise selection, retaining those with p < 0.05. Variables appearing in at least 50% of these analyses were retained in the final model, and the percentage of times they appeared was taken as a measure of variable selection reliability.
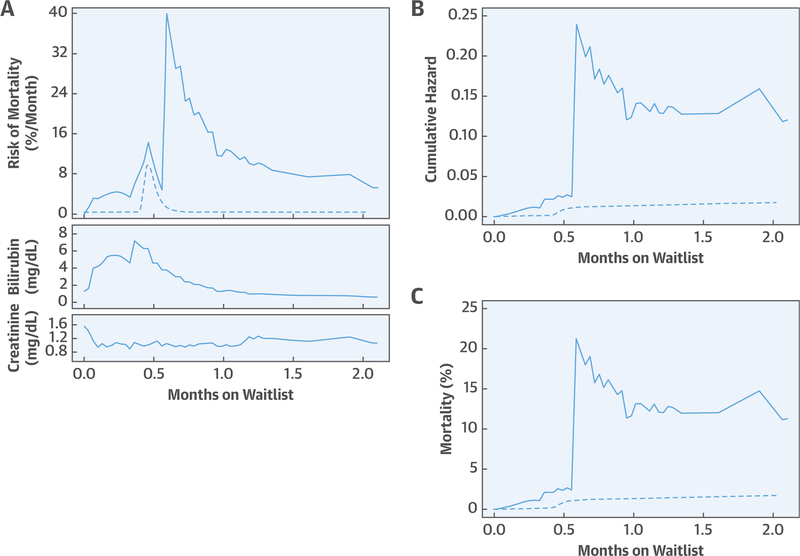
We then repeated this analysis, adding time-varying covariables. These included events occurring while awaiting transplantation, including time to each event relative to time zero and count of each distinct event at each reoccurrence, as well as laboratory measures at the time blood was drawn. For this model, we managed time-varying covariates in a simple way, as in a Cox model. That is, at the time of an event or change in value for a time-varying covariate, a step-function change in hazard occurred, and the magnitude of that change was dependent on the value and subsequent trajectory of the underlying hazard (Central Illustration). All clinical events, such as neurological events, were treated mathematically as single-state transitions; each laboratory measurement was treated as a single-state transition from 1 measured value to the next.
CENTRAL ILLUSTRATION. Method Used to Provide Continuously Updated Estimates of Mortality on the Heart Transplant Waitlist.
This patient was in acute-on-chronic heart failure and developed acute renal failure from poor kidney perfusion. Milrinone and nitroprusside replaced an angiotensin-converting enzyme inhibitor, aldactone, and digoxin. Five days later, the patient was listed for heart transplantation. The next day, the patient received a durable left ventricular assist device for deteriorating cardiac function. Two and one-half weeks later, the patient experienced a number of episodes of transient visual disturbances neurologists attributed to thromboembolism. (A) This episode is seen as a large increase in instantaneous risk of mortality at 18 days, or 0.59 months. The underlying risk of death if all risk factors are set to zero is shown by the dashed line. Also shown are frequent blood draws for creatinine and bilirubin. Every change in these levels increases or decreases estimated mortality risk as the graph is updated. (B) The area beneath the hazard function is the cumulative hazard function, and the dashed line is the underlying cumulative hazard. (C) An equation (see Online Appendix) transforms cumulative hazard into an estimate of mortality at each moment in time. Notice that the underlying risk of death continues smoothly upward, but this curve is constantly being modified by either events or measurements reflecting end-organ function.
Time-varying mortality risk.
To display a time-varying estimate of risk of death on the transplant waitlist, the equation resulting from the multivariable analysis was solved for each individual patient across time, updating the risk of mortality with the passage of time and every event, complication, and change in creatinine and bilirubin. For this, at each point in time, the equation generated a value for cumulative hazard that was transformed into a mortality probability as: [1 − exp (- cumulative hazard)] (Central Illustration, Online Appendix).
Missing values.
Baseline variables with missing values of 30% or greater were not used in the multivariable analyses. Variables with <30% missing values (see Table 1 for examples) had missing data imputed with 5-fold multiple imputation using a Markov Chain Monte Carlo technique to obtain final parameter estimates and a variance-covariance matrix (21).
RESULTS
DEATH ON THE TRANSPLANT WAITLIST.
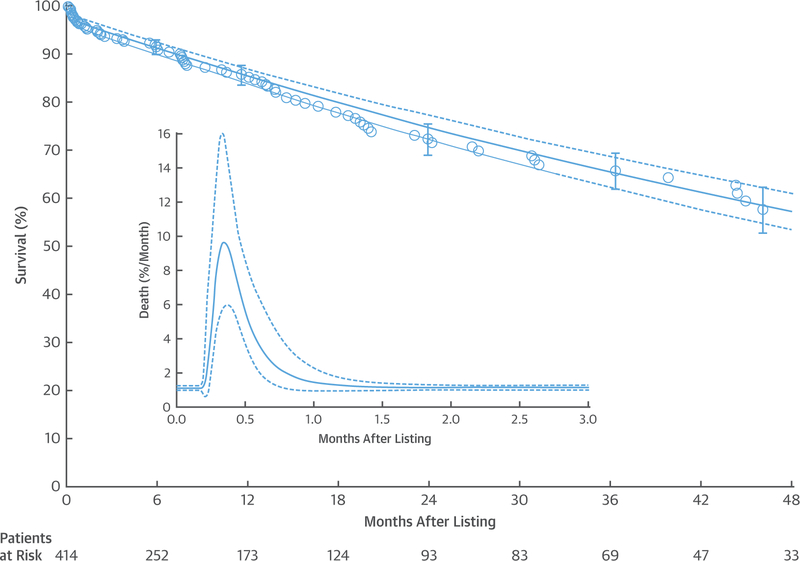
A total of 77 patients died on the heart transplant waitlist. Survival at 1, 3, and 6 months was 96%, 94%, and 91%, respectively, and at 1, 2, 3, and 4 years, it was 85%, 75%, 65%, and 57%, respectively (Figure 1). Instantaneous risk of death peaked at 9.5% per month (CL: 5.6 to 16) on day 10, then decreased to 6.8% per month (CL: 4.7 to 9.8) by 2 weeks, and stabilized at a constant rate of 1.1% per month (CL: 1.0 to 1.3) by 6 months (Figure 1, inset).
FIGURE 1. Overall Survival on the Heart Transplant Waitlist or After Insertion of a Durable Mechanical Circulatory Support Device as Bridge to Transplant Before Listing.
Each symbol represents a death positioned on the vertical axis by the Kaplan-Meier estimator; vertical bars are confidence limits equivalent to ±1 standard error. The solid line depicts parametric survival estimates enclosed within a dashed 68% confidence band equivalent to ±1 standard error. Numbers below the horizontal axis are patients remaining at risk. The inset shows an instantaneous risk of death (hazard function) on an expanded horizontal axis. The solid line depicts parametric estimates enclosed within a dashed 68% confidence band equivalent to ±1 standard error. Note the early peaking hazard and underlying constant hazard. Risk factors were simultaneously examined for each of these phases.
RISK FACTORS FOR MORTALITY.
After incorporating time-varying covariables for events and laboratory measurements while patients were awaiting transplantation, no baseline variable remained statistically significant; rather, risk factors for waitlist mortality were events or changes in laboratory values occurring during the waitlist period (Table 3), such as renal dialysis after initial listing for transplantation (Online Figure 2), new neurological events (Online Figure 3), respiratory complications (Online Figure 4), and changes in renal function (Online Figure 5) or hepatic function (Online Figure 6).
Table 3.
Incremental Risk Factors for Death on the Heart Transplant Waitlist
| Coefficient ± SE | Hazard Ratio (95% Cl) | p Value | Reliability (%)* | |
|---|---|---|---|---|
| Pre-listing | ||||
| None | - | - | - | - |
| Complications on waitlist | ||||
| New requirement for dialysis (Online Figure 2) | 1.3 ± 0.34 | 3.67 (1.88–7.14) | <0.0001 | 67 |
| Neurological events (Online Figure 3) | 2.6 ± 0.29 | 13.5 (7.63–23.8) | <0.0001 | 83 |
| More respiratory complications† (Online Figure 4) | 0.58 ± 0.19 | 1.79 (1.23–2.59) | 0.002 | 51 |
| Laboratory values on waitlist | ||||
| Higher creatinine | 0.53 ± 0.078 | (Online Figure 5) | <0.0001 | 72 |
| Higher bilirubin‡ | 1.1 ± 0.102 | (Online Figure 6) | <0.0001 | 97 |
Percent of times variable appeared in 500 bootstrap models.
Cumulative count at any given time of number of preceding respiratory events, defined for this study as tracheostomy and ventilation.
Log (bilirubin), logarithmic transformation.
CI = confidence interval; SE = standard error.
CONTINUOUSLY UPDATED TIME-VARYING MORTALITY RISK ILLUSTRATED.
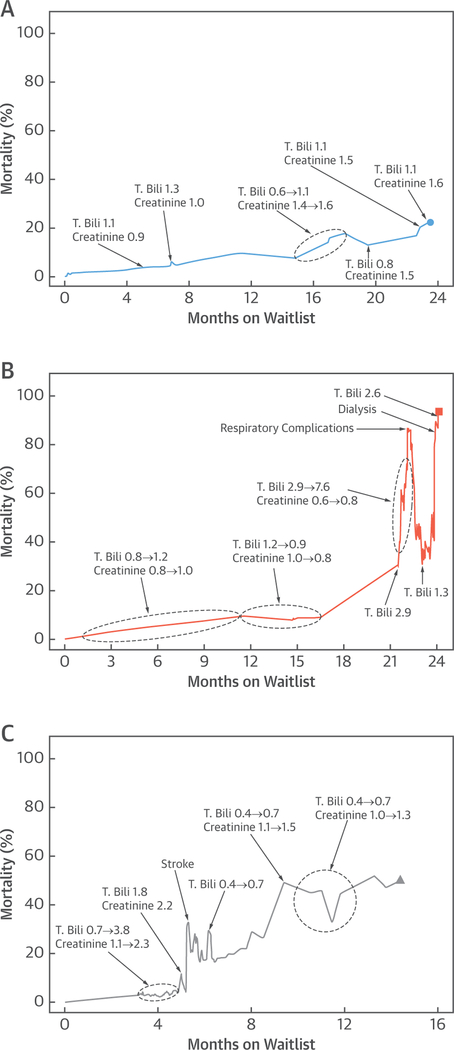
As in the Central Illustration, Figure 2 illustrates dynamic mortality estimates for 3 other patients on the basis of multivariable analysis (Table 3) and their actual events and laboratory values while awaiting transplantation. The first patient had a left ventricular assist device implanted 3 days after listing and experienced no complications. Risk of mortality rose gradually according to the underlying hazard, with small fluctuations reflecting changes in creatinine and bilirubin (Figure 2A). The second patient experienced no events until 21 months on the waitlist, when bilirubin rose, and the patient had to be mechanically ventilated. A left ventricular assist device was implanted, with normalization of hepatic function. However, renal dysfunction required dialysis, and the patient died 24 months after listing (Figure 2B). The third patient had a left ventricular assist device implanted 5 months after listing and shortly thereafter experienced a stroke that initially manifested as sudden onset of diplopia. Computed tomography revealed a subarachnoid hemorrhage that was stable on subsequent imaging after an elevated international normalized ratio was reversed. Both hepatic function and renal function fluctuated with increasing calculated risk of death until the patient underwent heart transplantation 14 months after listing (Figure 2C).
FIGURE 2. Individual Risk Profiles for Patients on the Heart Transplant Waitlist.
Superimposed are events or laboratory values leading to elevation of calculated risk of mortality. (A) Patient alive at end of follow-up. (B) Patient who died on the waitlist. (C) Patient who received a heart transplant. T. Bili. = total bilirubin.
Overall, patients who did not experience events, complications, or substantial laboratory value changes on the waitlist had mortality probabilities that slowly and nearly linearly increased with time (Online Figure 7A). Patients who died on the waitlist often had high values for the probability of death (Online Figure 7B). Those who underwent transplantation comprised a mix of patients with an elevated risk of death and those with a low risk (Online Figure 7C).
DISCUSSION
PRINCIPAL FINDINGS.
We have assessed risk factors dynamically affecting mortality on the heart transplant waitlist during the era of both temporary and durable mechanical support. We found that baseline characteristics, including year of listing, were not predictive of survival when changes in end-organ function and adverse events while waiting for transplantation were taken into account. This finding suggests that the patient’s urgency status for transplantation should depend on objective evidence of deterioration as long as the patient remains eligible for transplantation.
EXISTING HEART FAILURE SURVIVAL MODELS.
Existing heart failure survival models use information obtained at 1 time point—listing—to predict death (22–25). Often their formulation preceded the era of contemporary mechanical support. For example, the Heart Failure Survival Score was derived and validated in an advanced heart failure cohort before durable mechanical support (22). Data used to predict survival were collected after optimizing medications at a given time point. Although patients were awaiting heart transplantation, both the derivation and validation cohorts were ambulatory, and none had mechanical ventilation or data indicating changes in renal function or neurological status. The Seattle Heart Failure Model was derived from the PRAISE 1 (Prospective Randomized Amlodipine Survival Evaluation) cohort comparing amlodipine with a placebo for patients in New York Heart Association functional class IIb and IV (23). This study preceded the era of contemporary mechanical support and used baseline data that did not include dynamic changes in clinical status. This may explain why this model underestimated a combined endpoint of death, urgent transplantation, and MCS for patients with advanced heart failure at Cleveland Clinic (24). The Candidate Risk Score is 1 of the first heart allocation scores with a contemporary cohort (25). The survival model was derived from data obtained at initial listing for transplantation in France. It has not been validated in the United States using the national database because it contains variables not entered into the UNOS at listing, such as natriuretic peptide level and bilirubin.
DYNAMIC RISK ASSESSMENT IS NOT NEW.
The concept of combining physiological measurements to generate alerts or automatically implement treatment was recognized in the late 1960s in the care of critically ill patients. Sheppard et al. (26) introduced the concept of automated care in the cardiac surgical intensive care unit setting, where a sophisticated, rule-based, computerized, closed-loop monitoring and treatment system was used to automatically trigger all fluid, blood, and drug infusions and alert clinicians to events such as excessive bleeding or oliguria. In the early 1980s, the Acute Physiology and Chronic Health Evaluation (APACHE) score was introduced, incorporating laboratory tests, comorbidities, vital signs, and other measurements into a single score to help critical care clinicians evaluate patients and select appropriate management strategies (27). Multiple similar evaluation tools were later developed for critically ill patients, such as APACHE II, III, and IV and the Simplified Acute Physiology Score (SAPS) (28,29). Although some of these scores are recalibrated on a daily basis, they are generally short-term tools, whereas our goal was to develop a surveillance tool for use over an extended time while patients await heart transplantation.
In the field of transplantation, the Model for End-Stage Liver Disease (MELD) (30) and lung allocation scores (6,31) were developed to periodically assess patients listed for liver and lung transplantation. Both scores can be updated during the waitlist period, the MELD score by changes in laboratory values and the lung allocation score by underlying mortality risk and respiratory events. These allocation scores are available on the UNOS website as risk-prediction calculators (32). Our effort combined the elements of risk prediction for liver and lung: an underlying time-varying mortality model, events occurring while awaiting transplantation, and the longitudinal sequence of laboratory values reflecting end-organ function. Such a mortality model represents a possible approach to a continuously updated heart allocation score to replace the current tiered system (1,4).
STUDY LIMITATIONS.
This is a single-institution study with a relatively small number of adverse events and deaths; this means that the number of factors that can be incorporated into a multivariable risk-factor model could be no more than 7 to 8, thereby forcing us to develop a parsimonious model. For this reason, we were able to accommodate only 2 laboratory tests, and the remainder consisted of events that assessed pulmonary and neurological systems. Pre-operative comorbidities and MCS outcome variables, such as pump pocket infection, pump thrombosis, and others that are clinically important, were not statistically significant risk factors. This is as may be expected because when a patient is close to death, variables that change closer to death become more important than those assessed farther in the past.
With a larger population, more risk factors may be identified than those we identified, and the model may be more applicable to a broader group of patients, thus eventually leading to a true continuously updated heart allocation score when coupled with an assessment of post-transplant mortality. Because national transplantation and MCS databases do not capture events and laboratory data at this level of frequency, further development will require multi-institutional data, along with formal validation. Models in the future will also need to focus on the dynamic nature of allocation, including competing risks.
In modeling, once an event or complication occurred, it triggered a change in state with an effect on risk of death that remained constant until either it was superseded or the end of listing occurred (so-called “carried forward”). Using the industrial concept of modulated renewal or nonlinear semi-Markov multistate modeling with nonlinear transition rates, it may be possible to incorporate a complication-specific decay function.
Important and possibly controversial decisions had to be made concerning censoring at transplantation and delisting. To the extent that transplantation rescues some patients from impending death, our model underestimates waitlist mortality estimates. A future National Institutes of Health–funded endeavor is to estimate the magnitude of this informative censoring, and such models would require incorporating additional baseline variables such as blood type, longitudinal variables such as panel reactive antibody level, and events such as allocation status changes. We did not censor at delisting for recovery, change in suitability for transplantation, or other reasons. These patients continue to be followed up for heart failure, so we elected not to censor them at delisting.
Finally, we have proposed a tool that merely displays time-related changes in risk of mortality, not a complete decision-support system (33). For example, requirement for dialysis or occurrence of a life-threatening stroke will increase the calculated value for risk of mortality, but these events may make the patient unsuitable for transplantation, rather than triggering a need for urgent transplantation.
CONCLUSIONS
Patients with heart failure who are listed for transplantation comprise a heterogeneous group whose risk of mortality on the waitlist is primarily driven by adverse events and organ dysfunction that change over time, often dramatically. Clinical integration of these changes is challenging, and a tool that aggregates this complex information and displays it as a continuously updated mortality risk may aid in better appreciating patients’ dynamic clinical status. The methodology used in developing this model shows 1 way of creating an important portion of a heart allocation score that could provide a new approach to candidate evaluation and selection in an objective, flexible, and uniform way. This time-varying mortality, combined with clinical evaluation, may lead to dynamic candidate prioritization that could reduce mortality on the heart transplant waitlist.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE:
Baseline risk factors at time of listing for heart transplantation are eclipsed by subsequent events and by changes in laboratory values reflecting organ failure.
TRANSLATIONAL OUTLOOK:
Continuously updating the risk of mortality among patients awaiting transplantation could facilitate more objective allocation than the current tiered approach and reduce mortality. New methods are needed to estimate the magnitude and effect of informative censoring and refine predictors of time-varying risk among candidates for cardiac transplantation.
Acknowledgments
This study was supported in part by the Drs. Sidney and Becca Fleischer Heart and Vascular Education Chair (EHB). Data and methodology presented herein supported a grant application to the National Heart, Lung, and Blood Institute that is now funded as grant R01HL141892. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CL
confidence limit
- MCS
mechanical circulatory support
- UNOS
United Network for Organ Sharing
Footnotes
APPENDIX
For variables considered in multivariable analyses, model details, and supplemental figures, please see the online version of this paper.
REFERENCES
- 1.U.S. Department of Health and Human Services: Organ Procurement and Transplantation Network. OPTN/UNOS Thoracic Organ Transplantation Committee. Proposal to Modify the Adult Heart Allocation System. 2017. Available at: http://optn.transplant.hrsa.gov/media/1921/thoracic_adult_heart_allocation_modification_20160815.Pdf. Accessed July 2, 2018.
- 2.Hsich EM. Matching the market for heart transplantation. Circ Heart Fail 2016;9:e002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant 2015;34:1244–54. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services: Organ Procurement and Transplantation Network. Adult Heart Allocation Changes 2016. 2017. Available at: https://optn.transplant.hrsa.gov/governance/public-comment/adult-heart-allocation-changes-2016/. Accessed July 2, 2018.
- 5.U.S. Department of Health and Human Services: Organ Procurement and Transplantation Network. Policies. 2017. Available at: https://optn.transplant.hrsa.gov/governance/policies. Accessed June 13, 2018.
- 6.Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant 2012;12:3213–34. [DOI] [PubMed] [Google Scholar]
- 7.Colvin M, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: heart. Am J Transplant 2017;17 Suppl 1:286–356. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34: 1495–504. [DOI] [PubMed] [Google Scholar]
- 9.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370: 33–40. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Kormos RL, et al. Inter-agency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33: 12–22. [DOI] [PubMed] [Google Scholar]
- 11.Smedira NG, Blackstone EH, Ehrlinger J, et al. Current risks of HeartMate II pump thrombosis: non-parametric analysis of Interagency Registry for Mechanically Assisted Circulatory Support data. J Heart Lung Transplant 2015;34:1527–34. [DOI] [PubMed] [Google Scholar]
- 12.Jeffries N, Miller MA, Taddei-Peters WC, Burke C, Baldwin JT, Young JB. What is the truth behind pump thrombosis in the HeartMate II device? A National Heart, Lung, and Blood Institute perspective based on data from the Interagency Registry for Mechanically Assisted Circulatory Support. J Heart Lung Transplant 2015;34:1505–10. [DOI] [PubMed] [Google Scholar]
- 13.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant 2009;28:827–33. [DOI] [PubMed] [Google Scholar]
- 14.Blackstone EH. Sufficient data. J Thorac Cardiovasc Surg 2016;152:1235–6. [DOI] [PubMed] [Google Scholar]
- 15.Blackstone EH, Naftel DC, Turner ME Jr. The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc 1986;81: 615–24. [Google Scholar]
- 16.Lerner Research Institute, Cleveland Clinic. The Hazard Package. Available at: http://www.lerner.ccf.org/qhs/software/hazard. Accessed June 13, 2018.
- 17.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 2005; 59:301–7. [Google Scholar]
- 18.Breiman L Bagging predictors. Machine Learning 1996;24:123–40. [Google Scholar]
- 19.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 1992;11:2093–109. [DOI] [PubMed] [Google Scholar]
- 20.Rajeswaran J, Blackstone EH. Identifying risk factors: challenges of separating signal from noise. J Thorac Cardiovasc Surg 2017;153:1136–8. [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Multiple Imputation for Non-response in Surveys. New York, NY: Wiley, 1987. [Google Scholar]
- 22.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997; 95:2660–7. [DOI] [PubMed] [Google Scholar]
- 23.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–33. [DOI] [PubMed] [Google Scholar]
- 24.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail 2010;3:706–14. [DOI] [PubMed] [Google Scholar]
- 25.Jasseron C, Legeai C, Jacquelinet C, et al. Prediction of waitlist mortality in adult heart transplant candidates: the Candidate Risk Score. Transplantation 2017;101:2175–82. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard LC, Kouchoukos NT, Kurtts MA, Kirklin JW. Automated treatment of critically ill patients following operation. Ann Surg 1968;168: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerman JE, Kramer AA. Outcome prediction in critical care: the Acute Physiology and Chronic Health Evaluation models. Curr Opin Crit Care 2008;14:491–7. [DOI] [PubMed] [Google Scholar]
- 28.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957–63. [DOI] [PubMed] [Google Scholar]
- 29.Lorente JA, Vallejo A, Galeiras R, et al. Organ dysfunction as estimated by the sequential organ failure assessment score is related to outcome in critically ill burn patients. Shock 2009;31:125–31. [DOI] [PubMed] [Google Scholar]
- 30.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91–6. [DOI] [PubMed] [Google Scholar]
- 31.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6: 1212–27. [DOI] [PubMed] [Google Scholar]
- 32.United Network for Organ Sharing: Allocation Calculators. Available at: https://unos.org/transplantation/allocation-calculators/. Accessed June 13, 2018. [Google Scholar]
- 33.Reason JT. Human Error. Cambridge, MA: Cambridge University Press, 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.