Abstract
Inflammatory mediators released from activated microglia, astrocytes, neurons and mast cells mediate neuroinflammation. Parkinson’s disease (PD) is characterized by inflammation-dependent dopaminergic neurodegeneration in substantia nigra. 1-methyl-4-phenylpyridinium (MPP+), a metabolite of parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induces inflammatory mediators release from brain cells and mast cells. Brain cells interaction with mast cells are implicated in neuroinflammation. However, the exact mechanism involved are not yet clearly understood. Mouse fetal brain-derived cultured primary astrocytes and glia-neurons were incubated with mouse mast cell protease-6 (MMCP-6) and MMCP-7, and mouse bone marrow-derived mast cells (BMMCs) were incubated with MPP+ and brain protein glia maturation factor (GMF). Interleukin-33 (IL-33) released from these cells was quantitated by enzyme-linked immunosorbent assay. Both MMCP-6 and MMCP-7-induced IL-33 release from astrocytes and glia-neurons. MPP+ and GMF used as a positive control-induced IL-33 and reactive oxygen species expression in mast cells. Mast cell proteases and MPP+ activate p38 and extracellular signal-regulated kinases 1/2 (ERK1/2) mitogen-activated protein kinases (MAPKs) and transcription factor nuclear factor-kappa B (NF-κB) in astrocytes, glia-neurons or mast cells. Addition of BMMCs from wt mice and transduction with adeno-GMF show higher chemokine (C-C motif) ligand 2 (CCL2) release. MPP+ activated glial cells, and reduced microtubule associated protein 2 (MAP-2) expression indicating neurodegeneration. IL-33 expression increased in the midbrain and striatum of PD brains as compared with age and sex matched control subjects. Glial cells and neurons interact with mast cells and accelerate neuroinflammation and these interactions can be explored as a new therapeutic target to treat PD.
Keywords: Brain cells, Glia maturation factor, Interleukin-33, Mast cells, Mast cell proteases Parkinson’s disease
Introduction
Mast cells are multifunctional innate immune and inflammatory cells implicated in both physiological as well as pathological conditions by releasing proinflammatory and anti-inflammatory cytokines and chemokines and expression of various receptors [1-4]. Mast cells play important role in tissue homeostasis, host defense and tissue repair in normal conditions [1,5]. They also mediate anaphylaxis, autoimmune, allergic, and inflammatory and neuroinflammatory effects [6,7]. Mast cells protect body through immune surveillance and defense mechanisms [8,9]. Mast cell granules in the cytoplasm are filled with preformed and pre-activated immunomodulatory molecules such as proteases and tumor necrosis factor-alpha (TNF-α) [10,11]. Activated mast cells release these prestored mediators by degranulation process. Mast cells are also target and source of several neuropeptides in the central nervous system (CNS) and as well as in the peripheral system [12-14].
Neuroinflammation plays an important role in the pathogenesis of neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD) and autoimmune diseases such as Multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE) [15-17]. Others and we have previously shown that mast cells are implicated in the neurodegeneration in PD and other brain disorders by releasing several inflammatory mediators in the brain [18-21]. Brain inflammatory protein glia maturation factor (GMF) was first isolated, sequenced and cloned in our laboratory [22-25]. GMF is localized in glia and many neurons in the brains of neurodegenerative diseases [26]. Activated glial cells release GMF that can act on microglia and neurons and induce neurodegeneration in the brain [27-30]. Our previous in vitro studies have shown that GMF and 1-methyl-4-phenylpyridinium (MPP+), an active metabolite of parkinsonian neurotoxin 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP) activate mast cells to release neuroinflammatory molecules [31,32]. However, the molecular mechanism underlying GMF and MPP+-mediated activation of mast cells and mouse mast cell protease-6 (MMCP-6) and MMCP-7-mediated activation of astrocytes and glia-neurons remain poorly understood. Interleukin-33 (IL-33) is a member of the IL-1 family activates mast cells, T helper 1 (Th1), Th2, regulatory T (Treg), CD8+ and natural killer (NK) cells [33]. Mast cells respond to cell injury by detecting alarmin/cell injury associated cytokine IL-33 released from injured cells through IL-33 receptor ST2 expression [34]. Moreover, we have shown increased IL-33 expression in AD brains [35]. IL-33 is an important link between cell necrosis and the protective immune response in the body. However, over activation of immune system leads to vicious circle that can lead to chronic inflammatory reactions. The expression of mitogen-activated protein kinases (MAPKs) and nuclear factor- kappa B (NF-κB) are increased in neuroinflammatory disorders [36-38]. In the present study, we investigated the effect of MMCP-6 and MMCP-7 on mouse primary astrocytes and glia-neuronal cells as well as effect of MPP+ and GMF on mast cells to release neuroinflammatory mediators. Here, we report that mast cell proteases induce astrocyte and glia-neurons, and GMF and MPP’ induce mast cells to release inflammatory mediators through the activation of MAPK and NF-κB pathways.
Methods
Reagents and Antibodies
Dulbecco’s phosphate buffered saline (DPBS), Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (Ham) (DMEM F12) and fetal bovine serum (FBS) were purchased from Life Technologies (Grand Island, NY). Murine recombinant IL-3 was purchased from PeproTech (Rocky Hill, NJ). Cell culture flasks and tissue culture plates were purchased from Costar (Corning Incorporated, and Corning, NY). DuoSet enzyme-linked immunosorbent assay (ELISA) kits for mouse IL-33, mouse chemokine (C-C motif) ligand 2 (CCL2)/JE/MCP-1, human/mouse/rat-phospho-p38α, and human/mouse/rat-phospho-extracellular signal-regulated kinases (ERK1)/ERK2 were purchased from R&D Systems (Minneapolis, MN). C57BL/6 wild-type mice were purchased from Charles River (Wilmington, MA). Recombinant GMF used in this study was prepared at the Vector Core facility (University of Iowa, Iowa City, IA). Antibodies for total p38, phospho p38, total ERK1/ERK2, phospho ERK1/ERK2, NF-κB p65 and phospho NF-κB p65 were purchased from Cell Signaling Technology, Inc. (Danvers, MA) and goat anti-rabbit-IgG-horseradish peroxidase (HRP) were obtained from Santa Cruz Biotechnology (Dallas, TX). Reactive oxygen species (ROS) assay kit was purchased from Abcam (Cambridge, MA). Rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal antibody was from Chemicon International (Temecula, CA), Anti-microtubule associated protein 2 (MAP2) monoclonal antibody, toluidine blue, MPP+ and anti-mast cell tryptase mouse monoclonal antibody (Millipore Sigma, Burlington, MA) were purchased. IL-33 mouse monoclonal antibody was purchased from ThermoScientific (Rockford, IL). ImmPRESS polymer anti-mouse IgG peroxidase reagent (made in goat) kit, ImmPACT DAB peroxidase substrate kit and antifade mounting medium were purchased from Vector Laboratories (Burlingame, MA).
Mouse Primary Mast Cell Culture
Mouse bone marrow-derived mast cells (BMMCs) were cultured from adult wild type (wt) mice as well as from GMF-knockout (GMF-KO) mice as reported previously [18,32]. Our laboratory has previously developed GMF-KO mice and we maintain a colony of these mice for studies [30]. Briefly, mice were sacrificed by cervical dislocation and bone marrow cells were flushed-out of the femur using a syringe and cultured in DMEM supplemented with IL-3 (10 ng/ml), 10% heat-inactivated FBS, 1% penicillin-streptomycin, 20 μM 2-mercaptoethanol, 1% L-glutamine for 6-8 weeks in a 5% CO2 incubator at 37°C. Non-adherent cells were removed from each flasks twice a week with the addition of fresh culture medium. About >99% of the cells were mast cells after 5 weeks of culture as identified by 0.1% toluidine blue staining. Bone marrow collected from several mice were pooled together and cultured. Bone marrow obtained from wt and GMF-KO mice were cultured separately. The present study was conducted according to the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health (NIH). The Committee on the Ethics of Animal Experiments of the University of Missouri (Columbia, MO) approved this protocol.
Mouse Primary Astrocyte and Glia-Neuron Mixed Culture
C57BL/6 pregnant mice were sacrificed on day 16th-18th day of gestation. Astrocytes were isolated from the fetal brains and cultured in vitro, as we have previously reported [18]. Astrocytes were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a 5% CO2 and 95% air atmosphere in tissue culture flasks. These brain cells were grown in vitro using brains from wt mice as reported previously [39,40]. Glia-neurons (mixed culture) were grown in DMEM F-12 containing 5% FBS, 5% horse serum and 1% penicillin/streptomycin at 37 °C in a 5% CO2 and 95% air atmosphere in 25 cm2 or 75 cm2 tissue culture flasks as reported previously [41].
Cell Stimulation with MMCP-6, MMCP-7 and MPP+ and IL-33 Assay in the Cell Culture Supernatant
Astrocytes and glia-neurons were grown to confluence in tissue culture plates or tissue culture flasks and incubated with MMCP-6 (100 ng/ml) and MMCP-7 (100 ng/ml) for 24 h in 0.1% serum supplemented medium. The culture medium was removed, centrifuged and the supernatant medium was collected and stored at −80°C until used for IL-33 assay by ELIA using commercial kits. Mouse primary mast cells were plated at a concentration of 0.5×106 cells/ml in 48 wells and incubated with MPP+ (10 μM) or GMF (100 ng/ml) as a positive control for 6 h and 24 h in 1% serum containing medium. The culture medium was removed from the wells, centrifuged and the supernatant medium was collected, stored at −80°C until IL-33 assay by ELISA using a microplate reader (Molecular Devices; Sunnyvale, CA). The working concentrations of MMCP-6, MMCP-7, MPP+ and GMF were prepared in sterile 0.1% bovine serum albumin (BSA) in DPBS. The untreated control cells were incubated with equal volume of plain culture medium containing 0.1% BSA in DPBS. In another set of experiments, astrocytes and glia-neurons were grown in the tissue culture flasks (25 cm2) to confluence and then treated with mast cell proteases as described earlier and the supernatant medium was removed. After washing with DPBS, the cells were trypsinized using 0.25% trypsin-EDTA, the cell pellet was resuspended in lysis buffer (R&D Systems), and the cell lysates were prepared. Protein concentration was determined in the cell lysates by BCA method. The levels of phospho p38 and phospho ERK1/2 MAPKs were determined by using commercial ELISA kits (R&D Systems). Equal amount of proteins in lysates were used for p-p38 and p-ERK1/2 assays. The plates were read using a microplate reader as mentioned above using a microplate reader (VersaMax; Molecular Devices, Sunnyvale, CA). The results were expressed as pg/mg protein for p-p38 and ng/mg protein for p-ERK1/2.
Effect of MPP+ and GMF on ROS Generation in BMMCs
BMMCs were cultured for 8 weeks, washed and resuspended in 2 ml of 1% FBS containing BMMCs medium and counted. Cells were washed again, resuspended in 1 ml dichlorofluorescin diacetate (DCFDA), and incubated for 30 min at 37 °C incubator. Following this, DCFDA stain was removed by centrifugation with wash buffer. Pellet was resuspended in 1S supplementary buffer (18 ml of 1× buffer + 100 μl ml FBS), transferred 200 μl of cell suspension (0.2×l06 cells) to the wells of 96-well tissue culture plate and incubated with MPP+ or GMF as indicated in the Results and Legends. Then the plates were read at EX485/EM535 using a microplate reader for fluorescence intensity as described previously [42].
GMF Overexpression in BMMCs and Co-culture with Glia-Neurons
GMF overexpression in wt type BMMCs as well as GMF-KO BMMCs was carried out as we have previously shown [40]. We used a full-length rat GMF cDNA (Ad5CMV GMF) and a cytoplasmic lacZ cDNA (Ad5CMV cytolacZ). We incubated BMMCs with a viral dose of 20 multiplicity of infectivity (MOI) in serum-free as well as antibiotics-free DMEM medium for 4 h. Then the BMMCs were washed with plain DMEM and plated (0.2×106 cells/well) in 24 well flat bottom tissue culture plates and incubated with MPP+ (10 μM) for 24 h at 37 °C. Then the culture supernatant was collected by centrifugation and stored at −80 °C. CCL2 level was estimated in these media using commercial ELISA kit (R&D Systems).
Western Blot Analysis for NF-κB
Mast cells, astrocytes and glia-neurons were incubated with GMF, MPP+, MMCP-6 or MMCP-7 for the indicated times and dose. Cell pellets were sonicated (3 cycles) and cell lysates were prepared in RIPA cell lysis buffer with protease and phosphatase inhibitors. Protein concentration of the samples was determined as previously reported. Samples with 25 μg total protein were denatured and subjected to SDS-PAGE on 12% gels, then transferred to polyvinylidene difluoride (PVDF) membranes that were blotted against NF-κB p65 and p-NF-κB p65 (Cell Signaling Technology) and goat anti-rabbit-IgG-HRP secondary antibody (Cell Signaling Technology). Blots were developed using ECL plus Western blotting kit. The bands were visualized using ChemiDoc-It2 Imaging System (UVP LLC, Upland, CA).
Double Immunofluorescence Detection of GFAP and MAP2 in Glia-Neurons After Incubation with MPP+
Glia-neurons were grown in poly-D-lysine coated 24 well tissue culture plates and then incubated with MPP+ for 72 h. Following this, the cells were fixed with 4% paraformaldehyde and double immunofluorescence labelling was performed using the polyclonal antibody to GFAP (1:500 dilution) and monoclonal antibody to MAP2 (5 μg/ml; Millipore Sigma) as reported previously [43,44]. Briefly, after overnight incubation with primary antibodies, a cocktail of Alexa Flour 488 goat anti-rabbit IgG and Alexa Flour 568 goat anti-mouse IgG secondary antibodies (both at 1:500 dilutions) were used for double immunofluorescence labelling. After washing with PBS, the sections were mounted on to the slides, coversliped with mounting medium and viewed with Nikon fluorescence microscope and images were acquired.
Human PD Brain Tissues
Mid brain and striatum from post-mortem brains of PD patients (n=3) as well as age and sex matched non-PD control subjects (n=3) were obtained through Deeded body program at the University of Iowa (Iowa City, IA) and used as per the guidelines of the Institutional Review Board (IRB) of the University of Iowa and the University of Missouri (IRB #2008067; exempt application 224561). This study was conducted following the standard ethical guidelines and used appropriate personal protection safety procedures while using the human tissue samples.
Toluidine Blue and Tryptase Staining for Mast Cells Detection in PD Brain
Mast cells were identified in the mid brain sections (40 μm) from PD patients by staining with 0.1% toluidine blue for 2 min. Mast cells were identified by violet or shades of blue color as we have previously reported [45,46]. Mast cell degranulation was evaluated by the appearance of the mast cells and presence of extracellular granules under microscope. Additionally, mast cells were also identified by immunostaining for tryptase, a mast cell specific marker in human. Mast cells were also identified in the mid brain of PD patients by human mast cell specific protease tryptase immunohistochemistry as we have reported previously [47]. Briefly, mid brain sections from PD patients and age and sex matched control subjects were incubated with anti-mast cell tryptase mouse monoclonal antibody (1:1000 dilution) overnight at 4 °C. After washing with TBS, the sections were incubated with ultra-sensitive ready-to-use ImmPRESS polymer anti-mouse IgG peroxidase reagent and ImmPACT diaminobenzidine (DAB) peroxidase substrate as per the kit procedure. Development of brown color indicates a positive reaction for tryptase. Negative control staining was performed without the primary antibody.
Immunofluorescence Detection of IL-33 or GFAP and IL-33 in PD Brains
For IL-33 immunofluorescence staining, frozen sections from mid brain and striatum (40 μm) were washed in PBS, blocked with normal goat serum and incubated with IL-33 monoclonal antibody (20 μg/ml) overnight at 4 °C. Then the sections were washed with PBS and incubated with Alexa 568 conjugated secondary antibody (1:500 dilution) for 1 h at room temperature as we have reported previously [43]. Then the sections were washed and cover-slipped with antifade mounting medium (Vector Laboratories), observed under a confocal microscope (Leica Microsystems GmbH, Germany) and images were acquired. Following single immunofluorescence detection of IL-33, we also performed double immunofluorescence staining for the detection of IL-33 and GFAP in the sections from mid brain and striatum from PD patients and non-PD control subjects. Briefly, we incubated the sections in a mixture of IL-33 monoclonal antibody (20 μg/ml) and GFAP polyclonal antibody (1:500 dilution) followed by appropriate secondary antibodies (1:500 dilution) as we described above for the double immunofluorescence procedure and the images were obtained with a confocal microscope.
Statistics
All the results obtained from the experiments were analyzed by GraphPad InStat 3 software. Results were given as mean ± SEM. Data were analyzed using One-way Analysis of Variance (ANOVA) followed by post hoc test Tukey-Kramer multiple comparison analysis to determine statistically significant differences. Further, unpaired t-test was used when comparing only two groups. A p-value of <0.05 was considered statistically significant in all the comparisons.
Results
Mast Cell Proteases MMCP-6 and MMCP-7 Release IL-33 From Mouse Primary Astrocytes
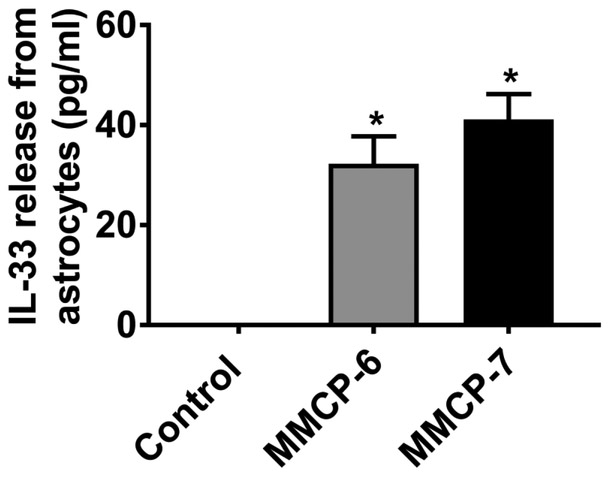
We first analyzed if mouse mast cell proteases MMCP-6 and MMCP-7 activate mouse primary astrocytes to release IL-33 in vitro (n=4). Our ELISA results show that mouse primary mast cells incubated with MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 24 h released significantly increased (*p<0.05 control vs treated; ANOVA and Tukey-Kramer post hoc analysis) amount of IL-33 as compared to untreated control cells in the cell culture medium (Fig. 1). These results show that mast cell proteases can activate astrocytes to release inflammatory mediators.
Fig. 1.
Mast cell proteases MMCP-6 and MMCP-7 release IL-33 from mouse primary astrocytes. Mouse primary astrocytes were incubated with MMCP-6 (100 ng/ml) and MMCP-7 (100 ng/ml) for 24 h and IL-33 released in the supernatant media was analyzed by ELISA kit (n=4). Both MMCP-6 and MMCP-7 significantly increased (*p<0.05 vs control, one-way ANOVA and Tukey-Kramer post hoc analysis) the release of IL-33 as compared to untreated control cells.
Mast Cell Proteases MMCP-6 and MMCP-7 Release IL-33 From Mouse Primary Glia-Neurons
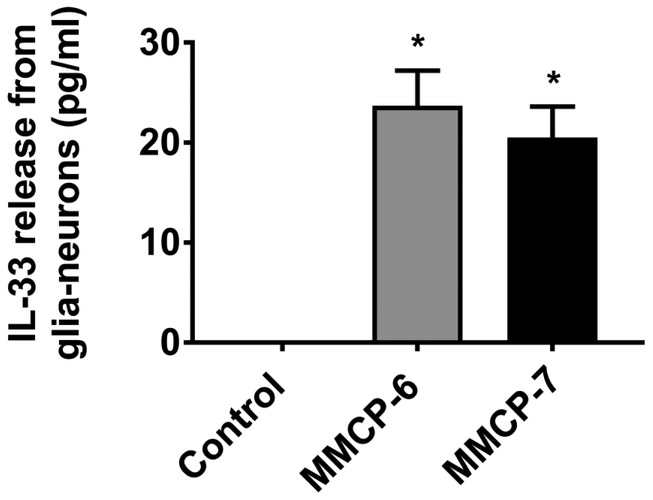
Next, we analyzed whether mouse mast cell proteases MMCP-6 and MMCP-7 also activate glia-neurons mixed culture to release IL-33 in vitro (n=3). We found that mouse primary glia-neurons incubated with MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 24 h released significantly increased (*p<0.05 control vs treated; ANOVA and Tukey-Kramer post hoc analysis) amount of IL-33 as compared to untreated control cells in the cell culture medium (Fig. 2). Our results show that mast cell proteases can activate glia-neurons to release proinflammatory mediators.
Fig. 2.
Mast cell proteases MMCP-6 and MMCP-7 release IL-33 from mouse primary glia-neurons. Mouse primary glia-neurons were incubated with MMCP-6 (100 ng/ml) and MMCP-7 (100 ng/ml) for 24 h and IL-33 level in the cell culture supernatant media was analyzed by ELISA kit (n=3). Mast cell proteases MMCP-6 as well as MMCP-7 significantly increased (*p<0.05 vs control, one-way ANOVA and Tukey-Kramer post hoc analysis) the release of IL-33 as compared to untreated control cells.
MPP+ Activates Mouse Mast Cells and Release IL-33
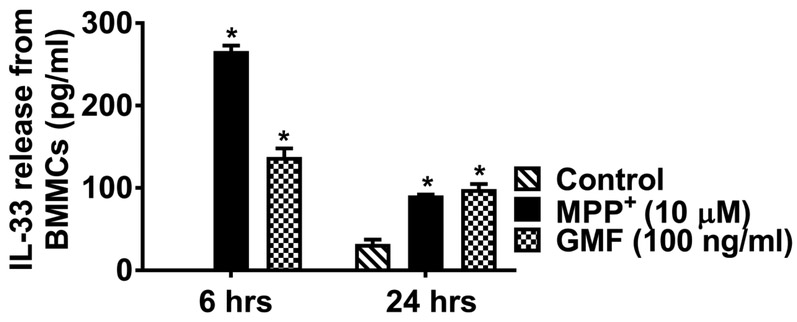
Further, we investigated PD related toxin MPP+ induces IL-33 release from mouse mast cells (n=3) that are implicated in neuroinflammation and PD. ELISA results show that BMMCs incubated with MPP+ (10 μM) for 6 h and 24 h showed significantly increased (p<0.05) release of IL-33 as compared to untreated control cells (Fig. 3). GMF (100 ng/ml) used as a positive control also showed significantly increased (*p<0.05 control vs treated; ANOVA and Tukey-Kramer post hoc analysis) release of IL-33 as compared to untreated control cells in the cell culture medium. These results demonstrate that MPP+ can activate mast cells to release IL-33.
Fig. 3.
MPP+ activate mouse mast cells and release IL-33. BMMCs were incubated with MPP+ (10 μM) for 6 h and 24 h and IL-33 released into the media was measured by ELISA kit (n=4). MPP+-induced significant release of IL-33 as compared with control unstimulated cells release. GMF used as a positive control also significantly increased (*p<0.05 control vs treated, one-way ANOVA and Tukey-Kramer post hoc analysis) the release of IL-33 as compared to untreated control cells.
MPP+ and GMF Increase ROS Generation in Mouse Mast Cells
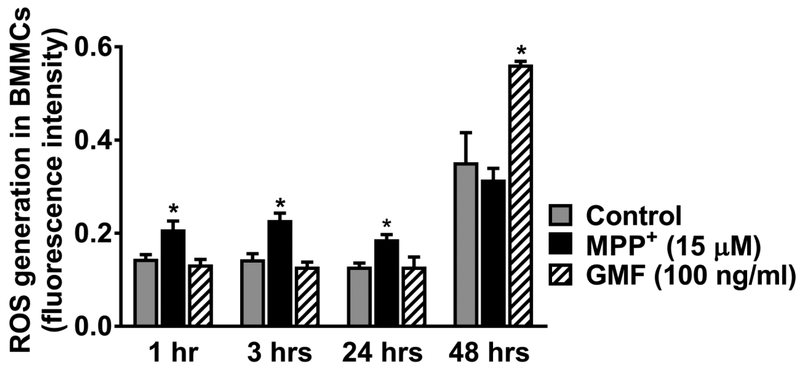
We investigated if MPP+ (15 μM) and GMF (100 ng/ml) activate BMMCs and increase the generation of ROS that is implicated in neurodegeneration. Results show that BMMCs incubated with MPP+ or GMF show increased ROS generation as compared with untreated control BMMCs (n=3). MPP+ increased the levels of ROS generation in BMMCs at 1, 3 and 24 h of incubation in vitro. GMF shows increased levels of ROS generation at 48 h of incubation (Fig. 4, *p<0.05 control vs treated; ANOVA and Tukey-Kramer post hoc analysis). These results show that MPP+ increases ROS generation that could induce neurodegeneration in the brain.
Fig 4.
MPP+ and GMF increase ROS generation in mouse primary mast cells. BMMCs were incubated with DCFDA 1, washed and resuspended in supplementary buffer containing FBS. Mast cells were incubated with MPP+ (15 μM) or GMF (100 ng/ml) for 1, 3, 24 and 48 h and measured the generation of ROS (N=3). The plates were read after 1, 3, 24 and 48 h using a micro plate reader for fluorescence intensity. BMMCs incubated with MPP+ show increased production of ROS at 1, 3 and 24 h of incubation. GMF show increased generation of ROS at 48 h of incubation as compared with untreated control BMMCs (*p<0.05 vs control, one-way ANOVA and Tukey-Kramer post hoc analysis).
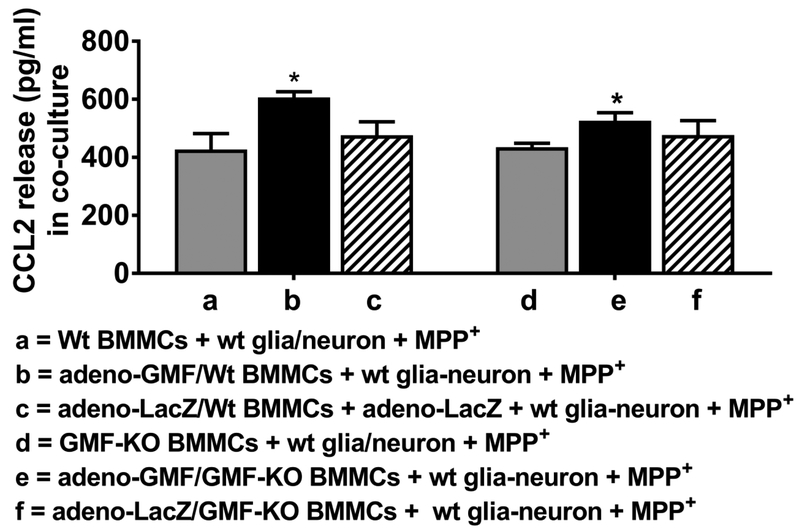
BMMCs Increase MPP+-mediated CCL2 Release From Mouse Glia-Neurons and Mast Cells in a Co-culture System
BMMCs from wt mice and GMF-KO mice were transduced with adeno-GMF for GMF overexpression or with control adeno-LacZ before adding to glia-neuron culture in vitro. Then MPP+ (10 μM) was added to these cultures for 24 h and the CCL2 release was measured in the supernatant media by ELISA (n=3). Results show that addition of wt BMMCs that were previously incubated with adeno-GMF for overexpression cause higher CCL2 release as compared to CCL2 released by BMMCs from GMF-KO mice transduced with adeno-GMF, indicating GMF-KO condition reduces the release of inflammatory mediators such as CCL2 after activation (Fig. 5, *p<0.05, t test).
Fig. 5.
BMMCs increases MPP+-mediated CCL2 release from mouse glia-neurons and mast cells co-culture. BMMCs from wt mice and GMF-KO mice were incubated with adeno-GMF or adeno-LacZ before adding to wt glia-neuron culture. Then MPP+ (10 μM) was added to these cultures for 24 h and CCL2 release was measured in the supernatant media by ELISA kit (N=3). Addition of BMMCs from wt mice that were incubated with adeno-GMF and further incubated with wt glia-neurons with MPP+ as compared to CCL2 released from BMMCs obtained from GMF-KO mice and treated with adeno-GMF (*p<0.05 t test).
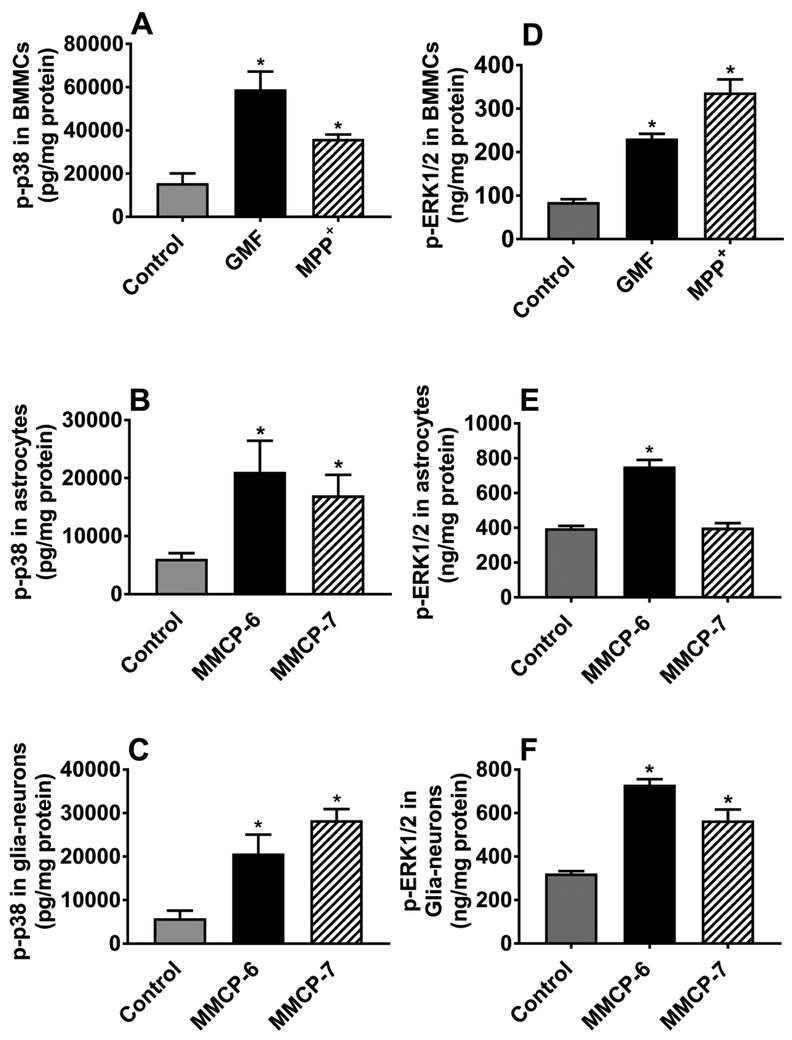
MPP+ and GMF Activate p38 and ERK1/2 MAPKs in Mouse Mast Cells, and Mouse Mast Cell Proteases Activate MAPKs in Astrocytes and Glia-Neurons
BMMCs were incubated with MPP+ (10 μM) or GMF (100 ng/ml), and astrocytes and glia-neurons were incubated with MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 15 min (n=3-4). Cell lysates were prepared from cell pellets and ELISA was performed for the expression of p-p38 and pERK1/2 (Fig. 6a-f). MPP+, GMF and mast cell proteases-induced phosphorylation of p38 and increased the expression of p-p38 in BMMCs (Fig. 6a), astrocytes (Fig. 6b) and glia-neurons (Fig. 6c). Further, GMF and MPP+ increased the expression of p-ERK1/2 in BMMCs (Fig. 6d). MMCP-6 increased the expression of p-p38 in astrocytes (Fig. 6e) and in glia-neurons (Fig. 6f), whereas MMCP-7 increased p-ERK1/2 expression in glia-neurons (Fig. 6f, *p<0.05 vs control. Overall, these results indicate that MPP+ and GMF activate p38 and ERK1/2 MAPKs in mouse mast cells, and MMCP-6 and MMCP-7 activate MAPKs in astrocytes and glia-neurons.
Fig. 6.
MPP+ and GMF activate MAPKs in mouse mast cells, and mouse mast cell proteases activate MAPKs in astrocytes and glia-neurons. BMMCs were incubated with MPP+ (10 μM) and GMF (100 ng/ml), and astrocytes and glia-neurons were incubated with MMCP-6 (100 ng/ml) and MMCP-7 (100 ng/ml) for 15 min. Cell lysates were prepared from cell pellets and the expression of p-p38 was assayed by ELISA (a-f). MPP+, GMF and mast cell proteases-induced phosphorylation of p38 and increased the expression of p-p38 in BMMCs (a; N=3), astrocytes (b; n=4) and glia-neurons (c; N=4). MPP+ and GMF-induced phosphorylation of ERK1/2 in BMMCs (d; N=3). Further, MMCP-6 increased the expression of p-ERK1/2 in astrocytes (e; N=3). Both MMCP-6 and MMCP-7 increased phosphorylation of ERK1/2 in glia-neurons (f; N=4). *p<0.05 vs control, one-way ANOVA and Tukey-Kramer post hoc analysis.
MPP+ and GMF Activate NF-κB in Mouse Mast Cells, and Mouse Mast Cell Proteases Activate NF-κB in Astrocytes and Glia-Neurons
BMMCs were incubated with MPP+ or GMF, and astrocytes and glia-neurons were incubated with MMCP-6 or MMCP-7 for 24 h. Cell lysates were prepared from cell pellets of these cells and the expression of total and p-NF-κB p65 were determined by Western blot (n=3). Our results show that MPP+, GMF or mast cell proteases-increased the expression of p-NF-κB p65 in BMMCs (Fig. 7a), astrocytes (Fig. 7b), and glia-neurons (Fig. 7c) as compared to untreated control cells. These results show that increased p-NF-κB p65 expression by MPP+ and GMF could be crucial in the upregulated neuroinflammation in PD.
Fig. 7.
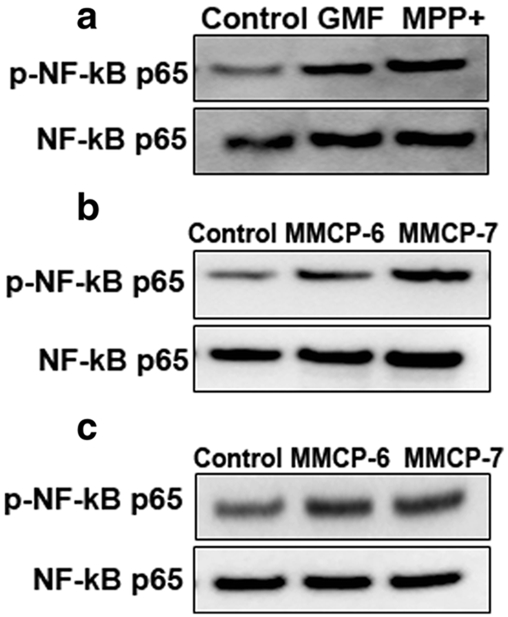
MPP+ and GMF activate NF-κB in mouse mast cells, and mouse mast cell proteases activate NF-κB in astrocytes and glia-neurons. BMMCs were incubated with MPP+ (10 μM) or GMF (100 ng/ml), and astrocytes and glia-neurons were incubated with MMCP-6 or MMCP-7 for 24 h (a-c). Cell lysates were prepared from cell pellets and the expression of total and p-NF-κB p65 was determined by Western blot (N=3). The bands were visualized using ChemiDoc-It2 Imaging System. Representative images show that MPP+, GMF and mast cell proteases-induced increased expression of p-NF-κB p65 in BMMCs (a), astrocytes (b) and glia-neurons (c)
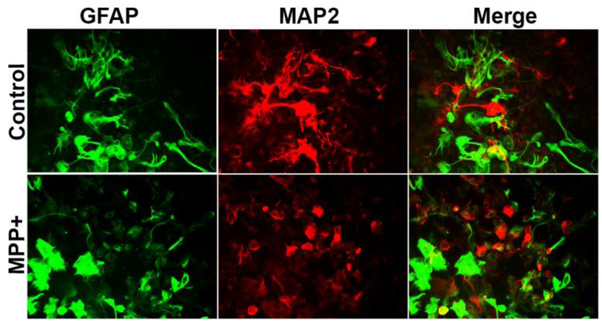
MPP+ Upregulated the Expression of GFAP and Reduced the Expression of MAP2 in Glia-Neurons as Detected by Double Immunofluorescence
Next, we investigated whether MPP+ affects the expression of GFAP and MAP 2 in glia-neurons by double immunofluorescence staining. Glia-neurons incubated with MPP+ for 72 h shows an activated morphology of astrocytes as seen by the increased expression of GFAP (green color), and reduced the expression of MAP-2 (red color) as compared with untreated control cells, indicating reduced neuronal processes due to neurodegeneration (Fig. 8). Our results show that MPP+ could activate astrocytes that is crucial in neuroinflammation.
Fig. 8.
Double immunofluorescence detection of GFAP and MAP2 in glia-neurons after incubation with MPP+. Glia-neurons were grown in 24 well tissue culture plate and incubated with MPP+ (15 μM) for 72 h. We then performed double immunofluorescence for the detection of GFAP and MAP2 using anti-GFAP polyclonal antibody (1:500 dilution) and anti-MAP2 monoclonal antibody (5 μg/ml) overnight at 4°C followed by Alexa 488 goat anti-rabbit IgG and Alexa 568 goat-anti-mouse IgG secondary antibodies. Then the sections were cover-slipped and observed under a microscope. Representative images show activated morphology of astrocytes (green color) and reduced MAP2 (red color) expression after incubation with MPP+. Original magnification = 200×.
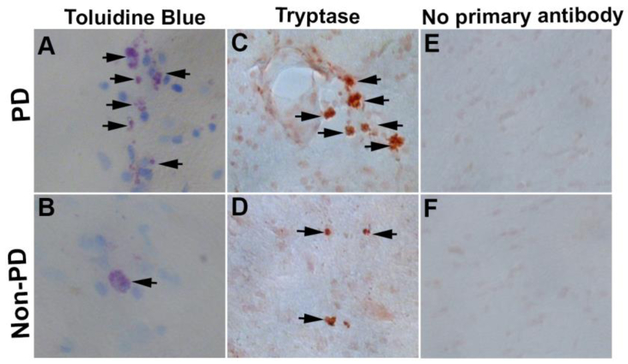
Toluidine Blue and Tryptase Staining for Mast Cells Detection in PD Brain
As we found that PD toxin MPP+ activates mast cells to release PD-relevant inflammatory mediators, we investigated mast cell activation status in the PD brains. We first stained mast cells using 0.1% toluidine blue in the mid brain of PD (n=3) and non-PD control subjects (n=3) brains. We found that PD brains show increased mast cells (arrows, blue/purple metachromatic color) as well as increased activation status as compared with non-PD control brains (Fig. 9a, b). Activated mast cells show extracellular granules and reduced blue color as seen in the PD brain. Additionally, we also immunostained tryptase, a mast cell specific protease in humans. Results show that tryptase-positive mast cells as well as their activation status were increased in PD (n=3) as compared with non-PD control subjects (n=3) brains (Fig. 9c, d, brown color, arrows). Negative control staining without using the primary antibodies did not show positive reaction for tryptase (Fig. 9e, f). These results show that increased mast cell activation could play an important role in the pathogenesis of PD.
Fig. 9.
Mast cell activation in PD brains. Mid brain sections from PD patients and non-PD control subjects were incubated with 0.1% toluidine blue to detect mast cells (N=3). PD brains show increased mast cells (arrows, blue/purple metachromatic color) as well as increased activation as compared with non-PD control brains (a, b). Degranulated mast cells show extracellular granules and reduced blue color as seen in the PD brain. Further, we immunostained human mast cell specific tryptase in these brain sections using tryptase monoclonal antibody and ready-to-use ImmPRESS polymer anti-mouse IgG peroxidase reagent and ImmPACT DAB peroxidase substrate. Development of brown color indicated a positive reaction for tryptase (c, d, arrows). Tryptase-positive mast cells and their activation status were increased in PD brains as compared to non-PD control subjects’ brains (c, d). Negative control staining performed without using the primary antibody did not show positive reaction for tryptase (e, f). Original magnification = 200×.
Immunofluorescence Detection of Increased Expression of IL-33 and GFAP in the Mid Brain and Striatum of PD Brain
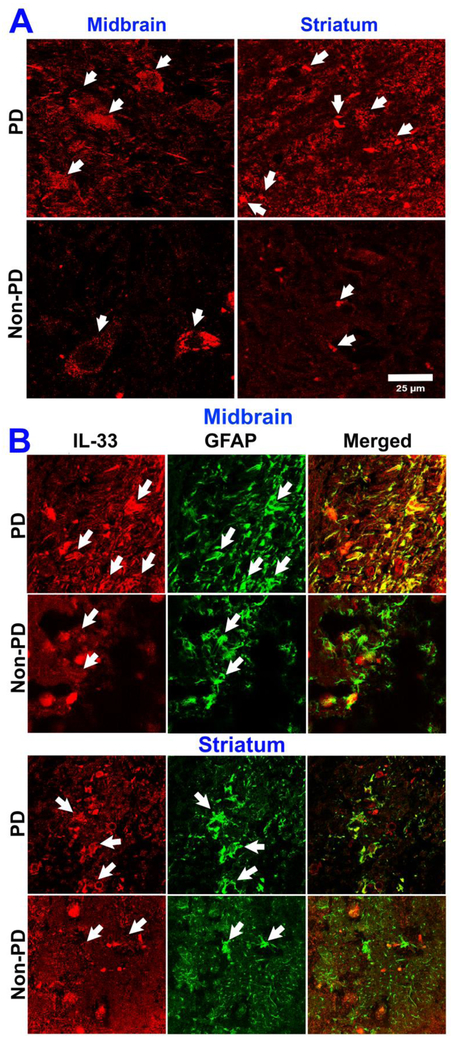
In order to know if inflammatory and cell injury-related cytokine IL-33 is increased in the PD brain, we performed immunofluorescence staining for IL-33 expression in PD and non-PD control subject brains. Our results show increased expression of IL-33 (red color, arrows) in the mid brain as well as in the striatum of PD brains as compared with the expression in the age and sex matched non-PD control subject’s mid brain and striatum (n=3) regions (Fig. 10a). This indicate that neuronal injury in PD could increase IL-33 expression in the brain. Next, we investigated the source of IL-33 by double immunofluorescence staining procedure for IL-33 as well as GFAP (marker for astrocytes) in the same sections (Fig. 10b). Our results show that IL-33 is increased in the mid brain as well as in the striatum of PD brains (n=3) as compared with IL-33 expression (red color, arrows) in non-PD control subjects (n=3) mid brain and striatum (Fig. 10b). Additionally, we found an increased expression of GFAP indicating activated/reactive astrocytes (green color, arrows) in the mid brain and striatum regions of PD brains as compared to the expression in non-PD control subjects brains (Fig. 10b). Merged images show co-localization of IL-33 and GFAP in the mid brain and striatum regions. Our current results demonstrate that increased IL-33 expression in PD could play a role in the pathogenesis of PD.
Fig. 10.
Increased expression of IL-33 and GFAP in human PD brains as detected by immunofluorescence staining. Sections (40 μm) from mid brain and striatum of PD patients (N=3) as well as age and sex matched non-PD control subjects (N=3) were incubated with IL-33 monoclonal antibody overnight at 4 °C and then with Alexa 568 conjugated goat anti-mouse IgG secondary antibody (1:500 dilution) for 1 h at room temperature. Then the sections were cover-slipped and observed under a confocal microscope using 63× oil immersion objective. Representative images from mid brain and striatum in PD brains show increased expression of IL-33 (arrows, red color) as compared with age and sex matched control subjects brains (a, scale bar = 25 μm). Further, we also investigated the source of IL-33 by double immunofluorescence staining using monoclonal antibody for IL-33 and polyclonal antibody for astrocyte marker GFAP along with Alexa Flour 488 goat anti-rabbit IgG and Alexa Flour 568 goat anti-mouse IgG secondary antibodies. After washing with PBS, the sections were mounted on to the slides, coversliped with mounting medium and viewed with a confocal microscope and images were acquired (b). Representative images show that IL-33 is increased in the mid brain and striatum of PD brains as compared with non-PD control subjects mid brain and striatum regions (b; arrows, red color). Additionally, expression of GFAP also increased indicating activated/reactive astrocytes (green color, arrows) in the mid brain and striatum regions of PD brains as compared to non-PD control subjects’ brains (b). Merged images show co-localization of IL-33 and GFAP in the mid brain and striatum regions (original magnification = 630×).
Discussion
We have investigated whether mouse mast cell proteases MMCP-6 and MMCP-7 activate mouse primary astrocytes and mouse primary glia-neurons to release proinflammatory cytokine IL-1 family member IL-33 from these cells. We found that both MMCP-6 and MMCP-7 significantly release IL-33 from astrocytes as well as glia-neurons in vitro. Further, we also show that neurotoxin MPP+ and GMF-induces upregulation of IL-33 and ROS expression in mouse mast cells in vitro. Additionally, MMCP-6, MMCP-7 and MPP+ upregulate the phosphorylation and expression of p38, ERK1/2 and NF-κB in astrocytes, glia-neurons and BMMCs. We also found that BMMCs obtained from wt mice with GMF overexpression and co-cultured with glia-neurons with MPP+ stimulation show increased CCL2 release as compared to the release from similarly treated BMMCs obtained from GMF-KO mice. MPP+ increased the activation of astrocytes as evidenced by the up-regulated expression of GFAP. MPP+ reduced the expression of MAP-2 in the neurons indicating reduced neuronal processes. Since our previous studies have shown that mast cells are implicated in PD [48,32], we examined mast cell number and its activation status in PD brains and compared with non-PD control subjects brains in the current study using toluidine blue staining as well as human mast cell specific marker tryptase by immunostaining. We found increased mast cells as well as its activation in the mid brain and striatum regions of PD brains as compared with non-PD control subjects’ brains. Further, our studies show increased expression of IL-33 and GFAP in the mid brain and striatum of PD patients as compared with age and sex matched control subjects. Increased GFAP shows increased activation of astrocytes/reactive astrocytes in PD brains as compared to non-PD control subjects’ brains as observed in our present results. Glial cells in the brain can produce IL-33 that can also activate glial cells to release other inflammatory cytokines and upregulate neuroinflammation [49,50]. IL-33 acts on other cells through its receptor ST2 and IL-1 receptor accessory protein (IL-1Racp) and plays an important role in several CNS disorders including AD, Multiple sclerosis, brain injuries, cerebrovascular diseases, chronic pain and neuroinflammation [50].
We have previously shown that amyloid beta (Aβ l-42)-induces the expression of IL-33 in mouse astrocytes in vitro [35]. IL-33 is highly expressed throughout the brain, as well as in the spinal cord [51]. CNS injury is devastating as it causes permanent impairment due to the lack of normal regeneration. Immune system detects CNS injury and responds rapidly and this response may be beneficial or detrimental in association with M2 (neurotoxic) and M1 (neuroprotective) microglia phenotypes. IL-33 exhibits both pro and anti-inflammatory properties as double-edged sword based upon the site and disease conditions [52]. Alarmin cytokine, IL-33 released from injured cells in the CNS acts on local microglia and astrocytes to release chemokine to attract immune cells into the site of injury to prevent further injury and inflammatory processes [53]. So, IL-33 is a neuroprotective as well as neurotoxic cytokine [50. If the concentration of IL-33 increases above the physiological level, it can induce proinflammatory reactions and may act as a prominent inflammatory mediator of neuroinflammation in the CNS as we have reported that IL-33 can induce neurodegeneration in vitro [35]. In the present study, we detected upregulated expression of IL-33 in the mid brain as well as in the striatum of PD patients’ brains. Increased expression of IL-33 in PD brains indicate that neuronal injury and neurodegeneration could have increased the release of IL-33 from glial cells and mast cells in the brain. IL-33 has been suggested as a new therapeutic target for autoimmunity and inflammation including neuroinflammation [54]. IL-33 is implicated in neuroinflammation through glial and mast cell activation, and is increased in the CNS injuries such as traumatic brain injury [49]. Moreover, recent studies have shown that IL-33 and ST2-axis are implicated in neurodegenerative diseases and other CNS disorders [50]. Another recent study has shown increased pain associated with high level of IL-33 and activation of C-Jun N-terminal kinase (JNK), ERK and NF-κB in rat lumbar disk herniation [55].
CCL2 is the best characterized chemokine as CCL2 suppression is important in therapeutics to reduce excessive accumulation of immune cells at the site of neuroinflammation and neurodegeneration [56]. CCL2 plays an important role in the pathogenesis of CNS disorders including AD, PD and brain injury by controlling the migration of immune cells and increasing blood-brain barrier permeability [57]. Results from our present study show that addition of BMMCs from wt mice that were already incubated with adeno-GMF for overexpression, co-cultured with wt glia-neurons and incubated with MPP+ show higher CCL2 release when compared with the release from BMMCs from GMF-KO mice incubated similarly. These results show that absence of GMF reduces the release of inflammatory mediators such as CCL2 from brain cells.
ROS are free radicals consisting of superoxide anion, hydroxyl radical and hydrogen peroxide (H2o2). Though ROS plays a role in normal cell functions, moderate levels cause cell death including neurodegeneration by oxidative stress. Oxidative stress-mediated redox reaction modulate protein, changes in cellular signaling pathways and NF-κB activation and neuronal death [58]. Previous studies have shown increased ROS expression in PD [58]. Both human and mouse mast cells are known to release ROS and hydrogen peroxide after its activation [59,60]. In the current study, we show that both MPP+ and GMF activate mast cells and induce the generation of ROS. Mast cell-derived ROS may contribute to the increased level of ROS in neuroinflammation and neurodegenerative diseases.
Neuroinflammation plays an important role in the neurodegeneration in several neurodegenerative diseases [61,37,62]. Cytokine networks play an important role in neuroinflammation [63]. Mast cells are heterogeneous multifunctional immune and inflammatory cells that contribute to the neuroinflammation process in the CNS by releasing several stored granules and newly synthesized neuroinflammatory mediators including tryptase, IL-1β, TNF-α, IL-6, IL-8, IL-33, matrix metalloproteinases (MMPs) and ROS [19,64-66]. Mast cells are implicated in the pathogenesis of PD, AD, MS and EAE [67,68,18,69,70]. Mast cells are increasingly considered as early responders in brain injury by releasing the prestored and preactivated inflammatory mediators such as TNF-α, and proteases such as MMCP-6 and MMCP-7 in mouse [71,72,11]. Mast cell degranulation release proteases and these proteases are known to activate glial cells as well as neurons to release inflammatory cytokines such as TNF-α and IL-6 via protease-activated receptor 2 (PAR-2) signaling pathways [73]. PAR-2 expressed on glia-neurons and mast cells play an important role in neuroinflammation [74-76,73]. PAR-2 also causes recruitment of mast cells at the site of inflammation [77]. Mouse mast cell proteases MMCP-6 and MMCP-7 upregulate PAR-2 expression in glia, neurons and mast cells [78]. Mast cells are implicated in neuroinflammation and AD in association with the detection of IL-33 [52]. Our present study also show similar results that IL-33 and mast cells are increased in PD brains. Moreover, increased release of protease tryptase could activate glial cells and neurons and mediate neurodegeneration in PD.
In the present study, we have investigated whether MMCP-6 and MMCP-7 activate glial cells and neuronal cells to release IL-33. We demonstrate that both MMCP-6 and MMCP-7 activate astrocytes and neurons and release IL-33 that are implicated in neurodegeneration, as we have reported previously [27]. This release involves activation of p38 and ERK1/2 MAPKs and NF-κB pathways as also reported previously for microglial activation by proteases [73], MAPKs are present in cell surface and transfer signals from the cell surface receptors to the nucleus. NF-κB is an important transcription factor implicated in the upregulation of inflammatory mediators, cytokines, chemokines and neurotoxic mediators in neurodegenerative diseases [36,37,79]. Normally, NF-κB is present in the cytoplasm in association with I-κB. The expression of proinflammatory mediators have been shown to be regulated by MAPKs through the activation of NF-κB. We have previously shown that upregulation of NF-κB p65 is associated with increased GMF expression in human AD brains [38]. Moreover, NF-κB is implicated in neuroinflammatory pathways in neurodegenerative diseases including PD and AD [36,80]. Our present findings indicate that mast cell-derived inflammatory mediators, brain protein GMF and PD relevant MPP+ can phosphorylate and upregulate the expression of p38, ERK1/2MAPKs and NF-κB in mast cells or brain cells in neurodegenerative diseases including PD.
Neurotoxin MPP+ is known to activate glia, neurons and mast cells to release neuroinflammatory mediators and induce neurodegeneration [31,32,18]. In the present study, we have demonstrated that MPP+ induces the release of IL-33 through the activation of ERK1/2, p38 and NF-κB. GMF was isolated, sequenced and cloned previously in our laboratory [22,23]. We have previously shown that GMF is implicated in neurodegeneration [28,29,27,81,44]. GMF also activates mast cells to release various inflammatory mediators in vitro [18,27]. In the present study, we have used GMF as a positive control for the stimulation and show that GMF-induced the release of IL-33 through the activation of MAPKs and NF-κB.
Conclusions
Mast cell proteases MMCP-6 and MMCP-7 activate astrocytes and neurons, and MPP+ activates mast cells to release IL-33 through the activation of ERK1/2, p38 MAPKs and NF-κB. Further, PD brains show increased mast cell number, its activation status, and expression of cell injury-associated cytokine IL-33 in the mid brain and striatum regions. We suggest that inhibition of glia-neuron and mast cell activation pathways may represent a new therapeutic target for neuroinflammatory diseases including PD.
Acknowledgements
This work was supported by Veteran Affairs Merit Award I01BX002477 and National Institutes of Health Grants AG048205 & NS073670 to AZ.
Footnotes
Ethics approval
The present study was conducted according to the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health (NIH). The Committee on the Ethics of Animal Experiments of the University of Missouri (Columbia, MO) approved this protocol.
Brains of PD patients and non-PD control subjects were obtained through Deeded body program at the University of Iowa (Iowa City, IA) and used as per the guidelines of the Institutional Review Board (IRB) of the University of Iowa and the University of Missouri (IRB #2008067; exempt application 224561).
Consent for publications
Not applicable
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arthur G, Bradding P (2016) New Developments in Mast Cell Biology: Clinical Implications. Chest 150 (3):680–693. doi: 10.1016/j.chest.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 2.DeBruin EJ, Gold M, Lo BC, Snyder K, Cait A, Lasic N, Lopez M, McNagny KM, Hughes MR (2015) Mast cells in human health and disease. Methods Mol Biol 1220:93–119. doi: 10.1007/978-1-4939-1568-2_7 [DOI] [PubMed] [Google Scholar]
- 3.Migalovich-Sheikhet H, Friedman S, Mankuta D, Levi-Schaffer F (2012) Novel identified receptors on mast cells. Front Immunol 3:238. doi: 10.3389/fimmu.2012.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao KN, Brown MA (2008) Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci 1143:83–104. doi: 10.1196/annals.1443.023 [DOI] [PubMed] [Google Scholar]
- 5.Gilfillan AM, Beaven MA (2011) Regulation of mast cell responses in health and disease. Crit Rev Immunol 31 (6):475–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita H, Saito H, Matsumoto K, Nakae S (2016) Regulatory roles of mast cells in immune responses. Semin Immunopathol. 38(5):623–629. doi: 10.1007/s00281-016-0566-0 [DOI] [PubMed] [Google Scholar]
- 7.Reber LL, Marichal T, Galli SJ (2012) New models for analyzing mast cell functions in vivo. Trends Immunol 33 (12):613–625. doi: 10.1016/j.it.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa E, Valitutti S (2017) New roles and controls of mast cells. Curr Opin Immunol 50:39–47. doi: 10.1016/j.coi.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 9.Weller CL, Collington SJ, Williams T, Lamb JR (2011) Mast cells in health and disease. Clin Sci (Lond) 120 (11):473–484. doi: 10.1042/CS20100459 [DOI] [PubMed] [Google Scholar]
- 10.Wernersson S, Pejler G (2014) Mast cell secretory granules: armed for battle. Nat Rev Immunol 14 (7):478–494. doi: 10.1038/nri3690 [DOI] [PubMed] [Google Scholar]
- 11.Yehya M, Torbey MT (2017) The Role of Mast Cells in Intracerebral Hemorrhage. Neurocrit Care. doi: 10.1007/s12028-017-0416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tore F, Tuncel N (2009) Mast cells: target and source of neuropeptides. Curr Pharm Des 15 (29):3433–3445 [DOI] [PubMed] [Google Scholar]
- 13.Mukai K, Tsai M, Saito H, Galli SJ (2018) Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 282 (1):121–150. doi: 10.1111/imr.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taracanova A, Alevizos M, Karagkouni A, Weng Z, Norwitz E, Conti P, Leeman SE, Theoharides TC (2017) SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci U S A 114 (20):E4002–E4009. doi: 10.1073/pnas.1524845114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molteni M, Rossetti C (2017) Neurodegenerative diseases: The immunological perspective. J Neuroimmunol 313:109–115. doi: 10.1016/j.jneuroim.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122 (4):1164–1171. doi: 10.1172/JCI58644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353 (6301):777–783. doi: 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- 18.Kempuraj D, Selvakumar GP, Zaheer S, Thangavel R, Ahmed ME, Raikwar S, Govindarajan R, Iyer S, Zaheer A (2017) Cross-Talk between Glia, Neurons and Mast Cells in Neuroinflammation Associated with Parkinson's Disease. J Neuroimmune Pharmacol. 13(1):100–112. doi: 10.1007/s11481-017-9766-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA (2017) Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev. 79:119–133. doi: 10.1016/j.neubiorev.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Raikwar SP, Iyer SS, Bhagavan SM, Beladakere-Ramaswamy S, Zaheer A (2017) Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer's Disease Pathogenesis. Front Neurosci 11:703. doi: 10.3389/fnins.2017.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A (2017) Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front Cell Neurosci 11:216. doi: 10.3389/fncel.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim R, Miller JF, Zaheer A (1989) Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci US A 86 (10):3901–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim R, Zaheer A (1991) Structure and function of glia maturation factor beta. Adv Exp Med Biol 296:161–164 [DOI] [PubMed] [Google Scholar]
- 24.Kaplan R, Zaheer A, Jaye M, Lim R (1991) Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem 57 (2):483–490 [DOI] [PubMed] [Google Scholar]
- 25.Zaheer A, Fink BD, Lim R (1993) Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem 60 (3):914–920 [DOI] [PubMed] [Google Scholar]
- 26.Wang BR, Zaheer A, Lim R (1992) Polyclonal antibody localizes glia maturation factor beta-like immunoreactivity in neurons and glia. Brain Res 591 (1):1–7 [DOI] [PubMed] [Google Scholar]
- 27.Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A (2013) Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. J Neuroimmune Pharmacol 8 (3):643–650. doi: 10.1007/s11481-013-9439-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaheer A, Zaheer S, Thangavel R, Wu Y, Sahu SK, Yang B (2008) Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res 1208:192–203. doi: 10.1016/j.brainres.2008.02.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaheer A, Knight S, Zaheer A, Ahrens M, Sahu SK, Yang B (2008) Glia maturation factor overexpression in neuroblastoma cells activates glycogen synthase kinase-3beta and caspase-3. Brain Res 1190:206–214. doi: 10.1016/j.brainres.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim R, Zaheer A, Khosravi H, Freeman JH Jr., Halverson HE, Wemmie JA, Yang B (2004) Impaired motor performance and learning in glia maturation factor-knockout mice. Brain Res 1024 (1–2):225–232. doi: 10.1016/j.brainres.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 31.Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan DA, Santillan MK, Zaheer A (2015) Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins alpha-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS One 10 (8):e0135776. doi: 10.1371/journal.pone.0135776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempuraj D, Thangavel R, Fattal R, Pattani S, Yang E, Zaheer S, Santillan DA, Santillan MK, Zaheer A (2016) Mast Cells Release Chemokine CCL2 in Response to Parkinsonian Toxin 1-Methyl-4-Phenyl-Pyridinium (MPP(+)). Neurochem Res 41 (5):1042–1049. doi: 10.1007/s11064-015-1790-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew FY, Girard JP, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16 (11):676–689. doi: 10.1038/nri.2016.95 [DOI] [PubMed] [Google Scholar]
- 34.Lunderius-Andersson C, Enoksson M, Nilsson G (2012) Mast Cells Respond to Cell Injury through the Recognition of IL-33. Front Immunol 3:82. doi: 10.3389/fimmu.2012.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, Zaheer A (2014) Alzheimer's Disease: Evidence for the Expression of Interleukin-33 and Its Receptor ST2 in the Brain. J Alzheimers Dis. 40 (2): 297–308. doi: 10.3233/JAD-132081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong JT (2017) NF-κB as a mediator of brain inflammation in AD. CNS Neurol Disord Drug Targets. doi: 10.2174/1871527316666170807130011 [DOI] [PubMed] [Google Scholar]
- 37.Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A (2016) Neuroinflammation Induces Neurodegeneration. J Neurol Neurosurg Spine 1 (1) [PMC free article] [PubMed] [Google Scholar]
- 38.Thangavel R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, Selvakumar GP, Iyer SS, Zaheer A (2017) Glia Maturation Factor and Mitochondrial Uncoupling Proteins 2 and 4 Expression in the Temporal Cortex of Alzheimer's Disease Brain. Front Aging Neurosci 9:150. doi: 10.3389/fnagi.2017.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaheer A, Yorek MA, Lim R (2001) Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res 26 (12):1293–1299 [DOI] [PubMed] [Google Scholar]
- 40.Zaheer A, Mathur SN, Lim R (2002) Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun 294 (2):238–244. doi: 10.1016/S0006-291X(02)00467-9 [DOI] [PubMed] [Google Scholar]
- 41.Lee Y, Aono M, Laskowitz D, Warner DS, Pearlstein RD (2004) Apolipoprotein E protects against oxidative stress in mixed neuronal-glial cell cultures by reducing glutamate toxicity. Neurochem Int 44 (2):107–118 [DOI] [PubMed] [Google Scholar]
- 42.Plastira I, Bernhart E, Goeritzer M, DeVaney T, Reicher H, Hammer A, Lohberger B, Wintersperger A, Zucol B, Graier WF, Kratky D, Malle E, Sattler W (2017) Lysophosphatidic acid via LPA-receptor 5/protein kinase D-dependent pathways induces a motile and pro-inflammatory microglial phenotype. J Neuroinflammation 14 (1):253. doi: 10.1186/s12974-017-1024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thangavel R, Bhagavan SM, Ramaswamy SB, Surpur S, Govindarajan R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, Selvakumar GP, Iyer SS, Zaheer A (2018) Co-Expression of Glia Maturation Factor and Apolipoprotein E4 in Alzheimer's Disease Brain. J Alzheimers Dis 61 (2):553–560. doi: 10.3233/JAD-170777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed ME, Iyer S, Thangavel R, Kempuraj D, Selvakumar GP, Raikwar SP, Zaheer S, Zaheer A (2017) Co-Localization of Glia Maturation Factor with NLRP3 Inflammasome and Autophagosome Markers in Human Alzheimer's Disease Brain. J Alzheimers Dis 60 (3):1143–1160. doi: 10.3233/JAD-170634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kempuraj D, Twait EC, Williard DE, Yuan Z, Meyerholz DK, Samuel I (2013) The novel cytokine interleukin-33 activates acinar cell proinflammatory pathways and induces acute pancreatic inflammation in mice. PLoS One 8 (2):e56866. doi: 10.1371/journal.pone.0056866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boucher WS, Letourneau R, Huang M, Kempuraj D, Green M, Sant GR, Theoharides TC (2002) Intravesical sodium hyaluronate inhibits the rat urinary mast cell mediator increase triggered by acute immobilization stress. J Urol 167 (1):380–384 [PubMed] [Google Scholar]
- 47.Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, Tomikawa M, Tachimoto H, Ebisawa M, Akasawa A, Miyagi T, Kimura H, Nakajima T, Tsuji K, Nakahata T (1999) Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood 93 (10):3338–3346 [PubMed] [Google Scholar]
- 48.Kempuraj D, Selvakumar GP, Zaheer S, Thangavel R, Ahmed ME, Raikwar S, Govindarajan R, Iyer S, Zaheer A (2018) Cross-Talk between Glia, Neurons and Mast Cells in Neuroinflammation Associated with Parkinson's Disease. J Neuroimmune Pharmacol 13 (1):100–112. doi: 10.1007/s11481-017-9766-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wicher G, Wallenquist U, Lei Y, Enoksson M, Li X, Fuchs B, Abu Hamdeh S, Marklund N, Hillered L, Nilsson G, Forsberg-Nilsson K (2017) Interleukin-33 Promotes Recruitment of Microglia/Macrophages in Response to Traumatic Brain Injury. J Neurotrauma 34 (22):3173–3182. doi: 10.1089/neu.2016.4900 [DOI] [PubMed] [Google Scholar]
- 50.Du LX, Wang YQ, Hua GQ, Mi WL (2017) IL-33/ST2 Pathway as a Rational Therapeutic Target for CNS Diseases. Neuroscience. 369:222–230. doi: 10.1016/j.neuroscience.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 51.Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, Wojciechowski S, Kanninen K, Koistinaho J, Malm T (2015) Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun 44:68–81. doi: 10.1016/j.bbi.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 52.Abd Rachman Isnadi MF, Chin VK, Abd Majid R, Lee TY, Atmadini Abdullah M, Bello Omenesa R, Osamah Ibraheem Z, Basir R (2018) Critical Roles of IL-33/ST2 Pathway in Neurological Disorders. Mediators Inflamm 2018:5346413. doi: 10.1155/2018/5346413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J (2015) The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85 (4):703–709. doi: 10.1016/j.neuron.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 54.Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P (2015) Targeting IL-33 in autoimmunity and inflammation. J Pharmacol Exp Ther 354 (1):24–31. doi: 10.1124/jpet.114.222505 [DOI] [PubMed] [Google Scholar]
- 55.Huang SJ, Yan JQ, Luo H, Zhou LY, Luo JG (2018) IL-33/ST2 signaling contributes to radicular pain by modulating MAPK and NF-kappaB activation and inflammatory mediator expression in the spinal cord in rat models of noncompressive lumber disk herniation. J Neuroinflammation 15 (1):12. doi: 10.1186/s12974-017-1021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madrigal JL, Caso JR (2014) The chemokine (C-C motif) ligand 2 in neuroinflammation and neurodegeneration. Adv Exp Med Biol 824:209–219. doi: 10.1007/978-3-319-07320-0_15 [DOI] [PubMed] [Google Scholar]
- 57.Bose S, Cho J (2013) Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res 36 (9):1039–1050. doi: 10.1007/s12272-013-0161-z [DOI] [PubMed] [Google Scholar]
- 58.Peterson LJ, Flood PM (2012) Oxidative stress and microglial cells in Parkinson's disease. Mediators Inflamm 2012:401264. doi: 10.1155/2012/401264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chelombitko MA, Fedorov AV, Ilyinskaya OP, Zinovkin RA, Chernyak BV (2016) Role of Reactive Oxygen Species in Mast Cell Degranulation. Biochemistry (Mosc) 81 (12):1564–1577. doi: 10.1134/S000629791612018X [DOI] [PubMed] [Google Scholar]
- 60.Suzuki Y, Yoshimaru T, Inoue T, Niide O, Ra C (2005) Role of oxidants in mast cell activation. Chem Immunol Allergy 87:32–42. doi: 10.1159/000087569 [DOI] [PubMed] [Google Scholar]
- 61.Schain M, Kreisl WC (2017) Neuroinflammation in Neurodegenerative Disorders-a Review. Curr Neurol Neurosci Rep 17 (3):25. doi: 10.1007/s11910-017-0733-2 [DOI] [PubMed] [Google Scholar]
- 62.McManus RM, Heneka MT (2017) Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther 9 (1):14. doi: 10.1186/s13195-017-0241-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17 (1):49–59. doi: 10.1038/nri.2016.123 [DOI] [PubMed] [Google Scholar]
- 64.Skaper SD, Facci L, Giusti P (2014) Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol Disord Drug Targets 13 (10):1654–1666 [DOI] [PubMed] [Google Scholar]
- 65.Nelissen S, Lemmens E, Geurts N, Kramer P, Maurer M, Hendriks J, Hendrix S (2013) The role of mast cells in neuroinflammation. Acta Neuropathol 125 (5):637–650. doi: 10.1007/s00401-013-1092-y [DOI] [PubMed] [Google Scholar]
- 66.Purcell WM, Atterwill CK (1995) Mast cells in neuroimmune function: neurotoxicological and neuropharmacological perspectives. Neurochem Res 20 (5):521–532 [DOI] [PubMed] [Google Scholar]
- 67.Shaik-Dasthagirisaheb YB, Conti P (2016) The Role of Mast Cells in Alzheimer's Disease. Adv Clin Exp Med 25 (4):781–787. doi: 10.17219/acem/61914 [DOI] [PubMed] [Google Scholar]
- 68.Sayed BA, Walker ME, Brown MA (2011) Cutting edge: mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis. J Immunol 186 (6):3294–3298. doi: 10.4049/jimmunol.1003574 [DOI] [PubMed] [Google Scholar]
- 69.Karagkouni A, Alevizos M, Theoharides TC (2013) Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev 12 (10):947–953. doi: 10.1016/j.autrev.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 70.Elieh-Ali-Komi D, Cao Y (2017) Role of Mast Cells in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Clin Rev Allergy Immunol 52 (3):436–445. doi: 10.1007/s12016-016-8595-y [DOI] [PubMed] [Google Scholar]
- 71.Traina G (2017) Mast cells in the brain - Old cells, new target. J Integr Neurosci. 16(s1):S69–S83. doi: 10.3233/JIN-170068 [DOI] [PubMed] [Google Scholar]
- 72.Gu Y, Yang DK, Spinas E, Kritas SK, Saggini A, Caraffa A, Antinolfi P, Saggini R, Conti P (2015) Role of TNF in mast cell neuroinflammation and pain. J Biol Regul Homeost Agents 29 (4):787–791 [PubMed] [Google Scholar]
- 73.Zhang S, Zeng X, Yang H, Hu G, He S (2012) Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem 29 (5-6):931–940. doi: 10.1159/000171029 [DOI] [PubMed] [Google Scholar]
- 74.Cottrell GS, Amadesi S, Schmidlin F, Bunnett N (2003) Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans 31 (Pt 6):1191–1197. doi: 10.1042/bst0311191 [DOI] [PubMed] [Google Scholar]
- 75.Saito T, Bunnett NW (2005) Protease-activated receptors: regulation of neuronal function. Neuromolecular Med 7 (1-2):79–99. doi: 10.1385/NMM:7:1-2:079 [DOI] [PubMed] [Google Scholar]
- 76.Rothmeier AS, Ruf W (2012) Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol 34 (1):133–149. doi: 10.1007/s00281-011-0289-1 [DOI] [PubMed] [Google Scholar]
- 77.Liu X, Wang J, Zhang H, Zhan M, Chen H, Fang Z, Xu C, Chen H, He S (2016) Induction of Mast Cell Accumulation by Tryptase via a Protease Activated Receptor-2 and ICAM-1 Dependent Mechanism. Mediators Inflamm 2016:6431574. doi: 10.1155/2016/6431574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui Y, Dahlin JS, Feinstein R, Bankova LG, Xing W, Shin K, Gurish MF, Hallgren J (2014) Mouse mast cell protease-6 and MHC are involved in the development of experimental asthma. J Immunol 193 (10):4783–4789. doi: 10.4049/jimmunol.1302947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kopitar-Jerala N (2015) Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front Mol Neurosci 8:73. doi: 10.3389/fnmol.2015.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pranski E, Van Sanford CD, Dalal N, Orr AL, Karmali D, Cooper DS, Gearing M, Lah JJ, Levey AI, Betarbet R (2013) NF-kappaB activity is inversely correlated to RNF11 expression in Parkinson's disease. Neurosci Lett 547:16–20. doi: 10.1016/j.neulet.2013.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selvakumar GP, Iyer SS, Kempuraj D, Raju M, Thangavel R, Saeed D, Ahmed ME, Zahoor H, Raikwar SP, Zaheer S, Zaheer A (2018) Glia Maturation Factor Dependent Inhibition of Mitochondrial PGC-1alpha Triggers Oxidative Stress-Mediated Apoptosis in N27 Rat Dopaminergic Neuronal Cells. Mol Neurobiol. doi: 10.1007/s12035-018-0882-6 [DOI] [PMC free article] [PubMed] [Google Scholar]