Abstract
Objective:
To assess the impact of chlorhexidine gluconate (CHG) bathing on skin bacterial burden in neonates
Study Design:
In this prospective observational study, arm and groin skin bacterial growth was measured in 40 CHG-exposed and non-exposed neonates admitted to the NICU. Exposed neonates received 2% CHG baths per protocol for central line-associated bloodstream infection (CLABSI) prevention or Staphylococcus aureus decolonization.
Results:
Forty neonates were enrolled, 18 of whom were CHG-exposed. Mean baseline Gram-positive (GP) bacterial burden was 2.19 log CFU/ml on the arm and 1.81 log CFU/ml on the groin. Bacterial burden decreased after the first bath, but returned to baseline by 72 hours. Residual skin CHG concentration declined over time, with a corresponding increase in GP bacterial burden.
Conclusions:
CHG bathing reduces skin bacterial burden, but burden returns to baseline after 72 hours. Twice weekly CHG bathing may be inadequate to suppress skin bacterial growth in hospitalized neonates.
Keywords: chlorhexidine gluconate, infection control, neonatal intensive care unit
Introduction
Neonates admitted to the Neonatal Intensive Care Unit (NICU) are uniquely vulnerable to infection, due to such factors as prematurity, critical illness, an immature immune system, and exposure to invasive procedures.1 The United States Centers for Disease Control and Prevention (CDC) estimates that there are 33,000 healthcare associated infections (HAI) per year in United States NICUs.2 Effective, safe infection prevention strategies are paramount in the NICU, and topical antisepsis is a key component of such strategies. The appropriate choice of antiseptic used in hospitalized neonates has been unclear, given unique concerns about immature skin increasing risk of dermatologic side effects, potential for systemic absorption, and uncertainty regarding appropriate dosing.1
Chlorhexidine gluconate (CHG) is a broad-spectrum topical antiseptic that binds to bacterial cells, affects membrane integrity, and results in cell death.3 CHG is active against Gram-positive (GP) and Gram-negative (GN) bacteria, as well as yeast and some viruses, and daily skin cleansing with CHG has been demonstrated to reduce density of potential pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE), rendering it particularly useful in preventing HAI.3–6 Common applications include antisepsis for central venous catheter (CVC) insertion and maintenance, pre-operative bathing, and daily bathing of patients with CVCs.3,7 CHG has been used successfully in low resource settings to reduce neonatal sepsis, primarily for cord care.8–10
CHG use in neonates is becoming more widespread, with 86% of responding institutions in a 2014 survey of United States NICUs reporting CHG use for antisepsis in neonates; these findings represented a significant increase in CHG use since a prior survey conducted in 2009.11,12 Whole body bathing with CHG is used in neonates to prevent central line-associated bloodstream infections (CLABSI), as well as for targeted MRSA decolonization.3,11 A Canadian study demonstrated a reduction in CLABSI in the NICU with implementation of CHG bathing.13 Despite the recent increase in use of CHG in neonates, there are no clear dosing guidelines in neonates; frequency of bathing varies widely, and institutions use a variety of age- and weight-based restrictions for CHG use.11 Such restrictions exist due to concerns for potential adverse dermatologic effects, including skin irritation and burns, and for potential systemic absorption in preterm neonates with immature skin.14
A 2012 study conducted in 20 adults admitted to the ICU and receiving daily CHG baths assessed the effect of CHG on skin bacterial burden by relating microbial skin density with residual CHG remaining on the skin, as measured by a colorimetric assay.15 The authors found that CHG concentration was inversely associated with skin bacterial burden. Additionally, residual antimicrobial persisted after 24 hours in patients followed after receiving their last bath; however, residual CHG concentration fell below the determined effective level after 1–3 days.
Data are not available regarding the impact of CHG on skin bacterial burden in neonates, and CHG dosing and frequency of bathing in this population is not evidence-based. Our objective was to measure microbial density and residual CHG remaining on the skin after the first CHG bath.
Materials and Methods
Study Design
We conducted a prospective observational study from March 2015 until October 2016 in a convenience sample of neonates admitted to NICU of the Johns Hopkins Bloomberg Children’s Center, a tertiary care center located in urban Baltimore, Maryland. The Johns Hopkins Children’s Center NICU is a 45-bed level IV unit, providing medical and surgical services for acutely ill neonates. Admitted neonates were screened for eligibility by review of the medical record for gestational age, chronologic age, and indications for CHG use, including presence of CVC or colonization with MRSA or methicillin-sensitive Staphylococcus aureus (MSSA), prior to initiation of CHG baths. Neonates enrolled in the study who were not CHG exposed met age-based criteria for CHG eligibility but otherwise did not meet criteria for CHG administration (i.e., no MRSA/MSSA colonization or CVC in place). This study was approved by the Johns Hopkins Medicine Institutional Review Board. Consent for study participation was obtained from authorized caregivers.
Chlorhexidine protocol
The Johns Hopkins Children’s Center NICU bathes hospitalized neonates meeting age-based criteria with 2% presoaked CHG cloths (Sage Products, Cary, IL) for CLABSI prevention and targeted MRSA/MSSA decolonization. Specifically, for CLABSI prevention, neonates with CVCs receive twice weekly CHG baths after 72 hours of life if ≥36 weeks gestation at birth or after 4 weeks of life if <36 weeks gestation at birth. After 2 months of life, all neonates with CVCs receive daily CHG bathing, regardless of gestational age at birth. For MRSA/MSSA decolonization, neonates found to be colonized on surveillance culture receive targeted decolonization with intranasal mupirocin (all neonates) and with CHG baths, twice 48 hours apart after 72 hours of life if ≥36 weeks gestation at birth or after 4 weeks of life if <36 weeks gestation at birth, or daily baths for 5 days in all neonates after 2 months of age, as previously described.16
Skin bacterial burden
Skin swabs were obtained prior to the first CHG bath to assess baseline bacterial growth on the skin in all neonates; skin swabs were repeated at 1, 24, 48, 72, and, if applicable, 96 hours after the first bath in CHG-exposed neonates, until the time of the second CHG bath. Neonates who had previously received a CHG bath were excluded, eliminating the possibility of residual CHG remaining on the skin at time of baseline assessment. Additionally, a group of non-exposed neonates were swabbed at baseline, and a subset of non-exposed neonates were swabbed 24, 48, and 72 hours after baseline ascertainment of skin bacterial burden. Swabs were obtained using the ESwab collection system (COPAN FLOQSwabs, 1 ml Liquid Amies medium) without lubrication or site preparation from the upper arm and groin. Swab collection was performed by a single study staff member throughout the study to standardize collection; an area of 2 cm2 was swabbed at both upper arm and groin sites. Samples were maintained in Liquid Amies medium and refrigerated until processing within 24–48 hours. After vortexing the Eswab collection system, 200 μl aliquots were inoculated onto blood agar (Remel, Lenexa, KS) and Columbia CNA agar (Remel) to isolate GP bacteria and yeast, and MacConkey agar (Remel) to isolate GN bacteria. Plates were incubated at 37°C for 24–48 hours. Bacterial and yeast growth was measured quantitatively in log CFU/ml, and unique species were identified by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry.17
Residual CHG
In CHG-exposed neonates, residual CHG concentration was measured at 1 hour following first bath and every 24 hours until the second bath (48–96 hours after first bath depending on CHG bathing schedule) at upper arm and groin sites. Sterile dry swabs (Arrowhead Forensics, Lenexa, KS) were collected adjacent to sites swabbed for bacterial culture. CHG concentration was assessed via colorimetric assay for CHG detection previously described in the literature.15 Swabs were placed into a freshly prepared solution of cetyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO) and sodium hypobromite (Fisher Scientific, Waltham, MA) and observed for color change, which was compared with swabs inoculated with known concentrations of CHG, diluted from 20% stock solution (Sage Products).
Outcomes of interest
The primary outcomes of interest for this study were arm and groin GP bacterial colonization, measured in log CFU/ml, at baseline and 1, 24, and, as applicable, 48, 72, and 96 hours after the first CHG bath. Secondary outcomes of interest included arm and groin GN colonization, also measured in log CFU/ml, at baseline and 1, 24, and, as applicable, 48, 72, and 96 hours after the first CHG bath. Variables of interest included gestational age, birth weight, mode of delivery, and other clinical and demographic factors, as well as residual CHG concentration measured at 1, 24, and, as applicable, 48, 72, and 96 hours after the first CHG bath.
Statistical analysis
Descriptive analysis was performed to characterize the study population, using Student t test for continuous variables and Pearson’s χ2 test for categorical variables. Skin bacterial burden data were log-transformed for statistical comparisons. Weighted-least squares linear regression models were fit separately to the arm and groin GP bacterial burden (log CFU/ml) as a function of time from CHG bathing. The models allowed for different variance estimates for each time point and an unstructured within neonate correlation structure. For all statistical tests performed, a p-value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata version 13.0 (Stata Corp., College Station, TX).
Results
Clinical characteristics of participants
A total of 40 neonates were enrolled, 18 of whom were CHG-exposed. The mean gestational age was 34.1 weeks, and the mean birth weight was 2332 grams (Table 1). There was no statistically significant difference in gestational age or birth weight in CHG-exposed and non-CHG exposed neonates. The majority of enrolled neonates were inborn (65%) and delivered via C-section (65%). Postpartum antibiotic exposure was ubiquitous in both groups, with 100% of CHG-exposed neonates and 81.8% of non-exposed neonates receiving antibiotics after delivery. At time of the first swab, 44.4% of CHG-exposed neonates and 18.2% of non-exposed neonates were receiving systemic antibiotics.
Table 1.
Baseline Characteristics by CHG Exposure
| CHG-exposed n=18 |
Non-exposed n=22 |
All n=40 |
P-value | |
|---|---|---|---|---|
| Male, n (%) | 8 (44.4%) | 13 (59.1%) | 21 (52.5%) | 0.36 |
| Gestational age in weeks, mean ± SD | 32.8 ± 6.0 | 35.2 ± 5.1 | 34.1 ± 5.6 | 0.17 |
| Birth weight in grams, mean ± SD | 2146.4 ± 1213.5 | 2484.3 ± 1047.5 | 2332.2 ± 1123.3 | 0.35 |
| Inborn, n (%) | 11 (61.1%) | 15 (68.2%) | 26 (65.0%) | 0.64 |
| C-section, n (%) | 11 (61.1%) | 15 (68.2%) | 26 (65.0%) | 0.64 |
| Antepartum antibiotics, n (%)1 | 14 (82.4%) | 16 (84.2%) | 30 (83.3%) | 0.88 |
| Postpartum antibiotics, n (%) | 18 (100%) | 18 (81.8%) | 36 (90.0%) | 0.06 |
| Current antibiotics, n (%) | 8 (44.4%) | 4 (18.2%) | 12 (30.0%) | 0.07 |
| Age in days at first swab, mean ± SD | 19.6 ± 13.4 | 20.2 ± 18.8 | 19.9 ± 16.4 | 0.90 |
| Baseline arm GP bacterial count, mean log CFU/ml (range) | 2.39 (1.30–5.86) |
2.03 (0–3.48) |
2.19 (0–5.86) |
0.29 |
| Baseline groin GP bacterial count, mean log CFU/ml (range | 1.69 (0–5.18) |
1.91 (0–5.17) |
1.81 (0–5.18) |
0.65 |
Notes. 1Antepartum antibiotics data are available for 17/18 CHG-exposed and 19/22 non-exposed neonates. Abbreviations: CHG = chlorhexidine gluconate; GP = Gram-positive; CFU = colony forming units.
Quantitative skin bacterial burden
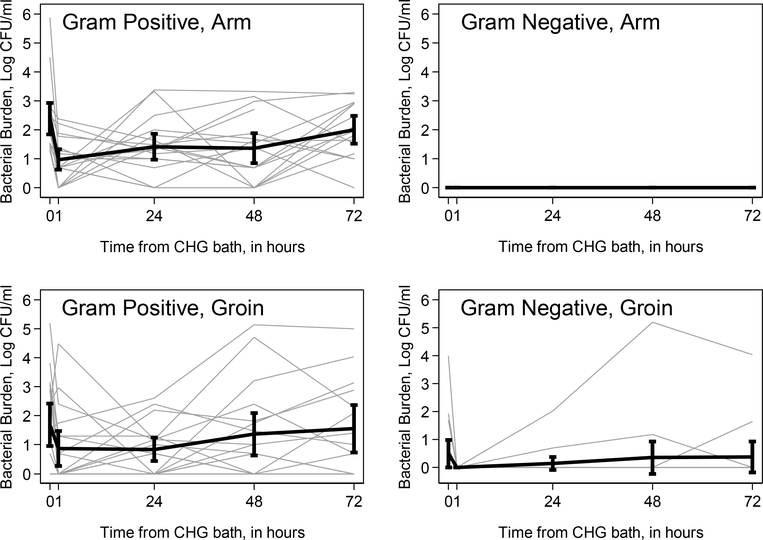
Mean baseline GP bacterial burden was 2.19 log CFU/ml (range 0–5.86) on the arm and 1.81 log CFU/ml (range 0–5.18) on the groin, with similar findings in CHG-exposed and non-exposed neonates (Table 1). In CHG-exposed neonates, mean arm GP bacterial burden decreased from 2.39 log CFU/ml (range 1.30–5.86) at baseline to 0.98 log CFU/ml (range 0–2.38) one hour after first bath (Table 2). Arm GP bacterial burden gradually increased at subsequent intervals, nearing baseline levels by 72 hours (Figure 1). Similarly, mean groin GP bacterial burden decreased from 1.69 log CFU/ml (range 0–5.18) at baseline to 0.87 log CFU/ml (range 0–4.48) one hour after first bath, and approached baseline levels by 72 hours (Figure 1). GN bacteria were not isolated on the arm of CHG-exposed neonates at baseline or at any time point following first bath (Figure 1). Mean groin GN bacterial burden decreased from 0.50 log CFU/ml (range 0–4.00) at baseline to zero at one hour, though GN growth was again detected in some participants at 24 hours and neared baseline by 72 hours (Figure 1). Only four neonates had a 96 hour measurement, due to the second scheduled bath occurring prior to this time point in most neonates.
Table 2.
Skin Bacterial Burden in CHG-exposed Neonates, by Site and over Time
| Baseline n = 18 |
1 Hour n = 18 |
24 Hour n = 18 |
48 Hour n = 18 |
72 Hour n = 14* |
96 Hour n = 4* |
|
|---|---|---|---|---|---|---|
| Arm | ||||||
| GP bacterial count, mean log CFU/ml (range) | 2.39 (1.30–5.86) |
0.98 (0–2.38) |
1.42 (0–3.38) |
1.36 (0–3.32) |
2.15 (1.00–3.30) |
0.88 (0–2.13) |
| GP species isolated, n (%) | CoNS = 18 (100%) Viridans Streptococci = 1 (5.6%) S. aureus = 2 (11.1%) E. faecalis = 1 (5.6%) |
CoNS = 13 (72.2%) Viridans Streptococci = 1 (5.6%) S. agalactiae = 1 (5.6%) E. faecalis = 1 (5.6%) |
CoNS = 15 (83.3%) Viridans Streptococci = 1 (5.6%) S. aureus = 2 (11.1%) E. faecalis = 3 (16.7%) |
CoNS = 13 (72.2%) S. aureus = 1 (5.6%) S. pneumoniae = 1 (5.6%) C. amycolatum = 1 (5.6%) E. faecalis = 1 (5.6%) |
CoNS = 12 (85.7%) Viridans Streptococci = 1 (7.1%) S. agalactiae = 1 (7.1%) S. aureus = 1 (7.1%) E. faecalis = 3 (21.4%) |
CoNS = 3 (75.0%) S. agalactiae = 1 (25.0%) |
| GN bacterial count, mean log CFU/ml (range) | 0 | 0 | 0 | 0 | 0 | 0 |
| GN species isolated, n (%) | -- | -- | -- | -- | -- | -- |
| Yeast count, mean log CFU/ml (range) | 0 | 0.04 (0–0.70) |
0 | 0 | 0 | 0 |
| Yeast species isolated, n (%) | -- | C. parapsilosis = 1 (5.6%) | -- | -- | -- | -- |
| Total count, mean log CFU/ml (range) | 2.40 (1.30–5.86) |
1.02 (0–2.38) |
1.42 (0–3.38) |
1.36 (0–3.32) |
2.15 (1.00–3.30) |
0.88 (0–2.13) |
| Groin | ||||||
| GP bacterial count, mean log CFU/ml (range) | 1.69 (0–5.18) |
0.87 (0–4.48) |
0.84 (0–2.60) |
1.37 (0–5.13) |
1.66 (0–5.00) |
1.18 (0–3.00) |
| GP species isolated, n (%) | CoNS = 11 (61.1%) Viridans Streptococci = 1 (5.6%) E. faecalis = 1 (5.6%) R. mucilaginosa = 1 (5.6%) |
CoNS = 7 (38.9%) E. faecalis = 1 (5.6%) |
CoNS = 8 (44.4%) S. aureus = 1 (5.6%) E. faecalis = 2 (11.1%) |
CoNS = 7 (38.9%) Viridans Streptococci = 2 (11.1%) S. agalactiae = 1 (5.6%) E. faecalis = 5 (27.8%) |
CoNS = 9 (64.3%) E. faecalis = 2 (14.3%) |
CoNS = 2 (50.0%) |
| GN bacterial count, mean log CFU/ml (range) | 0.50 (0–4.00) |
0 | 0.15 (0–2.02) |
0.35 (0–5.20) |
0.41 (0–4.04) |
0.71 (0–2.85) |
| GN species isolated, n (%) |
Klebsiella spp. = 4 (22.2%) E. aerogenes = 1 (5.6%) N. flavescens = 1 (5.6%) |
K. pneumoniae = 1 (5.6%) | K. pneumoniae = 2 (11.1%) |
E. coli = 1 (5.6%) K. pneumoniae = 2 (11.1%) B. firmus = 1 (5.6%) |
K. pneumoniae = 1 (7.1%) | E. cloacae = 1 (25.0%) |
| Yeast count, mean log CFU/ml (range) | 0.11 (0–2.06) |
0.10 (0–1.88) |
0 | 0 | 0 | 0 |
| Yeast, n (%) | C. parapsilosis = 1 (5.6%) | C. parapsilosis = 1 (5.6%) | -- | -- | -- | -- |
| Total count, mean log CFU/ml (range) | 1.81 (0–5.20) |
0.97 (0–4.48) |
0.95 (0–2.60) |
1.49 (0–5.20) |
1.68 (0–5.00) |
1.71 (0–3.00) |
Notes. Summary of colony counts and bacterial and yeast species identified in CHG-exposed neonates, by site (arm and groin) and over time.
1Due to bathing schedules per protocol, neonates receiving their second bath prior to 72 hours or 96 hours were not swabbed. Abbreviations: B. firmus = Bacillus firmus; CFU = colony forming units; C. amycolatum = Corynebacterium amycolatum; CHG = chlorhexidine gluconate; CoNS = coagulase-negative Staphylococcus; C. parapsilosis = Candida parapsilosis; E. aerogenes = Enterobacter aerogenes; E. cloacae = Enterobacter cloacae; E. coli = Escherichia coli; E. faecalis = Enterococcus faecalis; GN = Gram-negative; GP = Gram-positive; K. oxytoca = Klebsiella oxytoca; K. pneumoniae = Klebsiella pneumoniae; N. flavescens = Neisseria flavescens; R. mucilaginosa = Rothia mucilaginosa; S. agalactiae = Streptococcus agalactiae; S. aureus = Staphylococcus aureus; S. pneumoniae = Streptococcus pneumoniae
Figure 1. Skin bacterial burden on the arm and groin as a function of time from first CHG bath.
Spaghetti plot of skin bacterial burden in log CFU/ml as a function of time, by site and Gram classification. Left upper figure: arm Gram positive bacterial burden. Right upper figure: arm Gram negative bacterial burden. Left lower figure: groin Gram positive bacterial burden. Right lower figure: groin Gram negative bacterial burden. Gray lines represent individual patient measurements; black lines represent mean log CFU/ml with 95% confidence intervals at each time point. Abbreviations: CFU = colony forming units; CHG = chlorhexidine gluconate.
The results of the weighted-least squares models indicated that the mean GP bacterial burden differed for at least one time point, for both the arm (p < 0.001) and groin (p = 0.039), among CHG-exposed neonates (Table 3). Compared to the time of CHG bathing, there was a statistically significant reduction in the mean GP bacterial burden at the arm at 1 (p < 0.001), 24 (p = 0.005), and 48 (p = 0.001) hours. The mean arm GP bacterial burden did not differ statistically comparing the time of CHG bathing to 72 hours after bathing (p = 0.318). At the groin, there was a statistically significant reduction in the GP bacterial burden at 1 (p = 0.010) and 24 (p = 0.029) hours but not at 48 (p = 0.460) and 72 hours (p = 0.551). Weighted-least squares models were not performed for mean GN bacterial burden due to the significant number of samples with undetectable growth.
Table 3.
Change in Arm and Groin GP Bacterial Burden after First CHG Bath
| Reduction Relative to Baseline, mean log CFU/ml | P-value | 95% Confidence Interval | |
|---|---|---|---|
| Arm | |||
| 1 hour | −1.41 | <0.001 | −1.86, −0.96 |
| 24 hours | −0.97 | 0.005 | −1.65, −0.30 |
| 48 hours | −1.03 | 0.001 | −1.63, −0.42 |
| 72 hours | −0.35 | 0.318 | −1.03, 0.33 |
| Groin | |||
| 1 hour | −0.82 | 0.010 | −1.44, −0.20 |
| 24 hours | −0.85 | 0.029 | −1.61, −0.09 |
| 48 hours | −0.32 | 0.460 | −1.18, 0.53 |
| 72 hours | −0.26 | 0.551 | −1.10, 0.59 |
Notes. Weighted-least squares model assessing absolute reduction in mean skin GP bacterial burden on the arm and groin relative to baseline measurement in log CFU/ml obtained prior to first bath. Abbreviations: GP = Gram-positive; CHG = chlorhexidine gluconate; CFU = colony forming units.
A subset of non-exposed neonates had serial measurements of skin bacterial burden at 24 (n=4), 48 (n=3), and 72 hours (n=3). Specimens were not collected at 1 hour after baseline measurement, as these would not be expected to differ significantly from baseline. At 24 hours, mean arm GP bacterial burden was 2.56 log CFU/ml (range 1.30–5.03) and was stable at 48 hours (2.54 log CFU/ml, range 1.85–3.21) and 72 hours (2.63 log CFU/ml, range 1.7–4.00). Mean groin GP bacterial burden was 1.65 log CFU/ml (range 1.00–2.70) at 24 hours, 1.35 log CFU/ml (range 0–2.06) at 48 hours, and 1.85 log CFU/ml (range 0–3.78) at 72 hours. GN bacteria were not isolated on the arm of non-exposed neonates at 24 hours or 48 hours. Mean groin GN bacterial burden was 0.41 log CFU/ml (range 0–1.65) at 24 hours; no GN bacteria were isolated in three neonates at 48 hours and 72 hours.
Organisms isolated in CHG-exposed neonates
CHG-exposed neonates were most commonly colonized with coagulase-negative Staphylococcus species (CoNS), with 100% of neonates found to have at least one CoNS species on the arm at baseline; 50% of neonates had at least one CoNS species on the groin at baseline (Table 2). The most commonly isolated CoNS species was Staphyloccus epidermidis, though a variety of other CoNS species were present at each time point. Staphyolococcus aureus was present on the arm in two neonates at baseline (11.1%), none at 1 hour, two at 24 hours (11.1%), one at 48 hours (5.6%), one at 72 hours (7.1%), and none at 96 hours; only one neonate (5.6%) was found to have S. aureus at the groin, at 24 hours. Group B Streptococcus (GBS) was isolated in only one neonate, at 1 hour and at 72 hours on the arm, and at 48 hours on the groin. Enterococcus faecalis was isolated on the arm and the groin of at least one neonate at every time point except 96 hours.
As noted above, no CHG-exposed neonates were found to have GN bacteria on the arm at any time point (Table 2). Among groin specimens, the most commonly isolated GN organism was Klebsiella pneumoniae, with three neonates (16.7%) at baseline, one at 1 hour (5.6%), two at 24 hours (11.1%), two at 48 hours (11.1%), and one (7.1%) at 72 hours growing K. pneumoniae on culture. At baseline, the other organisms isolated included Klebsiella oxytoca, Enterobacter aerogenes, and Neisseria flavescens; none of these organisms were isolated at any time point after the first CHG bath. Escherichia coli was present in one neonate (5.6%) at 48 hours, Bacillus firmus in one neonate (5.6%) at 48 hours, and Enterobacter cloacae in one neonate (25.0%) at 96 hours.
Among CHG-exposed neonates, only one participant was found to be colonized with yeast; Candida parapsilosis was isolated at baseline on the groin and at one hour on the arm and groin (Table 2).
Residual CHG concentration and skin bacterial burden
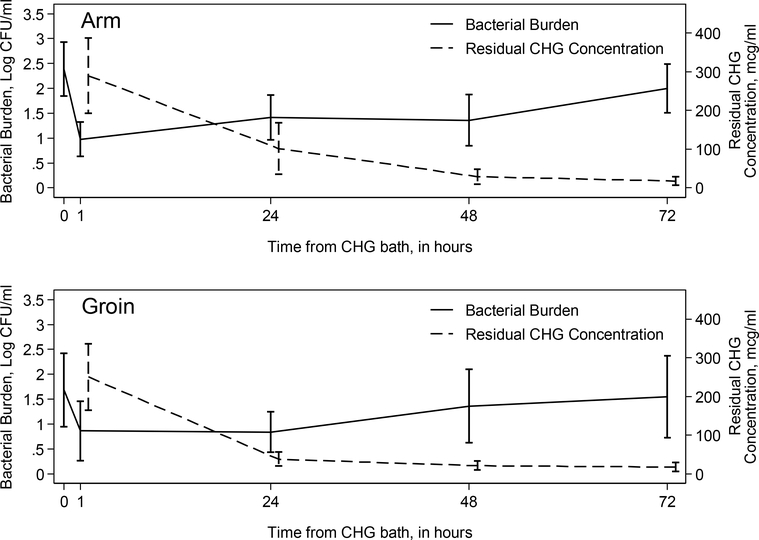
Residual CHG concentration was measured in at the same time points as skin bacterial burden in bathed neonates, at 1, 24, 48, and, if applicable, at 72 and 96 hours. As expected, peak concentrations were seen at 1 hour following first bath, with mean concentration of 290.0 mcg/ml (n=18) on the arm and of 250.7 mcg/ml (n=18) on the groin. On the arm, residual CHG concentration gradually decreased, to 100.6 mcg/ml at 24 hours (n=18), 29.3 mcg/ml at 48 hours (n=18), 17.8 mcg/ml at 72 hours (n=14), and 6.5 mcg/ml at 96 hours (n=3). Similarly, residual CHG concentration on the groin decreased over time, to 37.7 mcg/ml at 24 hours (n=18), 21.0 mcg/ml at 48 hours (n=17), 17.4 mcg/ml at 72 hours, and no detectable residual CHG at 96 hours (n=3). Over time, as residual CHG concentration on the skin falls, skin bacterial burden returns toward baseline at both arm and groin sites (Figure 2).
Figure 2: Mean Gram positive bacterial burden and residual CHG concentration as a function of time from CHG bath.
Mean Gram positive bacterial burden and mean CHG concentration with 95% confidence intervals at each time point, separately for the arm (upper figure) and groin (lower figure). Abbreviations: CFU = colony forming units; CHG = chlorhexidine gluconate.
Clinical outcomes
No neonate enrolled in this study was diagnosed with CLABSI during NICU admission. There were no dermatologic side effects noted in CHG-exposed neonates. One neonate enrolled in the study died after study completion of unrelated causes.
Discussion
In this observational study, bathing neonates admitted to the NICU with 2% CHG was associated with a statistically significant decrease in skin GP bacterial burden at 1, 24, and 48 hours after initial CHG bath, with gradual rebound toward baseline over the 72 hours following first bath as residual CHG concentration on the skin declined. In our study population, CHG bathing reduced bioburden of S. epidermidis, the most common causative pathogen in late onset sepsis and CLABSI in neonates in the United States, as well as other potential GP and GN pathogens. Presumptively, reduction in the quantitative skin bacterial burden reduces the risk of infection in these vulnerable neonates, though a threshold of adequate suppression to prevent infection has not been established.
Hospitalized adults and children are frequently bathed daily with CHG to reduce the risk of nosocomial infections, but the appropriate frequency of bathing in neonates has not been established.18,19 Some centers reduce CHG bathing frequency in neonates to twice weekly, hoping to reduce the risk of adverse dermatologic effects and the potential for systemic absorption; however, less frequent exposure may not be sufficient to reduce infection risk, as skin bacterial burden may not be adequately suppressed between baths.
Popovich and colleagues described a reduction of skin bacterial burden in critically ill adults bathed daily with CHG, with a similar initial dramatic decline in microbial density followed by a gradual increase over time.15 Higher CHG concentration, as measured by colorimetric assay, was also associated with lower GP colony counts. Of note, GP bioburden in adults at baseline was significantly lower than what we observed in neonates; however, adults included in the Popovich study had previously been exposed to CHG, perhaps reflecting residual suppression of bacterial growth from the prior bath.
Strengths of this study include its application of a colorimetric assay previously used in adult studies to a novel population, measurement of true baseline skin bacterial burden prior to any CHG exposure, and comparison of skin bacterial burden over time in CHG-exposed and non-exposed neonates. Of particular importance is the investigation of the effect of CHG bathing over time, as there are no clear guidelines on dosing and frequency of CHG bathing in neonates. Inclusion of a subset of non-exposed neonates demonstrated that skin bacterial burden remained relatively constant over time in neonates not bathed with CHG, whereas bathed neonates had a decrease following CHG exposure. Interpretation of the study results must be guided by the study design; the overall small sample size renders the study inadequately powered to assess certain outcomes of interest, including CLABSI rates. However, the reduction of CLABSI and other HAI with the introduction of CHG bathing has been demonstrated in other studies.5,13,18
CHG whole body bathing is a particularly promising intervention in settings where the incidence of neonatal sepsis is high. While a recent Cochrane review did not find the practice to have a conclusive benefit, included studies evaluated the benefit of a single bath.20 A 2016 randomized controlled trial by Gupta and colleagues conducted at a tertiary care center in India revealed a lower rate of culture-confirmed bacterial sepsis in neonates receiving daily chlorhexidine baths for the first seven days of life, 3.57% of neonates versus 6.85% of neonates receiving placebo; these findings did not reach statistical significance.21
More studies are needed to study the safety, efficacy, and dosing of CHG in high-risk neonates. However, clinicians considering the use of CHG for the reduction of skin bioburden in neonates admitted to the NICU should consider use of baths every 48–72 hours to achieve adequate reduction in infection risk.
Acknowledgements:
The authors wish to thank the study’s participants and their families, as well as Annie Voskertchian and Anne King for their study support, and Dr. Mary K. Hayden and her colleagues at Rush University for sharing their colorimetric assay protocol.
Financial support: This study was supported by an investigator-initiated research grant from Sage Products, LLC, and NIH Training Grant Award T32 HL 125239–1 (JJ).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Ponnusamy V, Venkatesh V, Clarke P. Skin antisepsis in the neonate: what should we use? Curr Opin Infect Dis 2014; 27: 244–250. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007; 122: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the armamentarium for control and prevention. Clin Infect Dis 2008; 46: 274–281. [DOI] [PubMed] [Google Scholar]
- 4.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006; 166: 306–312. [DOI] [PubMed] [Google Scholar]
- 5.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007; 167: 2073–2079. [DOI] [PubMed] [Google Scholar]
- 6.Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009; 37: 1858–1865. [DOI] [PubMed] [Google Scholar]
- 7.Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O’Grady NP et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35: 753–771. [DOI] [PubMed] [Google Scholar]
- 8.Mullany LC, Saha SK, Shah R, Islam MS, Rahman M, Islam M et al. Impact of 4.0% chlorhexidine cord cleansing on the bacteriologic profile of the newborn umbilical stump in rural Sylhet district, Bangladesh. Pediatr Infect Dis J 2012; 31: 440–450. [DOI] [PubMed] [Google Scholar]
- 9.Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomized trial. Lancet 2012; 379: 1022–1028. [DOI] [PubMed] [Google Scholar]
- 10.Sinha A, Sazawal S, Pradhan A, Ramji S, Opiyo N. Chlorhexidine skin or cord care for prevention of mortality and infections in neonates. Cochrane Database Syst Rev 2015; CD007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J, Bracken R, Tamma PD, Aucott SW, Bearer C, Milstone AM. Trends in chlorhexidine use in US Neonatal Intensive Care Units: Results from a follow-up national survey. Infect Control Hosp Epidemiol 2016; 37: 1116–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: results from a national survey. Infect Control Hosp Epidemiol 2010; 31: 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quach C, Milstone AM, Perpête C, Bonenfant M, Moore DL, Perreault T. Chlorhexidine bathing in a tertiary care neonatal intensive care unit: impact on central line-associated bloodstream infections. Infect Control Hosp Epidemiol 2014; 35: 158–163. [DOI] [PubMed] [Google Scholar]
- 14.Chapman AK, Aucott SW, Milstone AM. Safety of chlorhexidine gluconate used for skin antisepsis in the preterm infant. J Perinatol 2012; 32: 4–9. [DOI] [PubMed] [Google Scholar]
- 15.Popovich KJ, Lyles R, Hayes R, Hota B, Trick W, Weinstein RA et al. Relation of chlorhexidine gluconate skin concentration to microbial density on skin of critically ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol 2012; 33: 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popoola VO, Budd A, Wittig SM, Ross T, Aucott SW, Perl TM et al. MRSA transmission and infections in a neonatal intensive care unit despite active surveillance cultures and decolonization – challenges for infection prevention. Infect Control Hosp Epidemiol 2014; 35: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.S. Angeletti Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J Microbiol Methods 2017; 138: 20–29. [DOI] [PubMed] [Google Scholar]
- 18.Climo MA, Wong ES. Daily chlorhexidine bathing and hospital-acquired infection. N Engl J Med 2013; 368: 2332. [DOI] [PubMed] [Google Scholar]
- 19.Milstone AM, Elward A, Song X, Zerr DM, Orscheln R, Speck K et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicenter, cluster-randomised, crossover trial. Lancet 2013; 381: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankar MJ, Paul VK. Efficacy and safety of whole body skin cleansing with chlorhexidine in neonates – A systemic review. Pediatr Infect Dis J 2013; 32: e227–e234. [DOI] [PubMed] [Google Scholar]
- 21.Gupta B, Vaswani ND, Sharma D, Chaudhary U, Lekhwani S. Evaluation of efficacy of skin cleansing with chlorhexidine in prevention of neonatal nosocomial sepsis – a randomized controlled trial. J Matern Fetal Neonatal Med 2016; 29: 242–247. [DOI] [PubMed] [Google Scholar]