Abstract
Objectives
Ocular surface disease frequently co-exists with glaucoma and may be initiated or exacerbated by topical glaucoma medications. We performed a review of current literature to assess the prevalence, causes, and treatment of ocular surface disease in glaucoma patients, specifically those on topical therapy.
Methods
A Pubmed database search was conducted. A total of 720 articles published from 1972 to 2018 were found in relation to ocular surface disease, glaucoma, and glaucoma medications. Of these, 102 articles were included in this analysis. We included primary and empirical studies for patients on topical glaucoma medications. Exclusion criteria included case reports, non-English studies, and articles unrelated to the primary subject of this review.
Results
Ocular surface disease among normal and glaucomatous eyes was evaluated based on diagnostic testing including clinical exam and questionnaires to determine visual function and quality of life. Glaucoma medications can be associated with toxicities to the ocular surface, most often due to the nature of the preservative included in the medication; however, the incidence of toxicity can be mitigated by the use of preservative free medications, decreased preservative medications, or treatment of dry eye disease. Treatment of glaucoma with laser trabeculoplasty or minimally invasive glaucoma surgeries that spare the conjunctiva and the cornea may avoid or decrease reliance on topical glaucoma medications, potentially avoiding the initiation or progression of ocular surface disease.
Conclusions
Recognition and treatment of ocular surface disease in glaucoma patients may improve patient quality of life and medication adherence. This may ultimately improve glaucoma treatment outcomes.
Keywords: ocular surface disease, topical medications, glaucoma
Introduction
Ocular surface disease is a multifactorial disorder of the conjunctival epithelium, corneal epithelium, lacrimal glands, and meibomian glands that results in either deficient or inappropriate tear production and leads to decreased visual clarity and ocular discomfort through various inflammatory pathways1,2. Ocular surface disease can occur in conjunction with many other ocular conditions, and here we aim to focus on the coexistence of ocular surface disease with glaucoma.
Glaucoma is the second leading cause of blindness in the world and is expected to affect 79.6 million people by 2020. At present, 11% of the 5 million Americans over 50 who have dry eye disease also have glaucoma3–6. Topical medical therapy is the most common initial treatment for glaucoma, and 49-59% of glaucoma patients on topical anti-glaucomatous medications have ocular surface disease7–9. Ocular surface disease in these patients can be a pre-existing condition that is exacerbated by topical therapy or a novel disease that manifests after initiation of topical glaucoma therapy. Topical glaucoma medications can cause burning, irritation, itching, tearing, and decreases in visual acuity within three months of medication initiation2,11,12 .
Furthermore, untreated primary open angle glaucoma (POAG) patients have a higher risk of ocular surface disease in part due to a 22% lower basal tear turnover rate in comparison to patients without glaucoma13. The resulting ocular surface disease in patients with glaucoma can lead to poor medication compliance from the associated symptoms. In addition, ocular surface disease is also linked to a higher rate of failure in subconjunctival glaucoma surgery14–18. Thus, management of ocular surface disease in glaucomatous patients is important when trying to reduce further ocular morbidity and to improve the success of glaucoma therapy. We have performed a systemic review of the literature to describe the occurrence of ocular surface disease in conjunction with topical glaucoma medical therapy and the management of glaucoma when ocular surface disease exists.
Methods
A literature review was performed in collaboration with a research librarian using the PubMed Database to gather a complete list of studies in relation to ocular surface disease and glaucoma therapy. The PubMed database was searched using the following terminology: prevalence and epidemiology of ocular surface disease in glaucoma, preservative versus preservative-free eye drops, alternative treatments in ocular surface disease, alternative treatments in glaucoma, and glaucoma medication intolerance. In total, 720 articles published between 1972 to 2017 were reviewed and included or excluded from this analysis based on predetermined criteria. The titles and abstracts of the above studies were reviewed, and 95 studies were deemed appropriate to be included in the review. Inclusion criteria included primary and empirical studies of patients with glaucoma who are on topical glaucoma therapy. Exclusion criteria included animal studies that were not directly relevant to the subject matter, case reports, non-English language studies, and papers irrelevant to ocular surface disease and glaucoma.
Results
Current common therapies for the topical management of glaucoma include the use of prostaglandin analogs, beta-adrenergic antagonists, alpha-adrenergic agonists, and topical carbonic anhydrase inhibitors. Due to either the added preservative or the active ingredient of the medication itself, all of the above standard topical treatments for glaucoma can cause or worsen ocular surface disease. As the number of glaucoma medications required increases, both the prevalence and severity of dry eye also increase14,19–26. Correspondingly, ocular surface disease appears to increase in severity as the duration of therapy increases11,25. A large proportion of glaucoma and ocular hypertensive patients require multiple topical intraocular pressure (IOP) lowering agents, and 49% of ocular hypertensives will require at least two topical medications within five years of diagnosis, thus increasing the risk of ocular surface disease.
Each medication class has specific potential adverse effects on the cornea and ocular surface. Prostaglandin analogs are associated with both a higher prevalence and severity of obstructive meibomian gland dysfunction27. Furthermore, prostaglandin analog therapy was shown to cause a higher rate of meibomian gland dysfunction in patients already receiving non-prostaglandin analog ocular hypotensive therapy, possibly worsening ocular surface disease27,28. Beta blockers act on beta receptors in the lacrimal gland and reducing basal tear turnover rate29,30. Timolol has been found to alter the mucus composition in the tear film and also cause increased staining of the cornea and conjunctiva after one month of therapy31,32. The commonly used alpha-adrenergic agonist brimonidine tartrate has a significantly higher incidence of ocular allergy compared to other topical medications and may predispose patients to ocular allergy from additional topical antiglaucoma drops33. The carbonic anhydrase inhibitor dorzolamide has been found to increase corneal thickness, but the effect of dorzolamide on the corneal endothelium is still in question34. In vivo studies of human corneal epithelial cells show that pure glaucoma medications may decrease the viability of the corneal epithelial cells and have the capacity to induce apoptosis through C/EBP homologous protein recruitment35.
Pathology of Drop Toxicity
The pathology of drop toxicity can be a result of changes in various inflammatory pathways. Patients treated with long term topical antiglaucoma medications prior to glaucoma surgery had conjunctival and Tenon’s capsule biopsies with significantly more macrophages, mast cells, and lymphocytes compared to patients who only had primary glaucoma surgery, suggesting that antiglaucoma medications increase conjunctival inflammation36. Conjunctival cells of patients on multi-drug topical treatment for glaucoma showed significantly greater HLA-DR expression as compared to those on monotherapy37. Prolonged topical treatment for glaucoma significantly increased ocular surface inflammatory marker expression compared with untreated eyes, specifically IgE and Class II antigen HLA-DR17,28,37–39. Thus, both duration and quantity of topical antiglaucoma therapy are correlated with increased histological inflammation. These histological changes may explain clinically significant, toxicity-related symptoms including ocular hyperemia, chemosis, pruritis, periorbital edema, and erythema40,41.
Preservatives are regularly added to glaucoma medications at the lowest concentration possible to prevent microbial contamination of the drops42,43. At present, preservatives used in glaucoma medications include benzalkonium chloride (BAK), the sofZia preservative system [Alcon Laboratories, Fort Worth, TX] used in Travatan Z [Alcon Laboratories, Fort Worth, TX], stabilized oxychloro complex [Purite, Bio-Cide International Inc., OK, USA] used in Alphagan P [Allergan, Irvine, CA], and polyquaternium-1 [Polyquad, Alcon Laboratories, Fort Worth, TX] used in Travatan formulations outside the United States[Alcon Laboratories, Fort Worth, TX]42,43,43. In general these preservatives target bacterial cell walls and can increase drug penetration in the cornea42–48.
BAK, the most commonly used of these preservatives, has also been indicated in initiating or worsening ocular surface disease (Table 1). BAK is weakly allergenic with both time and dose-dependent toxicity on the conjunctiva and cornea. It may initiate or worsen ocular surface disease by destabilizing goblet cells and, subsequently, the tear film by inducing squamous metaplasia of the conjunctival epithelium, disrupting the corneal epithelium by reducing epithelial cell density, and increasing stromal keratocyte activation15, 29,42,49–51. Animal studies have shown that medications containing higher levels of BAK resulted in greater corneal damage and conjunctival infiltration than those preserved with Purite or lower levels of BAK52. Ocular surface effects are dependent on concentration, and low concentrations of BAK have been found to be similar in surface toxicity to newer preservatives in the same medication53. In comparison to BAK, one study found that Polyquad, Purite, and the sofZia preservative system have less toxic effects on the ocular surface with sofZia having the least amount of toxic effects40. However, while newer preservatives and lower concentration of BAK may result in less ocular surface toxicity, these additives are also less effective in inhibiting microbial growth in medications54. Thus, the benefits of ocular surface toxicity must also be carefully measured against drop safety for patients to gain the most benefit from these medications.
Table 1.
Pertinent studies assessing ocular surface disease in anti-glaucomatous formations preserved with BAK
| Reference | Type of Study | Patient Population | Medications being compared | Time frame of medication use | Results |
|---|---|---|---|---|---|
| Baudoin, 199839 | Crossover, randomized double blind study | 30 healthy volunteers | Topical 2% carteolol with and without preservative | Short-term use (3 days) | TBUT was significantly reduced at 3 hours and after 3 days in the PF carteolol No difference in Schirmer's test, corneal aesthesiometry, IOP lowering effect, subjective tolerance |
| Henry, 200871 | Prospective, multicenter, historical control study | 691 patients with ocular hypertension or primary open angle glaucoma | Switching from latanoprost or bimatoprost to travoprost BAK-free (Travatan Z, Alcon Laboratories, Inc., Fort Worth, TX, USA) | Intermediate-term use (3 months) | Mean OSDI scores were significantly improved, mean IOP was significantly decreased, conjunctival hyperemia significantly decreased in PF travoprost |
| Jaenen, 200712 | Multicenter cross-sectional epidemiologic survey in four European countries | 9658 patients with open angle glaucoma | Preservative vs. PF beta-blocking drops | Varies | Pain or discomfort during instillation, foreign body sensation, stinging or burning, dry eye sensation significantly less frequent in PF group |
| Januleviciene, 201251 | Prospective, observer-masked study. | 60 eyes of 30 open angle glaucoma patients | Switching from BAK-preserved latanoprost to PF tafluprost | Intermediate-term use (3 months) | Tear film osmolarity decreased significantly, mean TBUT increased significantly, abnormal fluorescein staining decreased significantly, subjective complaints decreased significantly in the PF group No statistically significant difference in IOP |
| Kanamoto, 2015103 | Prospective, randomized, observer unmasked, multicenter crossover trial | 174 glaucoma patients | Tafluprost with 0.001% BAK vs. travoprost with SofZia | Intermediate-term use (3 months) | Total superficial punctate keratopathy scores, conjunctival hyperemia scores were decreased in patients using SofZia-preserved travoprost No statistically significant difference in TBUT, IOP-lower effect, superficial punctate keratopathy scores of the superior/central/inferior areas. |
| Martone, 200929 | Retrospective, single-masked clinical study | 84 patients with primary open-angle glaucoma or ocular hypertension and 20 health age-matched volunteers | Untreated vs. timolol with 0.01% BAK vs PF timolol vs latanoprost with 0.02% BAK vs. timolol/latanoprost combination drop with 0.02% BAK vs. timolol with 0.01% BAK and latanoprost with 0.02% BAK separately | Long-term use (23 to 28.7 months) | Patients on glaucoma drops statistically more significant ocular surface disease than untreated eyes. Corneal sensitivity, Schirmer I test, TBUT, superficial epithelial cell density were significantly lower in the preservative medication groups compared to the preservative-free group Stromal keratocyte activation was higher in the preservative medication groups |
| Wong, 2018104 | Cross-sectional, investigator-masked, paired-eye comparison study | 33 patients with open angle glaucoma or ocular hypertension receiving medication in only one eye | 88% of study participants used prostaglandin analogues. 100% used BAK-containing eye drops. | Intermediate to long-term use (at least 6 months) | Treated eyes had statistically significant poorer tear film osmolarity, decreased TBUT, decreased tear meniscus height, and increased eyelid margin abnormality scores. No statistically significant difference in meibomian dropout, expressed meibum content, ocular surface staining. |
| Yamazaki, 201070 | Prospective multicenter, open-label uncontrolled study | 45 patients with open-angle glaucoma or ocular hypertension | Switching from BAK-preserved latanoprost to SofZia-preserved travoprost | Intermediate-term use (3 months) | Mean superficial punctate keratopathy score decreased significantly in the whole cornea after switching for SofZia-preserved travoprost |
Diagnostic Testing to Monitor Ocular Surface Complications
Determination of the effect of glaucoma drops on the ocular surface can be evaluated through clinical exam as well as through functional questionnaires to quantify the effects of ocular surface disease on patient quality of life. Slit lamp exam can reveal ocular surface issues with vital dye staining, determination of tear breakup time, Schirmer’s testing, conjunctival reaction, meibomian gland dysfunction, and evaluation of the corneal epithelium (Figure 1).
Figure 1.
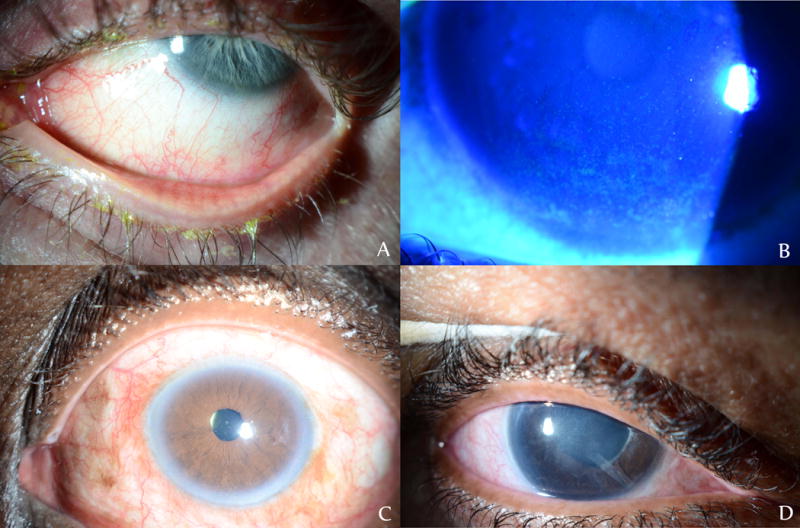
Examples of ocular surface disease in glaucoma patients poorly-tolerant to topical antiglaucomatous drops A) Evidence of telangiectatic vessels at lid margin, matting of lashes, and meibomian gland disease after separate trials of brimonidine tartrate/timolol maleate 0.2%/0.5%, latanoprost 0.005%, bimatoprost 0.01%, timolol 0.5% B) Diffuse punctate epithelial erosions under fluorescein staining in a patient on bimatoprost 0.01%, brimonidine tartrate 0.1%. C) Diffuse conjunctival hyperemia in a patient on travoprost 0.004% D) Diffuse conjunctival injection and subepithelial haze from persistent epitheliopathy after taking multiple BAK-containing glaucoma drops, including brimonidine tartrate/timolol maleate 0.2%/0.5%
Each additional drop of BAK-containing medication can double the likelihood of abnormal lissamine green staining20. One study found that patients on antiglaucoma medications with preservatives had significantly decreased Schirmer’s and tear break up time compared to those on preservative free glaucoma drops29. As the duration and number of topical glaucoma medications increases, tear break up time, Schirmers I testing, and corneal staining showed worsening signs of ocular surface damage25.
Quality of life can be determined with the use of the Ocular Surface Disease Index [OSDI questionnaire; Allergan, Irvine, CA], a 12 item questionnaire created to estimate the effect of dry eye symptoms on daily visual function and with the use of the Glaucoma Quality of Life-15 (GQL-15) form, a 15 item questionnaire created to estimate the impact of glaucoma on daily visual function14,55,56. Prior studies have used the OSDI questionnaire as a measure of ocular discomfort, function, and environmental triggers55,56. Higher OSDI scores in general are associated with worsening ocular surface symptoms and can be attributed to toxicity at the ocular surface, but higher scores may also be attributed to advancing visual field loss in glaucoma patients14,55,57,58. Lower GQL-15 scores are associated with more severe glaucoma and higher OSDI scores14. With respect to preservatives, BAK containing drops were more likely to result in abnormal OSDI scores than drops without BAK59. A daily dose of more than three BAK-containing drops was an independent risk factor for a higher OSDI score (Odds ratio: 2.61, 95% CI 1.52-4.48)14. Ultimately, determination of the specific effect of dry eye on the quality of life of glaucoma patients is difficult as both conditions can adversely affect visual function and quality of life.
Management of Ocular Surface Disease in Conjunction with Glaucoma Medications
Alternatives to conventional topical glaucoma therapy include alternative medications such as preservative free topical medications or surgical/procedural alternatives such as laser trabeculoplasty, minimally invasive glaucoma surgery, or novel forms of drug delivery that may result in improved outcomes for both ocular surface disease and glaucoma. Worsening of the ocular surface in patients with a history of ocular surface disease, conjunctivochalasis, or in elderly patients more susceptible to dry eye disease can lead to increasing patient anxiety and depression60,61. This change in mood can result in poor medication adherence and may in turn contribute to advancing glaucomatous disease14,15,21,49,62. Approximately 23 to 59% of patients on topical therapy can be non-adherent to prescribed treatment regimens63. Alternative options that can improve patient quality of life and medication compliance can include medical management using drops with less toxicity, intensive ocular surface disease treatment, and surgical management with both noninvasive glaucoma procedures and minimally invasive glaucoma surgery.
Medical Management
Prevention of ocular surface disease in patients on topical glaucoma therapy can be achieved by reducing exposure to BAK. This may involve the use of preservative free medications, glaucoma medications with a lower amount of BAK, alternative preservatives, or concurrent treatment of ocular surface disease. Reducing the eye’s exposure to surface damaging preservatives is the goal when attempting to prevent worsening of ocular surface disease and improving adherence to topical medications16,17,18,64.
Currently, most available generic eye drops in the United States contain BAK as a preservative. Recently, preservative-free formulations of glaucoma medications have become more popular to avoid the ocular surface irritation induced by BAK and other preservatives, and studies comparing the effectiveness of these formulations have shown an improvement in ocular surface symptoms when patients switched from BAK-preserved medications to preservative free glaucoma medications24,25,26,29,59,65–67. One study found that pain during drop instillation, foreign body sensation, burning, and dry eye sensation were significantly decreased in patients using topical preservative free beta blocker carteolol in comparison to BAK preserved carteolol65. Subjects in the BAK preserved carteolol group also had decreased TBUT from baseline, whereas those on preservative free carteolol had no change in TBUT65.
Higher BAK concentration in glaucoma medication has been found to be associated with greater ocular surface toxicity, and lower BAK concentration in glaucoma drops can result in a lower rate of ocular surface disease. A study in Japan found that a lower concentration of BAK in tafluprost ophthalmic solution (0.001%-0.003% compared to the normal 0.005%-0.01%) resulted in a level of corneal epithelial cell cytotoxicity comparable to preservative free tafluprost68. Similarly switching from latanoprost with 0.02% BAK, bimatoprost with 0.005% BAK, or tafluprost ophthalmic solution with 0.005%-0.01% BAK to tafluprost with 0.001%-0.003% BAK resulted in a significant reduction in superficial punctate keratitis at twelve weeks68.
BAK free preparations that utilize alternative preservatives have been shown in studies to result in improved ocular surface disease. Patients using polyquaternium-1 preserved travoprost had significantly lower OSDI scores at three months and six months compared to patients using BAK preserved travoprost, indicating a higher level of visual comfort and function with use of the alternative preservative69. SofZia reacted with cations on the ocular surface and formed non-toxic byproducts that resulted in less superficial punctate keratitis than BAK preserved travoprost40,70. Switching from BAK preserved latanoprost and bimatoprost to sofZia preserved travoprost (Travatan – Z; Alcon Laboratories, Fort Worth, TX) was noted to reduce OSDI scores, decrease conjunctival hyperemia, and improve visual acuity71. Of note, studies with BAK preserved and preservative free tafluprost, travoprost, betaxalol, topical 2% carteolol, and 0.1% timolol gel revealed no difference in terms of amount and duration of IOP reduction between preserved and preservative-free versions of the same topical medication 39,72–76.
Glaucoma medications with non-BAK preservatives and preservative free topical medications are not available in generic formulations in the United States, potentially increasing drug costs for patients40,69. One study created a questionnaire-based approach on recommending preservative free glaucoma treatments only to patients with a TBUT less than 10 seconds, marked corneal staining, hyperemia, and OSD complaints. This method may improve patient selection if cost is a barrier in patient care77. Notably, preservative free drops may have an increased risk of contamination especially in older patients who may have difficulty with appropriate drop instillation78. If no alternative is possible other than a BAK containing antiglaucoma medication, prostaglandins may be preferable because some evidence has suggested that these drops may cause less damage to the conjunctiva79,80.
Finally, maximizing the health of the ocular surface may be another treatment option that is viable for some patients. Utilizing artificial tears with sodium hyaluronate or hydroxypropyl-methycellulose/dextran, both ocular surface lubricants, can provide some relief of ocular surface disease symptoms. In a trial comparing safety and efficacy of hydroxypropyl-methycellulose/dextran with 0.18% sodium hyaluronate, glaucoma patients with ocular surface disease who used 0.18% sodium hyaluronate showed significantly more improvement in mean OSDI score, lid margin inflammation, and conjunctival injection81. For patients who are tolerant to BAK preserved anti-glaucoma medications, adding sodium hyaluronate containing artificial tears as an adjunct appears to decrease OSDI scores, increase goblet cell density, and increase TBUT82–84. Furthermore, visual field test parameters were improved in patients using artificial tears, highlighting the impact of a stable tear film and healthy corneal surface1.
Another preventative and ocular surface modifying measure in mild to moderate dry eye disease is the use of topical cyclosporine 0.05% twice daily in conjunction with glaucoma drops29. Topical cyclosporine 0.05% has been found to be beneficial for ocular surface symptoms following a trabeculectomy16. In glaucoma patients on chronic topical glaucoma therapy, taking topical cyclosporine for 6 months was found to significantly improve their sub-basal nerve fiber layer density and corneal sensitivity, helping to reverse the adverse surface toxicity from preserved anti-glaucoma medications29,85.
While studies are preliminary, additional supplements forskolin, vitamin A, and carbomer may benefit glaucoma patients with ocular surface disease. Forskolin is a plant-based product seen to induce conjunctival accessory lacrimal gland secretion and decrease intraocular pressure by reducing aqueous humor production through activation of the ciliary epithelial adenylate cyclase receptor complex86,87. Forskolin with rutin, vitamin B1, and vitamin B2 taken for thirty days was found to improve dry eye symptoms in 38 patients with POAG on chronic glaucoma medications by decreasing their OSDI score, increasing Schirmer’s test scores, and increasing TBUT86. Further studies are required to assess the specific effects of forskolin on both the ocular surface and intraocular pressure. In patients on long-term prostaglandin analogues, preliminary studies show that vitamin A palmitate and carbomer gel appeared to relieve dry eye symptoms by increasing conjunctival goblet cell density88, though larger studies are required for further assessment of this supplement.
Surgical Management
Practitioners treating glaucoma patients who have concomitant ocular surface disease should consider alternative ways to lower intraocular pressure to minimize potential ocular surface effects from topical medications. Studies have determined that laser trabeculoplasty is an effective first line therapy for glaucoma89. Specifically, the Glaucoma Laser Trial determined that argon laser trabeculoplasty was as effective as topical timolol for the first line treatment of glaucoma. Laser trabeculoplasty has the dual benefit of decreasing medication noncompliance as well as decreasing the incidence of ocular surface disease by potentially lowering the medication burden. Newer laser studies using micropulse diode laser trabeculoplasty (MDLT), titanium sapphire laser trabeculoplasty (TLT), pattern scan laser trabeculoplasty (PLT) are currently under investigation, and may broaden the clinical scope of laser trabeculoplasty and further glaucoma treatment90–92.
Filtration surgery may aid in better controlling glaucoma by decreasing the topical medication burden; however, patients post-glaucoma surgery can also experience conjunctival scarring and chronic ocular irritation16–18,93 (Figure 2). The Collaborative Initial Glaucoma Treatment study (CIGTS) evaluated the benefits of initial trabeculectomy treatment versus topical medical treatment and found that patients undergoing trabeculectomy had a higher rate of ocular irritation at five years while other studies have found no difference in dry eye symptoms between patients on chronic anti-glaucoma medications and those who had undergone trabeculectomy67,94.
Figure 2.
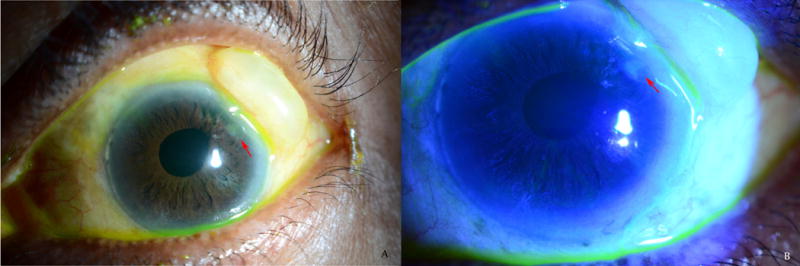
Example of ocular surface disease in glaucoma patient resulting from glaucoma surgery and large bleb A) White light photograph B) Epithelial defect anterior to bleb is highlighted with fluorescein staining (see red arrows)
While trabeculectomy may not be an ideal option for patients with pre-existing ocular surface disease, minimally invasive glaucoma surgery (MIGS) with less potential interaction with the ocular surface [iStent (Glaukos Corp., Laguna Hills, CA), Hydrus Microstent (Ivantis Inc., Irvine, CA), CyPass Microstent (Alcon, Fort Worth, TX), XEN Gel Stent (Allergan Plc, Dublin, Ireland), or ab interno trabeculotomy with the trabectome or Kahook Dual Blade (New World Medical, Rancho Cucamonga, CA)] may be more appropriate options for patients with concomitant ocular surface disease and glaucoma95–97. No current studies specifically examining the effect of these new technologies on the ocular surface exist to our knowledge, but given that these surgeries spare the conjunctiva and decrease the burden of topical glaucoma drops, they may result in less irritation to the ocular surface.
Future Directions
Two new drops have recently been approved in the United States for topical treatment of glaucoma. Netarsidil 0.02% (Rhopressa, Aerie Pharmaceuticals, Irvine, CA), recently approved for topical use in the United States, is a Rho-kinase inhibitor and norepinephrine transporter inhibitor that lowers intraocular pressure and also causes conjunctival hyperemia in 50 to 53% of patients98. This hyperemia, graded by investigators on a scale of 1 to 4 at worsening severities at each follow-up, did not cause significant ocular symptoms and may be due to the ability of Rho-kinase inhibitors to cause vasodilation in vascular smooth muscle98. The second agent newly available for topical use is 0.024% latanaprostene bunod (Vyzulta, Bausch and Lomb, Bridgewater, NJ). Latanaprostene bunod is a nitric oxide donating prostaglandin F2 analog. Patients using 0.024% latanaprostene bunod for a mean of 90 days did have an increase in conjunctival hyperemia (5.9%), eye pain (3.9%), and eye irritation (4.2%) from baseline in comparison to 0.5% timolol99.
While glaucoma is primarily treated with topical medications, introduction of new forms of drug delivery may revolutionize glaucoma care and also minimize ocular surface toxicity. Sustained release formulations allowing for a depot release of glaucoma medications that could last for weeks or months are currently in development. These include the bimatoprost ocular insert, latanoprost and travoprost punctal plugs, latanoprost-eluting contact lenses, bimatoprost and travoprost intraocular implants. Such formulations are applied in the subconjunctival space, inserted into the punctum, placed on the ocular surface, or injected intracamerally 100,101. Travoprost punctal plugs are able to slowly release the prostaglandin analog into patients’ tear film over a 90 day period and was found to not cause any hyperemia in a pharmaceutical phase 2b glaucoma clinical trial101. Notably, an early animal study using subconjunctival dorzolamide found post-injection subconjunctival inflammatory cells indicating that the subconjunctival application of these medications may still result in some clinical or subclinical ocular surface inflammation102. Further study is required to assess the potential benefit of decreased ocular surface toxicity with these new formulations.
Conclusion
The coexistence of glaucoma, glaucoma therapy, and ocular surface disease is an important consideration for patients and physicians. When prescribing medications, being conscious of potential ocular surface symptoms and patient risk factors for ocular surface disease may improve both patient and physician satisfaction with both current and new therapies. Further studies are needed to elucidate the complex relationship between these two ocular diseases. However, based on available literature, a variety of possible strategies reviewed here for reducing ocular morbidity from ocular surface disease in patients being treated for glaucoma can be employed while also improving adherence and success of glaucoma therapies.
Acknowledgments
Grants/Funding: Dr. Saeedi is funded by an NIH Career Development Award (K23 EY025014)
Footnotes
Conflicts of Interest: The authors listed have no conflicts of interest.
References
- 1.Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology. 2017;124(11S):S4–S13. doi: 10.1016/j.ophtha.2017.07.010. doi: S0161-6420(17)30580-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo MM. Ocular surface disease in glaucoma: Effect of polypharmacy and preservatives. Optom Vis Sci. 2015;92(9):e222–6. doi: 10.1097/OPX.0000000000000542. [doi] [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. doi: 90/3/262 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Methodologies to diagnose and monitor dry eye disease: Report of the diagnostic methodology subcommittee of the international dry eye WorkShop (2007) Ocul Surf. 2007;5(2):108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 5.Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28(4):267–282. doi: 10.2165/11588830-000000000-00000. [doi] [DOI] [PubMed] [Google Scholar]
- 6.Ferhina S, Ali BS, Esen K, Akpek MD. Glaucoma and dry eye. Ophthalmology. 2009;116(6):1232. doi: 10.1016/j.ophtha.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Prum BE, Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern((R)) guidelines. Ophthalmology. 2016;123(1):P41–P111. doi: 10.1016/j.ophtha.2015.10.053. [doi] [DOI] [PubMed] [Google Scholar]
- 8.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [doi] [DOI] [PubMed] [Google Scholar]
- 9.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2. [doi] [DOI] [PubMed] [Google Scholar]
- 10.Russ HH, Nogueira-Filho PA, de Barros JN, et al. Ocular surface evaluation in patients treated with a fixed combination of prostaglandin analogues with 0.5% timolol maleate topical monotherapy: A randomized clinical trial. Clinics (Sao Paulo) 2013;68(10):1318–1324. doi: 10.6061/clinics/2013(10)05. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi GC, Scudeller L, Rolle T, Pasinetti GM, Bianchi PE. From benzalkonium chloride-preserved latanoprost to polyquad-preserved travoprost: A 6-month study on ocular surface safety and tolerability. Expert Opin Drug Saf. 2015;14(5):619–623. doi: 10.1517/14740338.2015.1017467. [doi] [DOI] [PubMed] [Google Scholar]
- 12.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 13.Kuppens EV, van Best JA, Sterk CC, de Keizer RJ. Decreased basal tear turnover in patients with untreated primary open-angle glaucoma. Am J Ophthalmol. 1995;120(1):41–46. doi: 10.1016/s0002-9394(14)73757-2. [DOI] [PubMed] [Google Scholar]
- 14.Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9.e2. doi: 10.1016/j.ajo.2011.05.033. [doi] [DOI] [PubMed] [Google Scholar]
- 15.Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: The good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001. [doi] [DOI] [PubMed] [Google Scholar]
- 16.Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Experiment Ophthalmol. 2000;28(2):113–117. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 17.Broadway D, Hitchings R, Grierson I. Topical antiglaucomatous therapy: Adverse effects on the conjunctiva and implications for filtration surgery. J Glaucoma. 1995;4(2):136. doi: 00061198-199504000-00012 [pii] [PubMed] [Google Scholar]
- 18.Johnson DH, Yoshikawa K, Brubaker RF, Hodge DO. The effect of long-term medical therapy on the outcome of filtration surgery. Am J Ophthalmol. 1994;117(2):139–148. doi: 10.1016/s0002-9394(14)73068-5. [DOI] [PubMed] [Google Scholar]
- 19.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The ocular hypertension treatment study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. JAMA Ophthalmol. 2002;120(6):701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 20.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 21.Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1593–1601. doi: 10.1007/s00417-008-0881-9. [doi] [DOI] [PubMed] [Google Scholar]
- 22.Baudouin C, Renard JP, Nordmann JP, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. 2012:0. doi: 10.5301/ejo.5000181. doi: 7DCA742B-4568-4F77-A958-FA5574C8D807 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Lin C, Tsai Y, Kao C. Association between glaucoma medication usage and dry eye in taiwan. Optom Vis Sci. 2015;92(9):e227–e232. doi: 10.1097/OPX.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 24.Cvenkel B, Stunf S, Srebotnik Kirbis I, Strojan Flezar M. Symptoms and signs of ocular surface disease related to topical medication in patients with glaucoma. Clin Ophthalmol. 2015;9:625–631. doi: 10.2147/OPTH.S81247. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saade CE, Lari HB, Berezina TL, Fechtner RD, Khouri AS. Topical glaucoma therapy and ocular surface disease: A prospective, controlled cohort study. Can J Ophthalmol. 2015;50(2):132–136. doi: 10.1016/j.jcjo.2014.11.006. [doi] [DOI] [PubMed] [Google Scholar]
- 26.Labbe A, Terry O, Brasnu E, Van Went C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012;31(9):994–999. doi: 10.1097/ICO.0b013e31823f8cb6. [doi] [DOI] [PubMed] [Google Scholar]
- 27.Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, Karakaya J, Irkec M. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016 doi: 10.1097/IJG.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 28.Arita R, Itoh K, Maeda S, et al. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea. 2012;31(11):1229–1234. doi: 10.1097/ICO.0b013e31823f8e7d. [doi] [DOI] [PubMed] [Google Scholar]
- 29.Martone G, Frezzotti P, Tosi GM, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147(4):725–735.e1. doi: 10.1016/j.ajo.2008.10.019. [doi] [DOI] [PubMed] [Google Scholar]
- 30.Kuppens EV, Stolwijk TR, de Keizer RJ, van Best JA. Basal tear turnover and topical timolol in glaucoma patients and healthy controls by fluorophotometry. Invest Ophthalmol Vis Sci. 1992;33(12):3442–3448. [PubMed] [Google Scholar]
- 31.Thygesen J, Aaen K, Theodorsen F, Kessing SV, Prause JU. Short-term effect of latanoprost and timolol eye drops on tear fluid and the ocular surface in patients with primary open-angle glaucoma and ocular hypertension. Acta Ophthalmol Scand. 2000;78(1):37–44. doi: 10.1034/j.1600-0420.2000.078001037.x. [DOI] [PubMed] [Google Scholar]
- 32.Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99(7):1082–1088. doi: 10.1016/s0161-6420(92)31847-0. doi: S0161-6420(92)31847-0 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Osborne SA, Montgomery DM, Morris D, McKay IC. Alphagan allergy may increase the propensity for multiple eye-drop allergy. Eye (Lond) 2005;19(2):129–137. doi: 10.1038/sj.eye.6701441. [doi] [DOI] [PubMed] [Google Scholar]
- 34.Wirtitsch MG, Findl O, Heinzl H, Drexler W. Effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2007;125(10):1345–1350. doi: 10.1001/archopht.125.10.1345. doi: 125/10/1345 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Robciuc A, Witos J, Ruokonen S, et al. Pure glaucoma drugs are toxic to immortalized human corneal epithelial cells, but they do not destabilize lipid membranes. Cornea. 2017;36(10):1249–1255. doi: 10.1097/ICO.0000000000001322. [DOI] [PubMed] [Google Scholar]
- 36.Sherwood MB, Grierson I, Millar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and tenon's capsule in glaucomatous patients. Ophthalmology. 1989;96(3):327–335. doi: 10.1016/s0161-6420(89)32888-0. doi: S0161-6420(89)32888-0 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Baudouin C, Liang H, Hamard P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology. 2008;115(1):109–115. doi: 10.1016/j.ophtha.2007.01.036. doi: S0161-6420(07)00209-6 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Baudouin C, Garcher C, Haouat N, Bron A, Gastaud P. Expression of inflammatory membrane markers by conjunctival cells in chronically treated patients with glaucoma. Ophthalmology. 1994;101(3):454–460. doi: 10.1016/s0161-6420(94)31322-4. doi: S0161-6420(94)31322-4 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82(1):39–42. doi: 10.1136/bjo.82.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anwar Z, Wellik SR, Galora A. Glaucoma therapy and ocular surface disease: Current literature and recommendations. Curr Opin Ophthalmol. 2013;24(2):136–143. doi: 10.1097/ICU.0b013e32835c8aba. [DOI] [PubMed] [Google Scholar]
- 41.Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: An ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45(5):1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 42.Actis AG, Rolle T. Ocular surface alterations and topical antiglaucomatous therapy: A review. Open Ophthalmol J. 2014;8(1):67–72. doi: 10.2174/1874364101408010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur IP, Lal S, Rana C, Kakkar S, Singh H. Ocular preservatives: Associated risks and newer options. Cutan Ocul Toxicol. 2009;28(3):93–103. doi: 10.1080/15569520902995834. [doi] [DOI] [PubMed] [Google Scholar]
- 44.Majumdar S, Hingorani T, Srirangam R, Gadepalli RS, Rimoldi JM, Repka MA. Transcorneal permeation of L- and D-aspartate ester prodrugs of acyclovir: Delineation of passive diffusion versus transporter involvement. Pharm Res. 2009;26(5):1261–1269. doi: 10.1007/s11095-008-9730-0. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathore MS, Majumdar DK. Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS PharmSciTech. 2006;7(3):57. doi: 10.1208/pt070357. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pawar PK, Majumdar DK. Effect of formulation factors on in vitro permeation of moxifloxacin from aqueous drops through excised goat, sheep, and buffalo corneas. AAPS PharmSciTech. 2006;7(1):E13. doi: 10.1208/pt070113. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dave V, Paliwal S, Yadav S, Sharma S. Effect of in vitro transcorneal approach of aceclofenac eye drops through excised goat, sheep, and buffalo corneas. ScientificWorldJournal. 2015;2015:432376. doi: 10.1155/2015/432376. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuda M, Sasaki H. The transcorneal penetration of commercial ophthalmic formulations containing timolol maleate in rabbit eyes. J Ocul Pharmacol Ther. 2015;31(1):57–60. doi: 10.1089/jop.2014.0015. [doi] [DOI] [PubMed] [Google Scholar]
- 49.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2. [doi] [DOI] [PubMed] [Google Scholar]
- 50.Liang H, Pauly A, Riancho L, Baudouin C, Brignole-Baudouin F. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95(6):869–875. doi: 10.1136/bjo.2010.189449. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Januleviciene I, Derkac I, Grybauskiene L, Paulauskaite R, Gromnickaite R, Kuzmiene L. Effects of preservative-free tafluprost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin Ophthalmol. 2012;6:103–109. doi: 10.2147/OPTH.S28104. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23(5):490–496. doi: 10.1097/01.ico.0000116526.57227.82. doi: 00003226-200407000-00012 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Kanamoto T, Kiuchi Y, Tanito M, et al. Comparison of the toxicity profile of benzalkonium chloride-preserved tafluprost and sofzia-preserved travoprost applied to the ocular surface. J Ocul Pharmacol Ther. 2015;31(3):156–164. doi: 10.1089/jop.2014.0104. [DOI] [PubMed] [Google Scholar]
- 54.Ryan G, Jr, Fain JM, Lovelace C, Gelotte KM. Effectiveness of ophthalmic solution preservatives: A comparison of latanoprost with 0.02% benzalkonium chloride and travoprost with the sofZia preservative system. BMC Ophthalmol. 2011;11 doi: 10.1186/1471-2415-11-8. 8-2415-11-8. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 56.Gulati A, Sullivan R, Buring JE, Sullivan DA, Dana R, Schaumberg DA. Validation and repeatability of a short questionnaire for dry eye syndrome. Am J Ophthalmol. 2006;142(1):125–131. doi: 10.1016/j.ajo.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: Clinical implications. Acta Ophthalmol. 2014;92(2):161–166. doi: 10.1111/aos.12012. [doi] [DOI] [PubMed] [Google Scholar]
- 58.Mathews PM, Ramulu PY, Friedman DS, Utine CA, Akpek EK. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120(11):2241–2248. doi: 10.1016/j.ophtha.2013.03.045. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo MM. Ocular surface disease in glaucoma: Effect of polypharmacy and preservatives. Optom Vis Sci. 2015;92(9):e222–e226. doi: 10.1097/OPX.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 60.Szakats I, Sebestyen M, Nemeth J, Birkas E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res. 2016;41(8):1044–1049. doi: 10.3109/02713683.2015.1088955. [doi] [DOI] [PubMed] [Google Scholar]
- 61.Li M, Gong L, Sun X, Chapin W. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36(1):1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 62.Kocabeyoglu S, Mocan MC, Irkec M, Orhan M, Karakaya J. Conjunctivochalasis as a contributing factor for the development of ocular surface disease in medically treated glaucoma patients. J Glaucoma. 2014;23(5):333–336. doi: 10.1097/IJG.0b013e3182741f32. [doi] [DOI] [PubMed] [Google Scholar]
- 63.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl1):S57–68. doi: 10.1016/j.survophthal.2008.08.002. [doi] [DOI] [PubMed] [Google Scholar]
- 64.Fakhraie G, Lopes JF, Spaeth GL, Almodin J, Ichhpujani P, Moster MR. Effects of postoperative cyclosporine ophthalmic emulsion 0.05% (restasis) following glaucoma surgery. Clin Experiment Ophthalmol. 2009;37(9):842–848. doi: 10.1111/j.1442-9071.2009.02134.x. [doi] [DOI] [PubMed] [Google Scholar]
- 65.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 66.Rossi GC, Pasinetti GM, Scudeller L, Bianchi PE. Ocular surface disease and glaucoma: How to evaluate impact on quality of life. J Ocul Pharmacol Ther. 2013;29(4):390–394. doi: 10.1089/jop.2011.0159. [doi] [DOI] [PubMed] [Google Scholar]
- 67.Lee SY, Wong TT, Chua J, Boo C, Soh YF, Tong L. Effect of chronic anti-glaucoma medications and trabeculectomy on tear osmolarity. Eye (Lond) 2013;27(10):1142–1150. doi: 10.1038/eye.2013.144. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki K, Teranishi S, Sagara T, et al. Safety and efficacy of benzalkonium chloride-optimized tafluprost in japanese glaucoma patients with existing superficial punctate keratitis. J Glaucoma. 2015;24(6):e145–50. doi: 10.1097/IJG.0000000000000020. [doi] [DOI] [PubMed] [Google Scholar]
- 69.Sezgin Akcay BI, Guney E, Bozkurt TK, Topal CS, Akkan JC, Unlu C. Effects of polyquaternium- and benzalkonium-chloride-preserved travoprost on ocular surfaces: An impression cytology study. J Ocul Pharmacol Ther. 2014;30(7):548–553. doi: 10.1089/jop.2013.0248. [doi] [DOI] [PubMed] [Google Scholar]
- 70.Yamazaki S, Nanno M, Kimura T, Suzumura H, Yoshikawa K. Effects of switching to SofZia-preserved travoprost in patients who presented with superficial punctate keratopathy while under treatment with latanoprost. Jpn J Ophthalmol. 2010;54(1):7–14. doi: 10.1007/s10384-009-0754-8. [doi] [DOI] [PubMed] [Google Scholar]
- 71.Henry JC, Peace JH, Stewart JA, Stewart WC. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol. 2008;2(3):613–621. doi: 10.2147/opth.s3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denis P, Monoprost French Study Group Unpreserved latanoprost in the treatment of open-angle glaucoma and ocular hypertension. A multicenter, randomized, controlled study. J Fr Ophtalmol. 2016 doi: 10.1016/j.jfo.2016.05.006. doi: S0181-5512(16)30149-8 [pii] [DOI] [PubMed] [Google Scholar]
- 73.Easty DL, Nemeth-Wasmer G, Vounatsos JP, et al. Comparison of a non-preserved 0.1% T-gel eye gel (single dose unit) with a preserved 0.1% T-gel eye gel (multidose) in ocular hypertension and glaucomatous patients. Br J Ophthalmol. 2006;90(5):574–578. doi: 10.1136/bjo.2005.080424. doi: 90/5/574 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uusitalo H, Kaarniranta K, Ropo A. Pharmacokinetics, efficacy and safety profiles of preserved and preservative-free tafluprost in healthy volunteers. Acta Ophthalmol Suppl (Oxf) 2008;242:7–13. doi: 10.1111/j.1755-3768.2008.01380.x. [doi] [DOI] [PubMed] [Google Scholar]
- 75.Hamacher T, Airaksinen J, Saarela V, Liinamaa MJ, Richter U, Ropo A. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: Results from a pharmacodynamics analysis. Acta Ophthalmol Suppl (Oxf) 2008;242:14–19. doi: 10.1111/j.1755-3768.2008.01381.x. [doi] [DOI] [PubMed] [Google Scholar]
- 76.Gross RL, Peace JH, Smith SE, et al. Duration of IOP reduction with travoprost BAK-free solution. J Glaucoma. 2008;17(3):217–222. doi: 10.1097/IJG.0b013e31815a3472. [doi] [DOI] [PubMed] [Google Scholar]
- 77.Pfennigsdorf S, Eschstruth P. Preservative-free glaucoma treatment: Selection of the correct treatment in 1 min. Ophthalmologe. 2016;113(5):409–415. doi: 10.1007/s00347-015-0168-6. [doi] [DOI] [PubMed] [Google Scholar]
- 78.Kim MS, Choi CY, Kim JM, Chang HR, Woo HY. Microbial contamination of multiply used preservative-free artificial tears packed in reclosable containers. Br J Ophthalmol. 2008;92(11):1518–1521. doi: 10.1136/bjo.2008.144469. [doi] [DOI] [PubMed] [Google Scholar]
- 79.Zhu W, Kong X, Xu J, Sun X. Effects of long-term antiglaucoma eye drops on conjunctival structures: An in vivo confocal microscopy study. J Ophthalmol. 2015;2015:165475. doi: 10.1155/2015/165475. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demirel S, Doganay S, Gurses I, Iraz M. Toxic-inflammatory effects of prostoglandin analogs on the ocular surface. Ocul Immunol Inflamm. 2013;21(1):13–18. doi: 10.3109/09273948.2012.723106. [doi] [DOI] [PubMed] [Google Scholar]
- 81.Prabhasawat P, Ruangvaravate N, Tesavibul N, Thewthong M. Effect of 0.3% hydroxypropyl methylcellulose/dextran versus 0.18% sodium hyaluronate in the treatment of ocular surface disease in glaucoma patients: A randomized, double-blind, and controlled study. J Ocul Pharmacol Ther. 2015;31(6):323–329. doi: 10.1089/jop.2014.0115. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Yu FF, Zhong YM, Guo XX, Mao Z. Therapeutic effects of sodium hyaluronate on ocular surface damage induced by benzalkonium chloride preserved anti-glaucoma medications. Chin Med J (Engl) 2015;128(18):2444–2449. doi: 10.4103/0366-6999.164927. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86(2):181–184. doi: 10.1136/bjo.86.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pauloin T, Dutot M, Warnet JM, Rat P. In vitro modulation of preservative toxicity: High molecular weight hyaluronan decreases apoptosis and oxidative stress induced by benzalkonium chloride. Eur J Pharm Sci. 2008;34(4–5):263–273. doi: 10.1016/j.ejps.2008.04.006. [doi] [DOI] [PubMed] [Google Scholar]
- 85.Saini M, Dhiman R, Dada T, Tandon R, Vanathi M. Topical cyclosporine to control ocular surface disease in patients with chronic glaucoma after long-term usage of topical ocular hypotensive medications. Eye (Lond) 2015;29(6):808–814. doi: 10.1038/eye.2015.40. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nebbioso M, Rusciano D, Pucci B, Zicari AM, Grenga R, Pescocolido N. Treatment of glaucomatous patients by means of food supplement to reduce the ocular discomfort: A double blind randomized trial. Eur Rev Med Pharmacol Sci. 2013;17(8):1117–1122. doi: 3870 [pii] [PubMed] [Google Scholar]
- 87.Majeed M, Nagabhushanam K, Natarajan S, Vaidyanathan P, Karri SK, Jose JA. Efficacy and safety of 1% forskolin eye drops in open angle glaucoma - an open label study. Saudi J Ophthalmol. 2015;29(3):197–200. doi: 10.1016/j.sjopt.2015.02.003. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui X, Xiang J, Zhu W, et al. Vitamin A palmitate and carbomer gel protects the conjunctiva of patients with long-term prostaglandin analogs application. J Glaucoma. 2016;25(6):487–492. doi: 10.1097/IJG.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 89.Waisbourd M, Katz LJ. Selective laser trabeculoplasty as a first-line therapy: A review. Can J Ophthalmol. 2014;49(6):519–522. doi: 10.1016/j.jcjo.2014.10.003. [doi] [DOI] [PubMed] [Google Scholar]
- 90.Lee JW, Yau GS, Yick DW, Yuen CY. MicroPulse laser trabeculoplasty for the treatment of open-angle glaucoma. Medicine (Baltimore) 2015;94(49):e2075. doi: 10.1097/MD.0000000000002075. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaplowitz K, Wang S, Bilonick R, Oatts JT, Grippo T, Loewen NA. Randomized controlled comparison of titanium-sapphire versus standard Q-switched nd: YAG laser trabeculoplasty. J Glaucoma. 2016;25(7):e663–7. doi: 10.1097/IJG.0000000000000317. [doi] [DOI] [PubMed] [Google Scholar]
- 92.Mansouri K, Shaarawy T. Comparing pattern scanning laser trabeculoplasty to selective laser trabeculoplasty: A randomized controlled trial. Acta Ophthalmol. 2017;95(5):e361–e365. doi: 10.1111/aos.13280. [doi] [DOI] [PubMed] [Google Scholar]
- 93.Fakhraie G, Lopes JF, Spaeth GL, Almodin J, Ichhpujani P, Moster MR. Effects of postoperative cyclosporine ophthalmic emulsion 0.05% (restasis) following glaucoma surgery. Clin Experiment Ophthalmol. 2009;37(9):842–848. doi: 10.1111/j.1442-9071.2009.02134.x. [doi] [DOI] [PubMed] [Google Scholar]
- 94.Janz NK, Wren PA, Lichter PR, et al. The collaborative initial glaucoma treatment study: Interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108(11):1954–1965. doi: 10.1016/s0161-6420(01)00874-0. [DOI] [PubMed] [Google Scholar]
- 95.Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22(3):205–208. doi: 10.1097/IJG.0b013e3182311b92. [doi] [DOI] [PubMed] [Google Scholar]
- 96.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Fernandez-Perez C, Garcia-Sanchez J, Garcia-Feijoo J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br J Ophthalmol. 2012;96(5):645–649. doi: 10.1136/bjophthalmol-2011-300218. [doi] [DOI] [PubMed] [Google Scholar]
- 97.Hoeh H, Ahmed II, Grisanti S, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39(3):431–437. doi: 10.1016/j.jcrs.2012.10.040. [doi] [DOI] [PubMed] [Google Scholar]
- 98.Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.11.019. doi: S0002-9394(17)30513-5 [pii] [DOI] [PubMed] [Google Scholar]
- 99.Weinreb R, Liebmann J, Martin K, Kaufman P, Vittitow J. Latanoprostene bunod 0.024% in subjects with open-angle glaucoma or ocular hypertension: Pooled phase 3 study findings. J Glaucoma. 2018;27(1):7–15. doi: 10.1097/IJG.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franca JR, Foureaux G, Fuscaldi LL, et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In vitro and in vivo evaluation. PLoS One. 2014;9(4):e95461. doi: 10.1371/journal.pone.0095461. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aref AA, Sivaraman KR, Djalilian AR. Glaucoma drainage implant surgery and ocular surface transplant graft preservation. Semin Ophthalmol. 2015;30(3):210–213. doi: 10.3109/08820538.2013.835840. [doi] [DOI] [PubMed] [Google Scholar]
- 102.Fu J, Sun F, Liu W, et al. Subconjunctival delivery of dorzolamide-loaded poly(ether-anhydride) microparticles produces sustained lowering of intraocular pressure in rabbits. Mol Pharm. 2016;13(9):2987–2995. doi: 10.1021/acs.molpharmaceut.6b00343. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]


