Abstract
Nanotechnology is an emerging industry based on commercialization of materials with one or more dimensions of 100 nm or less. Engineered nanomaterials are currently incorporated into thin films, porous materials, liquid suspensions, or filler/matrix nanocomposites with future applications predicted in energy and catalysis, microelectronics, environmental sensing and remediation, and nanomedicine. Carbon nanotubes are one-dimensional fibrous nanomaterials that physically resemble asbestos fibers. Toxicologic studies in rodents demonstrated that some types of carbon nanotubes can induce mesothelioma, and the World Health Organization evaluated long, rigid multiwall carbon nanotubes as possibly carcinogenic for humans in 2014. This review summarizes key physicochemical similarities and differences between asbestos fibers and carbon nanotubes. The “fiber pathogenicity paradigm” has been extended to include carbon nanotubes as well as other high-aspect-ratio fibrous nanomaterials including metallic nanowires. This paradigm identifies width, length, and biopersistence of high-aspect-ratio fibrous nanomaterials as critical determinants of lung disease, including mesothelioma, following inhalation. Based on recent theoretical modeling studies, a fourth factor, mechanical bending stiffness, will be considered as predictive of potential carcinogenicity. Novel three-dimensional lung tissue platforms provide an opportunity for in vitro screening of a wide range of high aspect ratio fibrous nanomaterials for potential lung toxicity prior to commercialization.
Keywords: asbestos fibers, carbon nanotubes, high aspect ratio nanomaterials, fiber pathogenicity paradigm
1. Overview of nanotechnology and commercialization of carbon nanotubes
Nanotechnology has been described as “the next Industrial Revolution” that exploits engineering of matter at the nanoscale, defined as 100 nm or less in one or more dimensions (Hood, 2004). This new technology was envisioned by the physicist Richard Feynman in a lecture delivered in 1959 that challenged scientists to control matter at close to atomic scales to produce materials with unique physical, chemical, and electrical properties (Feynman, 1961). Government investments in this emerging industry, supported by the National Nanotechnology Initiative (NNI) in the United States beginning in 2000, as well as basic research funded by the National Science Foundation and industry (Hood, 2004), drove this emerging technology forward resulting in successful commercialization of engineered nanomaterials with ~300 nanoproducts marketed in 2006 (Maynard et al. 2006). However, even during the early stages of this emerging industry, environmental activists in Canada proposed a moratorium on nanotechnology in 2002 until potential health risks of engineered nanomaterials could be determined. Nanotechnologists also urged that this emerging industry consider potential environmental and safety impacts before nanoproducts are widely commercialized (Colvin, 2003). Toxicologists began to identify potential adverse health impacts of these emerging materials as described in a widely-cited review by Oberdörster et al. in 2005. Leaders in nanotechnology and nanotoxicology clearly articulated the need for systematic research on the risks, as well as the benefits, of nanotechnology and proposed five grand challenges for development of safe nanotechnology (Maynard et al., 2006). The Woodrow Wilson International Center for Scholars developed an inventory of commercial nano-enabled products; the most recent consumer products inventory lists 622 companies world-wide producing 1,814 products mostly based on metals (especially silver, titanium, zinc, and gold) and metal oxides followed by carbon-based and silicon-based materials (Vance et al., 2015). National and international consortia of researchers, industry leaders, government agencies, and consumer groups are now working together to develop approaches for assessing potential environmental and health risks of engineered nanomaterials (Olson and Gurian, 2012; Nel et al., 2013, Arts et al., 2014). Meanwhile, 3,400 nano-enabled consumer products are projected by 2020 (Woodrow Wilson Center, 2012) and Mulvaney and Weiss (2016) estimate that the nanotechnology industry will have a commercial value of $75 billion. Nanoscientists predict future growth especially in microelectronics, energy and catalysis, CO2 capture, consumer products, and nanomedicine (Kagan et al., 2016).
Carbon nanotubes are one example of the success of nanotechnology beginning with discovery and industrial synthesis near the end of the 20th century, leading to current large-scale production at a price of $200/kg (Mulvaney and Weiss, 2016). Global output has been projected to increase, with major applications in composite materials, thin coatings and films, microelectronics, energy storage, environmental remediation, and nanomedicine (Zhang et al. 2013; Vance et al. 2013; Sajid et al., 2016). In parallel with these developments, in 2004 the Royal Society and Royal Academy of Engineering recognized the physical similarities between carbon nanotubes and asbestos fibers and potential for human health risks (RS/RAE, 2004). Researchers also reflected on these concerns (Warheit, 2006; Muller et al., 2006; Kane and Hurt, 2008; Kostarelos, 2008; Parcurari et al., 2010). Toxicologic studies in rodents demonstrated that direct injection of multiwall carbon nanotubes induced mesothelioma (Sakamoto et al., 2009; Takagi et al., 2008; 2012) leading to the World Health Organization evaluation of long, rigid multiwall carbon nanotubes as possibly carcinogenic to humans (International Agency for Research on Cancer, IARC Group 2B; Grosse et al., 2014). The Mechanisms Subgroup for this IARC Working Group recently evaluated mechanistic evidence for potential carcinogenicity of carbon nanotubes and emphasized the importance of systematic investigation of physiochemical properties of the wide range of carbon nanotubes relevant for carcinogenicity (Kuempel et al., 2017). This paper summarizes key physicochemical similarities and differences between asbestos fibers and carbon nanotubes. The “fiber pathogenicity paradigm” (Donaldson et al., 2011) identified width, length, and biopersistence of natural and man-made fibers as critical determinants of lung disease following inhalation. In applying this paradigm to carbon nanotubes, as well as to other one dimensional fibrous nanomaterials, a fourth factor, mechanical bending stiffness, will be considered as predictive of pathogenicity and carcinogenicity.
2. Occupational exposure to carbon nanotubes
Carbon nanotubes are synthesized commercially by chemical vapor deposition (CVD), arc discharge, or laser ablation. The most widely-used method is CVD with decomposition of a carbon-containing organic vapor catalyzed by transition metals (Zhang et al., 2013). During the manufacturing and processing steps, carbon nanotubes can be released as dry powders (Maynard et al., 2004; Donaldson et al., 2006; Tantra & Cumpson, 2007). Inhalation exposure of workers is a major concern and the National Institute of Occupational Safety and Health (NIOSH) in the United States has proposed a recommended exposure limit of 1 μg/m3 (Dahm et al., 2015). Occupational exposure during subsequent stages in the product life cycle is also an emerging concern (Mitrano et al., 2015; Guseva Canu et al., 2016). For both carbon nanotube composites and asbestos-containing materials, high-temperature thermal incineration has been proposed to destroy the fibrous structures; however, potential toxicity of the released particles has not been thoroughly investigated (Wang et al., 2017). Occupational safety and health professionals advocate for establishment of timelines for investigation of key major areas including metrology, toxicology, exposure and risk assessment, risk management, and medical surveillance including epidemiology studies of workers (Schulte et al., 2014). NIOSH has initiated industry site visits to provide guidance on engineering controls and recommendations for personal protective equipment (Dahm et al., 2015). Their initial focus is on worker protection in the emerging nanotechnology industry in cooperation with business leaders, government, regulatory agencies, and consumers (Schulte et al., 2014; Liou et al, 2015). The ultimate goal is commercialization of nanomaterials in parallel with consideration of potential human health hazards and risk to avoid repeating the history of asbestos-related diseases (Sanchez et al., 2009).
3. Physical and chemical determinants of asbestos and carbon nanotube-induced toxicity
Regulated forms of asbestos refer to fibrous silicate minerals belonging to one of two classes: serpentines represented by chrysotile asbestos and amphiboles that include crocidolite, tremolite, amosite, actinolite, and anthophyllite. All regulated asbestos fibers have been classified as known human carcinogens (group 1) by IARC (volume 100C, 2012). Other fibrous materials including erionite and the non-commercial amphibole, fluoro-edenite are also classified as human carcinogens by IARC (2012 and Grosse et al., 2014). Other naturally-occurring fibrous silicates, including wollastonite, sepiolite, and talc not containing asbestiform fibers are not classifiable as to carcinogenicity to humans (Group 3; IARC, 1997). Man-made or synthetic vitreous fibers have been developed as substitutes for asbestos fibers including insulation fiber glass, glass wool, rock wool, and slag wool. These were designed to be less biopersistent than asbestos fibers, similar to wollastonite, and are also not classified as human carcinogens (IARC, 2002). A recent review of the toxicological and epidemiological evidence has confirmed that commercialization of wollastonite has not resulted in lung toxicity to workers (Maxim et al., 2014). Boffeta et al. (2014) also confirmed that manufacturing of synthetic vitreous fibers has not increased risk of mesothelioma in workers. Lippmann (2014) comprehensively reviewed evidence for lung toxicity of asbestos and erionite fibers, wollastonite, and synthetic vitreous fibers and concluded that fiber dimensions and biopersistence are key determinants of pathogenicity in support of the fiber pathogenicity paradigm (Donaldson et al., 2011).
The importance of biopersistence of particles or fibers following inhalation in the lungs originated from investigations of toxicity of ultrafine particles defined as <100 nm in diameter (see Oberdörster et al., 2007 for an historical perspective). Several experimental studies demonstrated a correlation between retention of ultrafine particles in the lungs, particle biopersistence, and inflammation (Oberdörster et al., 1994). Oxidative stress emerged as an important mechanism for injury and inflammation induced by asbestos fibers (Mossman and Landesman, 1983) that was extended to ultrafine particles (Donaldson et al., 1996) and ultimately to engineered nanoparticles (Oberdörster et al., 2005; Nel at al., 2006). With the development of less toxic man-made mineral fibers, also called synthetic vitreous fibers, a link between impaired clearance of long fibers, biopersistence, and lung toxicity was established and formed the basis for the fiber pathogenicity paradigm (Oberdörster et al., 2007). This paradigm was extended to include high-aspect-ratio nanomaterials (HARNs) including carbon nanotubes (Sanchez et al., 2009), nanowires, and nanorods regardless of the chemical composition (Donaldson et al., 2011).
In this review, the abbreviation HARNs will be used to refer to all naturally-occurring fibers with at least one dimension ≤100 nm (including asbestos) as well as one dimensional or fibrous nanomaterials classified as engineered HARNs (Tables 1 and 2). However, are all HARNs that conform to the fiber paradigm as toxic as asbestos fibers? Most studies have investigated carbon nanotubes and there are both similarities and differences in their physical and chemical properties (Table 3, Fubini et al., 2011; Donaldson et al., 2013). For example, single and multiwall carbon nanotubes can be long (up to 50-100 μm) and thin (0.4-200 nm in diameter; Bussy et al. 2013). Some types of carbon nanotubes that are long and rigid similar to asbestos fibers induce incomplete or frustrated phagocytosis by macrophages that impairs clearance from the lungs or pleural space and induces persistent inflammation (Poland et al., 2008; Donaldson et al., 2010b). Titanium dioxide nanorods also showed similar biological activity in alveolar macrophages (Hamilton et al., 2009). Metallic nanowires can be longer than asbestos fibers and carbon nanotubes and also impair phagocytosis and clearance from the pleural space (Schinwald et al., 2012). HARNs, both naturally-occurring asbestos fibers and engineered carbon nanotubes, have been compared with respect to potential carcinogenicity, especially induction of malignant mesothelioma following translocation to the pleura (Jaurand et al., 2009; Nagai and Toyokuni, 2010; Luanpitpong, 2016). Donaldson et al. (2011a) consider the pleural mesothelium as a “unique target for fibers including HARNs.” Although the route of translocation of HARNs from the lungs to the pleura is unknown, long, thin, biopersistent fibers are hypothesized to reach the pleural space, impair clearance through lymphatic stomata on the parietal pleura, and induce frustrated phagocytosis, oxidant production, and persistent inflammation leading to malignant mesothelioma (Figure 1; Donaldson et al., 2010). However, a closer examination of the properties of asbestos fibers and carbon nanotubes linked to the fiber pathogenicity paradigm reveals important differences (Table 3). For example, amphibole asbestos fibers are highly biopersistent while chrysotile fibers slowly leach surface Mg2+ ions in the lungs and split into smaller, thinner fibrils. Multiwall carbon nanotubes are also biopersistent; however, single wall carbon nanotubes, especially chemically functionalized varieties, can be degraded by peroxidases (Kotchey et al., 2013) or H2O2 at low pH (Liu et al., 2010). Naturally-occurring and engineered HARNs also differ in crystallinity, surface charge, and hydrophilicity/hydrophobicity: amphibole asbestos fibers have a positive surface charge, while chrysotile asbestos and carbon nanotubes typically have a negative charge unless specifically functionalized after synthesis. Pristine carbon nanotubes are hydrophobic, while asbestos fibers are hydrophilic. Carbon nanotubes have higher tensile strength than asbestos fibers (Virta, 2002; Bussy et al., 2013). Direct or indirect generation of reactive oxygen species (ROS) by asbestos particles or engineered nanoparticles with high surface area is a key mechanism linked to genotoxicity (Fubini et al., 2010); however, asbestos fibers and carbon nanotubes show important differences. Both amphibole asbestos and chrysotile directly catalyze generation of ROS (Fubini et al., 2011) in contrast to purified carbon nanotubes that may scavenge ROS (Fenoglio et al., 2006; Nymark et al., 2014). Asbestos fibers may contain redox-active iron, either in the crystal lattice or as substituted ions that are active in the iron-catalyzed Fenton reaction. Transition metal catalysts including iron or nickel may be presented in unpurified carbon nanotubes and participate in this reaction (Guo et al., 2007). Removal of metal catalyst residues from carbon nanotubes decreases the metal bioavailability (Liu et al., 2007; 2008).
Table 1.
Geometry of Engineered Carbon Nanomaterials
|
Spherical or Zero Dimensional Particles ex: fullerenes |
One Dimensional Fibers ex: carbon nanotubes |
Two Dimensional Sheets or Plates ex: graphene |
|---|---|---|
|
|

|
Carbon nanotubes belong to a large family of engineered carbon nanomaterials. Fullerenes are a spherical, hollow cage of carbon atoms; C60 fullerene is ~ 0.7nm in diameter. Pristine fullerenes so far have shown low toxicity to workers (Aschberger et al. 2010). Two dimensional sheets of hexagonal carbons form monolayer graphene 0.34 nm thick. Although there have been limited studies on toxicity of commercial forms of graphene, usually produced as few layer graphene or graphite nanoplatelets (reviewed by Sanchez et al., 2012), recent studies suggest that these two dimensional carbon nanomaterials show lower lung toxicity than carbon nanotubes (Ma-Hock et al., 2013; Roberts et al., 2016). Graphene has a high aspect ratio similar to carbon nanotubes and pristine graphite nanoplatelets are biopersistent in the lungs (Schinwald et al., 2014). This review will consider similarities and differences between carbon nanotubes and asbestos fibers that can also be considered high aspect ratio fibrous nanomaterials.
Table 2.
Classification of One-Dimensional Engineered HARNs
| Chemical Composition | Examples |
|---|---|
| Carbon-based materials | Carbon nanofibers Carbon nanotubes (single wall, double wall, multiwall) |
| Metals/metal oxides | Nanowires (Ag, Au, Ni, Pt) TiO2, ZnO, CeO2 nanorods or nanobelts |
| Semiconductors | Nanowires (Si, SiN) SiC nanowhiskers, nanotubes |
| Polymers | Cellulose nanocrystals Electrospun nanofibers Wormlike micelles |
Modified from Warheit et al., in press; Chu et al. (2013); Endes et al. (2016)
Table 3.
Properties of Carbon Nanotubes and Asbestos Fibers
| Property | Amphibole Asbestos | Chrysotile Asbestos | Carbon Nanotubes |
|---|---|---|---|
| Surface charge | Positive | Negative | Negative in as-produced materials |
| Hydrophilicity/hydrophobicity | Hydrophilic | Hydrophilic | Hydrophobic |
| Redox – active metals | Fe2+ and Fe3+ in crystal structure | Fe2+ substituted ions | Variable purity and bioavailability of Fe, Ni, Co catalyst residues |
| ROS generation | Positive | Positive | Low; net effect may be ROS scavenging |
| Tensile strength | 1-4 GPa | 1-4 GPa | 0.2–5 TPa |
| Biodurability | High | Slow leaching of Mg2+ ions and splitting into fibrils | Single wall carbon nanotubes degraded by peroxidase or H2O2 at low pH |
ROS – reactive oxygen species; Virta, 2002; Liu et al., 2010; Fubini et al., 2011; Donaldson et al., 2013; Kotchey et al., 2013; Bussy et al., 2013
Figure 1. Proposed mechanisms for carcinogenicity of asbestos fibres.
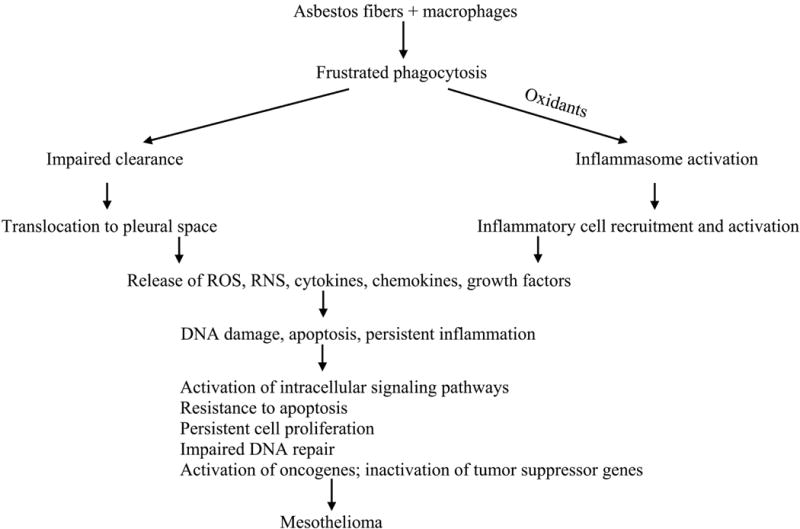
IL-1ß, interleukin - 1ß; IL-18, interleukin-18; RNS, reactive nitrogen species; ROS, reactive oxygen species Adapted with permission from IARC Monongraphs on the Evaulation of Carcinogenic Risks to Humans: Volume 100C. Arsenic, Metals, Fibres, and Dusts. IARC, Lyon, 2012.
As manufactured nanomaterials, carbon nanotubes are highly diverse depending on the producer, intended applications, post-production processing and functionalization, and even batch-to-batch variability (reviewed in Kuempel et al., 2017). In addition, the hydrophobicity of pristine, unfunctionalized carbon nanotubes (Table 3) impairs dispersion in biological media promoting their agglomeration into rope-like structures or tangled clumps. These agglomerated clumps, even after delivery by aerosolization rather than intratracheal instillation or oropharyngeal aspiration (reviewed by Warheit et al., in press), form discrete multifocal granulomas containing aggregates of macrophages and fibroblasts (see Ma-Hock et al., 2009 for a well-designed 90-day inhalation study in rats). These carbon nanotubes persisted in the lungs after 90 days but were also detected in mediastinal lymph nodes. If carbon nanotubes are better dispersed prior to instillation into the lungs, they are more potent in inducing lung inflammation and fibrosis (Wang et al., 2011). Individual carbon nanotubes may be released gradually from agglomerates in the lungs over time and translocate to the lung interstitium, pleura, and regional lymph nodes as described by Mercer et al. (2013a). These investigators found that well-aerosolized single wall carbon nanotubes (Shvedova et al., 2014) or multiwall carbon nanotubes delivered by inhalation (Mercer et al., 2013b) induced persistent inflammation, enhanced delivery of dispersed carbon nanotubes to the lung interstitium, and increased fibrosis up to one year post-exposure in mice.
Additional variables in the properties of carbon nanotubes complicate interpretation of toxicity testing and prediction of potential carcinogenicity (Kuempel et al., 2017). Different samples show a wide range in dimensions, especially in length that is important for successful uptake or phagocytosis by macrophages. Single and multiwall carbon nanotubes have different diameters and flexibilities: very thin nanotubes are more likely to form tangled agglomerates than thicker, more rigid multiwall nanotubes. Depending on the production conditions and post-processing, carbon nanotubes may have defects in the hexagonal carbon framework or be chemically functionalized by addition of charged groups to increase hydrophilicity, especially for biomedical applications. Finally, as discussed above, bioavailability of metal catalyst residues may contribute to redox activity (Fubini et al., 2011). These variables have resulted in conflicting evidence for carcinogenicity of carbon nanotubes in rodent assays as summarized next.
4. Carcinogenicity of carbon nanotubes
The International Agency for Research on cancer regularly convenes international experts in occupational health, epidemiology, and toxicology to evaluate potential carcinogenicity of chemicals, physical agents, drugs, and occupational exposures. On the basis of epidemiological evidence in humans, carcinogenicity assays in experimental animals, and other mechanistic data, these agents or exposures are classified into four groups: group 1 – carcinogenic to humans, group 2A – probably carcinogenic to humans, group 2B – possibly carcinogenic to humans, group 3 – not classifiable as to carcinogenicity in humans, and group 4 – probably not carcinogenic to humans (IARC, 2006). A working group was convened in 2014 and in the absence of any epidemiological data for humans, attempted to classify carbon nanotubes. Due to heterogeneity in the types of carbon nanotubes evaluated in experimental studies and mechanistic data gaps regarding their potential carcinogenicity, only one type of carbon nanotube, {a commercial product described in the report as “MWCNT-7”}, was classified in group 2B while other types of multiwall and single wall carbon nanotubes were classified as group 3 (Grosse et al., 2014). This classification was based on rodent carcinogenicity studies using MWCNT-7 samples, which are long, large-diameter, rigid multiwall tubes delivered by intraperitoneal (Takagi et al., 2008; Nagai et al., 2011) or intrascrotal injection (Sakamoto et al., 2009) to induce malignant mesothelioma. Another rat peritoneal assay tested different samples of multiwall carbon nanotubes (~ 11 nm in diameter and 0.7 μm in length) with different levels of structural defects (Muller et al., 2009). These very short, thin, tangled carbon nanotubes did not induce a significant increase in mesotheliomas. After the IARC evaluation, Rittinghausen et al. (2014) published a more extensive rat peritoneal assay with similar results: long, rigid multiwall carbon nanotubes were more potent than thinner, flexible or curved carbon nanotubes in inducing mesothelioma. These experimental studies using bolus delivery of carbon nanotubes to the peritoneum were confirmed by transtracheal intrapulmonary spraying in rats. Xu et al. (2014) showed that longer, rigid multiwall carbon nanotubes (~150 nm in diameter, ~8 μm in length) translocated to the parietal pleura and induced inflammation, fibrosis, and focal mesothelial cell proliferation to a greater extent than shorter, thinner agglomerates (~15 nm in diameter, 3 μm in length). Suzui et al. (2016) used the same exposure protocol and long, rigid multiwall carbon nanotubes (30-100 nm in diameter, 1-20 μm in length with < 0.05% iron content) similar in dimensions to MWCNT-7. After more than 100 weeks, mesotheliomas were induced in the pleural and pericardial linings as well as lung adenomas and carcinomas. A chronic inhalation assay in rats using MWCNT-7 induced lung adenomas and carcinomas at doses of 0.2 and 2 mg/m3 but no mesotheliomas (Kasai et al., 2016). Focal fibrosis and mesothelial cell proliferation were observed in the parietal pleura and single long, rigid carbon nanotubes were recovered following pleural lavage. These authors conclude that the number of nanotubes (~103 in the pleural lavage following exposure to 2 mg/m3) was not high enough to induce mesothelioma.
Overall, although there are a limited number of studies using inhalation to assess carcinogenicity of carbon nanotubes, these results confirm bolus delivery studies and provide support for the fiber pathogenicity paradigm (Donaldson et al., 2013).
There are, however, significant data gaps in our current understanding of mechanisms of carcinogenicity of asbestos fibers (Figure 1) as well as engineered HARNs. Given the significant physicochemical differences between carbon nanotubes and asbestos fibers (Table 3), heterogeneity in commercially-produced carbon nanotubes, and diversity of emerging classes of HARNs (Table 2), these data gaps must be addressed. There are also inconsistent results between carcinogenicity and genotoxicity assays noted by the IARC Working Group in 2014. For example, according to the fiber pathogenicity paradigm, long, biopersistent HARNs are more likely to induced frustrated phagocytosis, impaired clearance from the lungs and pleura, and persistent inflammation that are key events in lung carcinogenicity (Donaldson et al., 2013). However, in genotoxicity assays short multiwall carbon nanotubes (< 1 μm in length) can induce DNA damage (reviewed in Kuempel et al., 2017). The members of the mechanisms subgroup expressed different opinions regarding the strength of the mechanistic evidence in their overall evaluation, but they did agree on the following future research needs:
validation of in vitro cellular toxicity studies that incorporate new technologies and “omics” assays.
investigation of preneoplastic endpoints in animal studies, including definitions of hyperplastic and neoplastic lesions in animals in comparison with human lung pathology.
systematic dose-response relationships for development of preneoplastic and neoplastic endpoints in chronic bioassays.
systematic investigation of physicochemical properties of HARNs linked to lung toxicity and carcinogenicity.
development and validation of dosimetry models for both rodents and humans, especially routes and extent of translocation to the pleura.
biomonitoring of workers exposed to engineered HARNs to detect early evidence of potential adverse health impacts and to identify biomarkers that may be predictive of chronic endpoints including fibrosis and cancer.
In order to address the major mechanistic data gaps regarding the physicochemical properties of HARNs related to pleural translocation and carcinogenicity, a new theoretical framework is proposed next.
5. Testing strategies for high aspect ratio nanomaterials (HARNs)
Framing of the “asbestos-carbon nanotube analogy” paved the way for multidisciplinary national and international discussions on rational, cost-effective, rapid screening strategies for safety assessment of engineered nanomaterials. In recognition of the diversity of these materials, consensus gradually emerged about grouping of nanomaterials based on their solubility, biopersistence, surface reactivity, toxicity, and aspect ratio (Table 4; Arts et al., 2014). The purpose of this grouping is to provide a framework for prioritizing engineered nanomaterials for additional testing prior to widespread manufacturing and commercialization. Additional considerations include use, exposure scenarios, release following weathering, dustiness, and likelihood of aerosolization (Nowack et al., 2013). A testing scheme was developed specifically for the category of HARNs focusing on physicochemical properties including dimensions, agglomeration state, dissolution, biopersistence, and surface reactivity (Figure 2). Key threshold values for HARNs include aspect ratio ≥ 3:1, length > 5 μm, diameter < 3 μm, and lung half-life ≥ 40 days following intratracheal instillation (Arts et al., 2015). The importance of assessing of dissolution and biodurability of HARNs is based on development of synthetic vitreous fibers as asbestos fiber substitutes and this concept has been applied to engineered nanomaterials (Utembe et al., 2015). Carbon nanotubes are considered to be biopersistent following inhalation unless they are single-walled and have been deliberately functionalized (Fubini et al., 2011); however, secondary modifications in the lungs and translocation and biopersistence, especially in the pleura, have not been sufficiently investigated (Broaddus et al., 2011). In the assessment scheme proposed by Arts et al. (2014), additional testing is recommended for biopersistent HARNs that include assessment of chronic endpoints in the lungs including persistent inflammation, fibrosis, and cancer (Figure 2). Assessment of these chronic endpoints usually requires chronic inhalation assays in rodents. Alternative test strategies are required to accelerate screening and safety assessment of HARNs and to reduce or replace animal testing in nanotoxicology (Clift et al., 2011; Burden et al., 2017). Multiple stakeholders representing industry, consumers, regulatory agencies, and scientists agree that computational modeling combined with more sophisticated in vitro and in silico approaches are needed to guide safe product development and commercialization (reviewed in Nel et al., 2013). A major limitation of this alternative testing strategy based on carbon nanotubes as a case study is assessment of chronic endpoints, especially carcinogenicity, based on current in vitro and in silico approaches. Recent alternative in vitro testing platforms for lung toxicity have been developed. We propose to use these novel platforms to investigate the mechanistic role of mechanical bending stiffness or flexural rigidity that has been identified as a key factor in carcinogenicity of carbon nanotubes in the experimental animal bioassays summarized in section 4. Computational modeling of nanomechanical properties combined with experimental validation using novel in vitro lung toxicity testing platforms will be proposed as an alternative toxicity assessment strategy for HARNs.
Table 4.
Physicochemical Properties and Grouping of Engineered Nanomaterials
| Property | Classification |
|---|---|
| Soluble | Higher solubility, potential release of toxic ions |
|
| |
| Poorly-Soluble, Low Toxicity | Insoluble or poorly soluble, low inherent toxicity, lung inflammation determined by surface area |
|
| |
| Poorly-Soluble, High Toxicity | Biopersistent, specific toxicity related to surface reactivity and surface area |
|
| |
| High Aspect Ratio | Fibrous particles; lung toxicity related to biopersistence, translocation to pleura, genotoxicity |
Adapted from Arts et al., 2014 with permission from Elsevier Ltd. (pending).
Figure 2. Assessment Scheme for Potential Adverse Human Health Impacts of HARNs Following Inhalation.
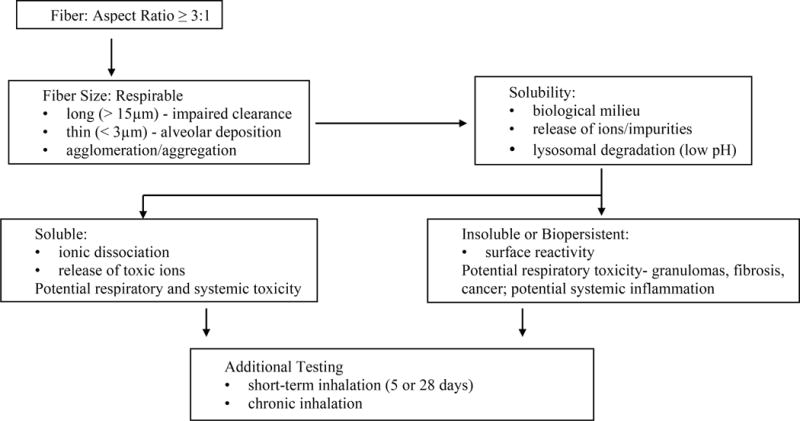
Adapted from Arts et al., 2014 with permission from Elsevier Ltd. (pending).
Although rodent inhalation assays are the “gold-standard” for toxicity testing of inhaled particles and HARNs (Arts et al., 2014), these assays are technically-demanding, expensive, and time consuming and anatomic differences between the lungs of rodents and humans, especially the pleura (Broaddus et al., 2011) limit extrapolation of results to humans (Han et al., 2012). Several new models, especially those based on tissue engineering, show potential for recapitulation of human lung structure, biomechanics, and physiology in vitro (reviewed by Patel et al., 2012). While traditional in vitro cellular toxicity assays are based on primary or immortalized cells lines in two-dimensional monolayer cultures, these assays are limited in duration and not suitable for assessment of chronic endpoints including fibrosis and cancer. The lung is composed of multiple cell types that interact spatially and temporally during the development of disease in a complex three-dimensional microenvironment (reviewed by Nichols et al., 2013). Clift et al. (2014) compared monocultures of human monocyte-derived macrophages, monocyte-derived dendritic cells, and lung epithelial cells with a triple cell co-culture model in transwells exposed to single or multiwall carbon nanotubes for 24 hours. Significant differences were found in release of proinflammatory mediators and oxidative potential in two-dimensional monocultures compared to triple cell co-cultures of the epithelial airway barrier. These investigators conclude that triple cell co-cultures of human cells in transwells are more appropriate for in vitro toxicity testing of nanoparticles than monocultures grown in monolayers (Clift et al., 2011). Recently, this model was adapted for a new nano aerosol exposure chamber capable of using a more physiological exposure route (Jeannet et al. 2014).
An alternative approach to model the three-dimensional architecture of the human lung are acellular human lung scaffolds as a platform for culturing human stem or progenitor cells (Nichols et al., 2012). These scaffolds have normal human extracellular matrix components that recapitulate the structure and mechanics of the native lung microenvironment. These acellular human lung scaffolds were designed as functional lung replacements; however, they also have potential for modeling of human lung deposition and translocation of carbon nanotubes. Natural or synthetic polymers have also been used as scaffolds for modeling the alveolus; for example, Zhang et al. (2011) recapitulated a differentiated alveolus using a collagen-Matrigel scaffold, murine fetal lung cells, and alginate-poly-L-lysine microcapsules to guide differentiation of type II epithelial cells. This bioengineered lung alveolus maintained viability and differentiation up to 14 days that could be useful for in vitro lung toxicity testing. More sophisticated microfabrication and microfluidic techniques have been used to reconstitute organs “on-a-chip”, including a model of the human alveolar-capillary functional unit (Hub et al., 2010). This microfluidic system used a poly(dimethylsiloxane) porous membrane coated with fibronectin or collagen and human alveolar epithelial or lung microvascular endothelial cells on opposite sides of the membrane. This microfluidic lung-on-a-chip had similar mechanical and functional properties as the human lung alveolus and was used to study delivery of silica nanoparticles applied to the lung at the air-liquid interface. Translocation of nanoparticles into endothelial channels was enhanced by mechanical stretching to simulate breathing motion. This human lung-on-a-chip provides proof-of-principle for development of novel alternatives to animal testing of engineered nanoparticles especially for short-term endpoints including release of proinflammatory mediators, disruption of lung epithelial barrier function, and increased lung vascular permeability (Hub et al., 2015).
What is the potential application of these novel three-dimensional lung tissue platforms for carcinogenicity testing of HARNs? The most obvious approach is assessment of genotoxicity, either direct genotoxicity induced by HARNs in relevant lung target cells, epithelial or mesothelial cells, or indirect genotoxicity associated with release of reactive oxygen or nitrogen species from macrophages as assessed in co-cultures (reviewed in Magdolenova et al., 2013). However, there are significant conceptual and technical difficulties in using acute genotoxicity assays to predict potential carcinogenicity, especially for cancers like malignant mesothelioma that has a long latent period up to 20-40 years in humans (reviewed in Kuempel et al., 2017). Technical caveats in application of widely-used genotoxicity endpoints include use of non-cytotoxic doses, sufficient exposure times to assess chromosomal damage induced by HARNs, particle interference with the assays, use of well-characterized positive and negative reference materials, and extrapolation of doses used in vitro to human chronic inhalation exposure (Magdolenova et al., 2013). Nevertheless, newer approaches to genotoxicity testing using transwell human lung cell triple co-cultures, three-dimensional spheroids or microtissues, or three-dimensional reconstructed lung tissues may provide more robust, reproducible in vitro models for genotoxicity testing of HARNs (Evans et al., 2017).
The initial barrier to translocation of carbon nanotubes into the lung interstitium from the airspaces is pulmonary surfactant at the air-liquid interface. Pulmonary surfactant (PS) spreads over the thin (~0.1 μm) lung lining fluid forming a film that reduces surface tension and is essential to sustain breathing (Siebert and Rugonyi, 2008). PS is composed of approximately 90% lipids (mainly dipalmitoylphosphatidylcholin or DPPC) and 10% proteins (Hidalgo et al., 2015). A lack or dysfunction of surfactant prevents the proper functioning of the alveoli and leads to severe lung diseases (Griese, 1999). PS also serves as the first line of defense against microorganisms, particles, or fibers deposited in the alveoli. For spherical microscopic particles, the surface tension force is found to dominate gravity and buoyancy forces in displacing latex (polystyrene) particles to the aqueous subphase. Particles in peripheral airways and alveoli likely are below the surfactant film and submerged in the subphase, which may promote clearance by macrophages (Schürch, 1990). In addition, particles displaced into the subphase are likely to contact and deform epithelial cells (Geiser, 2003b). For man-made vitreous fibers (MMVF), it is shown that the fibers were found on the surface of conducting airways and alveoli ~ 20 minutes following inhalation. Whether the fibers were totally submerged depends on the length and surface tension, and they are totally or partially covered by lining-layer materials (Figure 3A, B; Geiser, 2003a).
Figure 3. SEM Images of Fibrous Nanomaterial Deposition in Alveoli and Penetration into Alveolar Epithelial Cells.
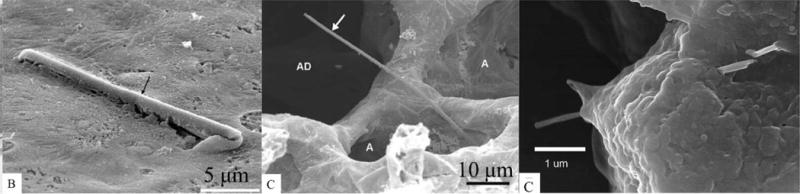
SEM images of (A) a fiber retained in an intrapulmonary conducting airway; the fiber is completely covered by the surface lining-layer (Geiser et al., 2003a), (B) a fiber retained in the gas exchanging compartment (Geiser et al., 2003a). Figures 3A, B reproduced with permission from Environmental Health Perspectives (Geiser et al., 2003a). The fiber touched the alveolar wall with one end only and the other end projected into the airspace and was not covered by lung lining layer. Abbreviations: A, alveoli; AD, alveolar duct. (C) FESEM of MWCNT penetration of alveolar epithelial cells (Mercer et al., 2010). Micrograph shows two MWCNTs passing through an alveolar epithelial cell 1 day after pharyngeal aspiration. Figure 3C reproduced with permission (Springer Open article, Mercer et al., 2010).
For carbon nanotubes, numerous in vivo experimental studies showed that carbon nanotubes can penetrate deep into the lung and reach the subpleural region (Ryman-Rasmussen et al., 2009; Li et al., 2007; Mercer, et al., 2010, 2011). Due to the importance of pulmonary surfactant as the first biological barrier to contact with airbone nanomaterials, extensive in vivo and in vitro experiments have also been done to study interaction between carbon nanotubes and PS monolayer at the air-water interface (Kapralov et al., 2012; Lee et al., 2013; Melbourne et al., 2015; Valle et al., 2015; Kadoya et al., 2016). Experimental studies of MWCNT-pulmonary surfactant interaction and its effect on PS functionality using a Langmuir-Blodgett trough show that the length and concentration of carbon nanotubes significantly influence the compression resistance of the film (Melbourne et al., 2015). In an in vitro study of pulmonary surfactants on a constrained drop surfactometer (CDS), compression-expansion loop shows a very high hysteresis when exposed carbon nanotubes, which could pose a danger to normal stable respiration (Valle et al., 2015).
Experimental in vivo studies have also been done to probe the effect of PS, for example, SWCNTs recovered from the bronchoalveolar lavage fluid (BALF) of mice were found to have absorbed surfactant lipids and related proteins forming an uninterrupted “coating” (Kapralov et al., 2012). This suggests a strong binding interaction of surfactant and SWCNTs, which has also been used to disperse SWCNTs (Wang et al., 2010). The preferred binding between surfactant proteins and carbon nanotubes could potentially damage lung immune defenses (Salvador-Morales et al., 2007). The pulmonary surfactant content of lung lavage fluid has been used as a measure of lung inflammation (Kadoya et al., 2016; Lee et al., 2013). Further studies show that the presence of the surfactant could markedly enhance in vitro uptake of SWCNTs by macrophages (Kapralov et al., 2012). After carbon nanotubes translocate through the pulmonary monolayer, experimental evidence shows that multiwall carbon nanotubes penetrated through alveolar epithelial cells (Figure 3C; Mercer et al., 2010, 2011), while dispersed single-wall carbon nanotubes are more prone to enter into the alveolar interstitium (Mercer et al., 2008).
Despite abundant experimental data showing adverse impacts of inhaled carbon nanotubes on the lungs, little is known about the mechanism by which the CNTs translocate through pulmonary surfactant and across alveolar epithelial cells. It will be crucial to investigate the underlying molecular mechanisms of CNTs translocation. Here we suggest two molecular dynamics simulations to explore the molecular mechanisms of CNT translocation into the alveolar wall.
(1) Molecular dynamics modeling of the mechanism of carbon nanotubes translocation through surfactant monolayer at the air-liquid interface
In vitro experiments show that the size and concentration of carbon nanotubes significantly change the mechanical response of the surfactant monolayer (Valle et al., 2015; Melbourne et al., 2015). Recent coarse-grained molecular dynamics simulations suggest that ultrashort SWCNTs (less than 5.5 nm) can insert into the surfactant monolayer via self-rotation (Yue et al., 2017), where the interaction morphology is determined by the length and diameter of SWCNTs and membrane tension of the surfactant (Xu et al., 2017). However, the detailed interaction between carbon nanotubes and lipid molecules could not be fully captured by coarse grained molecular dynamics simulations, and the energetics and molecular behaviors of lipids when carbon nanotubes translocate through the surfactant monolayer remain elusive.
Based on previous simulations and theoretical studies of interactions between CNTs and a lipid bilayer (Shi et al., 2011; Yi et al., 2014), the penetration angle, size and shape of the tip, as well as membrane tension could be important factors that influence the translocation behavior of carbon nanotubes through the surfactant monolayer. Systematic molecular dynamics simulations should be performed to investigate the roles of these parameters in carbon nanotube translocation through a surfactant monolayer. In such a study, the pulmonary membrane can be represented by a single layer of DPPC which separates the vacuum and water. Typical membrane tensions of 10 mN/m and 30 mN/m would be maintained to represent the compression and expansion states of the pulmonary wall (Xu et al., 2017). Mutiwalled carbon nanotubes of different diameters, with or without tip caps, would be placed in the air phase initially. Then they would be pulled into the liquid phase by steered molecular dynamics simulations at different penetration angles, and the preferred entry morphology determined though energy mapping and validated through a combination of molecular dynamics simulations and controlled experiments. The behavior of the lipid molecules in the surfactant monolayer, such as order parameters and diffusion constant, could be analyzed to reveal the molecular mechanisms of CNTs translocation though the surfactant.
(2) Molecular dynamics modeling of carbon nanotubes penetration through cell membranes
Experimental evidence show that MWCNTs migrate to the subpleural tissues and pleural space (Ryman-Rasmussen et al., 2009; Li et al., 2007), and also penetrate through alveolar epithelial cells (Figure 3C) (Mercer et al., 2010, 2011). It is important to investigate the mechanism by which carbon nanotubes penetrate through alveolar epithelial cells. A recent study indicates that persistent contact between a CNT tip and lipid bilayer leads to lipid extraction and membrane permeabilization (Zhu et al., 2016). This suggests that a CNT might be able to spontaneously penetrate through the cell membrane though persistent tip contact. To validate this hypothesis, full-atom molecular dynamics simulations could be useful to determine the energy barrier and detailed molecular interactions of the carbon nanotube and membrane during penetration. It is anticipated that the tip size and shape will significantly affect the interaction between the carbon nanotube and membrane, and that systematic molecular dynamics simulations could shed light on the detailed molecular mechanisms of this interaction. The predictions based on these theoretical modeling studies could be tested experimentally using transwell human lung cell cultures or human lung-on-a-chip devices (Clift et al., 2011; Hub et al., 2010).
An important potential application of human lung-on-a-chip devices or tissue engineered lung constructs is to assess potential translocation of HARNs to the lung interstitium and across the visceral pleura to reach the target site for development of mesothelioma, the parietal pleura (reviewed in Broaddus et al., 2011). The pleural space in humans is approximately 20 μm thick containing 0.5 – 2.0 ml of pleural fluid. The normal pleural pressure is −3 to −5 cm H2O and pleural fluid is drained through lymphatic stomata or openings on the parietal pleura (Akulian et al., 2013) into mediastinal lymph nodes (Warheit et al., in press). These lymphatic stomata are 3-10 μm in diameter and Donaldson et al. (2010) hypothesized that long, rigid HARNs that could not be efficiently cleared from the lungs by macrophages were translocated to the pleural space and trapped at lymphatic stomata on the parietal pleura where they could induce persistent inflammation, uptake by mesothelial cells, and development of malignant mesothelioma. These investigators used direct intrapleural injection of well-characterized metallic nanowires or carbon nanotubes in mice and demonstrated a threshold length of 4 μm for HARNs that are potentially pathogenic in the pleura (Schinwald et al., 2012). Induction of persistent inflammation and fibrosis in the pleural or peritoneal linings in response to direct injection of metallic nanowires, especially nickel nanowires (Poland et al., 2012) must be interpreted with caution because nickel compounds have been shown to induce mesotheliomas in rats (Pott et al., 1989). This caveat also extends to carbon nanotubes containing transition metal catalyst residues; these metals may be bioavailable in the lungs and contribute to lung toxicity as has been demonstrated for nickel-contaminated carbon nanotubes (Hamilton et al., 2012). However, the results with long, rigid carbon nanotubes in these direct pleural or peritoneal injection studies confirm the results of chronic carcinogenicity assays of similar carbon nanotubes summarized in section 4. There are two significant data gaps in our understanding about the mechanisms responsible for carcinogenicity of long, rigid carbon nanotubes. First, how do HARNs translocate to the target tissue in the parietal pleura? Second, is translocation related to the mechanical properties of HARNs, and do all fibrous nanomaterials have the potential to translocate to the pleura?
The translocation pathway for inhaled particles or HARNs from the lungs to the pleural space is unknown. Based on the fiber pathogenicity paradigm, long biopersistent HARNs are less efficiently cleared from the alveoli, induce lung injury and inflammation, penetrate into the interstitium, and reach the pleural space (Schinwald et al., 2012). Miserocchi et al. (2008; Figure 4) propose that lung inflammation increases lung alveolar capillary permeability resulting in increased interstitial pressure that facilitates translocation of biopersistent fibers into pulmonary lymphatics and across the visceral pleura. After particles gain access to pulmonary lymphatics, they are drained to hilar lymph nodes and this route of translocation has been confirmed in rodents exposed to asbestos fibers or carbon nanotubes by inhalation (Ma-Hock et al., 2009; Shvedoca et al., 2014; Mercer et al., 2013 a and b). Boutin et al. (1996) directly demonstrated the presence of carbonaceous soot particles as well as asbestos fibers at sites of lymphatic stomata on the parietal pleura of humans. The anatomy of lymphatic vessels in human lungs was recently re-visited by Sozio et al. (2012) using morphometry and immunohistochemistry to detect specific markers of lymphatics and endothelial cells. They demonstrated an abundant lymphatic network associated with bronchovascular bundles, especially in the intralobular peribronchiolar regions in the lungs (Figure 4). Occasional small lymphatics were identified in the lung interstitial region between alveoli. The lymphatics of the interlobular septa between the lobes of the lung connect to an abundant network of subpleural lymphatics underlying the thick fibroelastic connective tissue covered by a single layer of mesothelial cells in the visceral pleura covering the lungs. There are two significant anatomic barriers against direct penetration of inhaled particles into these lymphatic networks and into the pleural space (Figure 4). First, lymphatics associated with bronchovascular bundles are surrounded by connective tissue and smooth muscle accompanying the small bronchi, bronchioles, and parenchymal arteries and arterioles. Second, after particles gain access to the lung lymphatic network, they must penetrate dense subpleural fibroelastic tissue into the pleural space. Miserocchi et al. (2008) propose that particles are drained into the blood from lymphatics, then secondarily reach the pleural space from systemic capillaries in the parietal pleura. Alternatively, especially in the setting of fiber-induced injury to the alveolar-capillary unit and inflammation, biopersistent HARNs may cross the damaged air-liquid barrier into the interstitium where they may also gain access into pulmonary lymphatics and cross the visceral pleura.
Figure 4. Microscopic Anatomy of Pulmonary Lymphatics and Translocation of Fibers to the Pleural Space.
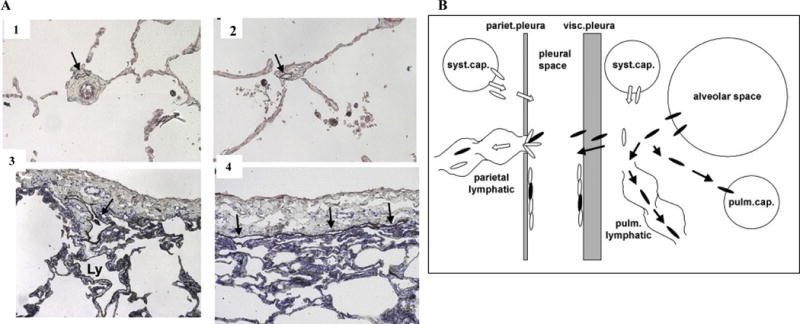
A. alveoli (panel A2). Lymphatics of the interlobular septa (Ly in panel A3) drain into lymphatics beneath the visceral pleura (arrows in panels A3, 4). Immunohistochemical detection of lymphatics with toluidine blue counterstain, original magnification × 10 (Sozio et al., 2012). Reprinted with permission from John Wiley and Sons.
B. Miserocchi et al. (2008) hypothesize that asbestos-induced pulmonary inflammation increases interstitial fluid pressure that allows biopersistent fibers to penetrate into pulmonary lymphatics and cross the visceral pleura. Fibers that are not cleared through lymphatic stomata are trapped at the parietal pleura leading to the development of mesothelioma as proposed in Figure 2. Reprinted with permission from an Open Access article (Miserocchi et al., 2008).
Is translocation of HARNs into the lung interstitium and pleural space determined by their mechanical properties? The lung is a highly-specialized organ that experiences significant mechanical forces during respiration (Roan and Waters, 2011). Lung mechanics has traditionally been described at the whole organ level using parameters based on alveolar pressure, pleural pressure, elastic recoil, and resistance to airflow assessed by pulmonary function testing. Lung mechanics may also be described at the level of the lung alveolus or microscale which is more relevant for particle translocation into the lymphatics and interstitium. Alveolar epithelial cells and fibroblasts secrete extracellular matrix proteins that endow the alveolus with its mechanical properties, especially collagen that resists mechanical loads and elastin that contributes to elastic recoil of the lung. At the microscale, the elastic modulus of the alveolus is ~5 kPa compared to an elastic modulus ~1 kPa in isolated alveolar epithelial cells. Mechanical deformation of the alveolus influences surfactant release, cellular responses to injury, and repair by fibrosis (Suki and Bates, 2008). In contrast to the remarkable viscoelastic properties of the lungs, pleural mechanics is governed by hydrodynamic lubrication forces. The single layer of flat, thin mesothelial cells lining the visceral and parietal pleural surfaces is covered by delicate microvilli supported by a basement membrane and a thin layer of connective tissue. Using atomic force microscopy, Kim et al. (2011) measured an elastic modulus of <1 kPa in the rat visceral pleura which is less than that of the alveolus, although the visceral pleura is thinner in rodents than in humans (Broaddus et al., 2011). Overall, the lung and pleura are biologically soft tissues that are orders of magnitude lower in stiffness or tensile strength than asbestos fibers (1-4 Gpa, Virta, 2002) or carbon nanotubes (0.2-5 TPa; Bussy et al., 2013).
The very high stiffness or tensile strength of carbon nanotubes in comparison to biologically soft tissue is an important mechanical property of these engineered nanomaterials. Some types are also flexible and resilient to breaking (Falvo et al., 1997). These stiffness values quoted above are the elastic (Young’s) modulus of the underlying material, which expresses the resistance of a material (here a fiber) to stretching forces. The ability of a fiber to translocate from the lung, however, it more likely related to bending rigidity, which describes how the fiber behaves under the compressive (pushing) forces associated with fiber penetration through soft biological tissue. Bending rigidity is determined not only by the Young’s modulus, but also by the fiber diameter. Fibers of low bending rigidity are string-like materials that may not be able to penetrate epithelial barriers, while fibers of high bending rigidity are needle-like materials that will be perceived as stiff by biological tissue.
The bending rigidity of fibrous materials can be described by the Euler buckling theory, which gives a critical threshold force for buckling described by the equation:
Where E = the elastic modulus, d = diameter, and L = length. We derived a generalized classification diagram for a range of HARNs include multiwall carbon nanotubes, metallic nanowires, metal oxide nanorods, and polymers (Figure 5, Zhu et al., 2014). This classification depends on the intrinsic stiffness or elastic modulus (E) of the nanomaterial, for example, wormlike micelles are quite soft (Chu et al., 2013) while metallic nanowires are stiffer. Most important, critical buckling load is inversely related to length and increases with the fourth power of the fiber diameter. Euler theory predicts that very long, thin HARNs will buckle more easily than shorter, thicker HARNs. For example, long single wall carbon nanotubes can be very thin, <<10 nm in diameter, with a high aspect ratio > 1,000. For this reason, they buckle easily and are observed to exist in curled and tangled states, and have been reported not to behave as rigid fibers in the same manner as thicker multiwall carbon nanotubes with lower aspect ratios between 10-100. We validated this classification using a well-characterized panel of thin, flexible and thicker, long, rigid multiwall carbon nanotubes experimentally and compared the results with other published studies (Zhu et al., 2014). This pathogenicity mechanism is based on lysosomal membrane damage induced by hydrophobic long, rigid carbon nanotubes (Palomäki et al., 2011) and is consistent with the lower pathogenicity of short or tangled carbon nanotubes summarized in section 2.
Figure 5. Pathogenicity Classification of HARNs.
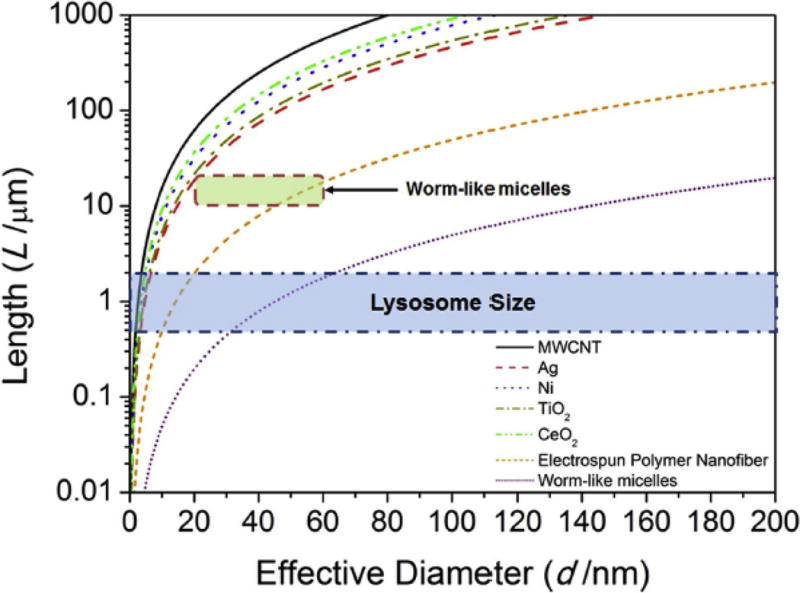
This pathogenicity classification is based on buckling of HARNs of different dimensions and Young’s moduli. For each material, the space above the curve is “biologically soft” and below the curve is “biologically stiff.” This classification is based on the mechanical pathway leading to lysosomal membrane damage. It is predicted the HARNs will induce lysosomal membrane permeability if their dimensions are in the region between the size range of the lysosome (in blue) and their buckling thresholds. Reproduced with permission (Zhu et al., 2016; National Academy of Sciences, USA).
Lysosomal membrane damage can be induced by other HARNs including asbestos fibers (Dostert et al., 2008) as well as titanium dioxide nanobelts but not nanospheres (Porter et al., 2013). The consequences of lysosomal membrane damage following phagocytosis of HARNs by macrophages include release of a protease, cathepsin B, from the lysosome that activates caspase-1 resulting in activation of the NALP3 (nucleotide binding domain leucine-rich repeat containing receptor, pyrin domain-containing-3) inflammasome in the cytoplasm (Sayan and Mossman, 2016). Inflammasome activation can trigger cell death by apoptosis or pyroptosis as well as processing and release of potent proinflammatory mediators, interleukin-1β and interleukin-18 (reviewed by Broz and Dixit, 2016). Cell death can also lead to release of additional inflammatory mediators and contribute to chronic inflammation in the lungs (Lee et al., 2015) in response to biopersistent HARNs (Figure 2). Evidence for the role of chronic inflammation triggered by interleukin-1β signaling was recently reported in a murine peritoneal model of mesothelioma induced by asbestos fibers (Kadariya et al., 2016). Development of malignant mesothelioma was delayed in Asc-deficient mice lacking a component of the NALP3 inflammasome complex or following treatment with anakinra, an inhibitor of the interleukin-1 receptor. Several mechanisms have been proposed to activate the NLRP3 inflammasome including mechanical disruption of the lysosomal membrane as proposed by Zhu et al. (2014), release of reactive oxygen species catalyzed by redox cycling of iron in asbestos fibers, or K+ ion efflux (Lee et al., 2016; Sayan and Mossman, 2016). Soluble nickel ions have also been reported to activate the NLRP3 inflammasome (Caicedo et al., 2009) and multiwall carbon nanotubes containing metallic nickel residues induced NLRP3 inflammasome activation in vitro and persistent lung inflammation following pharyngeal aspiration in mice (Hamilton et al., 2012).
Inhalation of asbestos fibers also induces lung cancer in humans which is a more common cancer than malignant mesothelioma (Markowitz, 2015). Cigarette smoking increases the risk of lung cancer in asbestos-exposed individuals in an additive or synergistic manner as described in a recent systematic review (Ngamwong, et al., 2015). Inhalation of multiwall carbon nanotubes also induces lung adenomas and carcinomas, but no malignant mesotheliomas, in rats (Kasai et al., 2016). The physicochemical and mechanical parameters of carbon nanotubes proposed for induction of malignant mesothelioma in this review may not apply to induction of lung cancer. Less rigid, tangled agglomerates of carbon nanotubes are more likely to deposit by interception at airway bifurcations and induce bronchial carcinomas (Mossman et al., 2011; Lippmann, 2014). In contrast, long, rigid asbestos fibers or carbon nanotubes are more likely to deposit in the alveoli and to translocate into the interstitium or to the pleura leading to development of malignant mesothelioma (Broaddus et al., 2011). Cigarette smoking may also be an important co-factor in inducing lung cancer in workers exposed to carbon nanotubes. Carbon nanotubes have a high surface area and are hydrophobic with potential to adsorb carcinogenic aromatic compounds in cigarette smoke (Brooks et al., 2011).
Asbestos fibers and carbon nanotubes may act as tumor promoters in the development of lung cancer and this mechanism has been proposed for increased growth and neoplastic progression of lung epithelial cells in mice exposed to methylcholanthrene as an initiator, followed by inhalation of multiwall carbon nanotubes (Sargent et. al., 2014). Inflammation and tissue injury induced by persistent asbestos fibers or carbon nanotubes in the lungs are potential mechanisms that contribute to development of lung cancer (Mossman et al., 2011; Rahman et al., 2017). These mechanisms were also hypothesized to contribute to the development of malignant mesothelioma induced by exposure to long, rigid carbon nanotubes that translocate to and persist in the pleura (Figure 1 and IARC, 2017).
6. Concluding Remarks
In summary, the asbestos-carbon nanotube analogy and the fiber pathogenicity paradigm have significantly shaped and informed the scientific debate about carbon nanotube health risks, but many questions remain. Carbon nanotubes are a diverse class of synthetic materials, and while one type of long, stiff, multiwall nanotubes has been classified as a possible human carcinogen, most varieties are not yet classifiable. One path to further progress is through new experimental and computational research on nanotube behavior in the lung and its relation to specific material properties.
A variety of in vitro and in vivo experimental studies provide support for physical or mechanical disruption of the lysosomal membrane by long, rigid carbon nanotubes. This mechanical mechanism for pathogenicity of HARNs depends on physical dimensions and aspect ratio and adds a fourth parameter to the fiber pathogenicity paradigm, mechanical bending stiffness, which is most pronounced for HARNs with aspect ratios between 10-100. Very long, thin HARNs tend to buckle, while very short HARNs (< 2 μm) do not exert sufficient contact forces on the inner leaflet to damage the lysosomal membrane (Zhu et al., 2014). Is a similar nanomechanical mechanism sufficient to explain the translocation of long, rigid HARNs into the lung interstitium, penetration into lymphatics, and accumulation at lymphatic stomata on the parietal pleura leading to the development of mesothelioma?
The nanomechanical properties related to interstitial penetration across the alveolar wall followed by lymphatic drainage and possible penetration from subpleural lymphatics across the visceral pleural connective tissue could be explored using in vitro three-dimensional models of the human lung alveolus and parietal pleura based on natural or synthetic lung scaffolds with similar mechanical properties in the range of ~1-5 kPa that have been measured for the alveolus (Roan and Waters, 2011) and the parietal pleura (Kim et al., 2011). Tissue-engineered models of the human alveolus and parietal pleura could be combined with microfluidics to model lymphatic flow in the normal or inflamed lung to test the hypothesis of Miserocchi et al. (2008) that persistent lung inflammation enhances translocation of asbestos fibers to the pleural space. These tissue-engineered models combined with a test panel of well-characterized HARNs could systematically test the roles of length, diameter, and nanomechanical properties in translocation from the lungs to the pleura and provide the basis for structure-activity relationships predictive of potential carcinogenicity. An integrated approach to incorporate novel testing strategies and validate structure-activity relationships in parallel with new product development and regulatory oversight will promote responsible and sustainable growth of nanotechnology (Crawford et al., 2017).
Highlights.
Engineered carbon nanotubes physically resemble asbestos fibers.
Long, rigid multiwall carbon nanotubes induce lung disease in rodents.
Length, width, and biopersistence of fibers are critical determinants of lung disease.
Mechanical bending stiffness is proposed as another factor relevant for toxicity.
Acknowledgments
The authors’ research is supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (Grant P42 ES013660) and the National Science Foundation (Grant CBET-1344097).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akulian J, Yarmus L, Feller-Kopman D. The Evaluation and Clinical Application of Pleural Physiology. Clinics in Chest Medicine. 2013;34:11–19. doi: 10.1016/j.ccm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Arts JHE, Hadi M, Irfan MA, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, Warheit D, Wiench K, Wohlleben W, Landsiedel R. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) Regulatory Toxicology and Pharmacology. 2015;71:S1–S27. doi: 10.1016/j.yrtph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Arts JHE, Hadi M, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, Warheit D, Wiench K, Landsiedel R. A critical appraisal of existing concepts for the grouping of nanomaterials. Regulatory Toxicology and Pharmacology. 2014;70:492–506. doi: 10.1016/j.yrtph.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Aschberger K, Johnston HJ, Stone V, Aitken RJ, Tran CL, Hankin SM, Peters SAK, Christensen FM. Review of fullerene toxicity and exposure – Appraisal of a human health risk assessment, based on open literature. Regulatory Toxicology and Pharmacology. 2010;58:455–473. doi: 10.1016/j.yrtph.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Donaldson K, Moolgavkar S, Mandel JS. A systematic review of occupational exposure to synthetic vitreous fibers and mesothelioma. Critical Reviews in Toxicology. 2014;44:436–449. doi: 10.3109/10408444.2014.899558. [DOI] [PubMed] [Google Scholar]
- Boutin C, Dumortier P, Rey FR, Viallat J, De Vuyst P. Black spots concentrate oncogenic asbestos fibers in the parietal pleura: Thoracoscopic and mineralogic study. Am J Respir Crit Care Med. 1996;153:444–449. doi: 10.1164/ajrccm.153.1.8542156. [DOI] [PubMed] [Google Scholar]
- Broaddus VC, Everitt JI, Black B, Kane AB. Non-Neoplastic and Neoplastic Pleural Endpoints Following Fiber Exposure. Journal of Toxicology and Environmental Health, Part B. 2011;14:153–178. doi: 10.1080/10937404.2011.556049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AJ, Lim HN, Kilduff JE. Adsorption uptake of synthetic organic chemicals by carbon nanotubes and activated carbons. Nanotechnology. 2012;23:294008. doi: 10.1088/0957-4484/23/29/294008. [DOI] [PubMed] [Google Scholar]
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- Burden N, Aschberger K, Chaudhry Q, Clift MJD, Doak SH, Fowler P, Johnston H, Landsiedel R, Rowland J, Stone V. The 3Rs as a framework to support a 21st century approach for nanosafety assessment. Nano Today. 2017;12:10–13. [Google Scholar]
- Bussy C, Ali-Boucetta H, Kostarelos K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Accounts of Chemical Research. 2013;46:692–701. doi: 10.1021/ar300199e. [DOI] [PubMed] [Google Scholar]
- Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. Journal of Orthopaedic Research. 2009;27:847–854. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
- Chu Z, Dreiss CA, Feng Y. Smart wormlike micelles. Chemical Society Reviews. 2013;42:7174–7203. doi: 10.1039/c3cs35490c. [DOI] [PubMed] [Google Scholar]
- Clift MJD, Endes C, Vanhecke D, Wick P, Gehr P, Schins RPF, Petri-Fink A, Rothen-Rutishauser B. A comparative study of different In vitro lung cell culture systems to assess the most beneficial tool for screening the potential adverse effects of carbon nanotubes. Toxicological Sciences. 2014;137:55–64. doi: 10.1093/toxsci/kft216. [DOI] [PubMed] [Google Scholar]
- Clift MJD, Gehr P, Rothen-Rutishauser B. Nanotoxicology: a perspective and discussion of whether or not in vitro testing is a valid alternative. Archives of Toxicology. 2011;85:723–731. doi: 10.1007/s00204-010-0560-6. [DOI] [PubMed] [Google Scholar]
- Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotech. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Hartung T, Hollert H, Mathes B, van Ravenzwaay B, Steger-Hartmann T, Studer C, Krug HF. Green Toxicology: a strategy for sustainable chemical and material development. Environmental Sciences Europe. 2017;29:16. doi: 10.1186/s12302-017-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Oberdörster G, Kane AB, Brown SC, Klaper RD, Hurt RH. Nanotoxicology, Chapter 28, Casarett & Doull’s Toxicology: The Basic Science of Poisons. (9th) (in press) [Google Scholar]
- Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon nanotube and nanofiber exposure assessments: An analysis of 14 site visits. Annals of occupational hygiene. 2015;59:705–723. doi: 10.1093/annhyg/mev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Volder MFL, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicological Sciences. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- Donaldson KH, Beswick PS, Gilmour P. Free radical activity associated with the surface of particles: A unifying factor in determining biological activity? Toxicology Lett. 1996;88:293–298. doi: 10.1016/0378-4274(96)03752-6. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine. 2010a;6:143–156. doi: 10.2217/nnm.10.139. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Particle and Fibre Toxicology. 2010b;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos — Similarities and differences. Advanced Drug Delivery Reviews. 2013;65:2078–2086. doi: 10.1016/j.addr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science (New York, NY) 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endes C, Camarero-Espinosa S, Mueller S, Foster EJ, Petri-Fink A, Rothen-Rutishauser B, Weder C, Clift MJD. A critical review of the current knowledge regarding the biological impact of nanocellulose. Journal of Nanobiotechnology. 2016;14:78. doi: 10.1186/s12951-016-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Clift MJD, Singh N, de Oliveira Mallia J, Burgum M, Wills JW, Wilkinson TS, Jenkins GJS, Doak SH. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis. 2017;32:233–241. doi: 10.1093/mutage/gew054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo MR, Clary GJ, Taylor RM, Chi V, Brooks FP, Washburn S, Superfine R. Bending and buckling of carbon nanotubes under large strain. Nature. 1997;389:582–584. doi: 10.1038/39282. [DOI] [PubMed] [Google Scholar]
- Fenoglio I, Tomatis M, Lison D, Muller J, Fonseca A, Nagy JB, Fubini B. Reactivity of carbon nanotubes: Free radical generation or scavenging activity? Free Radical Biology and Medicine. 2006;40:1227–1233. doi: 10.1016/j.freeradbiomed.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Feynman RP. There’s Plenty of Room at the Bottom. In: Gilbert HD, editor. Miniaturization. Reinhold; New York: 1961. [Google Scholar]
- Fubini B, Fenoglio I, Tomatis M, Turci F. Effect of chemical composition and state of the surface on the toxic response to high aspect ratio nanomaterials. Nanomedicine. 2011;6:899–920. doi: 10.2217/nnm.11.80. [DOI] [PubMed] [Google Scholar]
- Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4:347–363. doi: 10.3109/17435390.2010.509519. [DOI] [PubMed] [Google Scholar]
- Geiser M, Matter M, Maye I, Im Hof V, Gehr P, Schürch S. Influence of airspace geometry and surfactant on the retention of man-made vitreous fibers (MMVF 10a) Environmental Health Perspectives. 2003a;111:895–901. doi: 10.1289/ehp.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser M, Schürch S, Gehr P. Influence of surface chemistry and topography of particles on their immersion into the lung’s surface-lining layer. Journal of Applied Physiology. 2003b;94:1793–1801. doi: 10.1152/japplphysiol.00514.2002. [DOI] [PubMed] [Google Scholar]
- Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. European Respiratory Journal. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. The Lancet Oncology. 2014;15:1427–1428. doi: 10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- Guo L, Morris DG, Liu X, Vaslet C, Hurt RH, Kane AB. Iron bioavailability and redox activity in diverse carbon nanotube samples. Chemistry of Materials. 2007;19:3472–3478. [Google Scholar]
- Guseva Canu I, Bateson TF, Bouvard V, Debia M, Dion C, Savolainen K, Yu IJ. Human exposure to carbon-based fibrous nanomaterials: A review. International Journal of Hygiene and Environmental Health. 2016;219:166–175. doi: 10.1016/j.ijheh.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Buford M, Xiang C, Wu N, Holian A. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhalation Toxicology. 2012;24:995–1008. doi: 10.3109/08958378.2012.745633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Particle and Fibre Toxicology. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Corson N, Wade-Mercer P, Gelein R, Jiang J, Sahu M, Biswas P, Finkelstein JN, Elder A, Oberdörster G. Assessing the relevance of in vitro studies in nanotoxicology by examining correlations between in vitro and in vivo data. Toxicology. 2012;297:1–9. doi: 10.1016/j.tox.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Cruz A, Pérez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2015;95:117–127. doi: 10.1016/j.ejpb.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Hood E. Nanotechnology: Looking as we leap. Environmental Health Perspectives. 2004;112:A740–A749. doi: 10.1289/ehp.112-a740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. Silica, some silicates, coal dust and para-aramid fibrils. Vol. 68. Lyon, France: International Agency for Research on Cancer; 1997. IARC monographs on the evaluation of carcinogenic risks to humans. Available at: http://monographs.iarc.fr/ [PMC free article] [PubMed] [Google Scholar]
- IARC. IARC monographs on the evaluation of carcinogenic risks to human: Man-made vitreous fibres. Vol. 81. Lyon, France: International Agency for Research on Cancer; 2002. Available at: http://monographs.iarc.fr/ [PMC free article] [PubMed] [Google Scholar]
- IARC. Preamble to the IARC Monographs. Lyon, France: International Agency for Research on Cancer; 2006. Available from: http://monographs.iarc.fr/ [Google Scholar]
- IARC. IARC monographs: Arsenic, metals, fibres, and dusts Vol 100C A review of human carcinogens. Lyon, France: International Agency for Research on Cancer; 2012. Available from: http://monographs.iarc.fr/ [Google Scholar]
- IARC. IARC Monographs: Some nanomaterials and some fibres. Vol. 111. Lyon, France: International Agency for Research on Cancer; 2017. Available from: http://monographs.iarc.fr/ [Google Scholar]
- Jaurand MC, Renier A, Daubriac J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Particle and Fibre Toxicology. 2009;6:16–16. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet N, Fierz M, Kalberer M, Burtscher H, Geiser M. Nano aerosol chamber for In-vitro toxicity (NACIVT) studies. Nanotoxicology. 2015;9:34–42. doi: 10.3109/17435390.2014.886739. [DOI] [PubMed] [Google Scholar]
- Kadariya Y, Menges CW, Talarchek J, Cai KQ, Klein-Szanto AJ, Pietrofesa RA, Christofidou-Solomidou M, Cheung M, Mossman BT, Shukla A, Testa JR. Inflammation-related IL-1β/IL-1R signaling promotes the development of asbestos-induced malignant mesothelioma. Cancer Prevention Research (Philadelphia, Pa) 2016;9:406–414. doi: 10.1158/1940-6207.CAPR-15-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya C, Lee BW, Ogami A, Oyabu T, Nishi KI, Yamamoto M, Todoroki M, Morimoto Y, Myojo T. Analysis of pulmonary surfactant in rat lungs after inhalation of nanomaterials: Fullerenes, nickel oxide and multi-walled carbon nanotubes. Nanotoxicology. 2016;10:194–203. doi: 10.3109/17435390.2015.1039093. [DOI] [PubMed] [Google Scholar]
- Kagan CR, Fernandez LE, Gogotsi Y, Hammond PT, Hersam MC, Nel AE, Penner RM, Willson CG, Weiss PS. Nano day: Celebrating the next decade of nanoscience and nanotechnology. ACS Nano. 2016;10:9093–9103. doi: 10.1021/acsnano.6b06655. [DOI] [PubMed] [Google Scholar]
- Kane AB, Hurt RH. Nanotoxicology: The asbestos analogy revisited. Nat Nano. 2008;3:378–379. doi: 10.1038/nnano.2008.182. [DOI] [PubMed] [Google Scholar]
- Kapralov AA, Feng WH, Amoscato AA, Yanamala N, Balasubramanian K, Winnica DE, Kisin ER, Kotchey GP, Gou P, Sparvero LJ, Ray P, Mallampalli RK, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA, Kagan VE. Adsorption of surfactant lipids by single-walled carbon nanotubes in mouse lung upon pharyngeal aspiration. ACS Nano. 2012;6:4147–4156. doi: 10.1021/nn300626q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, Fukushima S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Particle and Fibre Toxicology. 2016;13:53. doi: 10.1186/s12989-016-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Butler JP, Loring SH. Probing softness of the parietal pleural surface at the micron scale. Journal of Biomechanics. 2011;44:2558–2564. doi: 10.1016/j.jbiomech.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarelos K. The long and short of carbon nanotube toxicity. Nat Biotech. 2008;26:774–776. doi: 10.1038/nbt0708-774. [DOI] [PubMed] [Google Scholar]
- Kotchey GP, Zhao Y, Kagan VE, Star A. Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo. Advanced Drug Delivery Reviews. 2013;65:1921–1932. doi: 10.1016/j.addr.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Geiser M. Nanoparticles in Medicine and Environment. Springer; Netherlands: 2010. Dosimetry of inhaled nanoparticles; pp. 145–171. [Google Scholar]
- Kuempel ED, Jaurand MC, Møller P, Morimoto Y, Kobayashi N, Pinkerton KE, Sargent LM, Vermeulen RCH, Fubini B, Kane AB. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Critical Reviews in Toxicology. 2017;47:1–58. doi: 10.1080/10408444.2016.1206061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BW, Kadoya C, Horie M, Mizuguchi Y, Hashiba M, Kambara T, Okada T, Myojo T, Oyabu T, Ogami A, Morimoto Y, Tanaka I, Uchida K, Endoh S, Nakanishi J. Analysis of pulmonary surfactant in rat lungs after intratracheal instillation of short and long multi-walled carbon nanotubes. Inhalation Toxicology. 2013;25:609–620. doi: 10.3109/08958378.2013.821562. [DOI] [PubMed] [Google Scholar]
- Lee S, Suh GY, Ryter SW, Choi AMK. Regulation and function of the nucleotide binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3 inflammasome in lung disease. American Journal of Respiratory Cell and Molecular Biology. 2016;54:151–160. doi: 10.1165/rcmb.2015-0231TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JG, Li WX, Xu JY, Cai XQ, Liu RL, Li YJ, Zhao QF, Li QN. Comparative study of pathological lesions induced by multiwalled carbon nanotubes in lungs of mice by intratracheal instillation and inhalation. Environmental Toxicology. 2007;22:415–421. doi: 10.1002/tox.20270. [DOI] [PubMed] [Google Scholar]
- Liou SH, Tsai CSJ, Pelclova D, Schubauer-Berigan MK, Schulte PA. Assessing the first wave of epidemiological studies of nanomaterial workers. Journal of nanoparticle research: an interdisciplinary forum for nanoscale science and technology. 2015;17:413. doi: 10.1007/s11051-015-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M. Toxicological and epidemiological studies on effects of airborne fibers: Coherence and public health implications. Critical Reviews in Toxicology. 2014;44:643–695. doi: 10.3109/10408444.2014.928266. [DOI] [PubMed] [Google Scholar]
- Liu X, Guo L, Morris D, Kane AB, Hurt RH. Targeted removal of bioavailable metal as a detoxification strategy for carbon nanotubes. Carbon. 2008;46:489–500. doi: 10.1016/j.carbon.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gurel V, Morris D, Murray DW, Zhitkovich A, Kane AB, Hurt RH. Bioavailability of nickel in single-wall carbon nanotubes. Advanced Materials. 2007;19:2790–2796. [Google Scholar]
- Liu X, Hurt RH, Kane AB. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon. 2010;48:1961–1969. doi: 10.1016/j.carbon.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luanpitpong S, Wang L, Davidson DC, Riedel H, Rojanasakul Y. Carcinogenic potential of high aspect ratio carbon nanomaterials. Environmental Science: Nano. 2016;3:483–493. doi: 10.1039/C5EN00238A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8:233–278. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- Ma-Hock L, Strauss V, Treumann S, Küttler K, Wohlleben W, Hofmann T, Gröters S, Wiench K, van Ravenzwaay B, Landsiedel R. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoplatelets and low surface carbon black. Particle and Fibre Toxicology. 2013;10:23–23. doi: 10.1186/1743-8977-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van Ravenzwaay B, Landsiedel R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 Months. Toxicological Sciences. 2009;112:468–481. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- Markowitz S. Asbestos-related lung cancer and malignant mesothelioma of the pleura: Selected current issues. Seminars in Respiratory and Critical Care Medicine. 2015;36:334–46. doi: 10.1055/s-0035-1549449. [DOI] [PubMed] [Google Scholar]
- Maxim LD, Niebo R, Utell MJ, McConnell EE, LaRosa S, Segrave AM. Wollastonite toxicity: an update. Inhalation Toxicology. 2014;26:95–112. doi: 10.3109/08958378.2013.857372. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdörster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handling of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. Journal of Toxicology and Environmental Health, Part A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Melbourne J, Clancy A, Seiffert J, Skepper J, Tetley TD, Shaffer MS, Porter A. An investigation of the carbon nanotube–lipid interface and its impact upon pulmonary surfactant lipid function. Biomaterials. 2015;55:24–32. doi: 10.1016/j.biomaterials.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Particle and Fibre Toxicology. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, Castranova V, Porter DW. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Particle and Fibre Toxicology. 2011;8(1):21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, Andrew M, Castranova V, Porter DW. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Particle and Fibre Toxicology. 2013b;10:33. doi: 10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter DW. Extrapulmonary transport of MWCNT following inhalation exposure. Particle and Fibre Toxicology. 2013a;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Mounting evidence that carbon nanotubes may be the new asbestos. Friends of the Earth Australia. 2008:1–16. [Google Scholar]
- Miserocchi G, Sancini G, Mantegazza F, Chiappino G. Translocation pathways for inhaled asbestos fibers. Environmental Health. 2008;7:4. doi: 10.1186/1476-069X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environment International. 2015;77:132–147. doi: 10.1016/j.envint.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Landesman JM. Importance of oxygen free radicals in asbestos-induced injury to airway epithelial cells. CHEST. 83:50S–51S. doi: 10.1378/chest.83.5.50s. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. Journal of toxicology and environmental health Part B. 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicological Sciences. 2009;110:442–448. doi: 10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Lison D. Respiratory toxicity of carbon nanotubes: How worried should we be? Carbon. 2006;44:1048–1056. [Google Scholar]
- Mulvaney P, Weiss PS. Have nanoscience and nanotechnology delivered? ACS Nano. 2016;10:7225–7226. doi: 10.1021/acsnano.6b05344. [DOI] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, Toyokuni S. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proceedings of the National Academy of Sciences. 2011;108:E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Toyokuni S. Biopersistent fiber-induced inflammation and carcinogenesis: Lessons learned from asbestos toward safety of fibrous nanomaterials. Archives of Biochemistry and Biophysics. 2010;502:1–7. doi: 10.1016/j.abb.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nel AE, Nasser E, Godwin H, Avery D, Bahadori T, Bergeson L, Beryt E, Bonner JC, Boverhof D, Carter J, Castranova V, DeShazo JR, Hussain SM, Kane AB, Klaessig F, Kuempel E, Lafranconi M, Landsiedel R, Malloy T, Miller MB, Morris J, Moss K, Oberdorster G, Pinkerton K, Pleus RC, Shatkin JA, Thomas R, Tolaymat T, Wang A, Wong J. A multi-stakeholder perspective on the use of alternative test strategies for nanomaterial safety assessment. ACS Nano. 2013;7:6422–6433. doi: 10.1021/nn4037927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JE, Niles JA, Cortiella J. Production and utilization of acellular lung scaffolds in tissue engineering. Journal of Cellular Biochemistry. 2012;113:2185–2192. doi: 10.1002/jcb.24112. [DOI] [PubMed] [Google Scholar]
- Nichols JE, Niles JA, Vega SP, Cortiella J. Novel in vitro respiratory models to study lung development, physiology, pathology and toxicology. Stem Cell Research & Therapy. 2013;4:S7–S7. doi: 10.1186/scrt368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers. Cincinnati, Ohio: US Department of Health and Human Services Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2013. (Publication No 2013-145). [Google Scholar]
- Ngamwong Y, Tangamornsuksan W, Lohitnavy O, Chaiyakunapruk N, Scholfield CN, Reisfeld B, Lohitnavy M. Additive synergism between asbestos and smoking in lung cancer risk: A systematic review and meta-analysis. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowack B, David RM, Fissan H, Morris H, Shatkin JA, Stintz M, Zepp R, Brouwer D. Potential release scenarios for carbon nanotubes used in composites. Environment International. 2013;59:1–11. doi: 10.1016/j.envint.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Nymark P, Jensen KA, Suhonen S, Kembouche Y, Vippola M, Kleinjans J, Catalán J, Norppa H, van Delft J, Briedé JJ. Free radical scavenging and formation by multi-walled carbon nanotubes in cell free conditions and in human bronchial epithelial cells. Particle and Fibre Toxicology. 2014;11:4. doi: 10.1186/1743-8977-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environmental Health Perspectives. 1994;102:173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Stone V, Donaldson K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology. 2007;1:2–25. [Google Scholar]
- Olson MS, Gurian PL. Risk assessment strategies as nanomaterials transition into commercial applications. Journal of Nanoparticle Research. 2012;14:786. [Google Scholar]
- Pacurari M, Castranova V, Vallyathan V. Single- and multi-wall carbon nanotubes versus asbestos: Are the carbon nanotubes a new health risk to humans? Journal of Toxicology and Environmental Health, Part A. 2010;73:378–395. doi: 10.1080/15287390903486527. [DOI] [PubMed] [Google Scholar]
- Palomäki J, Välimäki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 Inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Patel B, Gauvin R, Absar S, Gupta V, Gupta N, Nahar K, Khademhosseini A, Ahsan F. Computational and bioengineered lungs as alternatives to whole animal, isolated organ, and cell-based lung models. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2012;303:L733–L747. doi: 10.1152/ajplung.00076.2012. [DOI] [PubMed] [Google Scholar]
- Poland CA, Byrne F, Cho WS, Prina-Mello A, Murphy FA, Davies GL, Coey JMD, Gounko Y, Duffin R, Volkov Y, Donaldson K. Length-dependent pathogenic effects of nickel nanowires in the lungs and the peritoneal cavity. Nanotoxicology. 2012;6:899–911. doi: 10.3109/17435390.2011.626535. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nano. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Porter DW, Wu N, Hubbs AF, Mercer RR, Funk K, Meng F, Li J, Wolfarth MG, Battelli L, Friend S, Andrew M, Hamilton JR, Sriram K, Yang F, Castranova V, Holian A. Differential mouse pulmonary dose and time course responses to titanium dioxide nanospheres and nanobelts. Toxicological Sciences. 2013;131:179–193. doi: 10.1093/toxsci/kfs261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott FRR, Roller M, Csicsaky M, Rosenbruch M, Huth F. Proceedings of the International Conference on Heavy Metals in the Environment 2. Geneva: World Health Organization; 1989. Tumours in the abdominal cavity of rats after intraperitoneal injection of nickel compounds; pp. 127–129. [Google Scholar]
- Rahman LN, Jacobsen NR, Aziz SA, Wu D, Williams A, Yauk C, White P, Wallin H, Vogel U, Halappanavar SS. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: Investigating the mechanisms of pulmonary carcinogenesis. Mutation Research. 2017;823:28–44. doi: 10.1016/j.mrgentox.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Rittinghausen S, Hackbarth A, Creutzenberg O, Ernst H, Heinrich U, Leonhardt A, Schaudien D. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Particle and Fibre Toxicology. 2014;11:59. doi: 10.1186/s12989-014-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? American Journal of Physiology - Lung Cellular and Molecular Physiology. 2011;301:L625–L635. doi: 10.1152/ajplung.00105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, Mercer RR, Stefaniak AB, Seehra MS, Geddam UK, Chaudhuri IS, Kyrlidis A, Kodali VK, Sager T, Kenyon A, Bilgesu SA, Eye T, Scabilloni JF, Leonard SS, Fix NR, Schwegler-Berry D, Farris BY, Wolfarth MG, Porter DW, Castranova V, Erdely A. Evaluation of pulmonary and systemic toxicity following lung exposure to graphite nanoplates: a member of the graphene-based nanomaterial family. Particle and Fibre Toxicology. 2016;13:34. doi: 10.1186/s12989-016-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RS/RAE. Nanoscience and nanotechnologies. 2004 Available at: http://www.royalsoc.ac.uk/
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nature Nanotechnology. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajid MI, Jamshaid U, Jamshaid T, Zafar N, Fessi H, Elaissari A. Carbon nanotubes from synthesis to in vivo biomedical applications. International Journal of Pharmaceutics. 2016;501:278–299. doi: 10.1016/j.ijpharm.2016.01.064. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, Hirose A, Nishimura T, Ohashi N, Ogata A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. The Journal of Toxicological Sciences. 2009;34:65–76. doi: 10.2131/jts.34.65. [DOI] [PubMed] [Google Scholar]
- Salvador-Morales C, Townsend P, Flahaut E, Vénien-Bryan C, Vlandas A, Green ML, Sim RB. Binding of pulmonary surfactant proteins to carbon nanotubes; potential for damage to lung immune defense mechanisms. Carbon. 2007;45:607–617. [Google Scholar]
- Sanchez VC, Pietruska JR, Miselis NR, Hurt RH, Kane AB. Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos? Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2009;1:511–529. doi: 10.1002/wnan.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli LA, Siegrist KJ, Kashon ML, Mercer RR, Bauer AK, Chen BT, Salisbury JL, Frazer D, McKinney W, Andrew M, Tsuruoka S, Endo M, Fluharty KL, Castranova V, Reynolds SH. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Particle and Fibre Toxicology. 2013;11:3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayan M, Mossman BT. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Particle and Fibre Toxicology. 2016;13:51. doi: 10.1186/s12989-016-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinwald A, Murphy F, Askounis A, Koutsos V, Sefiane K, Donaldson K, Campbell CJ. Minimal oxidation and inflammogenicity of pristine graphene with residence in the lung. Nanotoxicology. 2014;8:824–832. doi: 10.3109/17435390.2013.831502. [DOI] [PubMed] [Google Scholar]
- Schinwald A, Murphy FA, Prina-Mello A, Poland CA, Byrne F, Movia D, Glass JR, Dickerson JC, Schultz DA, Jeffree CE, MacNee W, Donaldson K. The threshold length for fiber-induced acute pleural inflammation: Shedding light on the early events in asbestos-induced mesothelioma. Toxicological Sciences. 2012;128:461–470. doi: 10.1093/toxsci/kfs171. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Geraci CL, Murashov V, Kuempel ED, Zumwalde RD, Castranova V, Hoover MD, Hodson L, Martinez KF. Occupational safety and health criteria for responsible development of nanotechnology. Journal of Nanoparticle Research. 2014;16:2153. doi: 10.1007/s11051-013-2153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA, Roth G, Hodson LL, Murashov V, Hoover MD, Zumwalde R, Kuempel ED, Geraci CL, Stefaniak AB, Castranova V, Howard J. Taking stock of the occupational safety and health challenges of nanotechnology: 2000–2015. Journal of nanoparticle research: an interdisciplinary forum for nanoscale science and technology. 2016;18:159. doi: 10.1007/s11051-016-3459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respiration Physiology. 1990;80:17–32. doi: 10.1016/0034-5687(90)90003-h. [DOI] [PubMed] [Google Scholar]
- Shi X, von Dem Bussche A, Hurt RH, Kane AB, Gao H. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nature Nanotechnology. 2011;6:714–719. doi: 10.1038/nnano.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Yanamala N, Kisin ER, Tkach AV, Murray AR, Hubbs A, Chirila MM, Keohavong P, Sycheva LP, Kagan VE, Castranova V. Long-term effects of carbon containing engineered nanomaterials and asbestos in the lung: one year postexposure comparisons. American Journal of Physiology - Lung. Cellular and Molecular Physiology. 2014;306:L170–L182. doi: 10.1152/ajplung.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert TA, Rugonyi S. Influence of liquid-layer thickness on pulmonary surfactant spreading and collapse. Biophysical Journal. 2008;95:4549–4559. doi: 10.1529/biophysj.107.127654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozio F, Rossi A, Weber E, Abraham DJ, Nicholson AG, Wells AU, Renzoni EA, Sestini P. Morphometric analysis of intralobular, interlobular and pleural lymphatics in normal human lung. Journal of Anatomy. 2012;220:396–404. doi: 10.1111/j.1469-7580.2011.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suki B, Bates JHT. Extracellular matrix mechanics in lung parenchymal diseases. Respiratory Physiology & Neurobiology. 2008;163:33–43. doi: 10.1016/j.resp.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzui M, Futakuchi M, Fukamachi K, Numano T, Abdelgied M, Takahashi S, Ohnishi M, Omori T, Tsuruoka S, Hirose A, Kanno J, Sakamoto Y, Alexander DB, Alexander WT, Jiegou X, Tsuda H. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Science. 2016;107:924–935. doi: 10.1111/cas.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53+/− heterozygous mice. Cancer Science. 2012;103:1440–1444. doi: 10.1111/j.1349-7006.2012.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, p53+/−Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53+/−; mouse by intraperitoneal application of multi-wall carbon nanotube. The Journal of Toxicological Sciences. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- Tantra R, Cumpson P. The detection of airborne carbon nanotubes in relation to toxicology and workplace safety. Nanotoxicology. 2007;1:251–265. [Google Scholar]
- Utembe W, Potgieter K, Stefaniak AB, Gulumian M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Particle and Fibre Toxicology. 2015;12:11. doi: 10.1186/s12989-015-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle RP, Wu T, Zuo YY. Biophysical influence of airborne carbon nanomaterials on natural pulmonary surfactant. ACS Nano. 2015;9:5413–5421. doi: 10.1021/acsnano.5b01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF, Jr, Rejeski D, Hull MS. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein Journal of Nanotechnology. 2015;6:1769–1780. doi: 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virta RL. Asbestos: Geology, Mineralogy, Mining, and Uses. 2002. (US Geological Survery Open-File Report, 02-149). [Google Scholar]
- Wang J, Schlagenhauf L, Setyan A. Transformation of the released asbestos, carbon fibers and carbon nanotubes from composite materials and the changes of their potential health impacts. Journal of Nanobiotechnology. 2017;15:15. doi: 10.1186/s12951-017-0248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Castranova V, Mishra A, Chen B, Mercer RR, Schwegler-Berry D, Rojanasakul Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Particle and Fibre Toxicology. 2010;7:31. doi: 10.1186/1743-8977-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xia T, Ntim SA, Ji Z, Lin S, Meng H, Chung CH, George S, Zhang H, Wang M, Li N, Yang Y, Castranova V, Mitra S, Bonner JC, Nel AE. The Dispersal State of Multi-walled Carbon Nanotubes Elicits Pro-Fibrogenic Cellular Responses that Correlate with Fibrogenesis Biomarkers and Fibrosis in the Murine Lung. ACS Nano. 2011;5:9772–9787. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB. What is currently known about the health risks related to carbon nanotube exposures? Carbon. 2006;44:1064–1069. [Google Scholar]
- Warheit DB, Oberdörster G, Kane AB, Brown SC, Klaper RD, Hurt RH. Nanotoxicology, Chapter 28, Casarett & Doull’s Toxicology: The Basic Science of Poisons. (9th) 2001 (in press) [Google Scholar]
- Woodrow Wilson Center. 2012 Available at: http://www.nanotechproject.org/inventories/consumer/analysis_draft/
- Xu J, Alexander DB, Futakuchi M, Numano T, Fukamachi K, Suzui M, Omori T, Kanno J, Hirose A, Tsuda H. Size- and shape-dependent pleural translocation, deposition, fibrogenesis, and mesothelial proliferation by multiwalled carbon nanotubes. Cancer Science. 2014;105:763–769. doi: 10.1111/cas.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Luo Z, Li S, Li W, Zhang X, Zuo YY, Huang F, Yue T. Perturbation of the pulmonary surfactant monolayer by single-walled carbon nanotubes: a molecular dynamics study. Nanoscale. 2017;9:10193–10204. doi: 10.1039/c7nr00890b. [DOI] [PubMed] [Google Scholar]
- Yi X, Shi X, Gao H. A universal law for cell uptake of one-dimensional nanomaterials. Nano Letters. 2014;14:1049–1055. doi: 10.1021/nl404727m. [DOI] [PubMed] [Google Scholar]
- Yue T, Xu Y, Li S, Luo Z, Zhang X, Huang F. Ultrashort single-walled carbon nanotubes insert into a pulmonary surfactant monolayer via self-rotation: poration and mechanical inhibition. The Journal of Physical Chemistry B. 2017;121:2797–2807. doi: 10.1021/acs.jpcb.7b00297. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Huang JQ, Qian WZ, Zhang YY, Wei F. The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small. 2013;9:1237–1265. doi: 10.1002/smll.201203252. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Lin QX, Zhang Y, Liu CT, Qiu LY, Wang HB, Wang YM, Duan CM, Liu ZQ, Zhou J, Wang CY. The reconstruction of lung alveolus-like structure in collagen-matrigel/microcapsules scaffolds in vitro. Journal of Cellular and Molecular Medicine. 2011;15:1878–1886. doi: 10.1111/j.1582-4934.2010.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, von dem Bussche A, Yi X, Qiu Y, Wang Z, Weston P, Hurt RH, Kane AB, Gao H. Nanomechanical mechanism for lipid bilayer damage induced by carbon nanotubes confined in intracellular vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:12374–12379. doi: 10.1073/pnas.1605030113. [DOI] [PMC free article] [PubMed] [Google Scholar]


