Abstract
Background:
Histones are essential components of chromatin and mutations in histones lead to alterations in methylation and acetylation, which play an important role in tumorigenesis. Most of the chondroblastomas harbor H3K36M mutation. With the availability of mutation specific antibody, we aimed to assess the sensitivity of this antibody and the alterations of histone methylation in a series of chondroblastoma cases.
Design:
Immunohistochemical stains using antibodies for H3K36M, trimethylated histones (H3K27me3 and H3K36me3), and osteoblastic marker (SATB2) were performed on 27 chondroblastomas from 27 patients. The clinical and radiological characteristics of each patient was reviewed.
Results:
All 27 tumors showed typical radiological and histological features of chondroblastoma with a subset of cases showing secondary aneurysmal bone cyst changes (11/27), giant cell rich foci (4/27), and matrix rich areas mimicking chondromyxoid fibroma (1/27). All except 1 case (26/27, 96%) were positive for H3K36M immunostaining (nuclear). In majority of cases staining pattern was diffuse. Immunohistochemical expression of H3K27me3 and H3K36me3 revealed heterogeneous staining pattern in all cases regardless of mutation status. None of the cases showed loss or diffuse positivity. Focal or diffuse SATB2 expression was seen in 21 out of 26 tumors (81%).
Conclusion:
Our results demonstrate that vast majority of chondroblastomas are positive for H3K36M by immunohistochemical analysis, confirming its diagnostic value. The expression of H3K27me3 and H3K36me3 are heterogeneous in these tumors.
Keywords: chondroblastoma, histone, H3K36M, mutation, methylation, H3K27me3, H3K36me3, SATB2
Introduction
Histones are protein components of chromatin and their major role is to package the DNA into nucleosomes and regulate gene expression. There are 5 major types of histones: one linker histone, H1, and four core histones, H2A, H2B, H3, and H4. Post-translational modifications of histones, including methylation, acetylation, phosphorylation, ubiquitylation, and sumoylation, play an important role in epigenetic regulation of gene expression, leading to either gene activation or repression. Among all histones, H3 and H4 are most commonly affected by modifications. Mutations and post-translational modifications of histones are involved in tumorigenesis, drug resistance, and prognosis [1].
Recent studies have identified mutations of H3 in giant cell tumors of bone and chondroblastomas[2]. Majority of chondroblastomas harbor histone H3 lysine 36 to methionine mutation (K36M) in H3F3B and H3F3A and most of the giant cell tumors of bone contain G34W mutation in H3F3A [2]. These mutations may represent dominant driver events in these tumors and are rarely detected in other bone tumors [2]. Lu et al. further studied the underlying mechanism of H3K36M in tumorigenesis and found that H3K36M mutation impairs chondrocytic differentiation of mesenchymal progenitor cells [3]. Mesenchymal progenitor cells expressing H3K36M mutant histone, when transplanted into immunodeficient mice, can form tumors resembling human undifferentiated sarcoma [3]. The authors also found that histone methylation is driven by H3F3B mutation. H3K36M mutation leads to loss of H3K36me2/3 and gain in H3K27me2/3 [3], the latter functioning as repressor of gene transcription.
In this study, we retrospectively reviewed 27 cases of chondroblastoma that were treated from 2000 to 2013 at our institution. The clinicopathological features of these tumors were reviewed and immunohistochemical studies of mutant H3 (H3K36M) and methylated H3(H3 K36me3 and H3K27me3) were performed. The study was performed to confirm the presence of H3K36M mutation using mutant specific antibody in chondroblastomas and correlate the mutation status with clinicopathological features and the expression of H3K27me3 and H3K36me3.
Material and Methods
Patient selection and evaluation of clinical characteristics
Patients who underwent surgical treatment during the period from 2000 to 2013 with a final pathological diagnosis of chondroblastoma were included in this study. Twenty-seven patients were identified. Medical records were reviewed for the patients’ age, gender, site of involvement, radiological findings, treatment, follow-up, and recurrence of tumor. In selected cases radiology input was obtained from a musculoskeletal radiologist. The project have been approved by the Office of Clinical Research at Memorial Sloan Kettering Cancer Center (IRB -16–1490).
Histology and immunohistochemistry
The specimens were fixed in formalin and decalcified as needed. Routine H&E sections were prepared and reviewed by 3 pathologists independently. Immunohistochemical staining using antibodies specific for mutant H3K36M (RM193, Cayman Chemical, San Francisco, CA. 1:2500), methylated H3K27me3 (Cell Signaling Technology, Danvers, MA. 1:200), H3K36me3(MABI 0333, Active Motif, Carlsbad, CA. 1:500), and osteoblastic marker SATB2 (EP281, Cell Marque, Rocklin, CA. 1:400) was performed on either the Leica (H3K36M, H3K27me3 and H3K36me3) or Benchmark Ultra platform (SATB2). A known positive control was used for each antibody to ensure the quality of staining. Nuclear staining of H3K36M and SATB2 were interpreted as positive or negative regardless of percentage of tumor cell staining. The staining pattern of H3K27me3 and H3K36me3 was semi-quantitatively assessed using a method modified from the widely used H-Score [4]. Briefly, the staining intensity of H3K27me3 or H3K36me3 is graded as follows: 0 = negative; 1 = weak; 2 = intermediate; and 3 = strong. The percentage of positive tumor cells in the tumor tissue is then estimated. The modified H-score is calculated using the following equation: modified H-score = % of positive cells * staining intensity. Statistics was performed to determine the correlation of H-scores between H3K27me3 and H3K36me3 staining.
Results
Clinical presentation
The clinical features of the 27 cases are summarized in Table 1. The twenty-seven patients include 20 males and 7 females with ages ranging from 11 to 34 years (median 19.5 years). Sixteen patients were in their 2nd, eight in their 3rd, and three in their 4th decade of life.
Table 1.
Clinical features of the 27 cases of chondroblastoma
| Case # | age | Gender | Location | Laterality | Tumor size | F/U (months) | Recurrence or Metastasis | H3K36M | H3K27me3# | H3K36me3# | SATB2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | m | Femur, distal epiphysis | L | 1.1cm | 62 | n | pos | 120 | 120 | pos |
| 2 | 11 | m | Femur, distal epiphysis | R | 1.4 cm | 58 | n | pos | 240 | 270 | neg |
| 3 | 29 | m | Femur, distal epiphysis | R | 1.8 cm | 46 | n | pos | 100 | 80 | pos, focal |
| 4 | 22 | m | Femur, distal epiphysis | L | na | 129 | Local recurrence at 23 months | pos | 120 | 80 | pos, focal |
| 5 | 22 | f | Femur, great trochanter | R | 3.5 cm | 1 | n | neg | n/a | n/a | pos, focal |
| 6 | 33 | f | Femur, great trochanter | L | 4.1 cm | 54 | n | pos | n/a | n/a | pos |
| 7 | 24 | m | Femur, great trochanter | L | na | 10 | n | pos | 60 | 160 | neg |
| 8 | 17 | m | Humerus, proximal epiphysis | R | 3.5 cm | 110 | n | pos | 210 | 140 | pos, focal |
| 9 | 13 | m | Humerus, proximal epiphysis | R | na | 77 | n | pos | 140 | 240 | pos |
| 10 | 14 | f | Humerus, proximal epiphysis | L | 2.7cm | 57 | n | pos | 270 | 270 | pos, focal |
| 11 | 16 | m | Humerus, proximal epiphysis | L | 2.3 cm | na | na | pos | 180 | 210 | pos |
| 12 | 20 | m | Humerus, proximal epiphysis | L | 2.1 cm | 12 | n | pos | 80 | 180 | neg |
| 13 | 14 | m | Humerus, proximal epiphysis | L | 2.4 cm | 69 | n | pos | 160 | 120 | pos, focal |
| 14 | 23 | m | Humerus, trochlea | L | 3 cm | 16 | n | pos | 210 | 180 | pos, focal |
| 15 | 13 | m | Ischium | L | 5.2 cm | 11 | Metastasized to lung at 11 months | pos | 80 | 270 | rare |
| 16 | 19 | f | Scapula, acromion | R | 3.3 cm | 102 | n | pos | 270 | 240 | pos |
| 17 | 34 | m | Scapula, acromion | R | 3.4 cm | 17 | n | pos | 270 | 270 | neg |
| 18 | 21 | m | Scapula, coracoid process | L | 9.3 cm | 0 | n | pos | 270 | 270 | pos, focal |
| 19 | 27 | m | Talus | L | 2.8 cm | 44 | n | pos | 120 | 210 | n/a |
| 20 | 31 | m | Talus | R | 2 cm | 68 | n | pos | 240 | 240 | pos |
| 21* | 20 | f | Talus | L | 2.3 cm | 21 | n | pos | 40 | 180 | pos |
| 22 | 17 | m | Talus | R | 2.3 cm | 3 | n | pos | 80 | 150 | neg |
| 23 | 27 | m | Talus | R | 2.3 cm | 36 | n | pos | 240 | 210 | pos, focal |
| 24 | 13 | f | Tibia, proximal epiphysis | L | na | 25 | n | pos | n/a | n/a | pos |
| 25 | 17 | m | Tibia, proximal epiphysis | L | 1.5 cm | 30 | n | pos | 30 | 90 | pos |
| 26* | 11 | f | Tibia, proximal epiphysis | L | 2.5 cm | 18 | n | pos | 50 | 80 | pos, focal |
| 27 | 14 | m | Tibia, proximal epiphysis | L | 2 cm | 3 | n | pos | 120 | 210 | pos |
The patients presented to our hospital with recurrent chondroblastoma. Pos = positive; neg = negative; n/a = not available.
Modified H-score = % of positive cells * average staining intensity.
The locations of tumors included femur (n=7, 3 in greater trochanter and 4 in distal epiphysis), humerus (n=7, 6 in proximal epiphysis and 1 in trochlea), ischium (n=1), scapula (n=3, 2 in acromion and 1 in coracoid process), talus (n=5), and tibia (n=4, all in proximal epiphysis). Twenty-five tumors were primary and the other 2 were recurrent, of which the primaries were curetted at outside hospitals 8 and 18 months before surgery at our hospital. All tumors were treated by curettage with bone grafting or cementation. Selected cases were treated with pre-operative embolization or cryoablation during surgery. One patient was lost for follow up right after surgery and the rest 26 patients were followed for 1 to 129 months after surgery (median 30 months, mean 40 ± 35 months). One tumor had local recurrence at 23 months after primary surgery and the recurrent tumor was curetted again and no recurrence was seen after a follow-up of another 103 months. One patient had lung metastases at 12 months after the surgery and then was lost for follow-up.
Radiologic characteristics
The available imaging studies including plain radiographs, CT and MRI were reviewed for all the cases. Except for the one tumor that involved the left ischium, all the other 24 primary tumors were located in epiphyseal or apophyseal bone and showed typical radiological findings, including lytic lesion, well-circumscription, sclerotic rimming, and prominent edema (Fig. 1). Among these 24 tumors, one tumor showed cortical breakthrough (trochlea of humerus), the tumor was curetted, and the patient remained free of disease at 16 months after surgery.
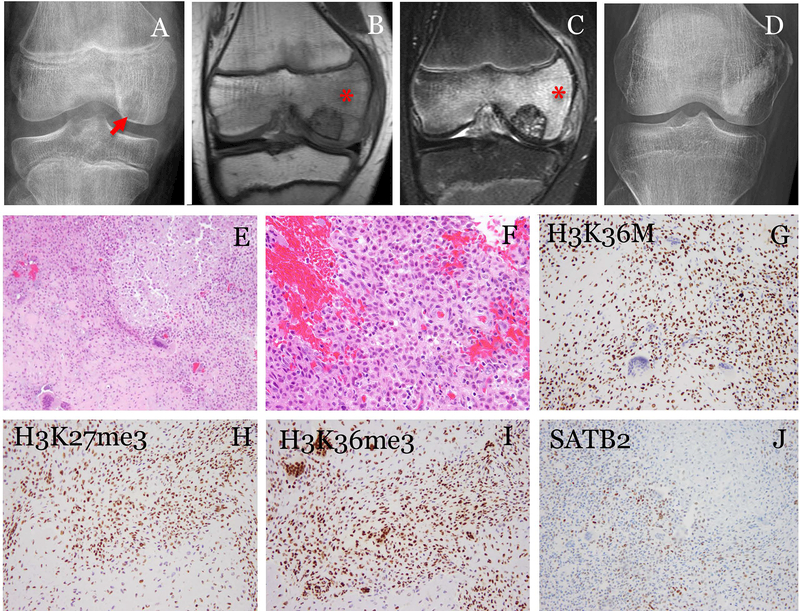
Fig. 1.
A representative case of chondroblastoma with positive H3K36M immunostaining. The patient was a 11-year-old male. (A) Radiograph showed an epiphyseal lytic tumor(arrow). (B) MRI T1- and (C) T2-weighted fat-suppressed images showed the tumor surrounded by edema (*). The tumor was curetted and cemented. The patient was followed for 58 months and (D) radiograph showed no recurrence. (E) Histologically, the tumor was composed of sheets of uniform cells with focal chondroid differentiation and scattered multi-nucleated giant cells (200x). (F) The tumor cells were oval to polygonal with well-defined pink cytoplasm and cleaved nuclei (400x). (G) The tumor cells showed strong nuclear staining of H3K36M (200x). Immunostaining for methylated histones, (H) H3K27me3 and (I) H3K36me3, showed heterogeneous staining in both the mononuclear tumor cells and the multi-nucleated giant cells (200x). (J) SATB2 was positive in a subset of the tumor cells (200x).
Pathological characteristic
All 27 tumors showed typical morphology of chondroblastoma, including sheets of polygonal cells with eosinophilic cytoplasm and nuclei with grooving and scattered osteoclast-type giant cells (Fig. 1). Primitive chondroid foci with chicken-wire calcification were frequently seen. Eleven tumors showed secondary aneurysmal bone cyst-like changes, 4 tumors showing giant cell rich foci mimicking giant cell tumor, and 1 tumor showing matrix rich areas mimicking chondromyxoid fibroma (Fig. 2).
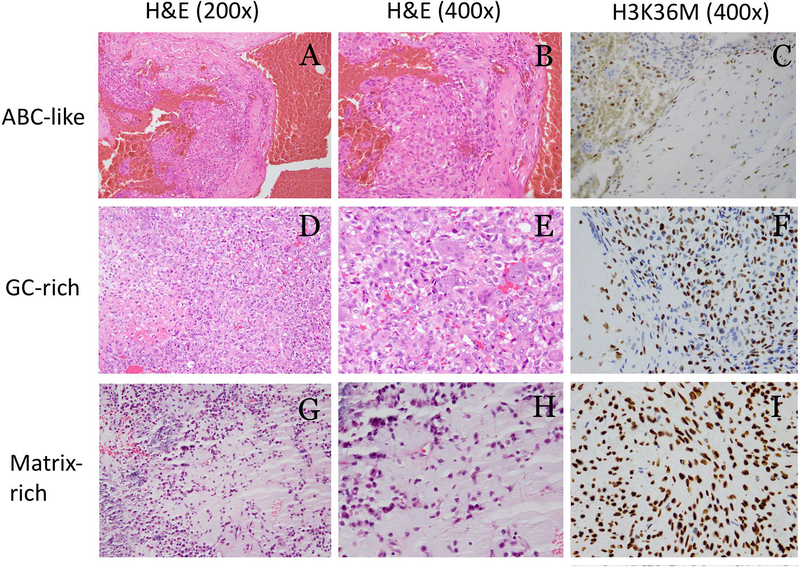
Fig. 2.
Chondroblastomas with focal aneurysmal bone cyst-like changes (A-C), giant cell rich area (D-F), and matrix rich area (G-I). All tumors were positive for H3K36M immunostaining. H3K36M positive cells are seen in ABC-like areas (arrow) (C).
Results of immunohistochemistry
Twenty six out of the 27 cases (96%) showed nuclear positivity for H3K36M antibody and the staining pattern was overall diffuse in majority of cases (Fig. 1 and Table 1). While internal non-neoplastic tissue, i.e. endothelial cells and fibrous tissue, showed consistent strong staining, the tumor cells exhibited a heterogenous pattern for both H3K27me3 and H3K36me3 immunostaining (Fig. 1 and Table 1). The modified H-scores of the H3K27me3 staining varied from 30–270 (median = 130) and the scores of the H3K36me3 staining varied from 80–270 (median = 195). Statistical analysis showed the H-scores of H3K27me3 and H3K36me3 staining are positively correlated and the Pearson correlation coefficient is 0.65 (P<0.001).
There was only one tumor that was negative for H3K36M antibody. The patient was a 22-year-old female and the tumor was located in the greater trochanter of right femur. The tumor showed typical radiologic and pathologic features of chondroblastoma and exhibited negative staining for H3K27me3 and focal weak staining for H3K36me3. It is possible that fixation and decalcification may have contributed to the absent staining in this case. The tumors with ABC-like, giant cell rich, and matrix rich foci were all diffusely positive for H3K36M immunostaining (Fig. 2). Immunostain for SATB2, a marker for osteoblastic differentiation was positive in the tumor cells in 21 out of 26 tumors (81%. Fig. 1), while 11 of them showed diffuse staining and the rest of the cases were focal (Table 1).
Chondroblastoma with lung metastasis
In this series, we have one rare case of lung metastasis. The patient was a 13-year-old boy with a large left ischial lesion. The lesion had been curetted twice at an outside hospital before the patient came to our hospital. Plain radiographs and CT scan showed a large expansile lytic lesion involving the left ischium. The lesion extended beyond the contour of normal cortex but appeared to be confined by a thin shell of bone (Fig. 3). On MRI, the lesion showed multi-cystic changes with fluid-fluid levels (Fig. 3). Chest radiograph was negative at the time of admission. The lesion was embolized, curetted, treated with liquid nitrogen and allograft. Pathological examination demonstrated a chondroblastoma with typical morphology and secondary aneurysmal bone cyst-like changes. The tumor was positive for H3K36M antibody supporting the diagnosis of chondroblastoma (Fig. 3). Three months later, evidence of local recurrence was again seen on MRI imaging and biopsy confirmed recurrence of chondroblastoma (Fig. 3). At 11 months after the primary surgery, chest x-ray revealed multiple nodules in both lungs. Biopsy of one of the nodules in the left lower lobe showed typical morphology of chondroblastoma. Tumor cells were positive for H3K36M immunostaining similar to the primary tumor. The patient decided to continue his treatment with his primary physician and was lost for follow-up (Fig. 3).
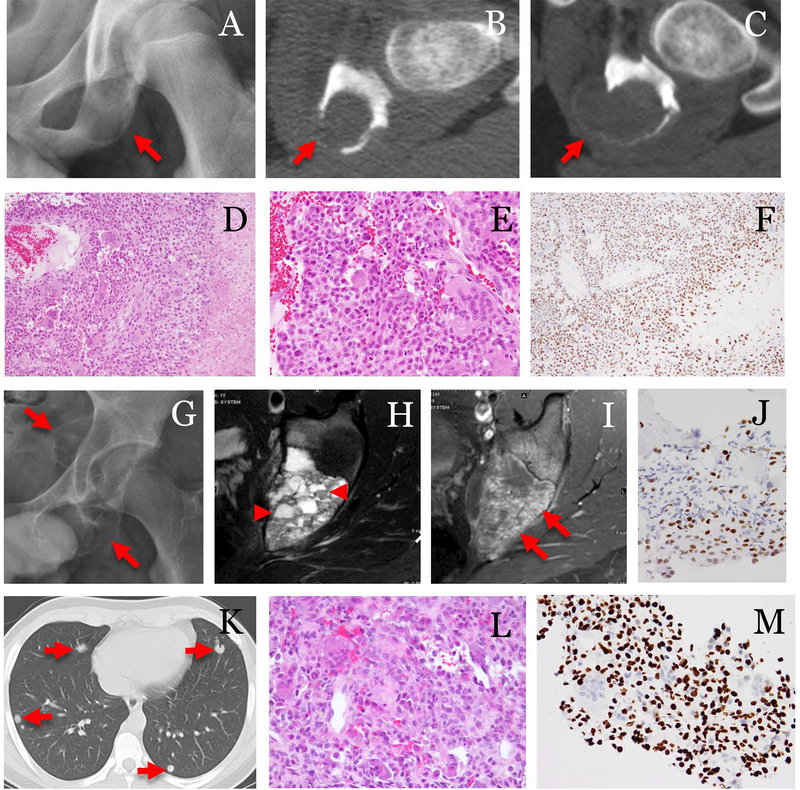
Fig. 3.
A case of chondroblastoma with lung metastasis. The patient was a 13-year-old male. (A) Pre-op radiograph showed a lytic ischial lesion(arrow). (B, C) Serial CT exams in 2 months showed increased size and cortical destruction of the tumor(arrows). The tumor was treated by embolization, curettage and bone grafting. (D) Histologically, the tumor was composed of sheets of uniform cells with chondroid differentiation, aneurysmal bone cyst-like change, and frequent multi-nucleated giant cells (100x). (E) The tumor cells showed typical morphology of chondroblasts (400x) and (F) were strongly positive for H3K36M (200x). Three months after curettage, (G) radiograph showed recurrent tumor (arrows). (H) MRI T2-weighted, and (I) T1-weighted FS post-contrast images showed fluid levels (arrowheads) and enhancing components(arrow) in the tumor. (J) The recurrence was biopsied and tumor was again positive for H3K36M immunostaining. At 11 months after the surgery, (K) CT showed pulmonary nodules(arrows). (L) Needle core biopsy of a lung nodule confirmed metastatic chondroblastoma, which showed similar morphology as that seen in the primary tumor (400x) and (M) tumor cells were positive for H3K36M immunostaining (400x).
Discussion
Chondroblastomas are most commonly seen in patients in their second decade of life. In a large series of 103 cases reported by Konishi et al., the ages ranged between 8–61 years[5]. Chondroblastomas in the first decade or older adults are relatively rare. Dahlin et al. reported chondroblastomas in two 5-year-old children [6]. In a group of patients with chondroblastoma in the hands or feet, the youngest patient was 7-year-old while the oldest was 52 [7]. Chondroblastomas at unusual locations such as ribs tend to affect older individuals [6]. The ages of our patients ranged from 11 to 34 years, within the reported age range.
Majority of the cases in our cohort had typical clinical, radiological, and pathological features of chondroblastoma. These cases involved the epiphysis or apophysis of long bones, metatarsals, scapula, and ischium. None of our cases involved the carpal bones. Most of the tumors presented as well-circumscribed lesions within the bone and cortical breakthrough was very uncommon.
Behjati et al. screened 77 chondroblastomas and found Histone- 3 mutations in 95% of the cases. All the mutations were K36M with a majority in H3F3B and the rest in H3F3A [2]. The authors also found 1 out 15 clear cell chondrosarcomas and 1 out of 75 conventional chondrosarcomas harboring mutations of H3K36M. Amary et al. further assessed the value of using immunohistochemistry for H3K36M (mutant specific antibody) and found that immunoexpression is highly specific for K36M mutation status and is a valuable diagnostic tool for chondroblastoma[8]. Our results confirmed the presence of H3K36M mutated protein in vast majority of chondroblastomas with typical morphology and immunoreactivity of H3K36M antibody is highly sensitive for chondroblastoma.
A few recent studies have examined the underlying mechanisms of tumorigenesis induced by H3K36M mutation [3,9]. One postulated mechanism is reprogramming the epigenome of chondrocytes by altering histone methylation especially reducing H3K36 methylation[9]. Lu et al. found that oncogenic H3K36M mutation is associated with H3K36 hypomethylation and H3K27 hypermethylation [3]. In the interplay of histone modifications, H3K27methylation is increased in the absence of H3K36 methylation [10]. In our current study, we examined the histone methylation status by immunohistochemistry using antibodies against H3K36me3 and H3K27me3. All H3K36M immunoreactive chondroblastomas showed heterogeneous expression (in a scale of 1+ to 3+) of H3K27me3 and H3K36me3. No complete loss of H3K36me3 methylation was observed. Similar to our study, Cleven et al found a mosaic type (defined as <50% of nuclei) in 6 of 10 chondroblastoma samples [11]. We had only one case of chondroblastoma that was negative for H3K36M immunostaining and we did not have enough material to assess the effect of H3K36M mutation on histone methylation in that case. Interestingly, when we performed semi-quantitative analysis using modified H-score and correlated the methylation patterns of H3K27me3 and H3K36me3, we found a positive correlation. Similar correlation study was not performed in Lu et al.’s study and our finding of positive correlation should not be interpreted as contradictory to their findings of hypomethylation of H3K36 and hypermethylation of H3K27 associated with H3K36M transgenes [3]. Whether this represents an aberrant methylation pattern is yet to be determined. and additional studies are needed which is beyond the scope of this study.
Our study showed that SATB2 is positive in majority of chondroblastomas. SATB2 is a transcription factor that plays an important role in osteoblast differentiation and bone formation [12]. The use of SATB2 immunohistochemistry as a marker of osteoblastic differentiation was shown by Conner et al. who demonstrated frequent nuclear staining of SATB2 in osteosarcoma and other bone forming tumors [13]. Non-specific staining, usually weak and focal, is seen in chondromyxoid fibroma, unclassified pleomorphic sarcomas, fibrosarcoma, monophasic synovial sarcoma, and rare sclerosing epithelioid fibrosarcoma [14, 15]. Chondroblastoma is classified as a chondrogenic tumor in the current WHO classification [16]. Previous immunohistochemical studies have shown that the tumor cells of chondroblastoma express SOX9 [17], S100, and proteoglycan [18], supporting the chondrogenic nature of this tumor. Yet, chondroblastomas can produce osteoid [19] and lack collagen type II expression and IDH mutation [17, 20]. In this accord, it is not surprising that SATB2 is positive in some cases. From a practical point, positivity of SATB2 should not be used as an evidence to exclude the diagnosis of chondroblastoma.
Metastatic chondroblastoma
Chondroblastoma can very rarely metastasize. In our cohort of 27 cases, we have one case that developed lung metastasis. Lin et al. studied 82 consecutive cases of chondroblastoma and identified 3 cases who had local recurrences and then distant metastases [21]. In majority of the reported larger series of chondroblastomas (up to 103 cases), metastases were seen in only one to two cases and always presented in the lungs [22–24].
Follow-up data on metastatic chondroblastoma is limited in the literature. The case reported by Sohn et al. was followed for 3 years with no recurrence or new metastasis [23]. While metastases tend to behave in a benign fashion, malignant transformation was seen in rare instances. Viswanathan et al. reported one case of malignant transformation after 3 local recurrences [24]. In the 3 cases of metastatic chondroblastoma reported by Lin et al., the tumors underwent local recurrence followed by malignant transformation and metastases [21].
In summary, our study confirmed that immunostaining of H3K36M is a useful ancillary study to establish a diagnosis of chondroblastoma. H3K27me3 and K36me3 antibodies show heterogeneous expression, making it difficult to evaluate the alterations of histone methylation in H3K36M mutated cases by immunohistochemical analysis. One should be aware of majority of chondroblastomas express SATB2 by immunohistochemical analysis.
Acknowledgement:
We would like to thank Rene N. Serrette for his excellent technical support. This work was supported by the Department of Pathology at Memorial Sloan Kettering Cancer Center Internal Research Fund and in part by the NIH/National Cancer Institute Cancer Center Support grant under award P30CA008748 (Disclosures: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH).
Footnotes
Statement of conflict of interest: There is no conflict of interest.
Reference:
- 1.Wilting RH and Dannenberg JH. Epigenetic mechanisms in tumorigenesis, tumor cell heterogeneity and drug resistance. Drug Resist Updat, 2012. 15(1–2): p. 21–38. [DOI] [PubMed] [Google Scholar]
- 2.Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet, 2013. 45(12): p. 1479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C, Jain SU, Hoelper D, et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science, 2016. 352(6287): p. 844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClelland RA, Finlay P, Walker KJ, et al. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res, 1990. 50(12): p. 3545–50. [PubMed] [Google Scholar]
- 5.Konishi E, Nakashima Y, Mano M, et al. Chondroblastoma of extra-craniofacial bones: Clinicopathological analyses of 103 cases. Pathol Int, 2017. 67(10): p. 495–502. [DOI] [PubMed] [Google Scholar]
- 6.Dahlin DC and Ivins JC. Benign chondroblastoma. A study of 125 cases. Cancer, 1972. 30(2): p. 401–13. [DOI] [PubMed] [Google Scholar]
- 7.Davila JA, Amrami KK, Sundaram M, et al. Chondroblastoma of the hands and feet. Skeletal Radiol, 2004. 33(10): p. 582–7. [DOI] [PubMed] [Google Scholar]
- 8.Amary MF, Berisha F, Mozela R, et al. The H3F3 K36M mutant antibody is a sensitive and specific marker for the diagnosis of chondroblastoma. Histopathology, 2016. 69(1): p. 121–7. [DOI] [PubMed] [Google Scholar]
- 9.Fang D, Gan H, Lee JH, et al. The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas. Science, 2016. 352(6291): p. 1344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Cooper S, and Brockdorff N. The interplay of histone modifications - writers that read. EMBO Rep, 2015. 16(11): p. 1467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleven AH, Hocker S, Briaire-de Bruijn I, et al. Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. Am J Surg Pathol, 2015. 39(11): p. 1576–83. [DOI] [PubMed] [Google Scholar]
- 12.Hassan MQ, Gordon JA, Beloti MM, et al. A network connecting Runx2, SATB2, and the miR-23a~27a~24–2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci U S A, 2010. 107(46): p. 19879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conner JR and Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology, 2013. 63(1): p. 36–49. [DOI] [PubMed] [Google Scholar]
- 14.Davis JL and Horvai AE. Special AT-rich sequence-binding protein 2 (SATB2) expression is sensitive but may not be specific for osteosarcoma as compared with other high-grade primary bone sarcomas. Histopathology, 2016. 69(1): p. 84–90. [DOI] [PubMed] [Google Scholar]
- 15.Wojcik JB, Bellizzi AM, Dal Cin P, et al. Primary sclerosing epithelioid fibrosarcoma of bone: analysis of a series. Am J Surg Pathol, 2014. 38(11): p. 1538–44. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick S and Romeo S. Chondroblastoma, in WHO Classification of Tumours of Soft Tissue and Bone, Fletcher CD, et al. , Editors. 2013, International Agency for Research on Cancer: Lyon, France: p. 262–263. [Google Scholar]
- 17.Konishi E, Nakashima Y, Iwasa Y, et al. Immunohistochemical analysis for Sox9 reveals the cartilaginous character of chondroblastoma and chondromyxoid fibroma of the bone. Hum Pathol, 2010. 41(2): p. 208–13. [DOI] [PubMed] [Google Scholar]
- 18.Soder S, Oliveira AM, Inwards CY, et al. Type II collagen, but not aggrecan expression, distinguishes clear cell chondrosarcoma and chondroblastoma. Pathology, 2006. 38(1): p. 35–8. [DOI] [PubMed] [Google Scholar]
- 19.Aigner T, Loos S, Inwards C, et al. Chondroblastoma is an osteoid-forming, but not cartilage-forming neoplasm. J Pathol, 1999. 189(4): p. 463–9. [DOI] [PubMed] [Google Scholar]
- 20.Damato S, Alorjani M, Bonar F, et al. IDH1 mutations are not found in cartilaginous tumours other than central and periosteal chondrosarcomas and enchondromas. Histopathology, 2012. 60(2): p. 363–5. [DOI] [PubMed] [Google Scholar]
- 21.Lin PP, Thenappan A, Deavers MT, et al. Treatment and prognosis of chondroblastoma. Clin Orthop Relat Res, 2005. 438: p. 103–9. [DOI] [PubMed] [Google Scholar]
- 22.Ozkoc G, Gonlusen G, Ozalay M, et al. Giant chondroblastoma of the scapula with pulmonary metastases. Skeletal Radiol, 2006. 35(1): p. 42–8. [DOI] [PubMed] [Google Scholar]
- 23.Sohn SH, Koh SA, Kim DG, et al. A case of spine origin chondroblastoma metastasis to lung. Cancer Res Treat, 2009. 41(4): p. 241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viswanathan S, Jambhekar NA, Merchant NH, et al. Chondroblastoma of bone--not a “ benign disease”: clinico-pathologic observations on sixty cases. Indian J Pathol Microbiol, 2004. 47(2): p. 198–201. [PubMed] [Google Scholar]