To the Editor:
Albuminuria occurs in 10–35% of children and up to 60% of adults with SCA.[1, 2] Annual screening of albumin to creatinine ratios (ACR) beginning at 10 years of age is recommended; however, evidence-based therapy for those identified to have albuminuria is lacking.[3] Low hemoglobin (Hb) and low fetal hemoglobin (HbF) have been associated with albuminuria in children with SCA, and hydroxyurea increases these levels. We hypothesized hydroxyurea may prevent the development of albuminuria and improve ACR in children with existing albuminuria.
Children (≤18 years of age) with SCA who had baseline (pre-hydroxyurea) and longitudinal ACR assessments from two IRB-approved studies [The Hydroxyurea Study of Long-Term Effects (HUSTLE; NCT00305175)[4] and the Sickle Cell Clinical Research and Intervention Program (SCCRIP; NCT02098863)[5] were analyzed. Hydroxyurea was escalated to a maximum tolerated dose (MTD). A previous analysis of 23 HUSTLE participants reported hydroxyurea did not improve ACR, but current analysis provides longer follow-up. [4]
Albuminuria was defined as an ACR of ≥30 mg/g. Demographics, hydroxyurea treatment history, and Hb, HbF, and mean corpuscular volume (MCV) levels were abstracted at baseline, at the time of ACR measurement closest to one year after initiation of hydroxyurea, and up to five years after initiation.
Summary statistics were reported and compared using appropriate statistical methods. Follow-up was censored at 5 years. We undertook three analytical approaches: 1) time-to-event analysis from baseline until the first albuminuria, 2) time-to-recurrent-event, and 3) comparison of the proportions of subjects with albuminuria at baseline, at up to 12 months (“Year 1”) (average of 7.7 months +/− 2.9 months) following hydroxyurea initiation, and beyond 12 months during chronic hydroxyurea therapy with ACR values closest to the 5-year censoring for each participant or closest to the first albuminuria which occurred post Year 1 treatment.
In time-to-event and time-to-recurrent event models, the cumulative incidence of albuminuria was estimated using the Kaplan-Meier method and Log-rank or Wilcoxon test were used to test the survival function between groups. Univariate and multivariate Cox proportional hazards model and shared frailty model, which accommodates interval-censored data, were used to calculate hazard rates (HR) and 95% confidence intervals (95% CI). Covariates included baseline age, baseline ACR, and Hb. For the third approach, the proportions of children with albuminuria at two time points were compared with the McNemar’s test. Statistical significance was defined as p-value <0.05.
Eighty-eight children with SCA, median (range) age 10.1 (2.1–17.6) years, initiated hydroxyurea and were followed for a median of 3.0 years (0.5–4.9 years), providing 222 patient-years of follow-up. Forty-six (52%) were male. The mean (SD) dose of hydroxyurea at MTD was 24.9 (±5.1) mg/kg/day with a range of 14 to 35 mg/kg/day at last follow-up. Baseline ACRs were normal in 45 (51%) and abnormal in 43 (49%) participants. At baseline, children with albuminuria had a lower mean Hb compared to those without albuminuria (7.8 vs. 8.4 g/dL, p= 0.03). Age, sex and other hematologic indices, including HbF, MCV and absolute reticulocyte count, were similar between groups.
After one year of hydroxyurea, 21 (47%) of 45 children identified without albuminuria at baseline had a repeat ACR obtained at one year; 19 (90%) continued to be free of albuminuria. Following up to five years of hydroxyurea, 23 children who did not have albuminuria at baseline continued without developing albuminuria while 10 children developed albuminuria. In univariate analysis, children who initiated hydroxyurea before 10 years of age were less likely (HR 0.49; 95% CI: 0.25–0.97, p=0.038) to develop albuminuria post initiation of hydroxyurea compared to those who initiated at 10 years or older (Figure 1a). Hemoglobin, HbF, and MCV were not associated with time to first abnormal ACR.
Figure 1.
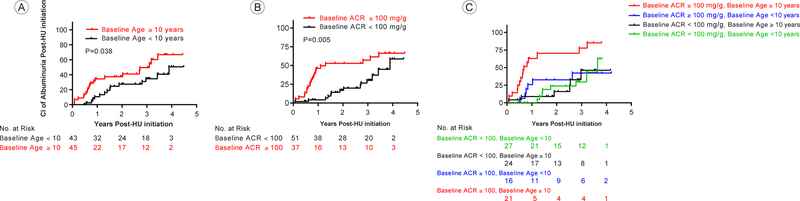
Probability of developing the first albuminuria episode after the initiation of hydroxyurea in children with SC.
Figure 1a: Cumulative Incidence of developing albuminuria post HU initiation by baseline HU initiation age of ≥ 10 years as compared to <10 years.
Figure 1b: Cumulative Incidence of developing albuminuria post HU initiation by baseline albumin to creatinine ratio of ≥ 100mg/g as compared to <100 mg/g.
Figure 1c: Cumulative Incidence of developing albuminuria post HU initiation by baseline age and albumin to creatinine ratio. P-values reported are compared to participants with baseline ACR ≥ 100mg/g and age ≥ 10 years. Baseline ACR ≥ 100mg/g and age < 10 years p=0.006, baseline ACR < 100mg/g and ≥ age 10 years p=0.001, and baseline ACR < 100mg/g and age < 10 years p=0.0006.
Following one year of hydroxyurea, 26 (60%) of 43 children who had baseline albuminuria had a repeat ACR performed; 10 (38%) had resolution and 16 (62%) had persistent albuminuria. Children with resolved albuminuria at one year received a higher dose of hydroxyurea (26.7±3.8 vs. 21.1±3.9mg/kg/day, p =0.003), had higher HbF (24.2 vs. 15.8%, p=0.036) and lower ARC (0.10 vs. 0.13 ×106/mm3, p=0.037), as well as a non-significantly higher Hb (10.3 vs. 8.8 g/dL, p= 0.054). Also, participants with resolved albuminuria at one year had lower ACRs at initiation of hydroxyurea (120 mg/g vs. 255 mg/g, p<0.03). In total, among the 47 children with or without albuminuria at baseline who had repeated ACR at up to one year, fewer children had albuminuria (18/47=38%) following one year of therapy compared to that at baseline (26/47=55%, p=0.02, McNemar’s test).
After adjusting for albuminuria at baseline, children who initiated therapy ≥10 years of age had a higher risk for albuminuria post initiation of hydroxyurea (HR: 2.3; 95%CI: 1.2 – 4.6, p=0.02). When controlling for baseline age, children with baseline albuminuria had a higher risk of albuminuria following hydroxyurea initiation (HR: 2.3; 95%CI: 1.1–4.8, p=0.02). In addition, when controlling for baseline age, children with a higher baseline ACR (ACR ≥100 mg/g versus <100 mg/g) had a higher risk of albuminuria post initiation of hydroxyurea (HR: 2.7; 95%CI: 1.4–5.3, p=0.005. Figure 1b). Combining age and higher baseline albuminuria (Figure 1c), participants who initiated therapy ≥10 years of age with a baseline ACR ≥100mg/g were more likely to have albuminuria post initiation of hydroxyurea as compared to patients who either initiated therapy <10 years of age with a baseline ACR of <100mg/g (HR: 0.22; 95% CI: 0.09–0.52, p=0.0006), or initiated therapy <10 years of age with a baseline ACR of ≥100mg/g (HR: 0.26; 95% CI: 0.10–0.68, p=0.006), or initiated therapy ≥10 years of age with a baseline ACR of <100mg/g (HR: 0.21; 95% CI: 0.08–0.54, p=0.001). In a time to recurrent event analyses, patients who initiated therapy ≥10 years of age had higher risk to develop albuminuria (HR: 2.2; 95%CI: 1.1 – 4.6, p=0.03) compared to those who initiated therapy < 10 years of age, when adjusted for baseline albuminuria (HR: 1.3; 95%CI: 0.7 – 2.6, p=0.41).
This study demonstrates several novel findings to improve patient care. First, hydroxyurea therapy escalated to MTD can prevent the onset of albuminuria, especially when initiated earlier in life. Second, one-third of children with albuminuria had resolution after initiation of hydroxyurea, especially when initiated before 10 years of age and at a lower level of baseline albuminuria. This finding supports earlier screening for albuminuria than the current NIH evidence-based guidelines recommendation, which states screening should begin at 10 years of age. Third, this study identified a novel high-risk patient population for persistent albuminuria, namely patients with ACR ≥100 mg/g and ≥10 years of age. Over 70% of patients with both risk factors had persistent albuminuria after initiation of hydroxyurea, suggesting the need for additional renoprotective agents. Potential interventions in these high-risk patients, based on moderate adult SCA evidence, are angiotensin converting enzyme inhibitors or angiotensin receptor blockers.[3] Novel agents with promising murine SCA data include statin therapy and endothelial receptor antagonists, but these should not be initiated outside of a clinical trial.
Some limitations of this study are worth noting. First, the SCCRIP and HUSTLE protocols vary in timing of ACR measurements as well as requirements for adherence to clinic visits. Second, the high acceptance rate of hydroxyurea precluded an adequate sample size for the control group. Third, the HUSTLE protocol only enrolled participants at the time of hydroxyurea initiation and therefore, we could not confirm the persistence of albuminuria prior to drug initiation. Lastly, we did not prospectively monitor adherence with hydroxyurea; however, the average medication possession ratio in the study was >93%.
In summary, these data support early initiation of hydroxyurea with escalation to MTD to reduce the incidence of albuminuria in children and treat patients that have developed albuminuria. Children older than 10 years of age who have ACRs of ≥100mg/g may be refractory to hydroxyurea alone and require adjunctive renoprotective medications. These data support a change in clinical practice to (1) perform annual urine ACR measurements prior to 10 years of age, (2) escalate hydroxyurea to MTD in young SCA children prior to the onset of albuminuria, and (3) investigate additional renoprotective therapy in SCA patients that do not respond to hydroxyurea alone. The SCCRIP study will prospectively assess the natural history of albuminuria and test novel strategies to slow progression to chronic kidney disease.
Acknowledgements
Analysis of this project was supported by the National Heart, Lung, and Blood Institute through grant R34 HL-127162 and by the American Society of Hematology Scholar Award (JHE). The SCCRIP study is supported by ALSAC. The authors would like to thank all staff at St. Jude Children’s Research Hospital for their support throughout the course of the HUSTLE and SCCRIP studies, especially Vanessa Howard, FNP; Nicole Dockery, PNP; Melissa Lee, PA; Tiana Thomas, CRAII; Madelene Wilson, CRA-RNII; Gail Fortner, CRA-RNII; Ashley Coley, Ivanka Rankovic, Jola Dowdy, Sr. Coordinator; Courtney Mays, CRAIII; Jason Hodges, PhD; Martha Villavicencio, PhD; and Teresa Carr. We acknowledge Terri Davis for her assistance with text formatting. Finally, we especially recognize the eager participation and time commitments made by the children and families who enrolled in these studies.
ClinicalTrials.gov identifier: NCT00305175, NCT02098863
JHE receives funding support from Pfizer and Eli Lilly and Co and serves as a consultant for Daiichi Sankyo and Global Blood Therapeutics. WCW and JSH receive funding support from Global Blood Therapeutics. JSH is a consultant for bluebird bio and NCQA. JDL receives funding for the NHLBI and Pfizer to support his research and serves as a consultant to Novartis.
Footnotes
Conflict of Interest Disclosures
The other authors declare no competing financial interests.
References
- 1.McPherson Yee M, Jabbar S, Osunkwo I, et al. Chronic Kidney Disease and Albuminuria in Children with Sickle Cell Disease. Clin J Am Soc Nephrol 2011. [DOI] [PMC free article] [PubMed]
- 2.Lebensburger J, Johnson SM, Askenazi DJ, et al. Protective role of hemoglobin and fetal hemoglobin in early kidney disease for children with sickle cell anemia. Am J Hematol 2011;86:430–432. [DOI] [PubMed] [Google Scholar]
- 3.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–1048. [DOI] [PubMed] [Google Scholar]
- 4.Aygun B, Mortier NA, Smeltzer MP, et al. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. American journal of hematology 2013;88:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankins JS, Estepp JH, Hodges JR, et al. Sickle Cell Clinical Research and Intervention Program (SCCRIP): A lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer 2018:e27228. [DOI] [PubMed]


