Abstract
Academic performance in adolescence strongly influences adult prospects. Intelligence quotient (IQ) has historically been considered a strong predictor of academic performance. Less objectively explored have been morphometric features. We analyzed brain MRI morphometry metrics in early adolescence (age 12–14 years) as quantitative predictors of academic performance over high school using a naïve Bayesian classifier approach with n = 170 subjects. Based on the mean GPA, subjects were divided into high (GPA ≥ 3.54; n=87) and low (GPA <3.54; n=83) academic performers. Covariance analysis was performed to look at the influence of subject demographics. We examined predictive features from the 343 available regions (surface areas, cortical thickness, and subcortical volumes) and applied 4 algorithms for selection and reduction of attributes using Weka. Cortical thickness measures performed better than surface areas or subcortical volumes as predictors of academic performance. We identified 15 cortical thickness regions most predictive of academic performance, three of which have not been described in the literature predictive of academic performance. These were in the left hemisphere fusiform, bilateral insula, and left hemisphere paracentral regions. Prediction had a sensitivity of 0.65 and specificity of 0.73 with independent validation. Follow-up independent t-test analyses between high and low academic achievers on 10 of 15 regions showed between-group significance at the p < 0.05 level. High achievers demonstrated thicker cortices than low achievers. These newly identified regions may help pinpoint new targets for further study in understanding the developing adolescent brain in the classroom setting.
Keywords: naïve Bayesian classifier, academic performance, adolescence, magnetic resonance imaging, cortical thickness
INTRODUCTION
High school academic performance likely impacts scholastic advancement, including the opportunity to attend college and other career-determining educational programming (Jung and Haier 2007; Shaw 2007).
Schnack and colleagues (Schnack et al. 2015) have looked at the relationship between intelligence, cortical thickness, and cortical surface area. They found that lower intelligence has been associated with slightly thinner cortices at a young age (10 years old) and thicker cortices have been associated with higher intelligence during a person’s forties (Schnack et al. 2015). In addition, higher intelligence has been associated with cortical surface area expansion being completed at a younger age (Schnack et al. 2015). However, localized regions of the brain involved in predicting academic performance have not been as extensively explored. By studying regions of the brain thought to predict exemplary academic performance, we sought to gain understanding into which neural regions and networks are most critical in the classroom setting.
Machine learning techniques (e.g., neural networks) have been widely used in other areas of research including structure prediction and have greater applicability to areas with large amounts of accumulated experimental data (Meruelo, Samish, and Bowie 2011). This quantitative approach may allow identification of critical brain regions better than other approaches by providing a systematic, quantitative, and non-biased identification of areas, in this case, associated with higher academic performance. In contrast, while naïve Bayesian classifier methods can identify non-linear relationships linear regression is limited to linear relationships and is sensitive to outliers (Gelman and Hill 2006). Naïve Bayesian learning methods also are able to identify significant relationships with less input data and less impact of confounding variables (Gelman and Hill 2006). Naïve Bayesian classifiers can be optimal even if the assumption of feature independence is violated (Zhang 2005; Domingos and Pazzani 1997). Thus, we employed a naïve Bayesian classifier and contrasted the results with those of traditional linear, hierarchical regression to see if there was agreement between the two methods. We employed cross-validation for each algorithm for the naïve Bayesian classifier to avoid common problems such as overfitting and bias (Kohavi 1995; Trippa et al. 2015). Knowledge of the identified brain regions may inform future targets for early developmental and behavioral interventions towards improving cognitive function in the academic environment, and academic achievement.
The aim of this paper is to quantitatively identify regions whose morphometry in early adolescence predicts later academic performance in high school. Several regions were examined. The basal ganglia have been shown to be involved in stepwise, feedback-based learning, while the medial temporal lobes are implicated in encoding of relationships between stimuli and facilitate application of relationships in new contexts (Shohamy et al. 2008). The lateral prefrontal cortex plays a major role in behavioral planning related to learning (Tanji, Shima, and Mushiake 2007). The anterior cingulate gyrus has been suggested as important locus for self-regulation, support cognitive and emotional functioning during task performance (Posner et al. 2007). The hippocampal role in long-term episodic memory has been related to constructing mental imagery (Bird and Burgess 2008). Language processing and social attention through eye gaze processing, essential to classroom learning, relies on the superior temporal sulcus (Redcay 2008). Finally, the amygdala appears important to processing positive and negative rewards, and reinforcement (Murray 2007).
For the present study, we hypothesized that frontal, basal ganglia, limbic, and temporal morphometry in early adolescence would predict academic performance in high school (Shohamy et al. 2008; Kéri 2008; Tanji, Shima, and Mushiake 2007; Berger et al. 2007; Murray 2007; Posner et al. 2007; Sandi and Pinelo-Nava 2007; Hargreaves et al. 2005; Bird and Burgess 2008; Ishai et al. 1999; Redcay 2008). We also wanted to ensure that potentially confounding factors (age, pubertal development, socioeconomic status, etc.) were accounted for, so covariance analysis was completed to examine these, as described below.
METHODS
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the UCSD under the UCSD Human Research Protections Program (Project #090269).
Informed consent
Informed consent was obtained from the parents/guardians/LAR for all participants included in the study.
Participants
Study participants (N = 288) were recruited from local San Diego area schools at age 12–14 years (average age 13.2 ± 0.8), defined as the baseline year. Longitudinal data including magnetic resonance imaging (MRI) and grade point average (or GPA) were collected throughout study through graduation from high school. Exclusionary criteria included history of psychiatric or neurological disorder, imaging data with resolution less than 3T, and for this study, incomplete GPA data through high school. Brain MRI data were collected at baseline and GPA at the 11th and 12th grade level (followup). These criteria resulted in a reduced total of N = 170 subjects. Demographics statistics for all subjects prior to exclusion have been included in Supplementary Table 2 for reference. Complete study procedure design, timeline, urine toxicology testing, and IRB in described in further detail in previous work (Jacobus et al. 2012).
Measures
The Customary Drinking and Drug Use Record was employed to assess lifetime alcohol, cannabis, tobacco, and other drug use defined as the cumulative use (e.g., cannabis) episodes at baseline (Brown et al. 1998). The Beck Depression Inventory (Beck, Steer, and Carbin 1988) and Spielberger State Trait Anxiety Inventory (Spielberger et al. 1983) were used to assess subject depression and state anxiety. The Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al. 2001; Shaffer et al. 1996) was obtained from child and parent to exclude those with Axis I disorders other than alcohol or cannabis use disorders at baseline. Family history of substance use disorders was obtained using the Family History Assessment Module (Rice et al. 1995). Grade point average and parental income were obtained during an interview prior to baseline imaging acquisition.
Due to the sample used for predictive purposes of high school performance consisting of 12–14 years olds at the time of baseline neuroimaging, substance use (cannabis, alcohol, and other dugs) was very low. Thus, neuroimaging results do not reflect the future use of alcohol or drugs on high school performance in adolescence.
Image Acquisition and Processing
Scans were acquired on a 3.0 T CXK4 short bore Excite-2 magnetic resonance system (General Electric, Milwaukee, WI) with an eight-channel phase array head coil at the UCSD Center for Functional MRI. Subjects were instructed to remain motionless while a high-resolution T1-weight anatomical spoil gradient recall (SPGR) scan was obtained (TE/TR = min full, field of view = 24 cm, resolution = 1 mm3, 170 continuous slices). Cortical thickness, area, and volume estimates were obtained in the same manner as previously published by our laboratory (Jacobus et al. 2014, 2015). FreeSurfer (version 5.1, surfer.nmr.mgh.harvard.edu) was used for cortical surface reconstruction and to obtain cortical thickness estimates (Fischl, Sereno, and Dale 1999; Dale, Fischl, and Sereno 1999). The cross-sectioning process, cortical thickness calculation, and parcellation procedure has previously been described in detail (Jacobus et al. 2015).
Data Analysis
For feature selection analysis, surface areas, cortical thickness, and subcortical volumes were extracted as input training data to distinguish those with exemplary versus lesser academic performance in high school. The mean GPA of subjects was determined to be 3.54 ± 0.60 (ranging from a low of 1.35 to a high of 5.00; these include AP courses for which students can achieve higher than GPA of 4.0) and consequently subjects were divided into high (GPA ≥ 3.54; n = 87) and low (GPA < 3.54; n = 83) academic performers.
To identify predictive features from the 343 available segmented brain regions (surface areas, cortical thickness, and subcortical volumes), we applied the CfsSubset with BestFirst algorithm for selection and reduction of attributes using Weka (M. Hall et al. 2009). CfsSubset evaluates the worth of a subset of attributes by considering the individual predictive ability of each feature along with the degree of redundancy between them; subsets of features that are highly correlated with the binary class while having low intercorrelation are preferred (M. A. Hall 1999). BestFirst searches the space of attribute subsets by greedy hillclimbing augmented with a backtracking facility (M. Hall et al. 2009). Subcortical and white matter volumes were normalized using regression-based intracranial volumes to account for intrasubject intracranial volume (ICV) differences. As ICV normalization methods vary in their ability to accurately capture morphometric measures, we also performed predictive analysis as described below without ICV correction and the results have been included in Supplemental Table 3 below.
Fifteen morphometric attributes were used to make predictions using a naïve Bayes classifier of high and low academic performance. Predictive attributes were then validated by dividing the sample in half with 50% used for training and 50% used for validation. Others have used similar approaches to prediction successfully in other major biological problems especially in the area of genomics (Douglass et al. 2016; Geng et al. 2015; Tian and Lim 2015).
Group differences between parameters of predictive regions for high v. low academic performers were identified by performing independent t-test comparisons using IBM SPSS v22. Significance was determined at the p < 0.05 level. Demographic analysis was completed using independent t-test comparisons between high and low academic performers across age, annual household income, mood scores at baseline, familial alcohol density, gender, ethnicity, lifetime substance use, and presence of DSM-5 (American Psychiatric Association 2013) psychiatric conditions (i.e., social phobia, panic disorder, generalized anxiety disorder, specific phobias, obsessive-compulsive disorder, major depressive disorder, manic disorder, schizophrenia, attention deficit hyperactive disorder (ADHD), oppositional defiant disorder, conduct disorder, alcohol use disorder, cannabis use disorder, substance use disorder, ADHD functional impairment, and separation anxiety disorder) using the Diagnostic Interview Schedule for Children (DISC) Predictive Scales (Leung et al. 2005; Lucas et al. 2001). Covariance analysis used StatPlus (http://www.analystsoft.com/en/products/statplus/) and Pearson correlation coefficients between longitudinal baseline age, socioeconomic status (SES), familial alcohol density, and pubertal developmental score, and 15 predictive cortical thickness regions.
RESULTS
Demographic analysis demonstrated similarity of low- and high-academic performers across a variety of measures including age, income, depressive and anxiety symptoms, cannabis and alcohol use, family history of alcoholism, incidence of developing psychiatric and substance use disorders, and impairment related to ADHD (see Table 1).
Table 1.
Demographic Characteristics at Baseline
| Mean±SD or % for academic performer group | ||
|---|---|---|
| High (n=84) | Low (n=77) | |
| Age | 13.1±0.7 | 13.2±0.8 |
| Annual household income ($K) | 93±109 | 78±85 |
| Familial alcohol use disorder density | 0.00±0.03 | 0.01±0.06 |
| % Male | 54 | 61 |
| % Hispanic/Latino | 13 | 39 |
| Race (%white) | 75 | 53 |
| Times used tobacco | 0.1±0.6 | 0±1 |
| Days used alcohol per month in past 3 months | 0.0±0.2 | 0.0±0.2 |
| Times used cannabis, lifetime | 0±0 | 0.1±0.4 |
| Times used other drug, lifetime | 0±0 | 0.0±0.1 |
| Beck Depression Inventory total | 1±2 | 2±4 |
| Spielberger State Anxiety T-score | 48±4 | 47±5 |
| Social phobia* | 0.1±0.4 | 0.2±0.4 |
| Panic disorder* | 0.1±0.2 | 0±0 |
| Generalized anxiety disorder* | 0.1±0.4 | 0.1±0.4 |
| Specific phobias* | 0.3±0.6 | 0.4±0.7 |
| Obsessive-compulsive disorder (OCD)* | 0.0±0.2 | 0.0±0.2 |
| Major depressive disorder (MDD)* | 0.3±0.8 | 0.1±0.6 |
| Manic disorder* | 0.1±0.3 | 0.1±0.3 |
| Schizophrenia* | 0.0±0.0 | 0.0±0.0 |
| Attention deficit hyperactivity disorder (ADHD) * | 0.3±0.8 | 1±2 |
| Oppositional defiant disorder* | 0.2±0.7 | 1±1 |
| Conduct disorder* | 0.1±0.2 | 0.3±0.7 |
| Alcohol use disorder* | 0.0±0.1 | 0.0±0.1 |
| Cannabis use disorder* | 0.0±0.0 | 0.0±0.0 |
| Substance use disorder* | 0.0±0.0 | 0.0±0.0 |
| ADHD functional impairment* | 0±1 | 1±2 |
| Separation anxiety disorder* | 0.0±0.2 | 0.2±0.6 |
Subscale or total score from DISC Predictive Scales(Lucas et al. 2001; Leung et al. 2005, 200)
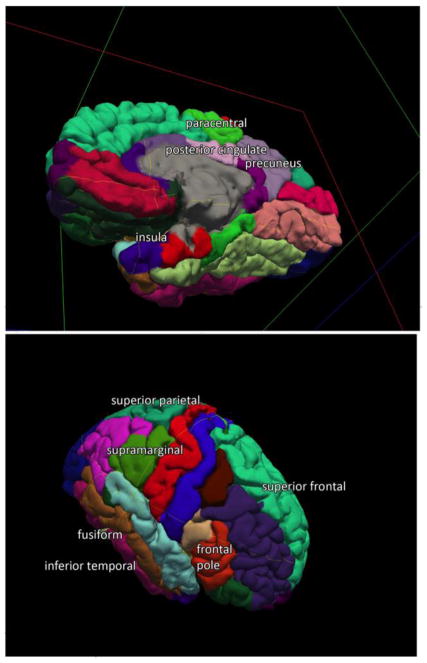
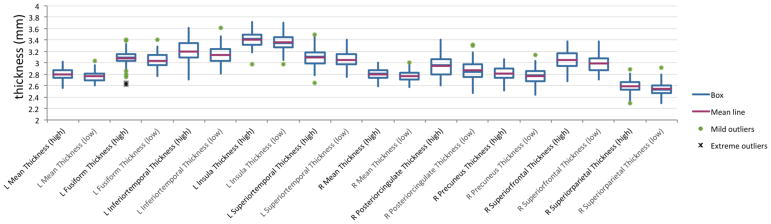
We found that 15 cortical regions distinguished high versus low academic performance at follow-up optimally, using a naïve Bayesian classifier approach with training on half of the dataset and validation on the remaining half of dataset, with a sensitivity of 0.65 and specificity of 0.73 without ICV correction (or a sensitivity of 0.60 and specificity of 0.64 with ICV correction). High performers, compared to low performers, showed greater baseline cortical thickness in: bilateral hemisphere mean thickness, bilateral insula, bilateral precuneus, left frontal pole, left fusiform, left inferior temporal, left paracentral, left superior temporal, left supramarginal, right posterior cingulate, right superior frontal, and right superior parietal cortices, as illustrated in Figure 1(a) and 1(b). Follow-up analyses confirmed group differences for 10 of 15 regions in independent t-tests (p<.05) (see Table 2 and Figure 2). Subcortical and white matter volumes were not found to be significantly predictive of outcome academic performance using the naïve Bayesian classifier approach. We also performed this analysis using data uncorrected for intracranial volume and found similar results (13 of 15 regions noted above remained the same) provided in Supplementary Table 3.
Fig. 1.
Predictive cortical thickness regions for academic performance. (A) Medial view, (B) Lateral view. This colored figure illustrates the 15 regions of the brain without respect to laterality whose cortical thickness predicts academic performance. Please refer to Table 2 for laterality.
Table 2.
Independent t-tests for Predictive Regions
| Mean for academic performer group | P-value | ||
|---|---|---|---|
| High (n=84) | Low (n=77) | (nonequal variances) | |
| L Mean Thickness (*) | 2.802 | 2.769 | 0.03 |
| L Frontal Pole Thickness | 3.214 | 3.188 | 0.59 |
| L Fusiform Thickness (*) | 3.083 | 3.037 | 0.03 |
| L Inferior Temporal Thickness (*) | 3.196 | 3.138 | 0.03 |
| L Insula Thickness (*) | 3.412 | 3.363 | 0.02 |
| L Paracentral Thickness | 2.755 | 2.738 | 0.45 |
| L Precuneus Thickness | 2.789 | 2.76 | 0.15 |
| L Superior Temporal Thickness (*) | 3.098 | 3.049 | 0.04 |
| L Supramarginal Thickness | 2.939 | 2.899 | 0.09 |
| R Mean Thickness (*) | 2.802 | 2.766 | 0.01 |
| R Insula Thickness | 3.408 | 3.378 | 0.21 |
| R Posterior Cingulate Thickness (*) | 2.942 | 2.869 | 0.01 |
| R Precuneus Thickness (*) | 2.817 | 2.77 | 0.02 |
| R Superior Frontal Thickness (*) | 3.058 | 2.984 | 0 |
| R Superior Parietal Thickness (*) | 2.591 | 2.544 | 0.01 |
Abbreviations: L, Left. R, Right.
p<.05 for group differences
Fig. 2.
Boxplot analysis for predictive regions of cortical thickness for high v. low academic performers
The largest bivariate correlations were found between pubertal developmental score and gender (expected), right hemisphere supramarginal volume and gender, left hemisphere isthmus cingulate volume and gender, right superior frontal thickness and age, right hemisphere supramarginal volume and SES, right hemisphere pars opercularis volume and gender, left hemisphere precuneus thickness and age, right hemisphere superior parietal thickness and age, left hemisphere fusiform thickness and age, left hemisphere insula thickness and age, left hemisphere isthmus cingulate volume and age, left hemisphere supramarginal thickness and age, right hemisphere supramarginal volume and age, left hemisphere paracentral thickness and age, left hemisphere paracentral thickness and SES, and right hemisphere pars opercularis volume and pubertal developmental score (see Supplementary Table 1).
DISCUSSION
We found that early adolescent thickness of cortical regions previously associated with learning and memory formation were key to subsequent academic functioning. This highlights the importance of cortical thicknesses over surface areas or subcortical volumes as predictors of academic performance in high school. We found agreement with previous reports (Karama et al. 2011) of increased cortical thickness in high performers compared to low performers in the left frontal pole, left inferior temporal, bilateral precuneus, left superior temporal, left supramarginal, right posterior cingulate, right superior frontal, and right superior parietal cortices.
These findings are consistent with Shaw et al. (Shaw et al. 2006) who found a negative correlation between intelligence (associated with higher academic performance) and cortical thickness in early childhood, yet a positive correlation from late childhood onwards. Shaw et al. (Shaw et al. 2008) also found that the medial occipitotemporal and anterior superior temporal areas are isocortical regions that don’t follow typical developmental trajectories.
We identified three regions predicting subsequent academic performance that were not previously reported in the literature: left fusiform, bilateral insula, and left paracentral regions. The fusiform gyrus is logical, given its importance in understanding language, learning, memory, and word and facial (e.g., social element of school) recognition (Devlin et al. 2006; Kanwisher and Yovel 2006; Peelen and Downing 2005). The insula is involved in salience for monitoring the environment and salience of selecting attention to achieve tasks (Menon and Uddin 2010). The paracentral region is known to be involved in somatosensory of distal limbs and motor coordination, but its role in cognition is not established (Spasojević et al. 2013).
The insula and anterior cingulate cortex appear to lack the typical growth pattern of stabilization during the first three decades of life (Shaw et al. 2008). Both the insula and anterior cingulate cortex follow a quadratic model with increases in cortical thickness observed between ages 5 to 17 and decreases thereafter (Shaw et al. 2008). The insula has not been previously identified as being predictive of academic performance.
Strengths, Weaknesses, and Limitations
One important caveat in interpreting the importance of these regions is that the regions identified are not exclusionary of other regions that may be equal or of lesser importance in prediction of academic performance. The regions identified are non-redundant ones that have been identified as most predictive of academic performance in combination, but do not exclude the possibility that there are other regions that are of equal or lesser importance that may also predict academic performance. For example, while the left fusiform, bilateral insula, and left paracentral regions are three of the 15 regions found to be most predictive of academic performance this does not preclude that regions corresponding to other areas of the brain other than these 15 may also predict academic performance.
Two major strengths of the study were the large sample size (n = 84 high performers and n = 77 low performers) and the strategy employed to objectively identify predictive regions of the brain for academic performance (naïve Bayesian classifier method). Many studies have hand-select regions using regions of interest or employ whole-brain analysis, but we were able to start with 343 brain features and reduce these to a non-redundant set of features that was most predictive across multiple feature reduction algorithms. There are few studies that start with such a large subset of features and narrow down analysis to such a small subset (15 features) that can predict the variation in academic performance as our study has done.
One limitation of our study findings is that the cohort was comprised of relatively high performing students from within a 50-mile radius of the university-based study site, as compared to schools systems nationally. GPA ranged from a low of 1.35 to a high of 5.00; these include advanced placement courses from which students can achieve grades as high as 5.0. With the increasingly common presence of advanced placement courses available in American high schools, those under 3.5 are relatively lower functioning than in decades past. Thus, findings do not address variability in those with significant learning disabilities or very poor academic performance, but contrast youth with high versus medium performance levels. Future studies should replicate these findings in samples that include lower performing students.
An important limitation to consider is the validity of GPA as a measure of intelligence in comparison with other measures such as neurocognitive test performance, intelligence quotient, or aptitude tests (e.g., SAT/ACT) (Coyle et al. 2011; Frey and Detterman 2004). GPA reflects intelligence, but also a variety of factors such as rule following, compliance, test-taking skills (Kuncel, Credé, and Thomas 2005), and possibly cultural bias (Kruse 2016). GPA can also vary based on difficulty of school curricula, though with standardized AP course offerings this variability has been reduced (Kuncel, Credé, and Thomas 2005). GPA was the primary measure available as part of the Youth-at-Risk longitudinal study, hence its choice as a measure.
Another limitation of our study is that the comparable reliability and validity of cortical thickness, cortical surface area, and subcortical volumes must be considered given that these metrics were all used as input for the machine learning approach taken (B. Fischl and Dale 2000; Clarkson et al. 2011; Cardinale et al. 2014). Based on a review of the literature, test-retested correlations, intraclass correlation coefficients, and percent differences for cortical thickness, cortical surface area, and subcortical volume were 0.82/0.88/0.88, 0.81/0.87/0.88, and 0.86/1.19/1.39, respectively (Iscan et al. 2015). This suggests relatively strong psychometrics across measures yet that subcortical volume values may be more reliable than cortical thickness values. However, our machine learning approach validated on an independent data set not previously trained on, demonstrated that, to the contrary, a combination of metrics across subcortical volumes, surface areas, and cortical thickness was more predictive than any particular subcortical volume parameter. This may be because, while generalizations can be made of the reliability of classes of metrics, some measures may be more predictive independent of the reliability of a particular type of index.’
In summary, these findings highlight 15 cortical thickness regions identified to be predictive of high versus low academic performance. These regions were more predictive than surface area or subcortical volume; agreement in the regions identified was found with previous work by Karame and colleagues (Karama et al. 2011). Findings are also consistent with the developmental trajectory related to cortical thickness establish by Shaw and coworkers (P. Shaw et al. 2006; Philip Shaw et al. 2008; Philip Shaw 2007). Three new regions predictive of academic performance were identified, creating new targets for further study in understanding the developing adolescent brain in the classroom setting. We hope to be able to expand academic predictive studies further by adding diffusion tensor imaging data, explore the influence of comorbid substance use, and look at gender-related differences. The identified neuroimaging regions may be useful in identifying those struggling academically at an early age, monitoring the impact of educational interventions on brain development, and exploring the role of substance use on the developing adolescent brain as related to academic performance in future studies.
Supplementary Material
Acknowledgments
Acknowledgement of funding: This work was made possible by the National Institute of Mental Health grant R25 MH101072 and the National Institute on Alcohol Abuse and Alcoholism grant R01 AA013419-14S1 that support Research Track Resident Alejandro Meruelo, MD, PhD. Data were collected through the Youth at Risk (YAR) project by means of research grant from the National Institute on Alcohol Abuse and Alcoholism R01 AA013419 (PI: Tapert).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Beck Aaron T, Steer Robert A, Carbin Margery G. Psychometric Properties of the Beck Depression Inventory: Twenty-Five Years of Evaluation. Clinical Psychology Review. 1988;8(1):77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- Berger Andrea, Kofman Ora, Livneh Uri, Henik Avishai. Multidisciplinary Perspectives on Attention and the Development of Self-Regulation. Progress in Neurobiology. 2007;82(5):256–86. doi: 10.1016/j.pneurobio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Bird Chris M, Burgess Neil. The Hippocampus and Memory: Insights from Spatial Processing. Nature Reviews. Neuroscience. 2008;9(3):182–94. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric Evaluation of the Customary Drinking and Drug Use Record (CDDR): A Measure of Adolescent Alcohol and Drug Involvement. Journal of Studies on Alcohol. 1998;59(4):427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Dale Anders M, Fischl Bruce, Sereno Martin I. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Devlin Joseph T, Jamison Helen L, Gonnerman Laura M, Matthews Paul M. The Role of the Posterior Fusiform Gyrus in Reading. Journal of Cognitive Neuroscience. 2006;18(6):911–22. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos Pedro, Pazzani Michael. On the Optimality of the Simple Bayesian Classifier Under Zero-One Loss. Mach Learn. 1997;29(2–3):103–130. doi: 10.1023/A:1007413511361. [DOI] [Google Scholar]
- Douglass Stephen, Hsu Ssu-Wei, Cokus Shawn, Goldberg Robert B, Harada John J, Pellegrini Matteo. A Naïve Bayesian Classifier for Identifying Plant microRNAs. The Plant Journal: For Cell and Molecular Biology. 2016;86(6):481–92. doi: 10.1111/tpj.13180. [DOI] [PubMed] [Google Scholar]
- Fischl Bruce, Sereno Martin I, Dale Anders M. Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gelman Andrew, Hill Jennifer. Data Analysis Using Regression and Multilevel/Hierarchical Models. 1. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Geng Haijiang, Lu Tao, Lin Xiao, Liu Yu, Yan Fangrong. Prediction of Protein-Protein Interaction Sites Based on Naive Bayes Classifier. Biochemistry Research International. 2015;2015:978193. doi: 10.1155/2015/978193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Mark, Frank Eibe, Holmes Geoffrey, Pfahringer Bernhard, Reutemann Peter, Witten Ian H. The WEKA Data Mining Software: An Update. SIGKDD Explor Newsl. 2009;11(1):10–18. doi: 10.1145/1656274.1656278. [DOI] [Google Scholar]
- Hargreaves Eric L, Rao Geeta, Lee Inah, Knierim James J. Major Dissociation between Medial and Lateral Entorhinal Input to Dorsal Hippocampus. Science (New York, NY) 2005;308(5729):1792–94. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed Representation of Objects in the Human Ventral Visual Pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):9379–84. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus Joanna, Goldenberg Diane, Wierenga Christina E, Tolentino Neil J, Liu Thomas T, Tapert Susan F. Altered Cerebral Blood Flow and Neurocognitive Correlates in Adolescent Cannabis Users. Psychopharmacology. 2012;222(4):675–84. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus Joanna, Squeglia Lindsay M, Meruelo Alejandro D, Castro Norma, Brumback Ty, Giedd Jay N, Tapert Susan F. Cortical Thickness in Adolescent Marijuana and Alcohol Users: A Three-Year Prospective Study from Adolescence to Young Adulthood. Developmental Cognitive Neuroscience, Substance Use and the Adolescent Brain: Developmental Impacts, Interventions, and Longitudinal Outcomes. 2015 Dec;16:101–9. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus Joanna, Squeglia Lindsay M, Sorg Scott F, Nguyen-Louie Tam T, Tapert Susan F. Cortical Thickness and Neurocognition in Adolescent Marijuana and Alcohol Users Following 28 Days of Monitored Abstinence. Journal of Studies on Alcohol and Drugs. 2014;75(5):729–43. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Rex E, Haier Richard J. The Parieto-Frontal Integration Theory (P-FIT) of Intelligence: Converging Neuroimaging Evidence. The Behavioral and Brain Sciences. 2007;30(2) doi: 10.1017/S0140525X07001185. 135-154-187. [DOI] [PubMed] [Google Scholar]
- Kanwisher Nancy, Yovel Galit. The Fusiform Face Area: A Cortical Region Specialized for the Perception of Faces. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361(1476):2109–28. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama Sherif, Colom Roberto, Johnson Wendy, Deary Ian J, Haier Richard, Waber Deborah P, Lepage Claude, Ganjavi Hooman, Jung Rex, Evans Alan C. Cortical Thickness Correlates of Specific Cognitive Performance Accounted for by the General Factor of Intelligence in Healthy Children Aged 6 to 18. NeuroImage. 2011;55(4):1443–53. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéri Szabolcs. Interactive Memory Systems and Category Learning in Schizophrenia. Neuroscience and Biobehavioral Reviews. 2008;32(2):206–18. doi: 10.1016/j.neubiorev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kohavi Ron. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. Proceedings of the 14th International Joint Conference on Artificial Intelligence - Volume 2; San Francisco, CA, USA: Morgan Kaufmann Publishers Inc; 1995. pp. 1137–1143. IJCAI’95. http://dl.acm.org/citation.cfm?id=1643031.1643047. [Google Scholar]
- Leung Patrick WL, Lucas Christopher P, Hung Se-fong, Kwong Shi-leung, Tang Chun-pan, Lee Chi-chiu, Ho Ting-pong, Lieh-Mak Felice, Shaffer David. The Test-Retest Reliability and Screening Efficiency of DISC Predictive Scales-Version 4.32 (DPS-4.32) with Chinese Children/Youths. European Child & Adolescent Psychiatry. 2005;14(8):461–65. doi: 10.1007/s00787-005-0503-6. [DOI] [PubMed] [Google Scholar]
- Lucas Christopher P, Zhang Haiying, Fisher Prudence W, Shaffer David, Regier Darrel A, Narrow William E, Bourdon Karen, et al. The DISC Predictive Scales (DPS): Efficiently Screening for Diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(4):443–49. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Menon Vinod, Uddin Lucina Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Structure & Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo Alejandro D, Samish Ilan, Bowie James U. TMKink: A Method to Predict Transmembrane Helix Kinks. Protein Science: A Publication of the Protein Society. 2011;20(7):1256–64. doi: 10.1002/pro.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray Elisabeth A. The Amygdala, Reward and Emotion. Trends in Cognitive Sciences. 2007;11(11):489–97. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Peelen Marius V, Downing Paul E. Selectivity for the Human Body in the Fusiform Gyrus. Journal of Neurophysiology. 2005;93(1):603–8. doi: 10.1152/jn.00513.2004. [DOI] [PubMed] [Google Scholar]
- Posner Michael I, Rothbart Mary K, Sheese Brad E, Tang Yiyuan. The Anterior Cingulate Gyrus and the Mechanism of Self-Regulation. Cognitive, Affective & Behavioral Neuroscience. 2007;7(4):391–95. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Redcay Elizabeth. The Superior Temporal Sulcus Performs a Common Function for Social and Speech Perception: Implications for the Emergence of Autism. Neuroscience and Biobehavioral Reviews. 2008;32(1):123–42. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, Begleiter H. Comparison of Direct Interview and Family History Diagnoses of Alcohol Dependence. Alcoholism, Clinical and Experimental Research. 1995;19(4):1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Sandi Carmen, Teresa Pinelo-Nava M. Stress and Memory: Behavioral Effects and Neurobiological Mechanisms. Neural Plasticity. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack Hugo G, van Haren Neeltje EM, Brouwer Rachel M, Evans Alan, Durston Sarah, Boomsma Dorret I, Kahn René S, Hulshoff Pol Hilleke E. Changes in Thickness and Surface Area of the Human Cortex and Their Relationship with Intelligence. Cerebral Cortex (New York, NY: 1991) 2015;25(6):1608–17. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Shaffer David, Fisher Prudence, Dulcan Mina K, Davies Mark, Piacentini John, Schwab-Stone Mary E, Lahey Benjamin B, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, Acceptability, Prevalence Rates, and Performance in the MECA Study. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(7):865–77. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual Ability and Cortical Development in Children and Adolescents. Nature. 2006;440(7084):676–79. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw Philip. Intelligence and the Developing Human Brain. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2007;29(10):962–73. doi: 10.1002/bies.20641. [DOI] [PubMed] [Google Scholar]
- Shaw Philip, Kabani Noor J, Lerch Jason P, Eckstrand Kristen, Lenroot Rhoshel, Gogtay Nitin, Greenstein Deanna, et al. Neurodevelopmental Trajectories of the Human Cerebral Cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal Ganglia and Dopamine Contributions to Probabilistic Category Learning. Neuroscience and Biobehavioral Reviews. 2008;32(2):219–36. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević Goran, Malobabic Slobodan, Pilipović-Spasojević Olivera, Djukić-Macut Nataša, Maliković Aleksandar. Morphology and Digitally Aided Morphometry of the Human Paracentral Lobule. Folia Morphologica. 2013;72(1):10–16. doi: 10.5603/fm.2013.0002. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tanji Jun, Shima Keisetsu, Mushiake Hajime. Concept-Based Behavioral Planning and the Lateral Prefrontal Cortex. Trends in Cognitive Sciences. 2007;11(12):528–34. doi: 10.1016/j.tics.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Tian Xue W, Lim Joon S. Interactive Naive Bayesian Network: A New Approach of Constructing Gene-Gene Interaction Network for Cancer Classification. Bio-Medical Materials and Engineering. 2015;26(Suppl 1):S1929–1936. doi: 10.3233/BME-151495. [DOI] [PubMed] [Google Scholar]
- Trippa Lorenzo, Waldron Levi, Huttenhower Curtis, Parmigiani Giovanni. Bayesian Nonparametric Cross-Study Validation of Prediction Methods. The Annals of Applied Statistics. 2015;9(1):402–28. doi: 10.1214/14-AOAS798. [DOI] [Google Scholar]
- Zhang Harry. Exploring Conditions for the Optimality of Naïve Bayes. International Journal of Pattern Recognition and Artificial Intelligence. 2005;19(2):183–98. doi: 10.1142/S0218001405003983. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.