Abstract
A seven day pretreatment course of an oral antibiotic cocktail (Ampicillin, Metronidazole, Neomycin Sulfate, and Vancomycin) was shown to induce changes in peripheral immune regulation and protect mice from signs of experimental autoimmune encephalomyelitis (EAE). To determine if a shorter course of antibiotic pretreatment could also protect the mice from EAE and induce regulatory immune cells, studies were conducted using the same oral antibiotic cocktail for three days. In addition, the CNS was examined to determine the effects of antibiotic pretreatment on EAE disease course and immune modulation within the affected tissue. The shorter three day pretreatment course was also significantly protective against severe EAE in C57Bl/6 mice. Moreover, our study found increased frequencies of regulatory cells and a decrease in the frequency of anti-inflammatory macrophages in the spleen of EAE protected mice. Additionally, a chemokine and chemokine receptor array run on mRNA from spinal cords revealed that genes associated with regulatory T cells and macrophage recruitment were strongly upregulated in the antibiotic pretreated mice. Additional RT-PCR data showed genes associated with anti-inflammatory microglia/macrophages were upregulated and pro-inflammatory genes were downregulated. This suggests the macrophages recruited to the spinal cord by chemokines are subsequently polarized toward an anti-inflammatory phenotype. These results lend strong support to the conclusion that a three day course of antibiotic treatment given prior to the induction of severe EAE profoundly protected the mice by inducing regulatory lymphocytes in the periphery and an anti-inflammatory milieu in the affected spinal cord tissue.
Keywords: Microbiota, Neuroinflammation, EAE, Antibiotic, Regulatory cells, CNS
Introduction
Multiple sclerosis (MS) is an autoimmune disease that results in chronic immune mediated demyelination of the central nervous system (CNS). Previous studies have demonstrated the important role that gut microbiota can have on disease progression (Berer et al. 2011; Erny et al. 2015; Lee et al. 2011; Mielcarz and Kasper 2015; Ochoa-Reparaz and Kasper 2014; Ochoa-Reparaz et al. 2009; Wang et al. 2014). Individuals with MS have gut dysbiosis that can exacerbate their symptoms (Freedman et al. 2018). It has been recognized that the gut microbiota can shape the immune system and its response (Palm et al. 2015). Animal studies in germ free mice raised in sterile conditions have shown these mice develop an anti-inflammatory T helper (Th) 2 response and demonstrate a significant reduction in a pro-inflammatory Th17 response compared to control mice with intact gut microbiota (Niess et al. 2008). Certain bacteria have even been shown to skew the immune system from a Th17 to a Treg response (Ivanov et al. 2008).
The administration of antibiotics to mice prior to the induction of experimental autoimmune encephalomyelitis (EAE) protects mice from developing severe disease that is seen in control mice. This protection was attributed to the induction of interleukin 10 (IL-10) producing FoxP3+ Treg cells. These cells were significantly reduced in the spleen but significantly increased in the cervical and mesenteric lymph nodes. The presence of these Treg cells significantly reduced pro-inflammatory cytokines, including interferon gamma (IFNγ) and IL-17 (Ochoa-Reparaz et al. 2009). In addition to inducing regulatory T cells, antibiotic pretreatment also induces Breg (CD19+ CD5+ CD1dhi)-type B10 cells (Ochoa-Reparaz et al. 2010). These cells are known to produce IL-10 and create anti-inflammatory immune environments (Iwata et al. 2011). This protective B cell subset is upregulated with estrogen pretreatment prior to EAE that results in complete protection of mice from EAE (Zhang et al. 2015).
The above mentioned studies demonstrate how oral pretreatment with an antibiotic cocktail (ABX) prior to the induction of EAE can protect mice from the severe form of the disease seen in control mice. While most of the studies were conducted in SJL mice, it was shown that female C57BL/6 mice were also protected with 7 days of ABX pretreatment (Ochoa-Reparaz et al. 2009; Ochoa-Reparaz et al. 2010). The current study was conducted to examine the effect of three days of oral ABX pretreatment on female C57BL/6 mice and how this protection affected other immune cells not previously evaluated in the spleen and mesenteric lymph nodes. Additionally this study looked at the anti-inflammatory/protective effect on the CNS with ABX pretreatment and how this treatment affected chemokines and chemokine receptors within the CNS.
Materials and Methods
Animals
Female wild type C57BL/6 (8–10 weeks old) mice were purchased from The Jackson Laboratory (Sacramento, CA). All mice came from the same room at Jackson Laboratory to limit the number of factors that could influence the microbiome. Mice were only housed together if they were in the same treatment group. Mice were given access to food and water ad libitum and kept on a 12 h light/dark cycle. This study was conducted in accordance with NIH guidelines for the use of experimental animals and the VAPORHCS Animal Care and Use Committee approved protocols.
Antibiotic treatment and induction of EAE
An antibiotic cocktail (ABX) consisting of Ampicillin (1g/L), Metronidazole (1g/L), Neomycin Sulfate (1g/L), and Vancomycin (0.5g/L) dissolved in water was used to deplete the microbiota of mice. Female mice were gavaged with 200ul of ABX once a day for three consecutive days. Mice were immunized after three days of antibiotic oral gavage or sham treatment with 200μg mouse MOG-35-55 peptide (PolyPeptide Laboratories, San Diego, CA) in 200μg Complete Freund’s adjuvant (Incomplete Freund’s adjuvant (IFA, Sigma-Aldrich, St. Louis, MO)) with heat-killed Mycobacterium tuberculosis (Mtb, Difco, Detroit, MI) subcutaneously along the flanks at four sites. Additionally, mice were administered 75ng of pertussis toxin (Ptx, List Biologicals, Campbell, CA) via an intraperitoneal (i.p.) injection on the day of immunization and 200ng i.p. two days later. All mice were monitored daily for weight loss and clinical signs of EAE disease. Mice were scored using the following scale: 0 = normal; 1 = limp tail or mild hind limb weakness; 2 = moderate hind limb weakness or mild ataxia; 3 = moderately severe hind limb weakness; 4 = severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5 = paraplegia with no more than moderate forelimb weakness; 6 = paraplegia with severe forelimb weakness or severe ataxia or moribund condition.
Leukocyte preparation from the spleen and mesenteric lymph nodes
All tissues were collected from mice 28 days post-immunization. Spleens were passed through a 100μm nylon mesh filter (BD Falcon, Bedford, MA) into RPMI 1640 to create a single cell suspension. Red cells were lysed with 1x red cell lysis buffer (eBioscience, Inc., San Diego, CA) and the cell suspension subsequently washed with RPMI 1640. Cells were then counted on a Cellometer Auto T4 cell counter (Nexcelom, Lawrence, MA). After counting, cells were centrifuged and resuspended in staining buffer (PBS with 0.1% NaN3 and 1% BSA) for staining.
Mesenteric lymph nodes (MLN) were processed by passing LN through a 100μm nylon mesh filter (BD Falcon) and washing cells with RPMI 1640. After centrifugation cells were resuspended in staining buffer for FACS analysis.
Flow cytometry
Cells were resuspended at a concentration of 1×106 cells/ml in staining buffer. All cells were stained for extracellular markers after being blocked with rat anti-mouse CD16/CD32 Mouse BD Fc Block™ (BD Bioscience, San Jose, CA). After blocking cells were incubated with fluorescently tagged antibodies and protected from light. The cell viability dye, 7-amino-actinomycin D (7AAD) was used to assess cell survival in some stains. Cells used for intracellular staining or transcription factor staining were fixed with 4% paraformaldehyde and washed. Intracellular staining was done by resuspending cells in permeabilization buffer (BD Bioscience) and then incubated with antibodies or isotype controls. Transcription factor staining (FoxP3, T-bet, and RORγ) was done with fixation/permeabilization reagents per the manufacturer’s instructions (eBioscience). All samples were then run on a BD Accuri™ C6 (BD Bioscience) with a four color (FITC, PE, PerCP Cy5.5, and APC) fluorescence flow cytometry analysis.
The following antibodies were used: CD4 (GK1.5), CD11b (M1/70), CD19 (1D3), CD8 (53-6.7), CD1d (1B1), CD138 (281-2), CD25 (PC61), CD86 (GL1), CD206 (CO68C2), CD122 (TM-β1) (BD Biosciences), CD44 (1M7), FoxP3 (FJK-16s), RORγ (AFKJS-9) (eBioscience), CD5 (53-7.3), T-bet (4B10) (Biolegend), IL-17, IFNγ (BD Pharmingen), and ARG1 (R&D Systems, Minneapolis, MN).
RNA Isolation
RNA was isolated from spinal cords using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Spinal cords were weighed and suspended in 10ul/mg of RLT buffer. 300ul of each sample was diluted 1:2 with RLT for a 30 mg/600ul mixture. 10ul/ml of BME was added to the samples. Spinal cords were then homogenized by gentle pipetting. Spinal cord lysates were transferred to Qiashredder tubes and centrifuged at 13,000 rpm for 15 seconds. 600 ul of 70% alcohol was added to each Qiashredder, spun at 13,000 rpm for 15 seconds, and transferred to separate RNAeasy columns. The RNAeasy columns were centrifuged twice for 15 seconds at 13,000 rpm, discarding the flow-through after each step. 700ul of Buffer wash RW1 were added to each column. Samples were spun 15 seconds at 13,000 rpm, with the flow-through discarded. Two successive washes of 500 ul of Buffer RPE were added to the samples and spun at 13,000 rpm for 2 minutes. The columns were placed in collection tubes, then washed with 50ul of RNase-free water to elute the RNA. RNA quantity (ng/ul) and quality (A260/280) were measured using a NanoDrop™ One/OneC Microvolume UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA).
cDNA Synthesis
cDNA was synthesized using the RT2 First Strand Kit (Qiagen) using the manufacturer’s protocol. Recommended amounts of Buffer GE, Buffer BC3, Control P2, Reverse Transcriptase Mix, and RNase-free water were used. The samples were incubated at 42°C for 15 minutes, then 95°C for 5 minutes. 91 ul of RNase free water was added to each sample.
Chemokine and Receptor Array
cDNA from three spinal cords per group were pooled for the array. cDNA mix (102 ul) was added to 1350 ul of RT2 SYBR Green ROX qPCR Mastermix (Qiagen), along with 1248 ul of RNase-free water. Twenty-five ul of the mastermixes were loaded into each well of RT2 Profiler™ PCR Array Mouse Chemokines & Receptors (Qiagen) and run on the Applied Biosystems StepOnePlus Real-Time PCR System. Thermocycler conditions were 1 cycle of 95 °C for 10 minutes, followed by 40 alternating cycles of 95°C for 15 seconds and 60 °C for 1 minute. mRNA expression was normalized using the expression of various housekeeping genes and compared to the data from the control group according to the 2−DDCT method (Livak and Schmittgen 2001). Some of the results were confirmed with RT-PCR on individual samples. The protocol and analyses were performed according to the manufacturer’s instructions.
Statistics
Data were analyzed using Prism software (GraphPad Software, La Jolla, CA) using Mann-Whitney U test for determining significance for disease course. All other data were analyzed using a Student’s t-test. A p value of ≤0.05 was considered significant. Data are represented as mean ± standard error of the mean (SEM). All analyses were carried out in blinded fashion.
Results
ABX pretreatment protects mice from developing severe EAE
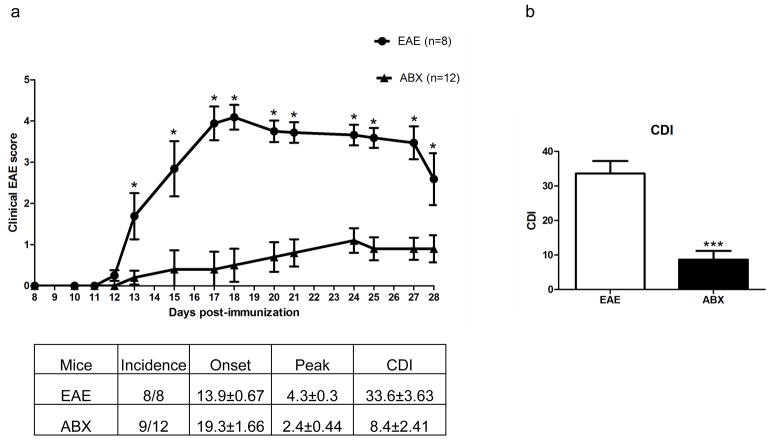
Pretreating mice for three days prior to EAE induction with an oral antibiotic cocktail significantly protected the mice from developing severe EAE. The incidence, disease onset, and peak disease scores were all lower in the ABX treated mice compared to control treated mice (Fig. 1A). The cumulative disease index (CDI) was also significantly lower in ABX mice compared to control mice (8.4±2.41 vs 33.6±3.63; p<0.001; Fig. 1B). Clinical disease scores were significantly lower starting at day 13 p.i. and remained significantly lower throughout to day 28 p.i. (p<0.05).
Fig. 1. ABX pretreatment protects mice from developing severe EAE.
Pretreating mice for three days prior to EAE induction with an oral antibiotic cocktail significantly protected the mice from developing severe EAE (p<0.05; A). The incidence, disease onset, and peak disease scores were all lower in the ABX treated mice (n=12) compared to control treated mice (n=8). The cumulative disease index (CDI) was also significantly lower in ABX mice compared to control mice (8.4±2.41 vs 33.6±3.63; p<0.001; B). Clinical disease scores were significantly lower starting at day 13 p.i. and remained significantly lower through day 28 p.i. (p<0.05)
The frequency of splenocyte cell types varies with ABX treatment prior to EAE
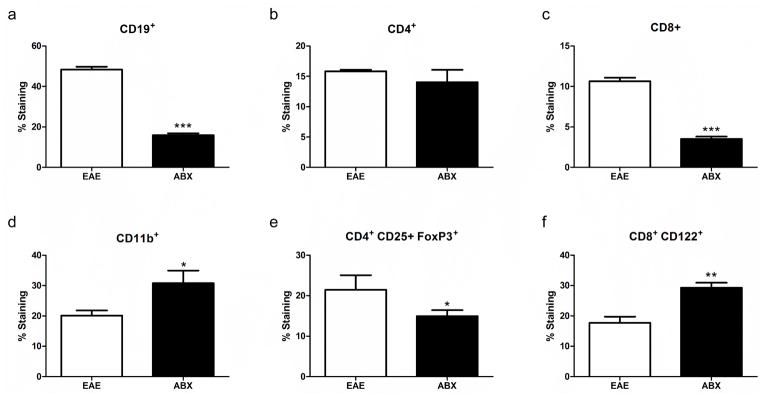
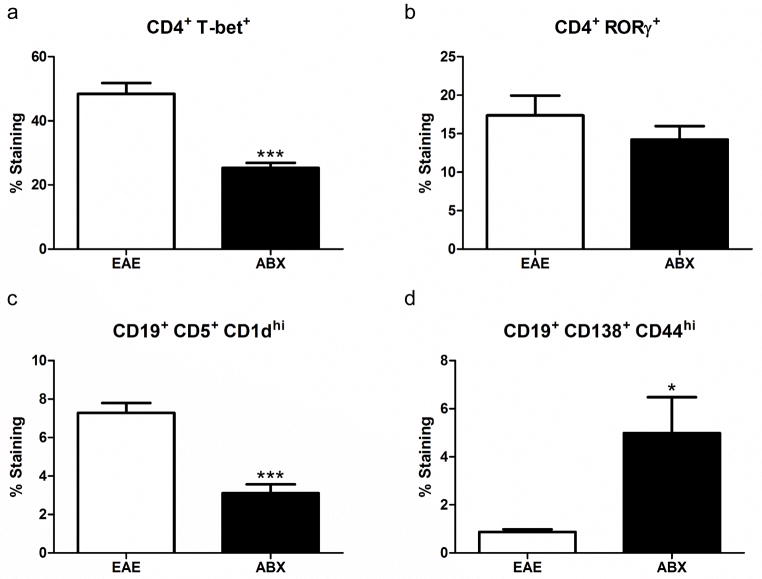
ABX pretreatment resulted in some spleen cell types being significantly lower than controls, while others were upregulated and some remained unchanged. The frequencies of CD19+ B cells, CD8+ T cells, and CD4+FoxP3+ Treg cells were all significantly decreased in spleens from ABX treated mice compared to controls whereas the frequencies of CD11b+ macrophages/monocytes and CD8+ Treg cells were both significantly increased. The CD4+ T cell frequency remained unchanged between the two groups (Fig. 2). Within the CD4+ T cell subsets, CD4+ T-bet+ Th1 cells were significantly decreased in frequency in the ABX treated mice, while the CD4+ RORγ+ Th17 cell frequency was unchanged between the two groups (Fig. 3A and B). Breg cells, which have been shown to be protective during EAE (Zhang et al. 2015), had some subsets that were significantly downregulated (B10 cells), and others that were significantly upregulated (plasmablasts, Fig. 3C and D) in spleens from ABX compared to control mice.
Fig. 2. The frequency of splenocyte cell types varies with ABX treatment prior to EAE.
ABX pretreatment resulted in some cell types being significantly lower than controls with others being upregulated or remaining unchanged. The frequency of CD19+ B cells (A), CD8+ T cells (C), and CD4+ FoxP3+ Treg cells (E) were all significantly decreased in ABX treated mice (n=7) compared to controls (n=5; p<0.05). CD11b+ macrophages/monocytes (D) and CD8+ CD122+ Treg cell (F) frequencies were both significantly increased in the spleens of ABX mice compared to controls (p<0.001). The CD4+ T cell (B) frequency remained unchanged between the two groups
Fig. 3. Th subsets and Breg cells in the spleen.
Within the CD4+ T cell subsets, CD4+ T-bet+ Th1 cells (A) were significantly decreased (p<0.001) in frequency in the ABX treated mice, while the CD4+ RORγ+ Th17 cell (B) frequency was unchanged between the two groups. Breg cells had some subsets (B10 cells; CD19+ CD5+ CD1dhi) that were significantly downregulated (p<0.001, C) and some (plasmablasts, CD19+ CD138+ CD44hi) that were significantly upregulated (p<0.05, D) in ABX mouse spleens compared to control mice
Anti-inflammatory macrophages are decreased in the spleen and increased in the spinal cord of ABX pretreated mice
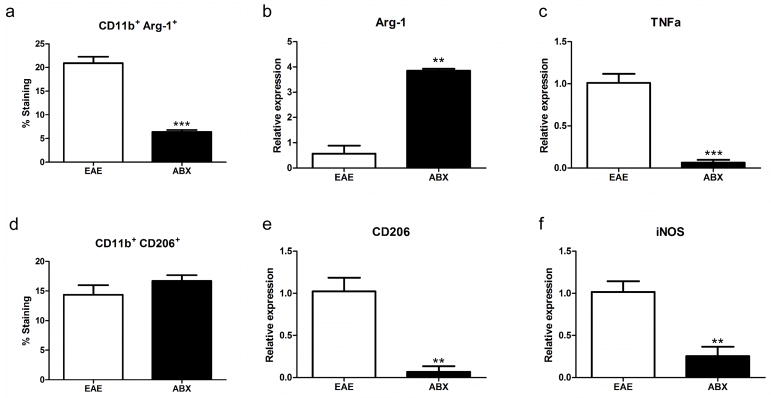
There was a significant decrease in the frequency of CD11b+ Arg-1+ cells (Fig 4A) in spleens from ABX treated mice compared to control mice and there was a corresponding significant increase in the relative expression of Arg1 in the spinal cords of ABX treated mice compared to controls (Fig 4B). Conversely, the frequency of CD11b+ CD206+ cells was unchanged in the spleen (Fig 4D) while there was a significant decrease in the relative expression of CD206 in the spinal cords of ABX mice compared to controls (Fig. 4E). The relative expression of markers of pro-inflammatory microglia/macrophages in the spinal cords of ABX pretreated mice (TNFα and iNOS) were significantly decreased compared to controls (Fig. 4C and F).
Fig. 4. Anti-inflammatory macrophages are decreased in the spleen and increased in the spinal cord of ABX pretreated mice.
In the spleen there was a significant decrease (p<0.001) in the frequency of CD11b+ Arg-1+ cells (A) in ABX treated mice (n=7) compared to control mice (n=5) and there was a corresponding significant increase (p<0.01) in the relative expression of Arg1 in the spinal cords of ABX treated mice (n=3) compared to controls (n=3; B). The frequency of CD11b+ CD206+ cells was unchanged in the spleen (D), although there was a significant decrease (p<0.01) in the relative expression of CD206 in the spinal cords of ABX mice compared to controls (E). Markers of pro-inflammatory microglia/macrophages in the spinal cords of ABX pretreated mice were significantly decreased (p<0.01) compared to controls, notably the relative expression of TNFα and iNOS (C and F) respectively
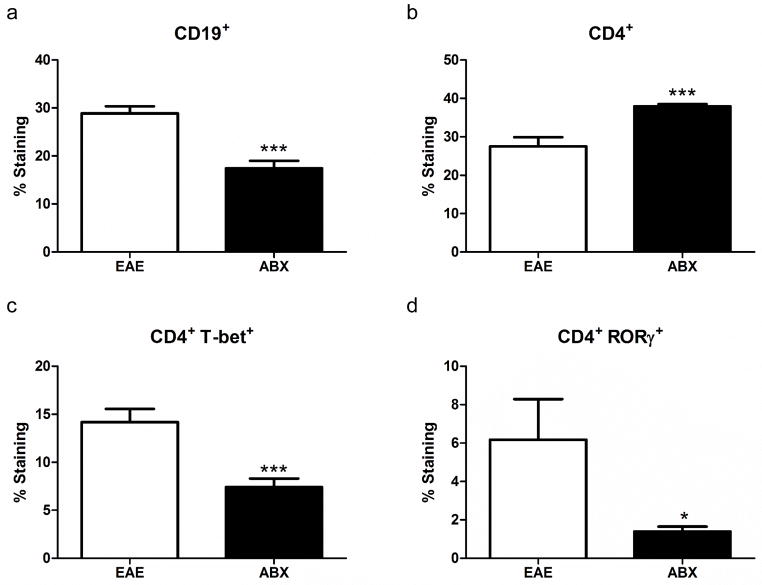
Mesenteric lymph node B cell frequencies are decreased while T cell frequencies are increased in ABX pretreated mice during EAE
Within the mesenteric lymph nodes the frequency of B cells was significantly decreased in ABX mice compared to controls (Fig 5A). The frequency of CD4+ T cells was significantly increased in the ABX mice although the CD4+ Th cell subsets were significantly decreased (Fig 5B), including both Th1 (CD4+ T-bet+) and Th17 (CD4+ RORγ+) cells in ABX pretreated mice (Fig 5C and D respectively).
Fig. 5. Mesenteric lymph node B cell frequencies are decreased while T cell frequencies are increased in ABX pretreated mice during EAE.
Within the mesenteric lymph nodes the frequency of B cells, CD19+, was significantly decreased (p<0.001) in ABX mice (n=7) compared to controls (n=5; A). The frequency of CD4+ T cells was significantly increased (p<0.001) in the ABX mice while the CD4+ Th cell subsets were significantly decreased (B), both Th1 (CD4+ T-bet+; p<0.001) and Th17 (CD4+ RORγ+; 0.05) in ABX pretreated mice (C and D) respectively
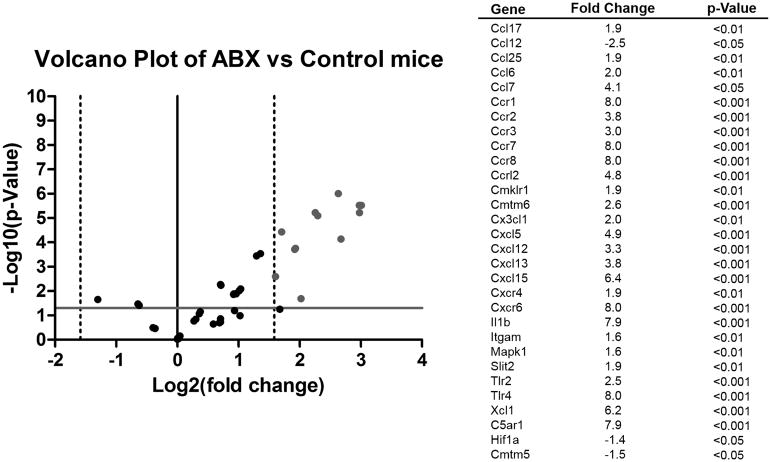
Upregulation of anti-inflammatory and regulatory chemokines and receptors in spinal cords of ABX mice
In addition to evaluating how the cellular immune response was modulated by ABX pretreatment, expression of chemokines and their receptors was also measured in the spinal cords of mice after EAE. Since chemokines are known to attract specific types of immune cells and to ask which recruitment signals may have accounted for the observed spinal cord immune cell subtypes, a panel of chemokines and their receptors was used to look at their expression in the spinal cord tissue from the mice. Of note, chemokines and their receptors associated with anti-inflammatory microglia/macrophages were upregulated in ABX spinal cords, particularly the CCR1/CCL7 pair, as well as the CC-type receptors CCR7 and CCR8. Additionally, the chemokine CXCL12 (SDF-1α) together with its receptor CXCR4 which is associated with regulatory Th cells (CD4+ FoxP3+) were upregulated in ABX pretreated mice compared to control mice (Fig. 6). In addition, CXCR6, a receptor associated with different T cell subsets, was found to be substantially upregulated, as was the B-cell chemokine CXCL13.
Fig. 6. Upregulation of anti-inflammatory and regulatory chemokines and receptors in spinal cords of ABX mice.
Chemokines and their receptors were measured in the spinal cords of mice after EAE. Since chemokines are known to attract specific types of immune cells a panel of chemokines and their receptors was used to look at their expression in the spinal cords of mice. cDNA from the spinal cords of mice were run on a RT2 Profiler™ PCR Array Mouse Chemokines & Receptors. Some of the results of the pooled samples were confirmed with RT-PCR on individual samples (n=3). Chemokines and receptors associated with anti-inflammatory microglia/macrophages were upregulated in ABX spinal cords, particularly CCR1 (p<0.001). Additionally, the chemokine CXCL12 (SDF-1α) which is associated with regulatory Th cells (CD4+ FoxP3+) was upregulated in ABX pretreated mice compared to control mice (p<0.001). Vertical dotted lines represent a threefold change in gene expression (threshold for fold difference). The solid gray line represents p=0.05, the cutoff for significance
Discussion
This study demonstrated that only three days of ABX pretreatment was necessary to provide significant protection to female mice from severe EAE seen in control mice. This finding is consistent with previous studies showing the same cocktail of antibiotics (Ampicillin, Metronidazole, Neomycin Sulfate, and Vancomycin) given orally prior to the induction of EAE results in a less severe disease (Ochoa-Reparaz et al. 2009; Ochoa-Reparaz et al. 2010). While metronidazole, at higher concentrations, is known to suppress the immune system (Fararjeh et al. 2008), with a low dosage and its short half-life suggests the immunosuppressive actions did not play a significant role in the protection from EAE. Similar to previous studies, there was a significant decrease in the frequency of CD4+ FoxP3+ Treg cells within the spleen. However, there was not an increase in CD19+ B cells in the spleen or mesenteric lymph nodes as previously reported (Ochoa-Reparaz et al. 2010), with B cell frequencies being significantly decreased in both tissues. There was also not a significant increase in B10 cells (CD19+ CD5+ CD1dhi) but rather a significant decrease in the spleen. However, there was a significant increase in another known Breg subset, the CD19+ CD138+ CD44hi plasmablasts, which also produces IL-10 and suppresses T cell functions (Matsumoto et al. 2014). The differences seen in B10 frequencies could be due to the different durations of ABX administration used in each study. One similarity is the presence of an IL-10 producing Breg cell subset. In addition to seeing an increase in plasmablasts there was also a significant increase in the frequency of CD8+ cytotoxic Treg cells (CD8+ CD122+) in the spleen, despite the significant decrease in the overall frequency of CD8+ cytotoxic T cells. CD8+ Treg cells have also been associated with increased IL-10 production and blunting of IFNγ production (Bodhankar et al. 2015; Shi et al. 2008).
The presence of several types of IL-10 producing regulatory lymphocytes in the spleen might explain the significant decrease in the frequency of Th1 (CD4+ T-bet+) cells in the spleen and mesenteric lymph nodes and the significant decrease in the frequency of Th17 (CD4+ RORγ+) cells in the mesenteric lymph nodes of ABX pretreated mice compared to control mice, even though overall CD4+ T cell frequencies were unchanged in the spleen and significantly increased in the mesenteric lymph nodes. The reduction of these cell types could explain why the ABX mice experienced less severe disease than control mice, as both IFNγ and IL-17 are known to drive CNS autoimmune reactions (Liu et al. 2008).
In addition to T cells and B cells, CD11b+ myeloid cells were also studied. The frequency of CD11b+ cells were significantly increased but the frequency of CD11b+ Arg-1+ anti-inflammatory cells was significantly decreased in the spleen. These cells either moved to the spinal cord or other cells induced the differentiation of anti-inflammatory Arg1 producing cells. In fact, there was a significant increase in the relative expression of Arg1 in the spinal cords of ABX mice compared to controls. There was also a significant decrease in the relative expression of tumor necrosis factor alpha (TNFα) and inducible nitric oxide synthase (iNOS) in the spinal cords of ABX mice compared to control mice. This suggests ABX pretreatment primed the immune system to respond in an anti-inflammatory manner towards the CNS and suppress pro-inflammatory responses from microglia and macrophages.
This hypothesis is further supported by the chemokines and chemokine receptors that were upregulated in ABX treated mice spinal cords compared to control mice. The chemokine receptor CCR1 was upregulated 8-fold and is known to bind chemokines that attract monocytes, e.g. CCL7. This could attract monocytes to the spinal cord and after entering the CNS the anti-inflammatory environment established by regulatory lymphocytes could cause the monocytes to differentiate into anti-inflammatory macrophages, increasing Arg1 expression. The chemokine CXCL12 (stromal cell-derived factor alpha, SDF-1α) was upregulated 3.2-fold and has been shown to polarize T helper cells towards Treg cells compared to Th1 cells through the induction of IL-10. This decreases both TNFα and IFNγ (Meiron et al. 2008). Of note, the cognate receptor of CXCL12, CXCR4, was also found to be upregulated in the spinal cord although only 2-fold. CXCR4 is expressed on various T cell subsets (Lim et al. 2008); thus, the joint upregulation of CXCL12 and CXCR4 would support the notion that this chemokine axis contributed to the observed increase in Treg cells within the affected spinal cord tissue. In addition to inducing Treg cells, CXCL12 is also expressed in the CNS and serves as a migratory factor for oligodendrocyte and neuronal precursors (Dziembowska et al. 2005) that could aid in the repair of damaged neural tissue. The chemokine array data also showed a marked upregulation of the CXC-type receptor CXCR6 and the CXC-type ligands CXCL5, CXCL13, and CXCL15. While CXCL13 may contribute to the recruitment of certain B cell subtypes (Kowarik et al. 2012; Rupprecht et al. 2009), the role of CXCR6 and CXCL5 and CXCL15 in EAE models is poorly understood. Together the analysis of the genes that were upregulated in the spinal cords of ABX pretreated mice suggests that there was a strong anti-inflammatory/protective environment established within the CNS that suppressed pro-inflammatory cells.
Conclusion
The primary purpose of this study was to determine how ABX pretreatment affected the immune system, particularly in the CNS. However, the microbiome was likely affected by the administration of antibiotics and therefore ongoing and future experiments will be conducted to study this interaction. Treatment with oral antibiotic cocktail for three days prior to the induction of EAE significantly reduced disease severity in female C57BL/6 mice, which is consistent with previous reports using a seven day oral antibiotic cocktail pretreatment that protected female mice and induced regulatory lymphocytes. The current study also found an increase in IL-10 regulatory B cells and a reduction in the spleen of regulatory T cells. While neither FoxP3 nor IL-10 were found in the spinal cords of ABX treated mice (data not shown), they likely were expressed earlier in the disease process prior to day 28 post immunization. That is, the chemokine and chemokine receptors that were upregulated in the spinal cords of ABX mice suggested the presence of these cells and the creation of a microenvironment to suppress pro-inflammatory cells. Additionally, the ABX mice did not have as severe EAE as control mice, with their disease process nearly resolved by day 28, whereas the control mice were just beginning to recover. ABX treatment thus modified the immune system to enhance levels of regulatory cells and cytokines/chemokines which likely protected the mice from developing a severe demyelinating disease of the CNS.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG) EXC1010 SyNergy (JB) and NIH National Institute Of Neurological Disorders And Stroke of the National Institutes R01NS080890 (HO). This material is also the result of work supported with resources and the use of facilities at the VA Portland Health Care Center in Portland, Oregon. The contents do not represent the views of the U.S. Department of Veterans Affairs, the United States Government or the official views of the National Institutes of Health.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (DFG) EXC1010 SyNergy (JB) and NIH National Institute Of Neurological Disorders And Stroke of the National Institutes R01NS080890 (HO). This material is also the result of work supported with resources and the use of facilities at the VA Portland Health Care Center in Portland, Oregon. The contents do not represent the views of the U.S. Department of Veterans Affairs, the United States Government or the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and/or animals
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
References
- Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Lapato A, Vandenbark AA, Murphy SJ, Saugstad JA, Offner H. Regulatory CD8(+)CD122 (+) T-cells predominate in CNS after treatment of experimental stroke in male mice with IL-10-secreting B-cells. Metab Brain Dis. 2015;30:911–924. doi: 10.1007/s11011-014-9639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fararjeh M, Mohammad MK, Bustanji Y, Alkhatib H, Abdalla S. Evaluation of immunosuppression induced by metronidazole in Balb/c mice and human peripheral blood lymphocytes. Int Immunopharmacol. 2008;8:341–350. doi: 10.1016/j.intimp.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Freedman SN, Shahi SK, Mangalam AK. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics. 2018;15:109–125. doi: 10.1007/s13311-017-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarik MC, et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation. 2012;9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol. 2015;17:344. doi: 10.1007/s11940-015-0344-7. [DOI] [PubMed] [Google Scholar]
- Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Kasper LH. Gut microbiome and the risk factors in central nervous system autoimmunity. FEBS Lett. 2014;588:4214–4222. doi: 10.1016/j.febslet.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clin Immunol. 2015;159:122–127. doi: 10.1016/j.clim.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht TA, et al. The chemokine CXCL13 is a key regulator of B cell recruitment to the cerebrospinal fluid in acute Lyme neuroborreliosis. J Neuroinflammation. 2009;6:42. doi: 10.1186/1742-2094-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Rifa’i M, Lee YH, Shiku H, Isobe K, Suzuki H. Importance of CD80/CD86-CD28 interactions in the recognition of target cells by CD8+CD122+ regulatory T cells. Immunology. 2008;124:121–128. doi: 10.1111/j.1365-2567.2007.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5:552–561. doi: 10.4161/gmic.29797. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab Brain Dis. 2015;30:1117–1127. doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]