Abstract
To improve the outcome of relapsed/refractory acute myeloid leukemia (AML), a randomized phase II trial of three novel regimens was conducted. Ninety patients were enrolled and were in first relapse or were refractory to induction/re-induction chemotherapy. They were randomized to the following regimens: carboplatin-topotecan (CT), each by continuous infusion for five days; alvocidib (formerly flavopiridol), cytarabine, and mitoxantrone (FLAM) in a timed sequential regimen; or sirolimus combined with mitoxantrone, etoposide, and cytarabine (S-MEC). The primary objective was attainment of a complete remission (CR). A Simon two-stage design was used for each of the three arms. The median age of the patients in the FLAM arm was older at 62 years compared to 55 years for the CT arm and the S-MEC arm. The overall response was 14% in the CT arm (5/35, 90% CI 7%−35%), 28% in the FLAM arm (10/36, 90% CI, 16%−43%), and 16% in the S-MEC arm (3/19, 90% CI, 4%−36%). There were nine treatment-related deaths, seven of which occurred in the FLAM arm with four of these in elderly patients. We conclude that the FLAM regimen had an encouraging response rate and should be considered for further clinical development but should be used with caution in elderly patients.
Keywords: acute myeloid leukemia, relapse, alvocidib, sirolimus
1. Introduction
Over the past 30 years, advances in treatment have enabled ≥50% of adult patients with acute myeloid leukemia (AML) to achieve a complete remission (CR). However, the median duration of CR is less than two years and is influenced heavily by risk factors, including age, cytogenetics, and molecular abnormalities.1 For patients with AML refractory to induction therapy or in relapse, outcomes are poor, with only approximately 10% of patients surviving long-term. While subsequent chemotherapy can induce remission in some of these patients, the only curative therapy is hematopoietic cell transplantation (HCT).2 There is an urgent need for more effective regimens to induce remissions in patients with refractory or relapsed disease.
The Leukemia Committee of the ECOG-ACRIN Cancer Research Group studied three different regimens piloted at ECOG-ACRIN institutions in a randomized phase II trial described here. The first regimen consisted of carboplatin and topotecan, two agents that had exhibited cytotoxic synergy when administered simultaneously to multiple cell lines in vitro or to tumor-bearing mice in vivo. Although the mechanism of synergy is not completely understood, studies have suggested that platination of DNA could enhance trapping of topoisomerase I-DNA covalent complexes and contribute to enhanced toxicity of the combination. A CR rate of 33% was reported at the highest three dose levels in a phase I trial when administered simultaneously by continuous infusion for five days.3
The second regimen incorporated alvocidib (formerly flavopiridol), a synthetic flavone that inhibits multiple cyclin-dependent kinases (CDKs).4 Studies of alvocidib alone and in combination with other agents have shown that alvocidib kills a variety of neoplastic cells and, when withdrawn, allows surviving cells to re-enter the cell cycle and become sensitive to S-phase poisons such as cytarabine.5 This observation led to serial studies combining alvocidib with cytarabine and mitoxantrone (FLAM) in a timed sequential fashion. A phase I study demonstrated the tolerability and the efficacy of this regimen with a response rate in AML patients of 31%.6 A subsequent phase II study demonstrated a CR rate of 75% (12/15) in patients with newly diagnosed secondary AML and also 75% (18/24) of patients with a first relapse.7
The third regimen combined sirolimus, an inhibitor of the mechanistic target of rapamycin (m-TOR), with a regimen of mitoxantrone, etoposide, and cytarabine (S-MEC). Previous studies have shown that combining an m-TOR inhibitor with etoposide led to a dramatic enhancement of cytotoxicity in AML ex vivo and prevented engraftment of primary AML samples in immunodeficient mice suggesting that inhibition of mTOR enhances response to chemotherapy and that survival of AML cells is disrupted.8 A phase I study of S-MEC regimen produced a 22% response rate.9
2. Patients, Materials, and Methods
2.1. Eligibility
Eligible patients had relapsed/refractory AML with ≥10% bone marrow blasts within two weeks prior to induction randomization. Patients with acute promyelocytic leukemia were excluded. Patients were stratified into those who had: a) relapse less than 6 months after first CR documentation, b) relapse between 6 and 12 months after first CR, or c) disease refractory to conventional induction chemotherapy (two courses or less of initial induction or one course of first reinduction). Patients who had received more than two prior regimens or had a prior HCT were not eligible. Patients had to be between the ages of 18 and 70 years of age.
2.2. Treatment
Patients were randomized to one of the following regimens (Supplemental Table 1). Carboplatin 150 mg/m2 per day intravenously by continuous infusion over 24 hours on days 1 to 5 combined with topotecan 1.6 mg/m2 per day over 24 hours by continuous infusion for five days given simultaneously (CT). The FLAM regimen consisted of alvocidib 90 mg/m2 per day given daily on days 1–3 as 30 mg/m2 infused intravenously over one-half hour (bolus) followed by 60 mg/m2 infused intravenously over four hours as well as cytarabine 667 mg/m2 intravenously over 24 hours daily for 72 hours (2 gm/m2 total) on days 6 to 8 and then mitoxantrone 40 mg/m2 infused intravenously over 60 to 120 minutes on day 9. The third arm consisted of sirolimus given orally in a loading dose of 12 mg on day 1, followed by 4 mg daily days 2 to 9. Starting 12 hours after the fourth dose of sirolimus, patients began mitoxantrone, 8 mg/m2 intravenously over 15 minutes daily for five days, combined with etoposide, 100 mg/m2 intravenously over one hour daily for five days and cytarabine 1000 mg/m2 intravenously over three hours daily for five days (S-MEC).
2.3. Study Design
The primary endpoint was achievement of a CR or a CR with incomplete blood count recovery (CRi). The rate of CR with cytogenetic remission (CRc) among those achieving CR or CRi was to be estimated. A total of 111 patients (99 eligible) were to be entered over 28 months and randomized equally to the three arms. A treatment regimen with complete remission (CR/CRi) rate of 35% or better was to be considered promising. The null hypothesis of ineffective treatment corresponded to a CR/CRi rate of 15%. A Simon two-stage design10 was used for each of the three arms. Eighteen patients (16 eligible) per arm were to be entered in the first stage. The arm(s) with two or less patients achieving CR or CRi would be stopped to accrual. The arm(s) with three or more patients achieving CR or CRi would accrue 19 additional patients (17 eligible) per arm. The arms with 8 or more CR or CRi patients among the 33 eligible patients were to be considered promising. This design had 91% power and 10% type I error rate. All patients gave written informed consent, and the protocol was approved by each participating institution’s Institutional Review Board. The study was registered at ClinicalTrials.gov as NCT00634244.
Further details on eligibility, treatment, supportive care, study design and statistical methods can be found in Supplemental Materials.
3. Results
3.1. Patient Characteristics
Between October 2008 and August 2013, a total of 92 patients were accrued to this trial. The trial was suspended for approximately one year from February 2011 to 2012 to evaluate response after meeting first stage accrual. Two patients were found to be ineligible for the trial. Therefore, this report summarizes the results of the 90 eligible patients. Thirty-five patients were accrued to the CT regimen, 36 to (the FLAM regimen), and 19 to (the S-MEC regimen) (Table 1). The median age of patients in the FLAM arm was somewhat older at 62 compared to 55 years on the CT arm and S-MEC arm. There were 54 males and 37 females. The disease status of the patients was well distributed between the three arms, with approximately 50% of patients having refractory AML, 26–33% having relapse less than six months after achievement of first CR, and 17–23% having relapse occurring 6 to 12 months after achievement of first CR.
Table 1:
Patient Characteristics at Study Entry
| Variable | Category | CT | FLAM | S-MEC | Total |
|---|---|---|---|---|---|
| Total | 35 | 36 | 19 | 90 | |
| Age | Median (Q1,Q3) | 55 (48,62) | 62 (52,67) | 55 (42,63) | 57 (48,64) |
| Gender | Male | 21(60%) | 22(61%) | 11(58%) | 54(60%) |
| Female | 14(40%) | 14(39%) | 8(42%) | 36(40%) | |
| Race | White | 33(94%) | 30(86%) | 13(72%) | 76(86%) |
| Black | 1(3%) | 4(11%) | 2(11) | 7(8%) | |
| Asian | 1(3%) | 1(3%) | 2(11%) | 4(5%) | |
| Multi-race | 0(0.0) | 0(0.0) | 1(6%) | 1(1%) | |
| Unknown/Missing | 0 | 1 | 1 | 2 | |
| Disease Status | < 6 mos after 1st CR |
9(26%) | 11(31%) | 6(33%) | 26(29%) |
| 6–12 mos after 1st CR |
8(23%) | 8(22%) | 3(17%) | 19(21%) | |
| Refractory | 18(51%) | 17(47%) | 9(50%) | 44(49%) | |
| Unknown/Missing | 0 | 0 | 1 | 1 | |
| ECOG Performance | 0 | 10(29%) | 11(31%) | 4(22%) | 25(28%) |
| Status | 1 | 19(54%) | 21(58%) | 12(67%) | 52(58%) |
| 2 | 6(17%) | 4(11%) | 2(11%) | 12(13%) | |
| Unknown/Missing | 0 | 0 | 1 | 1 | |
| Leukemia | FAB M1 | 3(9%) | 6(17%) | 3(17%) | 12(13%) |
| Classification | FAB M2 | 8(23%) | 2(6%) | 2(11%) | 12(13%) |
| FAB M4 | 2(6%) | 4(11%) | 3(17%) | 9(10%) | |
| FAB M5 | 4(11%) | 3(8%) | 0 | 7(8%) | |
| FAB M6 | 1(3%) | 2(6%) | 0 | 3(3%) | |
| FAB M0 | 2(6%) | 0 | 0 | 2(2%) | |
| Acute myeloid leukemia with multilineage dysplasia |
0 | 3(8%) | 2(11%) | 5(6%) | |
| Therapy-related acute myeloid leukemia, NOS |
1(3%) | 2(6%) | 0 | 3(3%) | |
| Acute myeloid leukemia, NOS |
14(40%) | 14(39%) | 8(44%) | 36(40%) | |
| Unknown/Missing | 0 | 0 | 1 | 1 | |
| Marrow Blast (%) | Median (Q1,Q3) | 58.0 (21.0,80.0) | 48.0 (21.0,66.5) | 40.0 (29.0,79.0) | 49.0 (22.0,78.5) |
| [Min, Max] | [10.0,95.0] | [6.0,97.0] | [15.0,95.0] | [6.0,97.0] | |
| Unknown/Missing | 1 | 1 | 2 | 4 | |
| WBC (× 103/mm3) | Median (Q1,Q3) | 5 (2,8) | 2 (1,5) | 3 (2,4) | 3 (2,6) |
| [Min, Max] | [0,119] | [0,134] | [0,73] | [0,134] | |
| Granulocytes | Median (Q1,Q3) | 1371 (312,2642) | 684 (210,2140) | 728 (265,1375) | 850 (210,2370) |
| (AGC/ANC) | [Min, Max] | [0,35070] | [0,17750] | [0,4900] | [0,35070] |
| Unknown/Missing | 3 | 2 | 0 | 5 | |
| Platelet (× 103/mm3) | Median (Q1,Q3) | 42 (22,79) | 32 (18,65) | 65 (26,175) | 42 (21,85) |
| [Min, Max] | [11,206] | [6,252] | [8,310] | [6,310] | |
| Hemoglobin (g/dl) | Median (Q1,Q3) | 10 (8,11) | 10 (9,10) | 10 (9,11) | 10 (9,11) |
| [Min, Max] | [7,15] | [8,15] | [8,13] | [7,15] | |
The subtypes of AML and hematological parameters are summarized in Table 2. The median number of marrow blasts was lower in the S-MEC arm at 40% compared to 48% in the FLAM arm and 58% in the CT arm. Median leukocyte counts were low at 5 × 103/mm3 or less in all arms, and platelets ranged from 32 × 109/L in the FLAM arm to 42 × 109/L in the CT arm and 65 × 109/L in the S-MEC arm.
Table 2:
Best Overall Response (eligible patients)
| CT | FLAM | S-MEC | |
|---|---|---|---|
| Complete Response (CR) | 2@ | 6* | 2^ |
| CRi | 3@ | 4* | 1^ |
| No change/Stable | 30 | 25 | 15 |
| Unevaluable | 0 | 1 | 1 |
| Total | 35 | 36 | 19 |
No evaluable patients in the CT arm with CR/CRi achieved a CRc.
2/6 evaluable patients in the FLAM arm with CR/CRi achieved a CRc.
1/3 evaluable patients in the S-MEC arm with CR/CRi achieved a CRc.
Of the 90 eligible cases enrolled to the trial, cytogenetic results were available in 77 patients at some point in the course of the study including 52 patients at the time of initial diagnosis, 70 patients at the time of study enrollment, 39 patients at the time of consolidation, and 4 patients at the time of relapse (Supplemental Table 2).
Forty patients had cytogenetic data at both diagnosis and enrollment. Clonal evolution was observed in 22 patients, including 9 patients with a complex karyotype at the time of diagnosis. In the remaining 18 patients, no clonal evolution was observed, including 10 patients with a normal karyotype at diagnosis, and patients with single chromosome abnormalities, such as del(5q), +8, del(11q) and +21.
Thirty-nine patients had cytogenetic results at pre-consolidation. Thirteen showed a complex karyotype, often evolving from diagnosis or enrollment, and 16 showed a normal karyotype. Four patients had cytogenetic data at the time of relapse and all four showed the same abnormal clones that were observed at the time of diagnosis or enrollment.
3.2. Response to Study Therapy
Among the first 16 eligible patients on each arm of the trial, there were 4, 4, and 2 patients achieving a CR+CRi on the CT, FLAM, and S-MEC arms, respectively. According to the two-stage design, the CT and FLAM arms met the pre-specified criterion of three or more responses in the first 16 patients to continue to the second stage of the study. The S-MEC arm did not meet this criterion and, therefore, closed with 19 eligible patients accrued.
The overall response for all eligible patients included two CR and three CRi in the CT arm, six CR and four CRi in the FLAM arm, and two CR and one CRi in the S-MEC arm (Table 2). All 10 of the CR/CRi patients in the FLAM arm were among the first 33 eligible patients enrolled on this arm, and this met the protocol prespecified criterion for a promising response rate (Table 2). The overall rates of achieving CR or CRi were 14% (90% CI 7%−35%) in the CT arm, 28% (90% CI 16%−43%) in the FLAM arm, and 16% (90% CI 4%−36%) in the S-MEC arm. In the FLAM arm, the response rate was higher in patients 60 or younger (40%, 90% CI, 19%−64%, n=15) compared to that of patients older than 60 of age (19%, 90% CI, 7%−38%, n=21), although the confidence intervals overlapped.
Among the 10 responders in the FLAM arm, 2 of 6 evaluable patients achieved a complete cytogenetic response, 1 did not, and 3 had normal cytogenetics at enrollment. In the other 4 patients on this arm, the cytogenetic response was unknown. For the CT arm, three of the five responders had normal cytogenetics at entry; one did not achieve a complete cytogenetic remission, and one was unknown. Of the three responders in the S-MEC arm, one achieved a complete cytogenetic response, one had normal cytogenetics at study entry, and one was unknown.
Patients were allowed to receive up to two additional courses of the same treatment that was given in induction but, in fact, few did for unclear reasons. In the CT arm, one patient received one course of consolidation, and two patients received two courses of consolidation. In the FLAM arm, two patients received one course of consolidation, and none received two; and in the S-MEC arm, only one patient received one course of consolidation.
Patients were also allowed to proceed to allogeneic HCT at the patient’s and/or investigator’s discretion. This, again, occurred in only a minority of patients and included five patients in the CT arm, two patients in the FLAM arm, and four patients in the S-MEC arm. Of these eleven patients, one was in CR, three in CRi, and 7 had stable disease. The reasons why so few patients proceeded to allogeneic HCT is not entirely clear as centers were only required to report that patients who went off protocol therapy were given alternate therapy.
A multivariable analysis of clinical variables that may be associated with response using logistic regression was performed. The analysis includes 89 of the 90 eligible patients with complete data. One patient in the S-MEC arm was missing ECOG performance status and was not included in this analysis. Variables assessed included: age, gender, ECOG performance status, disease status at study entry, and treatment arm. The association of these variables with response was not found to be statistically significant (data not shown).
3.3. Survival
Overall survival is shown in Figure 2. Median follow-up for the 11 patients still alive is 37.1 months (range 23.8 to 49.0 months). The median OS was 5.9 months (95% CI 4.3 to 12.2 months) for the CT arm, 4.4 months (95% CI 2.5 to 7.1 months) for the FLAM arm, and 8.3 months (95% CI 5.1 months to not reached) for the S-MEC arm.
Figure 2.
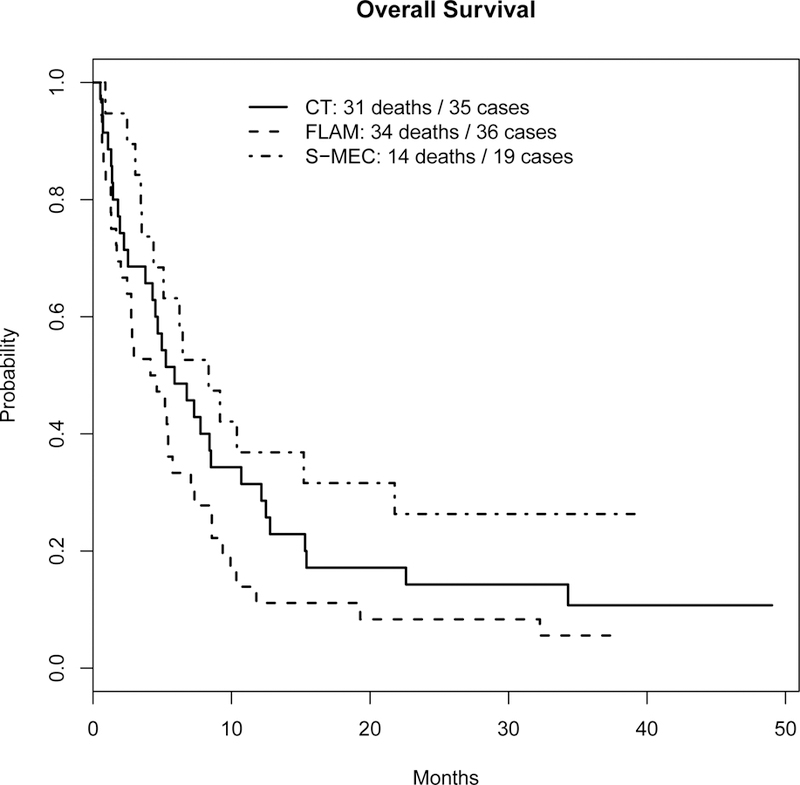
Overall Survival
Disease-free survival (DFS) for patients achieving CR or CRi is shown in Supplemental Figure 1a, and is defined to be time from CR/CRi to relapse or death without relapse. Those alive without relapse were censored at the date of last contact. The median DFS was 9.7 months (95% CI 3.6 months to not reached) for the CT arm, 4.6 months (95% CI 2.7 months to no reached) for the FLAM arm, and not reached for the S-MEC arm (95% CI 1.9 months to not reached). Survival for patients achieving CR or CRi is shown in Supplemental Figure 1b, and is defined to be time from CR/CRi to death. Those alive were censored at the date of last contact. The median survival for CR/CRi patients was 14.0 months (95% CI 7.8 months to not reached) for the CT arm, 7.3 months (95% CI 5.5 months to not reached) for the FLAM arm, and not reached (95% CI 1.9 months to not reached) for the S-MEC arm. In the FLAM arm, seven of the eight deaths were from AML and in the eighth patient was unknown.
3.4. Toxicity
Among the initial 27 patients accrued to the FLAM arm, there were 6 treatment-related deaths. In 5 of these 6 patients, death from septic shock or multi-organ failure occurred within one month of registration, and 4 of these 6 patients were over age 65. Therefore, in November, 2012, the upper age limit for the trial was lowered from 70 years of age to 65 years. One subsequent death was attributed to treatment after this amendment.
Grade 3 or higher adverse events were experienced in 72 patients. The most common hematologic events were cytopenias of one to three cell lines. Non-hematologic events included infections, gastrointestinal toxicities, and metabolic abnormalities. There were only three cases of tumor lysis syndrome, all in the FLAM arm. Despite concerns, electrolyte abnormalities were uncommon and primarily occurred in the FLAM arm consisting of grade 3 hypokalemia and grade 3 hypophosphatemia in 9 and 8 of the 36 accrued patients, respectively. Of ten cases of diarrhea, eight were in the FLAM arm and all were grade 3. Two cases of cytokine release syndrome occurred, one in the FLAM arm and one in the S-MEC arm and both were grade 3. In regard to the FLAM arm, grade 4 neutropenia and thrombocytopenia and grade 3 anemia were seen in almost all patients, but other non-hematologic toxicities were uncommon and no unexpected or unusual toxicities occurred. See Supplemental Table 3 for a complete list of toxicities and grades.
4. Discussion
This randomized phase II trial of three novel regimens developed at ECOG-ACRIN institutions showed that a regimen of alvocidib cytarabine, and mitoxantrone demonstrated an encouraging CR rate in high-risk AML patients. Alvocidib is a potent inhibitor of multiple CDKs, including CDK2 and CDK44,11–13 as well as CDK9, which forms a complex with cyclin T that is responsible for activating phosphorylation of RNA polymerase II in the C-terminal domains of this enzyme.14,15 Previous studies examining the effect of combining alvocidib with a variety of antineoplastic agents established the schedule dependency of its effects, with simultaneous administration antagonizing the effects of S-phase-dependent agents such as cytarabine because of cell cycle arrest. On the other hand, sequential combinations led to enhanced killing because cells that survive initial alvocidib treatment re-enter the cell cycle and become sensitive to S-phase poisons.5,16
Based on these in vitro observations, a phase I trial of alvocidib for three days, followed by a continuous intravenous infusion of cytarabine over 72 hours, starting on day 6 and mitoxantrone on day 9 was conducted.6 Doses of alvocidib administered were 40, 50, and 60 mg/m2. At the dose level of 60 mg/m2, two of the five patients treated had neutropenia lasting more than 40 days and an additional patient died of an acute non-ischemic cardiomyopathy. The CR rate was 23%, and partial remission (PR) rate was 8%. Pharmacokinetic analysis demonstrated a linear 2 compartment model with first order elimination of alvocidib. Multiple alvocidib-target proteins, including BCL-2, MCL-1, and phospho-RNA polymerase II, were shown to be downregulated.6
A phase II study of the FLAM regimen with alvocidib dosed at 50 mg/m2 per day in combination with the same doses of cytarabine and mitoxantrone in the phase I trial has been reported. A 50% decrease in peripheral blood blasts was seen in 44% of patients by median day 2 and ≥80% decrease in 26% by day 3. Self-limited tumor lysis was found in 53% of patients. Three patients died during therapy, but CR was achieved in 12 of 15 (75%) patients with newly diagnosed secondary AML, 18 of 24 (75%) with AML in first relapse after a short CR, but in only 2 of 13 (15%) of with primary refractory AML, and 0 of 10 with multiply refractory AML. The DFS for all CR patients was 40% at two years, and newly diagnosed patients had a two-year DFS of 50%.7
Based on studies in chronic lymphocytic leukemia demonstrating that a pharmacologically modeled “hybrid” schedule of alvocidib administration of a 30-minute bolus followed by a four-hour infusion is particularly efficacious in CLL,17 a phase I and pharmacokinetic study of bolus-infusion alvocidib followed by cytarabine and mitoxantrone in acute leukemia was conducted. Because of dose-limiting toxicity, which included tumor lysis syndrome, hyperbilirubinemia and mucositis, the recommended hybrid schedule was 30 mg/m2 followed by an infusion of 60 mg/m2 daily for three days, which is the regimen that was incorporated in the study reported here.18 In a more recent report, this regimen, at a slightly reduced dose of alvocidib, 30 mg/m2 over 30 minutes followed by 40 mg/m2 over four hours daily for three days, was compared to a bolus alvocidib schedule of 50 mg/m2 per day for three days in combination with cytarabine and mitoxantrone in a randomized phase II study. Response rates and DFS were similar between the two arms, and it was felt that, given the greater ease of the bolus administration schedule, this would be the preferred regimen.19 At the time the present study was designed, however, the data on the hybrid regimen of 30 mg/m2 over 30 minutes followed by 60 mg/m2 daily for three days was the preferred regimen and was the dose and schedule used here. While there was more toxicity in elderly patients with the FLAM regimen in the study reported here and therefore, more deaths, we identified this regimen as producing a higher CR rate than the others reported and therefore, worthy of further study.
Recently, a randomized multi-center phase II trial of the FLAM regimen with the bolus alvocidib dose of 50 mg/m2 per day for three days vs. cytarabine and daunorubicin in a 7+3 schedule was reported in newly diagnosed AML patients between the ages of 18 and 70. Patients with core-binding factor AML or acute promyelocytic leukemia were excluded. Patients were randomized in a 2:1 fashion to FLAM or 7+3. The CR rate with FLAM was 70% vs. 46% for 7+3 (p=0.003). No significant differences were found in overall or event-free survival, but post-induction treatment strategies were not standardized between the two arms.20
Despite encouraging results in a phase I trial,3 the combination of infusional topotecan and carboplatin did not produce a high response rate in this study. Combining sirolimus with the MEC regimen also achieved a disappointingly low response rate of 16%. These results with the CT and S-MEC regimens, while disappointing, are not dissimilar to what has been seen in other trials from our group in relapsed and refractory AML.21
Recent studies highlight the challenges of treating patients with relapsed/refractory AML. A recent international study randomized patients to a novel elaidic acid ester of cytarabine (elacytarabine) vs. investigators’ choice of one of seven commonly used salvage regimens for AML. No significant differences were seen in OS (3.5 vs. 3.3 months), response rate (23% vs. 21%), or relapse-free survival (5.1 vs. 3.7 months) between the elacytarabine and the control arms.22
Similarly, a recent phase III trial randomized 711 patients with relapsed and refractory AML to vosaroxin, a first-in-class anti-cancer quinolone derivative plus cytarabine vs. placebo plus cytarabine. The CR rate was 30% for vosaroxin plus cytarabine vs. 16% for patients receiving placebo plus cytarabine. Median OS was 7.5 months with vosaroxin plus cytarabine vs. 6.1 months for placebo plus cytarabine (p=0.06).23 The results obtained with vosaroxin + cytarabine regimen were similar to the results achieved with the FLAM regimen in the study presented here, although the mix of patients in the vosaroxin study was different with an older median age of 63, a lower percentage of refractory patients and including 22% of patients who had relapsed 12 months or more after achieving CR suggesting a more favorable risk group overall.
Even though the response rate with the FLAM regimen in the study presented here was encouraging, this regimen is not without toxicity. Of the nine treatment-related deaths, seven occurred in the FLAM arm, and four of these occurred in patients over the age of 65, which led to an amendment of the protocol during the course of the study to exclude patients over the age of 65. Non-lethal toxicities among patients in the three arms, however, were not significantly different.
Because of these toxicities, as well as somewhat limited efficacy, results with the FLAM regimen in this study remain far from ideal. The ability to define who can reap benefit from FLAM without incurring overwhelming toxicity is dependent both on clinical observations and molecular biomarkers.24,25 An international randomized trial of FLAM vs. cytarabine plus mitoxantrone (AM) without alvocidib (NCT02520011) is currently recruiting patients.
Much still needs to be done to improve outcomes in patients with relapsed and refractory acute myeloid leukemia. It is hoped that new agents targeting mutated genes in AML will bring further improvement in outcomes. Some of these agents have been recently reported and are in further development.26–28
4.1. Conclusions
We conclude that the FLAM regimen has an encouraging response rate, but poor overall survival because of increased toxicity, particularly in elderly patients. It should be considered for further clinical development with a focus on use in younger patients.
Supplementary Material
Figure 1.
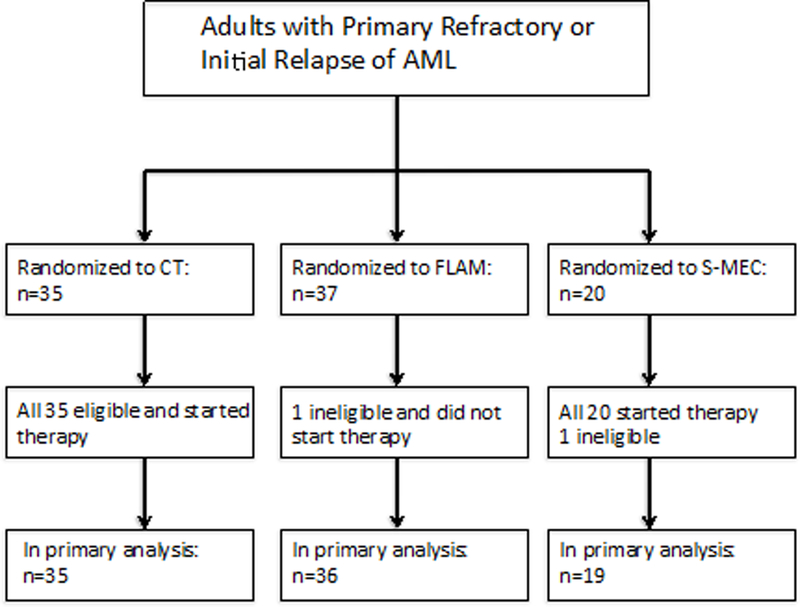
Consort diagram
Acknowledgements
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180790, CA180802, CA180853, CA189859, CA180795, CA180791, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The authors sincerely thank Ms. Denise P. Chase for her assistance with transcribing and editing the manuscript.
This manuscript is dedicated to the beloved memory of Robert L. Comis, MD, ECOG-ACRIN Cancer Research Group Co-Chair, who died unexpectedly May 10, 2017.
Footnotes
Conflict-of-Interest Statements
MRL, XVW, MPC, RPK, YZ, SHK, HML, SML, EMP, KWP, HWT, JKA, ERB, WBR, JMR and MST declare no conflicts of interest. JEK has served as a consult to Tolero Pharmaceuticals.
References
- 1.Estey EH. Acute myeloid leukemia: 2014 update on risk-stratification and management. Am J Hematol 2014;89(11):1063–1081. Prepublished on 2014/10/17 as DOI 10.1002/ajh.23834. PMID25318680. [DOI] [PubMed] [Google Scholar]
- 2.Ofran Y, Rowe JM. Treatment for relapsed acute myeloid leukemia: what is new? Curr Opin Hematol 2012;19(2):89–94. Prepublished on 2012/01/10 as DOI 10.1097/MOH.0b013e32834ff4e1. PMID22227525. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH, Karp JE, Letendre L, et al. Phase I and pharmacologic study of infusional topotecan and Carboplatin in relapsed and refractory acute leukemia. Clin Cancer Res 2005;11(18):6641–6649. Prepublished on 2005/09/17 as DOI 10.1158/1078-0432.CCR-05-0817. PMID16166443. [DOI] [PubMed] [Google Scholar]
- 4.Aleem E, Arceci RJ. Targeting cell cycle regulators in hematologic malignancies. Front Cell Dev Biol 2015;3:16 Prepublished on 2015/04/29 as DOI 10.3389/fcell.2015.00016. PMID25914884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86–8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res 1997;57(16):3375–3380. Prepublished on 1997/08/15 as DOI PMID9269999. [PubMed] [Google Scholar]
- 6.Karp JE, Passaniti A, Gojo I, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res 2005;11(23):8403–8412. Prepublished on 2005/12/03 as DOI 10.1158/1078-0432.CCR-05-1201. PMID16322302. [DOI] [PubMed] [Google Scholar]
- 7.Karp JE, Smith BD, Levis MJ, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res 2007;13(15 Pt 1):4467–4473. Prepublished on 2007/08/03 as DOI 10.1158/1078-0432.CCR-07-0381. PMID17671131. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood 2005;106(13):4261–4268. Prepublished on 2005/09/10 as DOI 10.1182/blood-2004-11-4468. PMID16150937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perl AE, Kasner MT, Tsai DE, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res 2009;15(21):6732–6739. Prepublished on 2009/10/22 as DOI 10.1158/1078-0432.CCR-09-0842. PMID19843663. [DOI] [PubMed] [Google Scholar]
- 10.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10(1):1–10. Prepublished on 1989/03/01 as DOI PMID2702835. [DOI] [PubMed] [Google Scholar]
- 11.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res 1996;56(13):2973–2978. Prepublished on 1996/07/01 as DOI PMID8674031. [PubMed] [Google Scholar]
- 12.Worland PJ, Kaur G, Stetler-Stevenson M, Sebers S, Sartor O, Sausville EA. Alteration of the phosphorylation state of p34cdc2 kinase by the flavone L86–8275 in breast carcinoma cells. Correlation with decreased H1 kinase activity. Biochem Pharmacol 1993;46(10):1831–1840. Prepublished on 1993/11/17 as DOI PMID8250970. [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Rahmani M, Dai Y, et al. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent process. Cancer Res 2003;63(8):1822–1833. Prepublished on 2003/04/19 as DOI PMID12702569. [PubMed] [Google Scholar]
- 14.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem 2001;276(34):31793–31799. Prepublished on 2001/06/30 as DOI 10.1074/jbc.M102306200. PMID11431468. [DOI] [PubMed] [Google Scholar]
- 15.Lam LT, Pickeral OK, Peng AC, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol 2001;2(10):RESEARCH0041. Prepublished on 2001/10/13 as DOI PMID11597333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karp JE, Ross DD, Yang W, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res 2003;9(1):307–315. Prepublished on 2003/01/23 as DOI PMID12538483. [PubMed] [Google Scholar]
- 17.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 2007;109(2):399–404. Prepublished on 2006/09/28 as DOI 10.1182/blood-2006-05-020735. PMID17003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp JE, Smith BD, Resar LS, et al. Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood 2011;117(12):3302–3310. Prepublished on 2011/01/18 as DOI 10.1182/blood-2010-09-310862. PMID21239698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karp JE, Garrett-Mayer E, Estey EH, et al. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica 2012;97(11):1736–1742. Prepublished on 2012/06/27 as DOI 10.3324/haematol.2012.062539. PMID22733022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeidner JF, Foster MC, Blackford AL, et al. Randomized multicenter phase 2 study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica 2015. September;100(9):1172–9. Prepublished on 2015/05/30 as DOI 10.3324/haematol.2015.125849. PMID26022709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litzow MR, Othus M, Cripe LD, et al. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: a report from the Eastern Cooperative Oncology Group. Br J Haematol 2010;148(2):217–225. Prepublished on 2009/10/07 as DOI 10.1111/j.1365-2141.2009.07917.x. PMID19804455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 2014;32(18):1919–1926. Prepublished on 2014/05/21 as DOI 10.1200/JCO.2013.52.8562. PMID24841975. [DOI] [PubMed] [Google Scholar]
- 23.Ravandi F, Ritchie E, Sayar H, et al. Improved Survival in Patients with First Relapsed or Refractory Acute Myeloid Leukemia (AML) Treated with Vosaroxin Plus Cytarabine Versus Placebo Plus Cytarabine: Results of a Phase 3 Double-Blind Randomized Controlled Multinational Study (VALOR) (Abstract LBA-6). Blood ASH Abstracts 2014;123(21). [Google Scholar]
- 24.Smith BD, Warner SL, Whatcott C, et al. An alvocidib-containing regimen is highly effective in AML patients through a mechanism dependent on MCL1 expression and function (2015 ASCO Annual Meeting. Abstract Number 7062. Poster Board Number 51). J Clin Oncol 2015;33(suppl):389. [Google Scholar]
- 25.Nelson DM, Joseph B, Hillion J, Segal J, Karp JE, Resar LM. Flavopiridol induces BCL-2 expression and represses oncogenic transcription factors in leukemic blasts from adults with refractory acute myeloid leukemia. Leuk Lymphoma 2011;52(10):1999–2006. Prepublished on 2011/07/07 as DOI 10.3109/10428194.2011.591012. PMID21728742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017. August 10;130(6):722–731. doi: 10.1182/blood-2017-04-779405. Epub 2017 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein EM, Garcia-Manero G, Rizzieri DA, et al. The DOT1L Inhibitor Pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood 2018. :prepublished blood-2017–12-818948; doi: 10.1182/blood-2017-12-818948 [DOI] [PMC free article] [PubMed]
- 28.Stein EM, Walter RB, Erba HP, et al. A phase 1 trial of vadastuximab talirineas monotherapy in patients with CD33-positive acute myeloid leukemia. Blood 2018. January 25;131(4):387–396. doi: 10.1182/blood-2017-06-789800. Epub 2017 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


