Abstract
Objective:
Decades-old, common ICU practices including deep sedation, immobilization, and limited family access are being challenged. We endeavoured to evaluate the relationship between ABCDEF bundle performance and patient-centered outcomes in critical care.
Design:
Prospective, multicenter, cohort study from a national quality improvement collaborative.
Setting:
68 academic, community, and federal ICUs collected data during a 20-month period.
Patients:
15,226 adults with at least one ICU day.
Interventions:
We defined ABCDEF bundle performance (our main exposure) in two ways: 1) complete performance (patient received every eligible bundle element on any given day) and 2) proportional performance (percentage of eligible bundle elements performed on any given day). We explored the association between complete and proportional ABCDEF bundle performance and three sets of outcomes: patient-related (mortality, ICU hospital discharge), symptom-related (mechanical ventilation, coma, delirium, pain, restraint use), and system-related (ICU readmission, discharge destination). All models were adjusted for a minimum of 18 a priori determined potential confounders.
Measurements and Results:
Complete ABCDEF bundle performance was associated with lower likelihood of seven outcomes: hospital death within 7 days (adjusted hazard ratio, 0.32; CI, 0.17–0.62), next-day mechanical ventilation (adjusted odds ratio [AOR], 0.28; CI, 0.22–0.36), coma (AOR, 0.35; CI, 0.22–0.56), delirium (AOR, 0.60; CI, 0.49–0.72), physical restraint use (AOR, 0.37; CI, 0.30–0.46), ICU readmission (AOR, 0.54; CI, 0.37–0.79), and discharge to a facility other than home (AOR, 0.64; CI, 0.51–0.80). There was a consistent dose-response relationship between higher proportional bundle performance and improvements in each of the above-mentioned clinical outcomes (all p < 0.002). Significant pain was more frequently reported as bundle performance proportionally increased (p = 0.0001).
Conclusions:
ABCDEF bundle performance showed significant and clinically meaningful improvements in outcomes including survival, mechanical ventilation use, coma and delirium, restraint-free care, ICU readmissions, and post-ICU discharge disposition.
INTRODUCTION
Critically ill patients experience a variety of distressing symptoms during their hospital stay including pain, agitation, delirium, weakness, and sleep deprivation (1–3). Because of the complexity of caring for ICU patients, these symptoms are often managed by keeping patients heavily sedated, immobilized, and often socially isolated (4−6). Historically, daily goals of care were organized according to specific organ dysfunction, rather than an integrated holistic approach, and team members worked in siloed care systems. Evidence increasingly suggests that symptoms, and the way the ICU team chooses to manage them, can have important negative prognostic implications (1−3). Given the aging population, rising healthcare costs, and increasing millions of patients who survive critical illness (7−10), society has an evergrowing public health problem related to ineffective symptom management that ultimately contributes to persistent and life-altering impairments in physical, mental, and cognitive health (often referred to as Post Intensive Care Syndrome, PICS) (11−13).
To our knowledge, few studies to date have specifically focused on evaluating integrated, interprofessional approaches to symptom management during critical illness. One such approach is known as the ABCDEF bundle (Assess, prevent, and manage pain; Both spontaneous awakening and breathing trials: Choice of Analgesia and Sedation; Delirium assess, prevent, and manage; Early Mobility and Exercise; Family engagement/empowerment) (14−18). The ABCDEF bundle differs from other evidence-based, multicomponent ICU interventions (19−23) in several ways. First, it is applicable to every ICU patient every day, regardless of mechanical ventilation status or admitting diagnosis. Second, as it focuses on symptom assessment, prevention, and management rather than disease processes, it is particularly relevant early during the course of critical illness and is suitable for use in conjunction with other life-sustaining therapies. The team-based ABCDEF bundle approach is also unique in that its ultimate goal is to produce patients who are more awake, cognitively engaged, and physically active, which ultimately serves to facilitate patient autonomy and the ability to express unmet physical, emotional, and spiritual needs.
While the safety and efficacy of the individual elements of the ABCDEF bundle are supported by dozens of peer-reviewed studies published in high-impact journals (14, 24, 25), only a handful of investigations to date have explored the effect of executing the collective interventions in a consistent and coordinated manner (17, 18, 26, 27). However, limitations of these studies include relatively small sample size, recruitment from a single-center or healthcare system, and retrospectively collected data. Moreover, some used older versions of the bundle that did not specifically focus on pain or the importance of family engagement and empowerment. It is plausible that these factors may partially explain why implementation of this evidence-based intervention on a global level remains suboptimal (28−33). Therefore, there is a clear need for large prospective studies that include a diversity of ICU practice settings and clearly operationalized ABCDEF bundle components, while exploring important yet previously uninvestigated outcomes.
This investigation provides clinical outcome results from the Society of Critical Care Medicine (SCCM) ICU Liberation Collaborative that was funded by the Gordon and Betty Moore Foundation. The collaborative included more than 15,000 patients from 68 community, academic, federal, and private ICUs (34). The quality improvement (QI) results of the ICU Liberation Project (i.e., whether collaborative participation resulted in increased adherence to the bundle and its individual elements over time) will be reported in a separate article. The primary objective of this current analysis was to examine the association between ABCDEF bundle performance and patient-, symptom-, and healthcare system-related outcomes. We hypothesized that complete and dose-related (i.e., proportional) performance of the ABCDEF bundle would be associated with improved clinical outcomes across these three domains.
MATERIALS AND METHODS
Overview
A description of the ICU Liberation Collaborative’s history, requirements for participation, and data collection procedures is provided in Supplemental Digital Content (SDC) Methods 1 and other publications (14, 34). Guided by the Consolidated Framework for Implementation Research (35) and based on published trials (14) and recommendations (2, 3, 36), a panel of 23 interprofessional ICU clinicians with expertise in QI and the bundle domains reached consensus on how the current version of the ABCDEF bundle should be operationally defined and measured in practice (SDC Table 1). The primary focus of the collaborative was to compile knowledge and toolkits for wide-scale dissemination and implementation of the ABCDEF bundle and to measure the relationship of the current expanded version of the bundle, which includes pain assessment and family engagement, with patient outcomes.
The ICU Liberation Collaborative ran from August 2015 to April 2017 and included 68 adult academic, community, and Veterans Administration ICUs from 29 states and Puerto Rico. The Vanderbilt University Medical Center Institutional Review Board (IRB) served as the coordinating center IRB and granted the QI project expedited approval. All participating sites acquired site-specific IRB evaluation and approval.
Data Collection
The collaborative used Research Electronic Data Capture (REDCap), a secure, web-based application for validated data entry, transmission, and storage. Site staff entered de-identified data into the collaborative’s database from their hospitals. A total of 20 months of data were collected per site which included 6 months of retrospectively collected data (January 2015−June 2015) and 14 months of prospectively collected data (January 2016–March 2017). During the retrospective period, staff from each site entered data on the first five consecutively admitted ICU patients each month (30 baseline patients per site). Throughout the prospective period, site staff collected data on the first 15 consecutively admitted patients per month. Data were collected for a maximum of seven ICU days (a limitation based on personnel available to conduct the collaborative) until patients either transferred out of the ICU, were designated non-ICU status, or died, whichever occurred first. Patients from both periods were included because the focus of this analysis was on the relationship between bundle performance and patient outcomes.
Participants
Adult patients, whether on or off mechanical ventilation, who were admitted to a participating medical, surgical, cardiac, or neurologic ICU were eligible to participate. We excluded patients who: 1) died or were discharged from the participating ICU within 24 hours of ICU admission or 2) were undergoing active life support withdrawal and/or “comfort care-only” within 24 hours of ICU admission.
Main Exposure
The primary independent variable for all analyses was ABCDEF bundle performance. There are six ABCDEF bundle elements. Because element B has two components, there are seven components in total. SDC Table 1 provides the operational definitions of performance for each ABCDEF bundle element. We defined “complete performance” as a patient-day in which every eligible element of the bundle was performed (i.e., 100% of the bundle versus anything less). We defined “proportional performance” as the percentage of eligible elements a patient received on a given day (i.e., “bundle dose”). We measured complete and proportional bundle performance only on the days that the patient was in the ICU for a full 24 hours.
Covariates
We adjusted all regression models for a minimum of 18 patient and institutional confounders chosen a priori because they previously helped explain variations in our selected outcomes. These confounders were demographic variables (age, sex, race, ethnicity, body mass index, residence before admission, mobility restriction before admission), admission features (diagnosis, hospital type [community vs. teaching], ICU type), and daily ICU characteristics on the day of bundle exposure (receipt of certain medications, including benzodiazepines, opioids, propofol, dexmedetomidine, typical or atypical antipsychotics; comfort care order; mechanical ventilation; coma). We also adjusted for delirium on the day of bundle exposure when analyzing the association between bundle performance and presence of delirium on the following day. Because the data on delirium exposure had a relatively high rate of missingness, we did not adjust for delirium in any other model; all other covariates had relatively little missing data. For ICU readmission and discharge destination models, we summarized ICU characteristics over the course of the original collaborative admission (e.g., adjusting for proportion of ICU days the patient received each medication, was on mechanical ventilation, experienced coma, or had comfort care orders). Because we did not have adequate severity of illness data to adjust for this covariate in the models, we conducted a “tipping point” sensitivity analysis (SDC Methods 2) (37, 38), which allowed us to quantify the amount of total unmeasured confounding that would be needed to render our analysis inconclusive. Additionally, we performed sensitivity analyses using the 6% (n = 950) of patients who had Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) III scores available. To do this we ran the same models for ABCDEF performance as in our original analysis except that, because of the lower numbers, we did not cluster by site or adjust for hospital type, and collapsed variables of age, race, admission reason, and ICU type (SDC Methods 3).
Outcomes and Statistical Analysis
Because rates of missing covariate data generally were low (SDC Figure 1), we chose to limit our analyses to complete cases and to not perform multiple imputation. We analyzed three sets of clinical outcomes using three types of multivariable regression models. First, we analyzed daily performance of the ABCDEF bundle and patient-related outcomes, including times to ICU discharge, hospital discharge, and death, all within the 7-day data collection period during the original collaborative ICU stay. We used Cox proportional hazards models with time-varying covariates for these outcomes. All patients with at least one 24-hour day in the ICU were included in the models. Next, we analyzed daily performance of the ABCDEF bundle and symptom-related outcomes the following day. The outcomes included significant pain episodes, coma, delirium, physical restraint use, and mechanical ventilation. We defined a significant pain episode as a recorded pain numeric rating scale score > 3, Behavioral Pain Scale (39) score > 5, or Critical Care Pain Observation Tool (40) score ≥ 3. We used logistic regression for these outcomes (where, for example, the outcome was on mechanical ventilation vs. not on mechanical ventilation). Only patients with at least two consecutive 24-hour days in the ICU were included in the models. We summarized ABCDEF bundle performance over the entire original collaborative ICU stay and, using logistic regression, analyzed the association between bundle performance, ICU readmission, and ICU discharge to a destination other than home among survivors (i.e., system-related outcomes). In all models, we used robust sandwich estimation, clustered by study site to adjust variances, accounting for correlation among observations from the same site. We used R Project for Statistical Computing software version 3.4 for all analyses (41).
RESULTS
Of the original 17,228 patients included in the collaborative, 2,002 had no full 24-hour days. Because the definitions used for bundle performance require a full ICU day, those patients were excluded from analysis, leaving 15,226 patients. Of those patients, 10,840 (72%) had two consecutive 24-hour ICU days and were thus eligible to be included in our symptom-related outcome models (33,689 patient-days); 12,756 (84%) survived hospitalization and thus were eligible for ICU readmission and discharge destination models.
The demographic, in-hospital, and discharge characteristics of the 15,226 patients eligible for any of the models are in Table 1. Most patients were 60 years of age or older (61%), male (58%), white (72%), and admitted to academic hospitals (63%). Admission diagnoses varied. The median length of time for the 54% that received invasive mechanical ventilation was 60 hours (interquartile range [IQR], 24–144) during their ICU stay. Median ICU and hospital length of stay were 3.5 (IQR, 2.5–6.0) and 9 (IQR, 5–15) days, respectively. More than 85% of patients were discharged alive from the hospital, most often to home (55% of survivors).
Table 1.
Baseline, Demographic, In-Hospital, and Discharge Characteristics of Cohort
| Patient Characteristics | n = 15,226 |
|---|---|
| Hospital type: academic, No. (%) | 9,519 (63) |
| Age category, No. (%) | |
| 18−29 | 789 (5) |
| 30−39 | 934 (6) |
| 40−49 | 1,397 (9) |
| 50−59 | 2,861 (19) |
| 60−69 | 3,889 (26) |
| 70−79 | 3,124 (21) |
| 80−89 | 1,811 (12) |
| 90+ | 3,632 (2) |
| Sex: female, No. (%) | 8,794 (58) |
| Race, No. (%) | |
| White | 11,025 (72) |
| Black/African-American | 1,986 (13) |
| Other/not specified | 1,396 (9) |
| Asian | 457 (3) |
| American Indian/Alaskan Native | 136 (1) |
| No race data entered | 98 (1) |
| Native Hawaiian/Pacific Islander | 92 (1) |
| Multiple races | 36 (0) |
| Hispanic, No. (%) | |
| Hispanic | 1,571 (10) |
| Non-Hispanic | 13,443 (88) |
| No ethnicity specified | 212 (1) |
| Body mass index, median (IQR) | 28 (24−34) |
| Residence, No. (%)a | |
| Living in a facility pre-admission | 2,974 (20) |
| Discharged to a facility | 5,677 (45) |
| Mobility restriction, No. (%)b | |
| At hospital admission | 4,686 (34) |
| At hospital discharge | 6,264 (53) |
| Primary admission diagnosis, No. (%) | |
| Sepsis/septic shock or ARDS | 3,393 (22) |
| Respiratory | 2,486 (16) |
| Other | 2,702 (18) |
| Neurologic | 1,534 (10) |
| Cardiac | 1,388 (9) |
| Gastrointestinal | 784 (5) |
| Trauma | 736 (5) |
| Genitourinary | 635 (4) |
| Surgery | 1,192 (8) |
| Overdose/withdrawal | 296 (2) |
| ICU type, No. (%) | |
| Mixed medical/surgical | 8,469 (56) |
| Medical | 2739 (18) |
| Surgical/trauma | 1,836 (12) |
| Neurologic | 767 (5) |
| Cardiac/surgical | 865 (6) |
| Cardiac | 550 (4) |
| On invasive MV for at least part of the time, No. (%) | 8,089 (54) |
| Time on invasive MV, median (IQR), hours | 60 (24–144) |
| ICU length of stay, median (IQR), days | 3.5 (2.5–6.0) |
| Hospital length of stay, median (IQR), days | 9 (5–15) |
| Readmitted to ICU at least once | 1,092 (7) |
| On “comfort care” at least part of the time, No. (%) | 564 (4) |
| Discharge status, No. (%) | |
| Died in ICU during the collaborative admission stay | 1,372 (9) |
| Died in ICU, but not during the collaborative admission stay | 310 (2) |
| Died during hospitalization but not in an ICU | 524 (4) |
| Discharged from hospital alive | 12,756 (85) |
Abbreviations: ARDS, Acute Respiratory Distress Syndrome; IQR, interquartile range; MV, mechanical ventilation.
Facility was defined as residence in 1) assisted living, rehabilitation center, long-term acute care hospital, nursing home, skilled nursing facility, another acute care hospital, hospice, or inpatient psychiatric unit.
Mobility restriction was defined as patient being unable to walk independently without the use of assistive devices, including cane, walker, or wheelchair.
A description of ABCDEF bundle-related metrics for all 24-hour days patients spent in the ICU is shown in SDC Table 3. Episodes of significant pain, coma, delirium, and/or use of physical restraint were documented on 49%, 15%, 29%, and 33% of all ICU days, respectively. As a proportion of ICU days, psychoactive medication exposure was common: opioids (63%), propofol (23%), benzodiazepines (21%), dexmedetomidine (9%), and antipsychotics (7%).
Table 2 shows the adjusted hazard ratios (AHRs) and adjusted odds ratios (AORs) for outcomes of patients with complete ABCDEF bundle performance (versus anything less), adjusting for covariates. Patients with complete ABCDEF bundle performance on a given day (8% of all ICU days, SDC Table 4) had a higher likelihood of ICU discharge (AHR, 1.17; CI, 1.05–1.30) and hospital discharge (AHR, 1.19; CI, 1.01–1.40) and a lower likelihood of death (AHR, 0.32; CI, 0.17–0.62) at any given time (within the up-to-7-day observation period) compared with patients who did not receive 100% of all eligible bundle elements on that day. A patient with complete ABCDEF bundle performance on a given day also had a significantly lower likelihood of mechanical ventilation (AOR, 0.28; CI, 0.22–0.36), coma (AOR, 0.35; 95% CI, 0.22–0.56), delirium (AOR, 0.60; CI, 0.49–0.72) or physical restraint (AOR, 0.37; CI, 0.30–0.46) on the following day. Table 2 also shows that hospital survivors with complete bundle performance (vs. any other patients) had a 46% lower likelihood of ICU readmission (AOR, 54%) and a 36% lower likelihood (AOR, 64%) of discharge to a destination other than home after adjusting for covariates.
Table 2.
Outcomes for Patients With Complete (vs Incomplete) ABCDEF Bundle Performance: Data are Adjusted Hazard Ratios (AHRs) and Adjusted Odds Ratios (AORs)
| Outcomes | Complete Bundle Performance |
p Value |
|---|---|---|
| Patient-Related Outcomes | AHR (95% CI) | |
| ICU dischargea | 1.17 (1.05–1.30) | < 0.004 |
| Hospital dischargeb | 1.19 (1.01–1.40) | < 0.033 |
| Deathc | 0.32 (0.17–0.62) | < 0.001 |
| Symptom-Related Outcomesd | AOR (95%CI) | |
| Mechanical ventilation | 0.28 (0.22–0.36) | < 0.0001 |
| Coma | 0.35 (0.22–0.56) | < 0.0001 |
| Delirium | 0.60 (0.49–0.72) | < 0.0001 |
| Significant pain | 1.03 (0.88–1.21) | 0.7000 |
| Physical restraints | 0.37 (0.30–0.46) | < 0.0001 |
| System-Related Outcomes | Adjusted OR (95%CI) | |
| ICU readmissione | 0.54 (037–0.79) | < 0.001 |
| Discharge destinationf | 0.64 (0.51–0.80) | < 0.001 |
Clinical interpretation (example): The ICU discharge AHR of 1.17 indicates that a patient who had complete bundle performance on a given day on average had a 17% higher likelihood of ICU discharge at any point within the 7 days of data collection versus an otherwise identical patient with incomplete bundle performance. Likewise, the AHR of death of 0.32 indicates that a patient who had complete bundle performance on a given day on average had only 32% the risk of death at any point within the 7 days of data collection versus an otherwise identical patient with incomplete bundle performance.
Results from the ICU discharge analysis are from a Cox proportional hazards model that allowed time-dependent covariates. Covariates included demographic variables (age, sex, race, ethnicity, body mass index, residence before admission, mobility restriction before admission), admission features (diagnosis, hospital type [community vs. teaching], and ICU type), and daily ICU characteristics on the day of bundle exposure (receipt of medications, including benzodiazepines, opioids, propofol, dexmedetomidine, typical/atypical antipsychotics; comfort care order; mechanical ventilation; coma). Patients who died before ICU discharge were censored at the time of death; patients who remained in the ICU after day 7 were censored at day 8 because, at most sites, data collection on the ABCDEF bundle elements stopped on ICU day 7. The final model included 12,255 patients, with 9,236 ICU discharges within 7 days.
Results from the hospital discharge analysis are from a Cox proportional hazards model that allowed time-dependent covariates. Covariates included demographic variables (age, sex, race, ethnicity, body mass index, residence before admission, mobility restriction before admission), admission features (diagnosis, hospital type [community vs. teaching], and ICU type), and daily ICU characteristics on the day of bundle exposure (receipt of medications, including benzodiazepines, opioids, propofol, dexmedetomidine, typical/atypical antipsychotics; comfort care order; mechanical ventilation; coma). Patients who died before hospital discharge were censored at the time of death; patients who remained in the hospital after day 7 were censored at day 8 because, at most sites, data collection on ABCDEF bundle elements stopped on ICU day 7. The final model included 12,212 patients, with 4,185 hospital discharges within 7 days. The tipping point analysis presented in the supplement digital content caused us to treat this outcome (and ICU discharge) with less emphasis, removing it from the abstract and overall conclusions of the manuscript.
Results from the mortality analysis are from a Cox proportional hazards model that allowed time-dependent covariates. Covariates included demographic variables (age, sex, race, ethnicity, body mass index, residence before admission, mobility restriction before admission), admission features (diagnosis, hospital type [community vs. teaching], and ICU type), daily ICU characteristics on the day of bundle exposure (receipt of medications, including benzodiazepines, opioids, propofol, dexmedetomidine, typical/atypical antipsychotics; comfort care order; mechanical ventilation; coma). Patients discharged before 7 days were censored at the time of discharge; patients who remained in the hospital after day 7 were censored at day 8 because data collection on ABCDEF bundle elements stopped on ICU day 7. The final model included 12,266 patients with 758 deaths occurring within 7 days.
For symptom-related outcomes, we assessed the relationship between ABCDEF bundle performance on a given ICU day and each of the outcomes the following ICU day using logistic regression models. Variance was adjusted using Huber-White sandwich estimation clustered by patient study site to account for correlation within each collaborative site. We allowed continuous variables to have a nonlinear association with our outcomes using restricted cubic splines with three knots. The covariates used were demographic variables (age, sex, race, ethnicity, body mass index, residence before admission, mobility restriction before admission), admission features (diagnosis, hospital type [community vs. teaching], and ICU type), and daily ICU characteristics on the day of bundle exposure (receipt of medications including benzodiazepines, opioids, propofol, dexmedetomidine, typical/atypical antipsychotics; comfort care order; mechanical ventilation; coma). We also adjusted for delirium on the day of bundle exposure when looking at the association between the bundle and delirium.
We used a logistic regression model for the ICU readmission system-related outcome to assess the relationship between ABCDEF bundle performance during the entire collaborative ICU stay (i.e., complete performance for 100% of ICU days vs. 0% of ICU days meeting “complete” criteria) and the likelihood of subsequent readmission to the ICU among patients who survived the collaborative ICU stay and had at least one full day in the ICU. We excluded patients who died during the initial ICU stay since they did not have the same opportunity for ICU readmission. The final model included 11,118 patients; 825 had an ICU readmission while 10,293 did not. We summarized ICU characteristics over the course of the original collaborative admission, i.e., adjusting for proportion of ICU days the patient received each medication, comfort care orders, or mechanical ventilation or experienced coma.
For the discharge destination system-related outcome, we used a logistic regression model to assess the relationship between ABCDEF bundle performance during the entire collaborative ICU stay (i.e. complete performance for 100% of ICU days versus 0% of ICU days meeting “complete” criteria) and the likelihood of discharge to any type of facility versus discharge home, among patients who survived their hospitalization and had at least one full day in the ICU. The final model included 10,503 patients: 4,701 were discharged to a facility while 5,802 were not. We summarized ICU characteristics over the course of the original collaborative admission, i.e., adjusting for proportion of ICU days on which the patient received each medication, comfort care orders, or mechanical ventilation or experienced coma.
As is shown in Figures 1 and 2, higher proportional performance of the ABCDEF bundle was consistently and strongly associated with significant improvements in most patient-, symptom-, and system-related outcomes after controlling for covariates. On any given observation day, after adjusting for covariates, a higher dose of ABCDEF bundle performance (i.e., a greater percentage of eligible bundle components were completed) yielded an increased likelihood of discharge from the ICU and/or hospital and a lower likelihood of death compared with days when none of the bundle elements were performed (p < 0.0001, p < 0.002, and p < 0.0001, respectively) (Figure 1). An increasing proportion of eligible ABCDEF bundle elements performed on a given day was also associated with a significantly decreased likelihood of mechanical ventilation, coma, delirium, or physical restraint the following day; the increased dose was also associated with more significant pain episodes (all p < 0.0001) (Figure 2).
Figure 1.

Association between proportional performance of the ABCDEF bundle and patient-related outcomes. Each panel shows the adjusted hazard ratio and 95% CI for the specified outcome, comparing patients with a given proportion of eligible ABCDEF bundle elements performed on a given day with patients with none of the bundle elements performed that day. The gray line at 1.0 indicates no association. Hazard ratios are adjusted for baseline, ICU admission characteristics, and daily covariates, measured the same day as bundle performance. For example, assuming all other covariates are equal, a patient who had 60% of the ABCDEF bundle elements for which he/she was eligible has on average about 1.4 times the likelihood of being discharged from the ICU on a given day as a patient with none of the bundle elements performed. All three outcomes were significant (p < 0.0001). The covariates adjusted for include demographic variables (age, sex, race, ethnicity, body mass index, residence before admission, mobility restriction before admission), admission features (diagnosis, hospital type [community vs. teaching], and ICU type), and daily ICU characteristics on the day of bundle exposure (receipt of medications, including benzodiazepines, opioids, propofol, dexmedetomidine, typical/atypical antipsychotics; comfort care order; mechanical ventilation; coma). We also adjusted for delirium on the day of bundle exposure when looking at the association between the bundle and delirium. Patients were “eligible to receive” elements A, C, D, and E on all ICU days. Patients were eligible for element B if sedated (part 1, SAT) and/or mechanically ventilated (part 2, SBT), and were eligible for element F if family or another caregiver was present. Therefore, patients were eligible for a maximum of seven and a minimum of four elements on any given day; proportion of elements performed is the number of elements performed, divided by the elements the patient was eligible to receive.
Figure 2.

Association between proportional performance of the ABCDEF bundle and symptom-related outcomes. These data represent the relationship between the proportion of eligible ABCDEF bundle elements performed on a given day and the probability of a daily clinical outcome the following day. For example, the upper left-hand panel represents the relationship between proportion of eligible elements performed on a given day and the probability that the patient would be mechanically ventilated the following day. Lines and confidence bands represent the probability of the outcomes and the 95% CI, adjusted for potential confounders measured at baseline, ICU admission, and daily [Au: daily what?] while in the ICU. Relationships between proportion of elements performed and each outcome were significant (all p < 0.0001). Patients were “eligible to receive” elements A, C, D, and E on all ICU days. Patients were eligible for element B if sedated (part 1, SAT) and/or mechanically ventilated (part 2, SBT), and were eligible for element F if family or another caregiver was present. Therefore, patients were eligible for a maximum of seven and a minimum of four elements on any given day; proportion of elements performed is the number of elements actually performed divided by the elements the patient was eligible to receive.
Figure 3 shows ICU survivors’ adjusted probabilities of ICU readmission and discharge to a destination other than home according to the percentage of total eligible ABCDEF bundle elements performed during the patient’s 7 days in the ICU (or until hospital discharge, if before 7 days). With more ABCDEF bundle elements performed, the risk of survivor readmission to an ICU or discharged to a facility significantly decreased (p < 0.002 and p < 0.0001, respectively).
Figure 3.
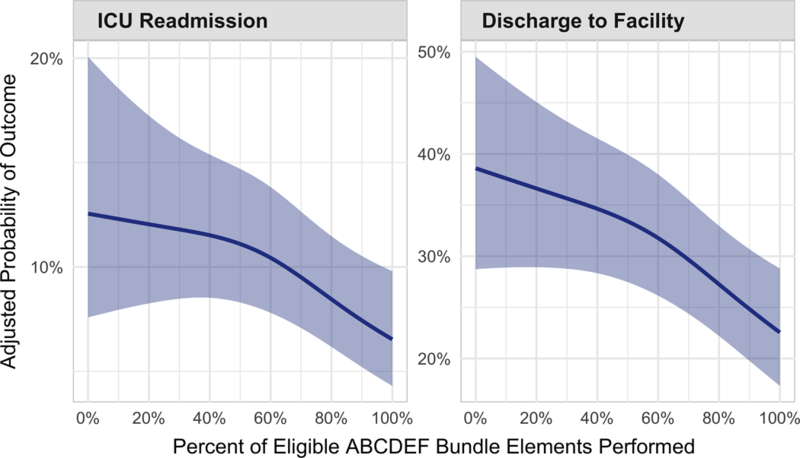
Association between proportional performance of the ABCDEF bundle and system-related outcomes. These data show the adjusted probabilities of ICU readmission (p = 0.002) and discharge to a facility versus home (p < 0.0001), respectively, among ICU survivors, according to what proportion of eligible ABCDEF bundle elements were performed during the first 7 days of a patient’s ICU stay. Probabilities are adjusted for baseline, ICU admission, and summary ICU characteristics (e.g., total proportion of ICU days the patient received benzodiazepines). Patients were “eligible to receive” elements A, C, D, and E on all ICU days. Patients were eligible for element B if sedated (part 1, SAT) and/or mechanically ventilated (part 2, SBT), and were eligible for element F if family or another caregiver was present. Therefore, patients were eligible for a maximum of seven and a minimum of four elements on any given day; proportion of elements performed is the number of elements actually performed during the entire ICU stay (up to 7 days) divided by the elements the patient was eligible to receive during that time.
The full results of our tipping point analysis are presented in SDC Table 2. An example of what the analysis showed is provided by looking at the outcome of mechanical ventilation. An odds ratio (OR) of 0.08 between a one-unit change in severity of illness and mechanical ventilation would be needed to tip our observed results to inconclusivity (closer to 1, or a null result)–an extremely large and unrealistic effect size. Using a more conservative approach (SDC Methods 2), we would still need an unlikely strong OR of 0.13 to move our original results to inconclusivity (closer to 1).
The results of the sensitivity analysis adjusting for severity of illness among the 950 patients with APACHE III scores available are reported in SDC Table 3. This sensitivity analysis demonstrated statistically significant relationships between ABCDEF Bundle performance and the odds of remaining on mechanical ventilation, in delirium, or receiving restraints. The findings along with ICU discharge and significant pain were qualitatively similar to the original analyses reported above for the entire cohort. No statistically significant associations were seen in the sensitivity analysis between ABCDEF bundle use and hospital discharge or discharge disposition. The absence of a statistically significant difference in the latter outcomes in this small subgroup analysis could be due to lack of power in the reduced subset or a true confounding effect from severity of illness.
DISCUSSION
The goal of this study was to evaluate the relationship between ABCDEF bundle performance and patient-centered outcomes from a diverse set of ICUs that participated in the ICU Liberation Collaborative. We sought to determine whether the bundle benefits reported in other, smaller cohorts (17, 18) would be reproducible in this larger and more diverse cohort that included multiple ICU types (medical, surgical, neurological, trauma) and academic, community, and federal hospitals throughout the United States and Puerto Rico. These data from over 15,000 patients in 68 ICUs showed a consistent signal of improved outcomes regardless of whether bundle performance was complete or proportional (i.e., across a “dose” range). Patients who received more of the ABCDEF bundle elements each day had a large and significantly improved likelihood of surviving; having less coma, delirium, and physical restraint; being liberated from ventilation; avoiding ICU readmission; and being discharged home.
Considering the burden that PICS imposes on ICU survivors, their family members, and society as a whole (5, 7, 42−45), there is a driving unmet need to improve both ICU structure and culture for the more than five million patients admitted to ICUs in the United States each year (46−51). One obvious tactic is to bundle proven interventions together. While some bundles and toolkits have been successful in improving patient outcomes (19−21), others have not (22). When assorted interventions that had proven effective individually (i.e., low tidal volume ventilation, moderate sedation, central venous and urinary catheter use, head of bed elevation, thromboembolism prophylaxis, and nutrition) were bundled together and implemented in 118 ICUs in Brazil, patient outcomes did not change (22). By contrast, the philosophy behind building the ABCDEF bundle was that the features had to be interdependent and clinically synergistic.
Despite early signals that the bundle would be advantageous, we know that clinical reproducibility in medical research is often poor (52, 53). When translating animal models to human studies, the most consistent predictor of reproducibility is the dose-response effect of an intervention (54). Guyatt emphasized that finding a dose-response gradient in clinical investigations upgrades the quality of evidence (55). Our investigation showed clear dose-response relationships between daily ABCDEF bundle performance and outcomes (Figures 1−3) considered important to ICU patients, families, and the clinicians caring for them. Similar dose-response relationships were found in a 6,000-patient cohort study that showed that every 10% increase in ABCDEF bundle compliance independently predicted a 15% improvement in both survival and days without coma and delirium (18).
In our cohort, the likelihood of a patient experiencing significant episodes of pain varied with bundle performance (Figure 2). Interestingly, the complete bundle performance analysis did not show this relationship, which could be a type II error. However, a more likely explanation is that, once sites had implemented bundle element A and started systematically assessing patients for pain using appropriate tools, significant pain that would otherwise have gone undetected was identified more frequently (i.e., a reporting bias as performance of bundle element A increased). It is also plausible that patients who received different elements of the bundle (i.e., proportions of the bundle rather than all or none) were at risk for significant pain. For example, if a patient was not receiving adequate pain assessments but was receiving early mobilization, staff members may not have recognized and managed pain appropriately. Finally, it could also be possible that patients with significant pain (which includes moderate and severe pain) might be more likely to have more of the bundle elements completed. Future research is needed in this area to better understand this relationship.
There are six main study limitations. First, this was not a randomized study design nor did we have access to concurrent controls. Therefore, unmeasured covariates may influence the observed associations between ABCDEF bundle performance and outcomes. For example, the bundle components introduce many elements of human connectedness (waking patients, holding their hand and walking with them, and their regaining a sense of agency) that could influence the outcomes and cannot be captured quantitatively. Future randomized controlled studies of this bundle intervention are being planned (56) .
Second, the ICU Liberation Collaborative intentionally included a variety of ICU types as part of a larger effort to understand the impact of the ABCDEF bundle on various types of critically ill patients, as well as to gain better understanding of implementation strategies that are unique to each setting. While this current report includes a minority of patients from neurologic, trauma, and cardiac settings, the results are consistent with those from the original ABCDE Bundle study (17), which included all medical, surgical, trauma, neurologic, and cardiac patients. The consistency further supports the message that the ABCDEF bundle can apply to all critical care patients. However, future inquiry is still needed to explore the full impact of the ABCDEF bundle in these specific populations as well as particular implementation challenges.
Third, our patient-level outcomes are not wholly independent of one another (i.e., there is a relationship between analyses of hospital death and discharge), and are assessed within a very short time frame, during which many of our patients did not experience these outcomes (requiring them to be censored). Future work could consider a longer follow-up period alongside competing risks regression to account for patients who, for example, die before they are discharged.
Fourth, similarly to other collaboratives and QI projects and many studies, the ICU Liberation Collaborative did not have the funds to support data accuracy auditing. While all sites were provided with a detailed standard operating procedures manual, offered formal data collection training, and were provided with ongoing as needed support, it is possible that errors may have occurred during the data collection process, introducing the possibility of reporting bias.
Fifth, this cohort analysis is from patient data collected within the scope of a large QI project that collected a minimum and de-identified dataset, both of which limited our ability to answer certain questions. The site personnel in the ICU Liberation Collaborative were unpaid and time constraints mandated that we collect data on a limited number of consecutively admitted ICU patients at each participating institution for a limited period (up to 7 days). These data therefore may not apply to patients with longer ICU stays and especially those who develop chronic critical illness. Additionally, data abstraction for these bundle elements is cumbersome because individual elements of the ABCDEF bundle are often separate and disconnected in current designs of electronic medical record (EMR) systems (e.g., EPIC and CERNER) which often have siloed screens and standard views that vary significantly depending on the user and institution. User-friendly EMR platforms that are easily adaptable would better support ongoing QI and research in this area (26, 34, 56−58). Additionally, data collected by multiple team members should be seamlessly displayed on integrated EMR dashboards accessible by all team members so that patients’ ABCDEF bundle progress can be monitored in a collaborative way (e.g., one-stop dashboard screen access for all bundle elements).
Finally, this initiative did not collect uniform severity of illness data because of funding limitations. Only 6% of patients had severity of illness scores from the same scoring system and those patients were all from six sites that already tracked the scores. This precluded directly adjusting for this covariate, which would help understand the very large effect sizes we have found previously from this bundle (18). Our sample size (over 15,000 ICU patients and nearly 50,000 patient-days of data) and the inclusion of 18 covariates chosen a priori, adjusting as possible for patient characteristics and measures of baseline health and acuity, are robust but do not completely remove the potential benefit of adjusting for severity of illness.
However, because of the importance of this limitation, we conducted two additional sensitivity analyses. First, we conducted a “tipping point” analysis (37, 38) which is described and presented in SDC Table 2. That type of analysis allowed us to quantify the amount of total unmeasured confounding needed to render our analysis inconclusive. The sensitivity analysis indicated that even if the true adjusted associations between ABCDEF bundle performance and all five in-ICU outcomes were smaller than observed after adjusting for severity of illness, they were still likely to be clinically relevant.
Additionally, in a very small (6%) subgroup of patients who had available APACHE III scores reported, we conducted a sensitivity analysis that directly incorporated severity of illness as an additional covariate into the original modeling (SDC Table 3). While obviously limited in size and power, the analysis found similar changes in endpoints of ICU discharge, mechanical ventilation, delirium, significant pain, physical restraints, and discharge destination, all of which were consistent with the results of the main analysis. The likelihood of hospital discharge, although not significant, showed an inverse relationship with bundle compliance. With that exception, these two sensitivity analyses were generally consistent with and thus support the validity of the main findings of this report.
This cohort analysis from the ICU Liberation Collaborative demonstrates that the performance of the ABCDEF bundle results in significant and dose-related improvements in outcomes, including better survival, duration of mechanical ventilation, brain organ dysfunction (i.e., delirium and coma), physical restraint use, ICU readmission rates, and discharge disposition of ICU survivors. Additional unmeasured benefits often expressed during the collaborative represent excellent points for future work, such as the effect that full integration of the ABCDEF bundle has on making ICU care more collaborative, holistic, and patient centered, with an eye toward returning patients to their previous lives.
Supplementary Material
ACKNOWLEDGMENTS
The following sites and members at each site participated in the ICU Liberation Collaborative and generated the data upon which this report is based: Advocate Christ Medical Center: Charles Alex, Nadia Abdessalam, George Gavrilos, Jill Sweeney; Atrium Health: Jaspal Singh, Julia Retelski, Lauren Macko, Brianne Riegel, Jennifer Cline; Aultman Hospital: Nihad Boutros, Amy Hiner, Jonas Sykes, Kim Dougan; Avera McKennan Hospital: Kari Taggart, Carol Leiferman, Fady Jamous, Kristy Colford; Banner University Medical Center Tucson: Alicia Johnson, Larry Deluca; Baptist Memorial Hospital Memphis: Jeffery Wright, Carole Schuh, Maria Zhorne; Berkley Medical Center (WV university): Phillip Aguila, Angela Girod, Chuck Steg, Donnie Kees, David Fillman; Cape Fear Valley Health System: Felicia McGarry, Samuel Wamathai Kimani, Kerstin Hudgins, Esteban Mery-Fernandez, Claudette Fragueiro, Lynn Bass; Cedars Sinai Medical Center: Bahar Mjos, Alice Chan, Robert Fellin, Michael Nurok, Todd Griner; Cleveland Clinic: Faith Factora, Kathleen Hill, Karoline Lubbeck, Roxanne Eaton, Dianne Havanchak; Columbia St. Mary’s: Antonio Salud II, Anne Putzer, Sara Harwood, Andi Gust, Cindy Keller; Community Regional Medical Center: Kim Pope, Krista Kaups, Jose Rendon, Catrina Cullen, Alice Evans, Melissa Reger, Paul Smith; Corona Regional Medical Center: Aimee French, Fernando Fierro, Kaveh Rezvan, Joon Kim; Edward Elmhurst Hospital: Mara Chiocca, Kim Clohecy, Keith Nguyen, Amy Rowe, Mohammed Sajed; Emory University Hospital: Jonathan Sevransky, Carolyn Holder, Stacey Campbell; Franciscan Health-Indianapolis: Imad Shawa, Kimberly Durham, LeeAnn McGinley-Wright, Cheryl Wolverton, Frank Lucas, Karen Hunt; Hannibal Hospital: Pranav Parikh, Kim Runquist, Pam Guilfoyle, Patti Gilbert; Harrison Medical Center: Griffith Blackmon, Patricia Hetrick, Cheryl Christian, Rima Kim, Len Schulmeister; HIMA San Pablo Caguas Hospital: Dra.Gloria Rodríguez Vega, Dr. Hector L Peniston Feliciano, Arlene Rivera, Eniliz E. Gerena, Jahaira Rentas, Ana M. Rodriguez, Wilma González, José R. Rodríguez; University of Puerto Rico, Medical Sciences Campus-SON: Milagros Figueroa-Ramos, C. Mabel Arroyo-Novoa; Houston Methodist Hospital: Christopher Cortes, Teal Riley, Rajashree Mondkar; Indiana University Health Arnett Hospital: Jennifer Hittle, Muhammad Ali, Katherine Douglass, Erin Hoag; Iowa Methodist Medical Center Unity Point: Sheryl Sahr, Sarah Pandullo, Lisa Kingery; Keck Hospital of USC: Perren Cobb, Kathrine Winnie, Geoff Cariker; Kettering Medical Center: Doug Paul, Delaine Adrian, Carol Severance, Melody Campbell; LAC+USC Medical Center: Santhi Kumar, Eileen Friesen-Mosher, Stephanie Summerville; Lake Cumberland Hospital: Sandra Schuldheisz, Courtney Troxell, Katrina Mounce; Lake Regional General Hospital: Kamen Rangelov, Stephanie Wheeler, Michael Smith; Northwestern Memorial Hospital: Jacqueline Kruser, Bryan Lizza, Megan Oakford, Leigh Anne Wild, Chris French; Novant Health Forsyth Medical Center: Christina Cassidy, Sandy Hunter, Barry Sigal, Sharon Cox, Lawson Millner; Novant Health PMC Presbyterian Medical Center: Wheeler Jervis, Deborah Briese, Laura Frantz; Oklahoma University Medical Center: Regina Ketts, Kammie Monarch, Pamela R. Roberts, Ruben Villanueva, Kris Wallace; Orange Regional Medical Center; Pali Momi Medical Center: Emilio Ganitano, Lorna Coloma, Jackie Scotka; Parkland Health & Hospital System: Brian Williams, Natalie Provenzale, Stacey Barker, Katherine Mapula; Parkview Community Hospital: Ahmed El Bershawi, Marlena DeMicco, Leonard Carreathers; Providence Portland Medical Center: David Hotchkin, Bev Lorhman, Julie Martinez; Providence St. Patrick Hospital: Will Surber, Nicole Marks, Marian Maxwell; Riverside University Health Systems: Arriti (Nikki) Mitial, Gigi McNicholl, Allison Flores, Walter Klein, Leah Patterson; Ronald Reagan UCLA Medical Center: Joseph Meltzer, Katrine Murray, Sheila Shirzi; WVU Medicine – Critical Care and Trauma Institute: Greg Schaefer, Shanna Watson, Karen Petros; Rush University Medical Center: Nicholas Panos, Sayona John, Valerie Musolf, Ankeet Patel, Payal Gurnani, Lillian Hall, Elizabeth Day, Barbara Gulczynski, Mark Yoder, Brenda Koverman, Diane Genaze, Michele Simler, Ruth Kleinpell, David Gurka, Gourang Patel, Brandon Bell, Maria Goetz, Amy Blackwood, Joseph Ceisel, Timothy Carrigan; Sarasota Memorial: Kirk Voelker, Karen Reynolds, Anit Legare, Melinda Bacallao; Scripps Mercy Hospital Chula Vista: John Perri, Deena Drake, Valeska Cid Donat, Terry Taylor, Alessio Bloesch; Sharp Grossmont Hospital: Barzan Mohedin, Ani Harter, Kareem Dally, Jill Limberg; South County Hospital: Bobbie Fay, Bashar Bash, Ellen Fales, Lisa Chatowsky, Siobhan Ryan; Spectrum Health Butterworth Hospital: Greg Marco, Nancy Bekken, Nick Ames, Stephen Fitch; St. Luke’s Hospital: Paula Brown, Hope Cranston Damato, Thomas Simon, Heather Thompson, Bhavna Desai; Sutter Health Memorial Medical Center: Roger Elias, Terry Lynch, Abigail Kurtz, Monika Rebalska, Pete Tomaino, Vio Burciu; Tennessee Valley Health System VA: Kelly Drumright, Margaret Russell, Shawn Sells, Julie Bastarache, John Barwise; The Ohio State Wexner Medical Center: Michele L. Weber, Kari Cape, Matthew Exline, Cindy Byrd, Connie McCarthy; Thomas Jefferson University Hospital: Michael Baram, Julie Rogan, Cara McDaniel, Miranda Tan; University of California San Francisco: Matthew Aldrich, Heidi Engel, Joyce Chang, Denise Barchas, Kathleen Puntillo; University of Michigan Hospital & Health Center: Connie Rickelmann, Megan Klei, James Miller, Jessica Cusac, Sharon Dickinson, Nikki Werner, Adam Carter, Lena Napolitano, Pauline Park; University of North Carolina Chapel Hill: Lydia Chang, Mikey Jernigan, Allison Driver; University of Wisconsin Hospital: Hee Soo Jung, Anna Krupp, Jeff Fish; VA Palo Alto Healthcare System: Juli Barr, Andrea Saito, Mylinh Ho, Elayne Rodriguez, Laura Zimmerman; Virginia Mason Medical Center: Aneal Gadgil, Markie Baxter, Ernesto Lopez; Washington Hospital Healthcare System: Carmen Agcaoili, Kathy Weinberg, Bhav Kaur, Elvie Ballar, Alisa Curry; Wooster Community Hospital: Bruce W. Arthur, Joann Panno, Jennifer Peterson, Karen Steiner.
Funding for the ICU Liberation ABCDEF collaborative was provided by the Gordon and Betty Moore Foundation and the Society of Critical Care Medicine. Grant support for REDCap UL1 TR000445 from NCATS/NIH.
Dr. Pun has received honoraria from Society of Critical Care Medicine (SCCM), American Association of Critical Care Nurses, and Michigan Hospital Association. Dr. Balas has received honoraria from SCCM, Michigan Hospital Association, Hospital of the University of Pennsylvania, and ANZICS/ACCCN Intensive Care ASM. Dr. Balas is currently receiving a grant from the American Association of Critical Care Nurses and is on the speaker’s bureau for the Medical Education Speaker’s Network. Dr. Ely receives ongoing NIH and VA funding (from the Geriatric Research Education Clinical Center, GRECC) and having conducted CME activities that provided honoraria from SCCM, Pfizer, Orion, and has been on a Scientific Advisory Board for Masimo, Inc. Dr. Aldrich receives NIH funding. Dr. Devlin has received research funding from the National Institute of Aging, National Heart, Lung and Blood Institute, the Canadian Institute of Health Research, and Astra Zeneca; he is on the editorial board of Critical Care Medicine and is the president of the American Delirium Society. Dr. Carson receives grants from the NIH and the Biomarck Corporation. Dr. Barr is an Advisory Board Member for Medasense Biometrics, Ltd and a Scientific Advisor for Masimo, Inc. Dr. Schweickert reports being a consultant for Arjo Inc.
Drs. Pun, Aldrich, Engel, Stollings, and Ely received funding from the SCCM/the Gordon and Betty Moore Foundation. Drs. Balas, Thompson, Byrum, Millner, Morse, Perme, Posa, Puntillo, and Schweickert received funding from SCCM. Dr. Balas’ institution received funding from the American Association of Critical Care Nurses (AACN) and Select Medical, and she received support for article research from the Gordon and Betty Moore Foundation. Dr. Aldrich received funding from the NIH/National Heart, Lung, and Blood Institute (NHLBI), Dannemiller Anesthesiology Review Course, and National Association for Medical Direction of Respiratory Care (travel support and lecture honorarium), and he has past relationships with the following institutions (honoraria paid for visiting lectures): Massachusetts General Hospital,University of Manitoba, University of Calgary, and Washington Hospital, Fremont, CA. Dr. Barr received funding from Masimo Inc, Medasense, and Dignity Health. Dr. Byrum received funding from AACN (board member of the Certification Corporation [travel expenses to meetings] and annual conference honorarium and travel expenses). Dr. Carson’s institution received funding from the NIH, SCCM, and Biomarck Corporation. Ms. Harmon’s, Ms. Hielsberg’s, Dr. Kumar’s, and Dr. Kelly’s institutions received funding from the Gordon and Betty Moore Foundation. Drs. Jackson and Ely received support for article research from the NIH. Dr. Perme received funding from American Hospital Association-HRET and Michigan Hospital Association (both as subcontractor on the HIIN CMS contract). Dr. Puntillo received funding from the Geneva Foundation (consulting) and the University of Oslo (consulting). Dr. Schweickert received funding from Arjo and the American College of Physicians. Dr. Ely’s institution received funding from the NIH and from VA Salary Support; he received other support from the Gordon and Betty Moore Foundation; he received funding from Pfizer and Orion for CME activities; and he has disclosed government work.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Devlin JW, Skrobik Y, Gelinas C, et al. : Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018; 46(9):e825–e873 [DOI] [PubMed] [Google Scholar]
- 2.Barr J, Fraser GL, Puntillo K, et al. : Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41(1):263–306 [DOI] [PubMed] [Google Scholar]
- 3.Martin J, Heymann A, Basell K, et al. : Evidence and consensus-based German guidelines for the management of analgesia, sedation and delirium in intensive care−short version. Ger Med Sci 2010; 8:Doc02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herridge MS, Tansey CM, Matte A, et al. : Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304 [DOI] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, et al. : Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shehabi Y, Bellomo R, Reade MC, et al. : Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med 2012; 186(8):724–731 [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, et al. : Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304(16):1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaukonen KM, Bailey M, Suzuki S, et al. : Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014; 311(13):1308–1316 [DOI] [PubMed] [Google Scholar]
- 9.Carson SS, Cox CE, Holmes GM, et al. : The changing epidemiology of mechanical ventilation: a population-based study. Journal of Intensive Care Medicine (Sage Publications Inc) 2006; 21(3):173–182 [DOI] [PubMed] [Google Scholar]
- 10.Halpern NA, Pastores SM: Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med 2010; 38(1):65–71 [DOI] [PubMed] [Google Scholar]
- 11.Elliott D, Davidson JE, Harvey MA, et al. : Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med 2014; 42(12):2518–2526 [DOI] [PubMed] [Google Scholar]
- 12.Needham DM, Davidson J, Cohen H, et al. : Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509 [DOI] [PubMed] [Google Scholar]
- 13.Davidson JE, Jones C, Bienvenu OJ: Family response to critical illness: postintensive care syndrome-family. Crit Care Med 2012; 40(2):618–624 [DOI] [PubMed] [Google Scholar]
- 14.Ely EW: The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit Care Med 2017; 45(2):321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morandi A, Brummel NE, Ely EW: Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care 2011; 17(1):43–49 [DOI] [PubMed] [Google Scholar]
- 16.Bassett R, Adams KM, Danesh V, et al. : Rethinking critical care: decreasing sedation, increasing delirium monitoring, and increasing patient mobility. Jt Comm J Qual Patient Saf 2015; 41(2):62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balas MC, Vasilevskis EE, Olsen KM, et al. : Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014; 42(5):1024–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes-Daly MA, Phillips G, Ely EW: Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: Implementing PAD Guidelines Via the ABCDEF Bundle in 6,064 Patients. Crit Care Med 2017; 45(2):171–178 [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Dellinger RP, Townsend SR, et al. : The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38(2):367–374 [DOI] [PubMed] [Google Scholar]
- 20.Klompas M, Anderson D, Trick W, et al. : The Preventability of Ventilator-associated Events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med 2015; 191(3):292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pronovost P, Needham D, Berenholtz S, et al. : An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355(26):2725–2732 [DOI] [PubMed] [Google Scholar]
- 22.Cavalcanti AB, Bozza FA, Machado FR, et al. : Effect of a Quality Improvement Intervention With Daily Round Checklists, Goal Setting, and Clinician Prompting on Mortality of Critically Ill Patients: A Randomized Clinical Trial. JAMA 2016; 315(14):1480–1490 [DOI] [PubMed] [Google Scholar]
- 23.Vasilevskis EE, Ely EW, Speroff T, et al. : Reducing iatrogenic risks: ICU-acquired delirium and weakness−crossing the quality chasm. Chest 2010; 138(5):1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pileggi C, Mascaro V, Bianco A, et al. : Ventilator Bundle and Its Effects on Mortality Among ICU Patients: A Meta-Analysis. Crit Care Med 2018; 46(7):1167–1174 [DOI] [PubMed] [Google Scholar]
- 25.Nassar Junior AP, Besen B, Robinson CC, et al. : Flexible Versus Restrictive Visiting Policies in ICUs: A Systematic Review and Meta-Analysis. Crit Care Med 2018; 46(7):1175–1180 [DOI] [PubMed] [Google Scholar]
- 26.Costa DK, White MR, Ginier E, et al. : Identifying Barriers to Delivering the Awakening and Breathing Coordination, Delirium, and Early Exercise/Mobility Bundle to Minimize Adverse Outcomes for Mechanically Ventilated Patients: A Systematic Review. Chest 2017; 152(2):304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nydahl P, Sricharoenchai T, Chandra S, et al. : Safety of Patient Mobilization and Rehabilitation in the Intensive Care Unit. Systematic Review with Meta-Analysis. Ann Am Thorac Soc 2017; 14(5):766–777 [DOI] [PubMed] [Google Scholar]
- 28.Nydahl P, Ruhl AP, Bartoszek G, et al. : Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med 2014; 42(5):1178–1186 [DOI] [PubMed] [Google Scholar]
- 29.Miller MA, Govindan S, Watson SR, et al. : ABCDE, but in that order? A cross-sectional survey of Michigan intensive care unit sedation, delirium, and early mobility practices. Ann Am Thorac Soc 2015; 12(7):1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrothers KM, Barr J, Spurlock B, et al. : Contextual issues influencing implementation and outcomes associated with an integrated approach to managing pain, agitation, and delirium in adult ICUs. Crit Care Med 2013; 41(9 Suppl 1):S128–S135 [DOI] [PubMed] [Google Scholar]
- 31.Bakhru RN, McWilliams DJ, Wiebe DJ, et al. : Intensive Care Unit Structure Variation and Implications for Early Mobilization Practices. An International Survey. Ann Am Thorac Soc 2016; 13(9):1527–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakhru RN, Wiebe DJ, McWilliams D, Spuhler VJ, Schweickert WD: An International Survey of Early Mobilization Practices In. American Thoracic Society Abstract; 2014. p. A.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morandi A, Piva S, Ely EW, et al. : Worldwide Survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) Bundle. Crit Care Med 2017; 45(11):e1111–e1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes-Daly MA, Pun BT, Harmon LA, et al. : Improving Health Care for Critically Ill Patients Using an Evidence-Based Collaborative Approach to ABCDEF Bundle Dissemination and Implementation. Worldviews Evid Based Nurs 2018; 15(3):206–216 [DOI] [PubMed] [Google Scholar]
- 35.Damschroder LJ, Aron DC, Keith RE, et al. : Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009; 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson JE, Aslakson RA, Long AC, et al. : Guidelines for Family-Centered Care in the Neonatal, Pediatric, and Adult ICU. Crit Care Med 2017; 45(1):103–128 [DOI] [PubMed] [Google Scholar]
- 37.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998; 54(3):948–963 [PubMed] [Google Scholar]
- 38.D’Agostino McGowan L Improving Modern Techniques of Causal Inference: Finite Sample Performance of ATM and ATO Doubly Robust Estimators, Variance Estimation for ATO Estimators, and Contextualized Tipping Point Sensitivity Analyses for Unmeasured Confounding In: Vanderbilt University Electronic Theses and Dissertations; March 23, 2018. [Google Scholar]
- 39.Payen JF, Bru O, Bosson JL, et al. : Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001; 29(12):2258–2263 [DOI] [PubMed] [Google Scholar]
- 40.Gelinas C, Fillion L, Puntillo KA, et al. : Validation of the critical-care pain observation tool in adult patients. Am J Crit Care 2006; 15(4):420–427 [PubMed] [Google Scholar]
- 41.R Core Team R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 42.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 2010; 153(3):204–205 [DOI] [PubMed] [Google Scholar]
- 43.Iwashyna TJ, Cooke CR, Wunsch H, et al. : Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwashyna TJ, Netzer G: The burdens of survivorship: an approach to thinking about long-term outcomes after critical illness. Semin Respir Crit Care Med 2012; 33(4):327–338 [DOI] [PubMed] [Google Scholar]
- 45.Ehlenbach WJ, Hough CL, Crane PK, et al. : Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010; 303(8):763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halpern NA, Goldman DA, Tan KS, et al. : Trends in Critical Care Beds and Use Among Population Groups and Medicare and Medicaid Beneficiaries in the United States: 2000–2010. Crit Care Med 2016; 44(8):1490–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coopersmith CM, Wunsch H, Fink MP, et al. : A comparison of critical care research funding and the financial burden of critical illness in the United States. Crit Care Med 2012; 40(4):1072–1079 [DOI] [PubMed] [Google Scholar]
- 48.Adhikari NK, Fowler RA, Bhagwanjee S, et al. : Critical care and the global burden of critical illness in adults. Lancet 2010; 376(9749):1339–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wunsch H, Angus DC, Harrison DA, et al. : Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med 2011; 183(12):1666–1673 [DOI] [PubMed] [Google Scholar]
- 50.Wunsch H, Wagner J, Herlim M, et al. : ICU occupancy and mechanical ventilator use in the United States. Crit Care Med 2013; 41(12):2712–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angus DC, Barnato AE, Linde-Zwirble WT, et al. : Use of intensive care at the end of life in the United States: An epidemiologic study. Crit Care Med 2004; 32(3):638–643 [DOI] [PubMed] [Google Scholar]
- 52.Ioannidis JP: Why most published research findings are false. PLoS Med 2005; 2(8):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannidis JP: Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005; 294(2):218–228 [DOI] [PubMed] [Google Scholar]
- 54.Hackam DG, Redelmeier DA: Translation of research evidence from animals to humans. JAMA 2006; 296(14):1731–1732 [DOI] [PubMed] [Google Scholar]
- 55.Guyatt GH, Oxman AD, Kunz R, et al. : What is “quality of evidence” and why is it important to clinicians? BMJ 2008; 336(7651):995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sosnowski K, Mitchell ML, White H, et al. : A feasibility study of a randomised controlled trial to examine the impact of the ABCDE bundle on quality of life in ICU survivors. Pilot Feasibility Stud 2018; 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eakin MN, Ugbah L, Arnautovic T, et al. : Implementing and sustaining an early rehabilitation program in a medical intensive care unit: A qualitative analysis. J Crit Care 2015; 30(4):698–704 [DOI] [PubMed] [Google Scholar]
- 58.Krumholz HM, Terry SF, Waldstreicher J: Data Acquisition, Curation, and Use for a Continuously Learning Health System. JAMA 2016; 316(16):1669–1670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


