Abstract
Swallow and breathing are highly coordinated behaviors reliant on shared anatomical space and neural pathways. Incremental ascent to high altitudes results in hypoxia/hypocapnic conditions altering respiratory drive, however it is not known whether these changes also alter swallow. We examined the effect of incremental ascent (1,045m, 3,440m and 4,371m) on swallow motor pattern and swallow-breathing coordination in seven healthy adults. Submental surface electromyograms (sEMG) and spirometry were used to evaluate swallow triggered by saliva and water infusion. Swallow-breathing phase preference was different between swallows initiated by saliva versus water. With ascent, saliva swallows changed to a dominate pattern of occurrence during the transition from inspiration to expiration. Additionally, water swallows demonstrated a significant decrease in submental sEMG duration and a shift in submental activity to earlier in the apnea period, especially at 4,371m. Our results suggest that there are changes in swallow-breathing coordination and swallow production that likely increase airway protection with incremental ascent to high altitude. The adaptive changes in swallow were likely due to the exposure to hypoxia and hypocapnia, along with airway irritation.
Keywords: High altitude ascent, Electromyograms, Airflow, Apnea duration, Airway protection, Swallow coordination
1. Introduction
Swallow and breathing are highly coordinated airway protective behaviors. Swallow is a multi-phase event, however the pharyngeal phase presents the highest risk for aspiration (Paydarfar et al., 1995). During the pharyngeal phase, supra-laryngeal/hyoid musculature moves the larynx superiorly and anteriorly resulting in closure of the airway and a functional apnea (German et al., 2009; Wheeler Hegland et al., 2011; Wheeler Hegland et al., 2009).
The expiratory phase of breathing is the preferred phase for swallow to occur, likely due to the limited inspiratory airflow (Martin-Harris et al., 2003). The central mechanism is thought to be due to interactions of breathing and swallow pattern generators (Dick et al., 1993; Miller, 1982), however this preference can be modified by peripheral feedback (Pitts et al., 2015b) and disease (Brodsky et al., 2010; Leslie et al., 2002; Troche et al., 2011). Specifically, alterations in respiratory mechanics due to chronic obstructive pulmonary disease (Nagami et al., 2017; Pinto et al., 2017) and/or upper abdominal laparotomy can shift swallow occurrences to inspiration, potentially increasing risk of aspiration (Pitts et al., 2015b). Additionally, there is also limited evidence that alterations in blood gasses (i.e., oxygen [O2] and carbon dioxide [CO2]) can also increase the likelihood that swallow will occur during inspiration (D’Angelo et al., 2014), (Ghannouchi et al., 2013).
Incremental ascent to high altitudes (>2,000m) produces hypoxia (low O2) induced hyperventilation, resulting in hypocapnia (low CO2) (Huang et al., 1984; Weil, 1986). As climbers acclimatize to high altitude they can reach a new “steady-state chemoreflex drive” in which balance is achieved between hypoxia and hypocapnia, while ventilation parameters can return to near baseline conditions (Bruce CD, 2018; Pfoh et al., 2017). Additionally, healthy individuals that are not acclimatized to high altitude conditions can have changes in pulmonary mechanics due to interstitial pulmonary edema, which can be accompanied with accumulation of fluid within and around the airway walls (Cremona et al., 2002; Pratali et al., 2010; Schoene et al., 1988). Early symptoms such as shortness of breath and cough are often overlooked leading to mortality (Dunin-Bell and Boyle, 2009).
Due to the significant coordination necessary for swallow and breathing, it is likely that conditions which significantly alter respiratory drive and mechanics would also affect swallow production and swallow-breathing coordination. We hypothesized that with incremental ascent to high altitude there would be a decrease in swallow duration, and a shift in swallow phase preference to inspiration.
2. Methods
Ethics and Participant Recruitment
This study abided by the Canadian Government Tri-Council policy on research ethics with human participants (TCPS2) and the Declaration of Helsinki, except for registration in a database. Ethical approval was received in advance through Mount Royal University Human Research Ethics Board (Protocol 100012) and was harmonized with the Nepal Health Research Council (Protocol 109–2017). Participants were recruited via email correspondence or direct verbal communication, and provided written, voluntary, informed and ongoing consent.
Ten participants were recruited for the study, while only seven (two males, five females) completed the study. One participant voluntarily withdrew from the study during ascent, another was excluded following baseline data acquisition due to a persistent cough and a third was excluded due to complications with data acquisition. Exclusion criteria included facial hair, as electrodes were unable to effectively adhere to skin, and health status (e.g., persistent cough, severe altitude illness). No pre-existing medical conditions were reported by any participants. Participants avoided rigorous exercise for at least 12 hours prior to data collection.
Incremental ascent to high altitude
Baseline measurements were recorded at 1,045m (Calgary) prior to the departure to Nepal. Following arrival in Kathmandu (1,400m), participants spent up to 3 days in Kathmandu before flying to Lukla (2,860m) where the trek to high altitude commenced (Figure 1). Consecutive measurements were obtained on rest days at 3,440m (Namche; day 3 at altitude) and 4,371m (Pheriche; day 5 at altitude) on every second day following arrival in Lukla (Figure 1), following one night sleep at each respective altitude.
Figure 1.
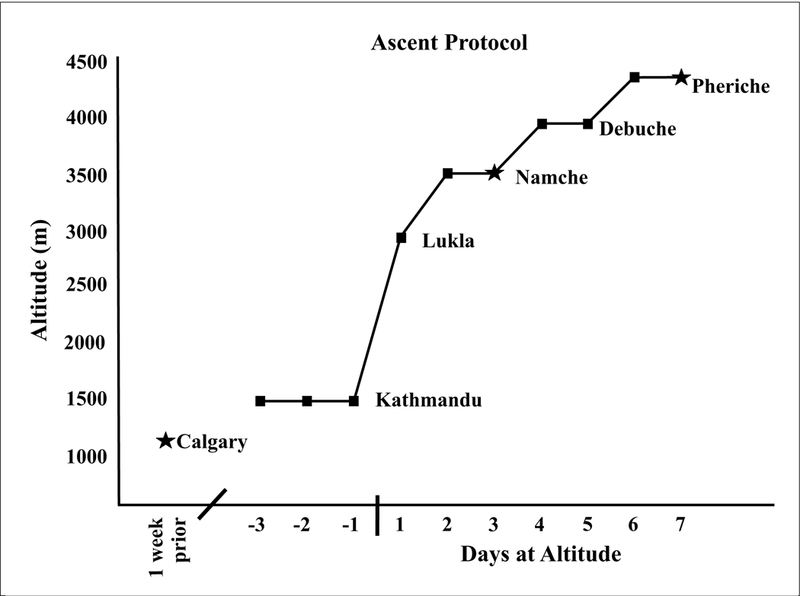
Timeline of travel, ascent, and recording locations. The (★) represents where data was collected, and (✈) indicates flights.
Data Collection
Data acquisition was performed using an analog to digital data acquisition system [Powerlab/16SP ML880; AD Instruments (ADI), Colorado Springs, CO, USA], and data was collected, archived and analyzed offline using commercially available software (LabChart Pro software version 8) and a personal laptop computer. Surface electromyogram (sEMG) (ADI MLA2503 & ADI FE132) electrodes were placed approximately 3 cm posterior to the mental region of the mandible, on each side of the midline, capturing the submental complex. The grounding electrode was placed inferior to the participant’s left clavicle. Voluntary swallow was performed in advance to ensure an adequate electrical signal through the sEMG electrodes.
A pneumotachometer (800L flow head; Series 3813; Hans Rudolph Inc.) and spirometer amplifier (ADI ML141) were used to monitor respiratory variables using a mouthpiece and nose-clip. Calibration of the flow head was performed with a 3L calibration syringe before data acquisition in each participant. Respiratory flow (L/s) was measured directly by the pneumotachograph. Inspired volume (VTI; L) and respiratory frequency (ƒR; min−1) were derived from respiratory flow. The product of VTI and ƒR was used to determine instantaneous minute ventilation (; L/min). The pressure of end-tidal PETCO2 was measured using a portable, calibrated capnograph (Masimo EMMA, Danderyd, Sweden) with a personal mouthpiece and nose clip and peripheral oxygen saturation (SpO2) was measured with a portable finger pulse oximeter (Masimo SET® Rad-5, Danderyd, Sweden). Electrocardiography (ECG; ADI MLA2503 & ADI FE132; lead II configuration) was utilized to derive instantaneous heart rate (HR; 1/R-R Interval in min−1). The protocol was carried out with participants sitting comfortably in a dark, quiet room with ear plugs and eyes closed. Resting ventilation at each altitude was analyzed from a one-minute representative period near the end of a 10-min baseline period, whereas PETCO2 and SpO2 measures were obtained after stability was achieved.
Swallow stimulation
1. Swallows produced during the baseline respiratory data via normal saliva collection in the mouth, termed saliva swallows.
2. Water swallows were trigged via water delivery from a 250 mL wash bottle (Nalgene 2089–0008 Narrow-Mouth Economy Bottle; Thermo Scientific, Waltham, MA, USA) inserted approximately 5 cm into the participant’s mouth, lateral to the pneumotachometer mouthpiece. The wash bottle was positioned by each participant to ensure comfort with the water delivery. The infusion protocol began by recording a thirty-second baseline with all instrumentation in place. Following this baseline, water was infused at ~1 mL/second for 30 seconds into the participants’ mouths. Finally, a 30 second washout was conducted after all instrumentation remaining in place. In all instances, participants were instructed before the introduction of water to swallow normally as needed.
Statistical Analysis
Data was analyzed from seven participants (5 female and 2 male) ages 19–23 at 1,045m (Calgary), 3,440m (Namche; day 3 at altitude), and 4,371m (Pheriche; day 7 at altitude) (Figure 1). All results were expressed as means ± standard deviation (SD) using SPSS software (IBM).
To examine changes in swallow phase preference the following designations were used for respiratory phase: A) transition from inspiration to expiration (In-Ex); within expiration (Ex-Ex); transition from expiration to inspiration (Ex-In); and within inspiration (In-In). Then the following assigned coding system was used with In-Ex = 1; Ex-Ex = 2; Ex-In = 3; and In-In = 4 to categorize where each swallow occurred (Table 1). Finally, Wilcoxon signed ranks tests were run to determine changes across swallow-type and altitude, as we have previously used (Pitts et al., 2015b).
Table 1.
Percent of swallow occurrence during breathing across the three levels of ascent.
| In-Ex | Ex-Ex | Ex-In | In-In | |
|---|---|---|---|---|
| Saliva Swallow | ||||
| 1,045m | 43 | 9 | 27 | 21 |
| 3,440m | 76 | 12 | 6 | 6 |
| 4,371m | 55 | 15 | 30 | 0 |
| Water Swallow | ||||
| 1,045m | 79 | 9 | 9 | 2 |
| 3,440m | 69 | 14 | 11 | 6 |
| 4,371m | 76 | 11 | 11 | 2 |
Swallow apnea duration was measured as the period of zero airflow in the event of a swallow (Figure 2). The apnea duration then was divided into three sub-phases: a) pre-swallow apnea, b) duration of submental sEMG, and c) post-swallow apnea (Figure 2). Pre-swallow apnea began at the time of zero airflow before the submental activation. Submental sEMG duration was measured as the activation and inactivation of submental sEMG. Post-swallow apnea was measured as the zero airflow after the inactivation of submental complex (Figure 2). Additionally respiratory rate, heart rate, mean arterial pressure (MAP), I, SpO2, PETCO2 and steady-state chemoreflex drive (SS-CD) were measured. The SS-CD was computed by calculating a stimulus index (SI; PETCO2/SpO2), and then comparing minute ventilation against SI (Bruce CD, 2018; Pfoh et al., 2017). A repeated measures ANOVA was used to determine differences in swallow motor pattern and respiratory parameters across the three elevations with significance at p ≤ 0.05, and if significance was met the LSD post-hoc test was used. A p ≤ 0.07 was designated as “approaching significance”.
Figure 2.
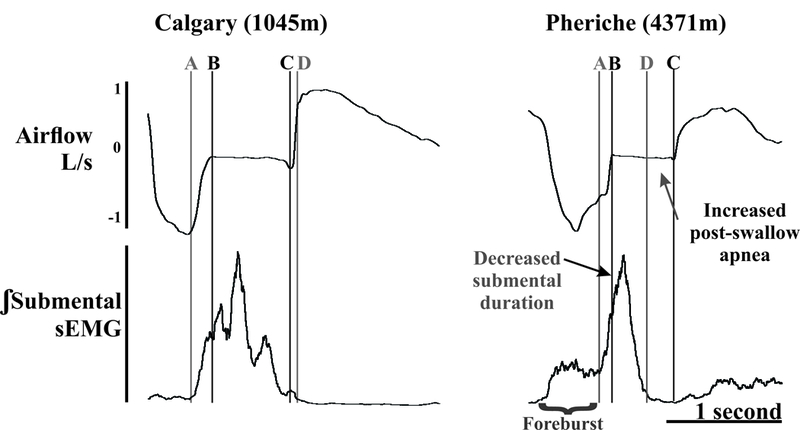
Example of submental sEMG and airflow from the same participant from Calgary (1,045m) and Pheriche (4,371m) during the water swallow protocol. B to C marks the swallow apnea period. A to B is the pre-swallow submental activity, A to D is the submental duration and C to D is the post-swallow apnea period. At 4,371m, there was a significant increase in the post-swallow apnea as well as a decrease submental duration. The “foreburst” is activity related to water being introduced to the oral cavity.
3. Results
Swallow was present during baseline respiratory measurements (saliva swallows), and reliably elicited with infusion of water in all subjects (water swallows). A total of 379 swallows (122 saliva and 257 water) were analyzed across the three altitudes (142 at 1,045m; 121 at 3,440m; and 116 at 4,371m).
Swallow-breathing coordination
Table 1 reports percent of swallow occurrences across each respiratory phase/transition. Water swallows had a strong In-Ex phase preference (69–79%) which was maintained through the ascent protocol. For saliva swallows at 1,045m only 43% occurred during In-Ex [significantly different than water (Z = −3.3, p < 0.001)], but this shifted at 3,440m with 76% of swallows occurring during In-Ex [significantly different than 1,045m (Z = −3.3, p < 0.001)]. At the highest altitude 4,371m the percent of swallows which occurred during the In-Ex transition reduced to 55% (p = 0.07). Interestingly, at 1,045m 21% of saliva swallows occurred during inspiration (In-In), which reduced to 6% at 3,440m and at 4,371m none occurred. In contrast <6% of water swallows occurred during inspiration (Table 1).
Change of swallow motor pattern with increasing altitude
Figure 3 demonstrates changes in pre-swallow apnea, submental duration, and post swallow apnea plotted by subjects across the three altitude locations. For swallows elicited by water, the average submental duration (ms) approached significance [1170 ± 539, 1038 ± 218, and 710 ± 227 respectively (F2, 12 = 4.19, p = 0.07)]. As elevation increased pre-swallow apnea duration (ms) significantly decreased [−256 ± 236, −115 ± 99, and −5 ± 172 respectively (F2, 12 = 4.218, p = 0.06)], and post-swallow apnea duration (ms) significantly increased [56 ± 109, 111 ± 171, and 241 ± 218 (F2, 12 =6.137, p < 0.05)] (Table 2 and Figure 2 and 3). Of note, pre-swallow submental sEMG activity was seen during swallows at each elevation and of each type (Figure 2). For saliva swallows there was no significant change in submental sEMG and apnea duration, or swallow frequency (Table 2).
Figure 3.
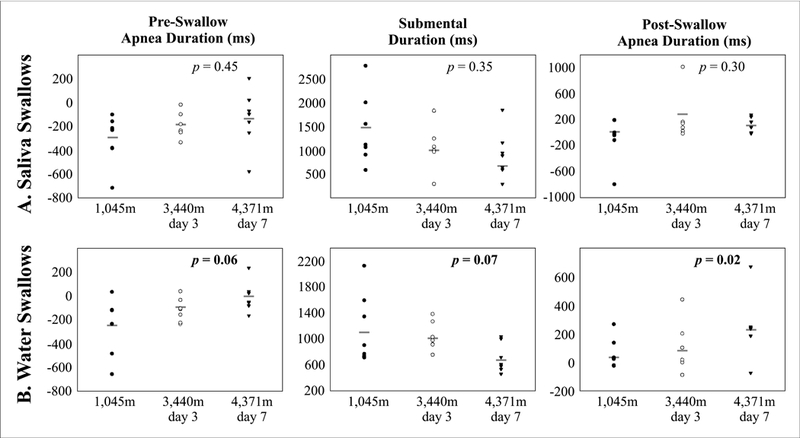
Scatter plot of duration measures (pre-swallow, submental and post-swallow) for each subjects across th e recording locations for the saliva (A) and water (B) swallow tasks. Repeated measures ANOVA p-value reported for each dependent measure, and gray line represents group mean.
Table 2.
Means, standard deviations (SD), and p-values comparing ventilatory, cardiac and acclimation values, as well as saliva and water swallows at the three different elevations are shown in this table. Resting respiratory rate (RR), mean arterial pressure (MAP), peripheral O2 saturation (SpO2), end tidal CO2 pressure (PETCO2), instantaneous minute ventilation (I), and steady-state chemoreflex drive (SS-CD) are recorded. Submental (swallow) duration, swallow apnea duration, pre-swallow apnea and post-swallow apnea (Figure 2) are recorded in both saliva and water conditions. Figure 3 displays swallow data by participant.
| Calgary (1,045m) | Namche (3,440m) | Pheriche (4,371m) | p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| Resting RR (min−1) | 14.0 | ± | 6.0 | 15.2 | ± | 3.9 | 15.5 | ± | 5.2 | 0.42 |
| Resting Heart Rate | 81.6 | ± | 9.5 | 97.8 | ± | 7.9 *** | 93.5 | ± | 5.8 ** | 0.004 |
| MAP (mm Hg) | 90.4 | ± | 8.4 | 96.0 | ± | 6.5 ** | 99.1 | ± | 9.2 *** | 0.004 |
| SpO2 (%) | 96.2 | ± | 1.0 | 88.1 | ± | 2.3 *** | 83.3 | ± | 5.3 ***†† | <0.001 |
| PETCO2 (Torr) | 31.1 | ± | 4.2 | 25.9 | ± | 2.7 ** | 21.3 | ± | 2.3 ***††† | 0.001 |
| (L/min) | 11.9 | ± | 2.7 | 14.2 | ± | 2.6 | 14.7 | ± | 3.8 | 0.07 |
| SS-CD (VI/SI) | 36.8 | ± | 8.5 | 49.3 | ± | 12.7 * | 58.7 | ± | 19.5 **† | 0.02 |
| Saliva Swallow Data | ||||||||||
| Submental Duration (ms) | 1480 | ± | 804 | 1070 | ± | 490 | 1015 | ± | 457 | 0.35 |
| Swallow Apnea Duration (ms) | 1088 | ± | 433 | 1121 | ± | 253 | 1010 | ± | 374 | 0.77 |
| Pre-Swallow Apnea (ms) | −296 | ± | 220 | −183 | ± | 113 | −141 | ± | 265 | 0.45 |
| Post-Swallow Apnea (ms) | −99 | ± | 363 | 233 | ± | 385 | 174 | ± | 101 | 0.30 |
| Water Swallow Data | ||||||||||
| Submental Duration (ms) | 1170 | ± | 539 | 1038 | ± | 218 | 710 | ± | 227 *††† | 0.07 |
| Swallow Apnea Duration (ms) | 973 | ± | 398 | 1030 | ± | 165 | 946 | ± | 285 | 0.60 |
| Pre-Swallow Apnea (ms) | −256 | ± | 236 | −115 | ± | 99 | −5 | ± | 126 * | 0.06 |
| Post-Swallow Apnea (ms) | 56 | ± | 109 | 111 | ± | 171 | 241 | ± | 218 **† | 0.02 |
Reported p-values are for repeated measures oneway ANOVA and significant values are bolded
Significant difference from Calgary *p≤0.06,
p<0.05
p<0.01
Significant difference between Namche and Pheriche † p≤0.06
p<0.05
p<0.01
Breathing related variables
Table 2 also illustrates resting minute ventilation , the pressure of end-tidal PETCO2, peripheral oxygen saturation (SpO2), stimulus index (SI) and measurement of steady-state chemoreflex drive (SS-CD) during incremental ascent to high altitude. All variables changed in predictable ways with incremental ascent. Heart rate [81.6 ± 9.5, 97.8 ± 7.9, and 93.5 ± 5.8 respectively (F2,12 =10.29, p < 0.05)], MAP [90.4 ± 8.4, 96.0 ± 6.5, and 99.1 ± 9.2 respectively (F2,12 = 11.88, p < 0.05)] and SS-CD significantly increased as altitude increased [36.8 ± 8.5, 49.3 ± 12.7, and 58.7 ± 19.5 respectively (F2,12 = 7.41, p < 0.05)]. SpO2 [96.2 ± 1.0, 88.1 ± 2.3, and 83.3 ± 5.3 respectively (F2,12 = 37.44, p < 0.001)] and PETCO2 [31.1 ± 4.2, 25.9 ± 2.7, and 21.3 ± 2.3 respectively (F2,12 = 31.61, p = 0.001)] significantly decreased as altitude increased. Additionally, respiratory rate and instantaneous minute ventilation remained stable across all elevations (Table 2).
4. Discussion
This is the first evidence of a significant change in swallow-breathing coordination as well as swallow production during incremental ascent to high altitude. There was a significant change in swallow phase preference comparing saliva to water swallows during baseline and approached significance at the highest elevation (4,371m). This was due to a shift in the dominance of the In-Ex pattern seen during water swallows and at 3,440m for saliva swallows. Additionally, in the water trials there was a significant increase in the post-swallow apnea period and a decrease (approaching significance) in the submental duration and pre-swallow apnea, while the overall swallow apnea duration did not change.
Phase Preference
Swallow phase preference has been intensely studied in humans (Martin-Harris, 2008; Martin-Harris et al., 2008; Martin-Harris et al., 2003; Martin-Harris and McFarland, 2013; Pratali et al., 2010; Wheeler Hegland et al., 2011; Wheeler Hegland et al., 2009), as well as in cats (Dick et al., 1993; Pitts et al., 2015a; Pitts et al., 2013; Pitts et al., 2015b), goats (Bonis et al., 2011; Feroah et al., 2002a; Feroah et al., 2002b), and rats (Saito et al., 2002a, b). However, all the peripheral stimulations and/or central mechanisms which regulate their interactions are not entirely understood. In the present study there was not a strong expiratory phase preference (~80%) which is observed in single swallow studies in which a 5 or 10 mL bolus is placed in the mouth (Wheeler Hegland et al., 2009). Saliva swallows (probably most akin to the typical single swallow task) demonstrated only 9% occurred during expiration, with 43% occurring in the transition of In-Ex, and of great interest is that 21% of these swallows occurred during inspiration (Table 1).
The dominance of In-Ex preference may be due in part to the mouthpiece which forces an “open mouth” swallow. It has been shown that muscle spindle afferents, in the masseter muscle, increase in discharge frequency during active opening of the jaw (Taylor et al., 1997). It has also been shown that input of muscle spindle afferents influence other central pattern generators [i.e. locomotion (Pearson, 1995)], and has been speculated that muscle spindle afferents influence mastication CPG output (Kolta et al., 1990; Lund, 2011). This information allows speculation that position of the jaw, indicated by proprioception of muscle spindle afferents can modulate the interaction between the swallow and breathing CPGs.
These changes could also be related to the effects of hypoxia and/or hypocapnia on swallow. Although there are limited studies, there are also conflicting results. In mice an increase in swallow frequency was reported (Khurana and Thach, 1996), no change in rat (Ghannouchi et al., 2013), and a decrease in the cat (Nishino et al., 1986). Hypoxia has also been studied in nonnutritive swallow in newborn lambs which showed a decrease in frequency during quiet sleep (Duvareille et al., 2007). Interestingly, hypercapnia shifts swallows towards In and Ex-In (D’Angelo et al., 2014) while we found that hypocapnia with hypoxia shifts swallow toward In-Ex. In light of the present data, further studies may need to investigate swallow-breathing coordination not only with variation of respiratory drive but swallow drive as well. We speculate that the water trials increased swallow excitability, which likely altered and stabilized its relationship with breathing.
Swallow motor pattern
In contrast to the swallow-breathing coordination data, the largest changes in the swallow motor pattern with ascent were on the water swallows, with a 39% decrease in the submental duration (Figure 2–3) at the highest altitude (compared to Calgary). This effect has been demonstrated in cats when swallow was coordinated with cough (airway irritation discussed below) (Leow et al., 2006); however we could find no study demonstrating a decrease in submental sEMG in healthy adults when using a mechanical/cold stimulus on the back of the mouth (Sciortino et al., 2003) or altering oral stimulation with taste (Leow et al., 2006).
To protect the airway during the pharyngeal phase of swallow the vocal folds must be adducted (zero flow; swallow apnea) during the laryngeal exposure to the bolus (Butler et al., 2004; Chi-Fishman and Sonies, 2000; Ding et al., 2003; Kijima et al., 1999; Martin-Harris et al., 2003; Martin et al., 1994; Paydarfar et al., 1995; Wheeler Hegland et al., 2011). In a review by Martin-Harris (2008), she stated that increases in the timing from the onset of the submental activity to the apnea period is related to significant clinical risk for aspiration. Evidence of this has been demonstrated in patients with Parkinson’s disease with dysphagia (Ertekin, 2014). Based on this current data, we speculate that the decrease in submental sEMG and the shift in its activity to closer to the start of the swallow apnea period could increase airway protection. Of note, Ertekin and colleagues (Ertekin, 2014; Gürgör et al., 2013) demonstrated an activation of the submental complex during the pre-swallow respiratory phase that is likely related to infusion of water into the mouth (termed foreburst). Figure 2 demonstrates the difference between swallow-related and pre-swallow submental activity.
Airway Irritation
Exposure to high altitude conditions is also associated with airway irritation from dry air and insensible water loss, which results in a chronic cough (Freer, 2004). The most common diagnosis in the Nepal Himalaya is “Khumbu cough”, also known as “high altitude hack” (Freer, 2004), thought to be caused by dry air, sub-zero temperatures, dust, and exposure to yak dung stoves in the lodges (Linoby et al., 2013). There is evidence that dry air increases airway responsiveness (Van Oostdam et al., 1986), and prolonged exposure results in an inflammatory response, desquamation of the epithelium, and edema of submucosa (Florey et al., 1932). While each subject did have evidence of coughing across the recording period, none were actively coughing during the measurement period. It is possible that activation of irritant receptors can alter swallow production without cough as a presenting feature.
Respiratory Drive
The changes in swallow and swallow-breathing coordination were also accompanied by changes/adaption of the chemoreflexes driving breathing. It is known that these reflexes become more dynamic as individuals acclimatize to their respective environment (Pfoh et al., 2017) (Steinback and Poulin). To asses this adaptation, Pfoh and colleagues (2017) created an index of steady-state chemoreflex drive (SS-CD), taking into account resting ventilation indexed against the overall contributions of both low O2 and low CO2 during exposure to hypoxia. Based on the magnitude of this index the significant change in the SS-CD from 1,045m to 3,440m is evidence of respiratory acclimatization in our participants [see also (Huang et al., 1984)].
Blood levels of O2 and CO2 are maintained in part by central (brainstem) and peripheral (carotid body) chemoreceptors. Central chemoreceptors, located throughout brainstem, detect PCO2/[H+] accumulation (Guyenet and Bayliss, 2015). Peripheral chemoreceptors located bilaterally within carotid bodies detect rapid changes in both O2 and CO2 synergistically (Fitzgerald and Parks, 1971; Lahiri and DeLaney, 1975; López-Barneo et al., 2016). A primary location for integrating these signals is in the nucleus tractus solitarius (NTS) (Jordan and Spyer, 1986; Paton et al., 2001). Due to the overlap in sensory integration in the NTS for breathing and swallow (Jean, 1984, 2001), this may be a site of shared central excitability which affects both respiratory and swallow central pattern generators.
Clinical Implications
Altitude exposure has inherent risks with 1–2% experiencing high altitude pulmonary edema (HAPE) (Houston, 1960; Hultgren, 1969; Schoene et al., 1986), a form of high altitude sickness, and of those 65% are diagnosed with a concomitant respiratory infection (most commonly pneumonia) (Leshem et al., 2008). It would be of interest to know if climbers with pneumonia display the same adaptations in swallow, especially in light of our knowledge of pneumonia rates with dysphagia.
5. Conclusions
Our results suggest that there are changes in swallow-breathing coordination and swallow motor production that increase airway protection with incremental ascent to high altitude. In conclusion, we suspect the adaptive changes in swallow were likely due to the exposure to superimposed hypoxia and hypocapnia, along with the increased airway irritation.
Acknowledgements:
R00-HL 111215, The Kentucky Spinal Cord and Head Injury Trust, The Commonwealth of Kentucky Challenge for Excellence, Natural Sciences and Engineering Research Council of Canada Discovery grant (RGPIN-2016–04915). We also want to thank the guides and research subjects we are grateful for your participation in this project.
Footnotes
Conflict of Interest: None of the authors have a conflict of interest to report.
References
- Bonis J, Neumueller S, Marshall B, Krause K, Qian B, Pan L, Hodges M, Forster H, 2011. The effects of lesions in the dorsolateral pons on the coordination of swallowing and breathing in awake goats. Respiratory Physiology and Neurobiology 175, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky MB, McFarland DH, Dozier TS, Blair J, Ayers C, Michel Y, Gillespie MB, Day TA, Martin-Harris B, 2010. Respiratory-swallow phase patterns and their relationship to swallowing impairment in patients treated for oropharyngeal cancer. Head Neck 32, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CD SG, Pfoh JR, Leacy JK, Zouboules SM, Peltonen JDB, Linares AM, Chiew AE, O’Halloran KD, Sherpa MT, Day TA, 2018. What is the Point of The Peak? Assessing Steady-State Chemoreflex Drive in High Altitude Field Studies. Adv Exp Med Biol. In Press. [DOI] [PubMed] [Google Scholar]
- Butler SG, Postma GN, Fischer E, 2004. Effects of viscosity, taste, and bolus volume on swallowing apnea duration of normal adults. Otolaryngology Head and Neck Surgery 131, 860. [DOI] [PubMed] [Google Scholar]
- Chi-Fishman G, Sonies BC, 2000. Motor strategy in rapid sequential swallowing: new insights. Journal of Speech, Language and Hearing Research 43, 1481. [DOI] [PubMed] [Google Scholar]
- Cremona G, Asnaghi R, Baderna P, Brunetto A, Brutsaert T, Cavallaro C, Clark TM, Cogo A, Donis R, Lanfranchi P, 2002. Pulmonary extravascular fluid accumulation in recreational climbers: a prospective study. The Lancet 359, 303–309. [DOI] [PubMed] [Google Scholar]
- D’Angelo OM, Diaz-Gil D, Nunn D, Simons JC, Gianatasio C, Mueller N, Meyer MJ, Pierce E, Rosow C, Eikermann M, 2014. Anesthesia and increased hypercarbic drive impair the coordination between breathing and swallowing. Anesthesiology: The Journal of the American Society of Anesthesiologists 121, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick T, Oku Y, Romaniuk J, Cherniack N, 1993. Interaction between central pattern generators for breathing and swallowing in the cat. The Journal of Physiology 465, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, Logemann JA, Larson CR, Rademaker AW, 2003. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: a surface electromyographic study. Journal of Speech, Language and Hearing Research 46, 977. [DOI] [PubMed] [Google Scholar]
- Dunin-Bell O, Boyle S, 2009. Secondary prevention of HAPE in a Mount Everest summiteer. High altitude medicine & biology 10, 293–296. [DOI] [PubMed] [Google Scholar]
- Duvareille C, Lafrance M, Samson N, St-Hilaire M, Pladys P, Micheau P, Bournival V, Langlois C, Praud J-P, 2007. Effects of hypoxia and hypercapnia on nonnutritive swallowing in newborn lambs. Journal of Applied Physiology 103, 1180–1188. [DOI] [PubMed] [Google Scholar]
- Ertekin C, 2014. Electrophysiological Evaluation of Oropharyngeal Dysphagia in Parkinson’s Disease. Journal of Movement Disorders 7, 31–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feroah TR, Forster H, Fuentes CG, Lang IM, Beste D, Martino P, Pan L, Rice T, 2002a. Effects of spontaneous swallows on breathing in awake goats. Journal of Applied Physiology 92, 1923–1935. [DOI] [PubMed] [Google Scholar]
- Feroah TR, Forster H, Fuentes CG, Wenninger J, Martino P, Hodges M, Pan L, Rice T, 2002b. Contributions from rostral medullary nuclei to coordination of swallowing and breathing in awake goats. Journal of Applied Physiology 93, 581–591. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Parks DC, 1971. Effect of hypoxia on carotid chemoreceptor response to carbon dioxide in cats. Respiration physiology 12, 218–229. [DOI] [PubMed] [Google Scholar]
- Florey H, Carleton H, Wells A, 1932. Mucus secretion in the trachea. British journal of experimental pathology 13, 269. [Google Scholar]
- Freer L, 2004. Descriptive report of experience designing and staffing the first-ever medical clinic at Mt. Everest base camp, 2003. High altitude medicine & biology 5, 89–90. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Thexton AJ, 2009. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannouchi I, Duclos C, Marie J, Verin E, 2013. Modification in swallowing and ventilation co‐ordination during hypercapnia, hypoxia, and tachypnea in unrestrained animals. Neurogastroenterology & Motility 25, 308. [DOI] [PubMed] [Google Scholar]
- Gürgör N, Arıcı Ş, Incesu TK, Seçil Y, Tokuçoğlu F, Ertekin C, 2013. An electrophysiological study of the sequential water swallowing. Journal of Electromyography and Kinesiology 23, 619–626. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, 2015. Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CS, 1960. Acute pulmonary edema of high altitude. New England Journal of Medicine 263, 478–480. [DOI] [PubMed] [Google Scholar]
- Huang S, Alexander J, Grover R, Maher J, McCullough R, McCullough R, Moore L, Sampson J, Weil J, Reeves J, 1984. Hypocapnia and sustained hypoxia blunt ventilation on arrival at high altitude. Journal of Applied Physiology 56, 602–606. [DOI] [PubMed] [Google Scholar]
- Hultgren HN, 1969. High altitude pulmonary edema. Biomedicine of high terrestrial elevations, 131–141. [Google Scholar]
- Jean A, 1984. Control of the central swallowing program by inputs from the peripheral receptors. A review. Journal of the autonomic nervous system 10, 225–233. [DOI] [PubMed] [Google Scholar]
- Jean A, 2001. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiological Review 81, 929–969. [DOI] [PubMed] [Google Scholar]
- Jordan D, Spyer KM, 1986. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res 67, 295–314. [DOI] [PubMed] [Google Scholar]
- Khurana A, Thach BT, 1996. Effects of upper airway stimulation on swallowing, gasping, and autoresuscitation in hypoxic mice. Journal of applied physiology (Bethesda, Md. : 1985) 80, 472–477. [DOI] [PubMed] [Google Scholar]
- Kijima M, Isono S, Nishino T, 1999. Coordination of swallowing and phases of respiration during added respiratory loads in awake subjects. Am J Respir Crit Care Med 159, 1898–1902. [DOI] [PubMed] [Google Scholar]
- Kolta A, Lund J, Rossignol S, 1990. Modulation of activity of spindle afferents recorded in trigeminal mesencephalic nucleus of rabbit during fictive mastication. Journal of neurophysiology 64, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney R, 1975. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respiration physiology 24, 249–266. [DOI] [PubMed] [Google Scholar]
- Leow L, Huckabee ML, Sharma S, Tooley T, 2006. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chemical senses 32, 119–128. [DOI] [PubMed] [Google Scholar]
- Leshem E, Pandey P, Shlim DR, Hiramatsu K, Sidi Y, Schwartz E, 2008. Clinical features of patients with severe altitude illness in Nepal. Journal of travel medicine 15, 315–322. [DOI] [PubMed] [Google Scholar]
- Leslie P, Drinnan MJ, Ford GA, Wilson JA, 2002. Swallow respiration patterns in dysphagic patients following acute stroke. Dysphagia 17, 202–207. [DOI] [PubMed] [Google Scholar]
- Linoby, A.F., Nias, M.A., Ahmad, B.E., Zaki, S., Canda, R., Sariman, H., Azam, Z., Amat, A., 2013. Physiological Responses and Adaptations to Exposure from Moderate to Extreme Altitude: A Case Study of the Youngest Malaysian Climber to Scale Mt. Everest.
- López-Barneo J, González-Rodríguez P, Gao L, Fernández-Agüera MC, Pardal R, Ortega-Sáenz P, 2016. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. American Journal of Physiology-Cell Physiology 310, C629–C642. [DOI] [PubMed] [Google Scholar]
- Lund JP, (2011). Chapter 15 - Chew before you swallow, in: Gossard JP, Dubuc R, Kolta A (Eds.), Progress in Brain Research. Elsevier, pp. 219–228. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B, 2008. Clinical implications of respiratory–swallowing interactions. Current opinion in otolaryngology & head and neck surgery 16, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J, 2008. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia 23, 392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B, 2003. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. Journal of Applied Physiology 94, 1735. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B, McFarland DH, (2013). Coordination of Deglutition and Respiration, Principles of Deglutition. Springer, pp. 25–34. [Google Scholar]
- Martin B, Logemann J, Shaker R, Dodds W, 1994. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. Journal of Applied Physiology 76, 714. [DOI] [PubMed] [Google Scholar]
- Miller AJ, 1982. Deglutition. Physiological Review 62, 129–184. [DOI] [PubMed] [Google Scholar]
- Nagami S, Oku Y, Yagi N, Sato S, Uozumi R, Morita S, Yamagata Y, Kayashita J, Tanimura K, Sato A, Takahashi R, Muro S, 2017. Breathing–swallowing discoordination is associated with frequent exacerbations of COPD. BMJ Open Respiratory Research 4, e000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T, Kohchi T, Honda Y, Shirahata M, Yonezawa T, 1986. Differences in the effects of hypercapnia and hypoxia on the swallowing reflex in cats. BJA: British Journal of Anaesthesia 58, 903–908. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Li YW, Kasparov S, 2001. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neuroscience 105, 231–248. [DOI] [PubMed] [Google Scholar]
- Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF, 1995. Respiratory phase resetting and airflow changes induced by swallowing in humans. The Journal of physiology 483, 273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, 1995. Proprioceptive regulation of locomotion. Current Opinion in Neurobiology 5, 786–791. [DOI] [PubMed] [Google Scholar]
- Pfoh JR, Steinback CD, Berg ERV, Bruce CD, Day TA, 2017. Assessing chemoreflexes and oxygenation in the context of acute hypoxia: Implications for field studies. Respiratory physiology & neurobiology 246, 67–75. [DOI] [PubMed] [Google Scholar]
- Pinto CF, Balasubramanium RK, Acharya V, 2017. Nasal airflow monitoring during swallowing: Evidences for respiratory-swallowing incoordination in individuals with chronic obstructive pulmonary disease. Lung India : official organ of Indian Chest Society 34, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Gayagoy A, Rose M, Poliacek I, Condrey J, Musslewhite M, Shen T, Davenport P, Bolser D, 2015a. Suppression of Abdominal Motor Activity during Swallowing in Cats and Humans. PloS one 10, e0128245–e0128245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Rose MJ, Mortensen AN, Poliaček I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC, 2013. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respiratory physiology & neurobiology 189, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Rose MJ, Poliacek I, Condrey J, Davenport PW, Bolser DC, 2015b. Effect of laparotomy on the swallow-breathing relationship in the cat. Lung 193, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratali L, Cavana M, Sicari R, Picano E, 2010. Frequent subclinical high-altitude pulmonary edema detected by chest sonography as ultrasound lung comets in recreational climbers. Critical care medicine 38, 1818–1823. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I, 2002a. Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat. J Physiol 544, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I, 2002b. Swallowing-related activities of respiratory and non-respiratory neurons in the nucleus of solitary tract in the rat. Journal of Physiology 540, 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene RB, Hackett PH, Henderson WR, Sage EH, Chow M, Roach RC, Mills WJ, Martin TR, 1986. High-altitude pulmonary edema. Jama 256, 63–69. [PubMed] [Google Scholar]
- Schoene RB, Swenson ER, Pizzo CJ, Hackett PH, Roach RC, Mills WJ Jr, Henderson W Jr, Martin T, 1988. The lung at high altitude: bronchoalveolar lavage in acute mountain sickness and pulmonary edema. Journal of Applied Physiology 64, 2605–2613. [DOI] [PubMed] [Google Scholar]
- Sciortino KF, Liss JM, Case JL, Gerritsen KGM, Katz RC, 2003. Effects of Mechanical, Cold, Gustatory, and Combined Stimulation to the Human Anterior Faucial Pillars. Dysphagia 18, 16–26. [DOI] [PubMed] [Google Scholar]
- Steinback CD, Poulin MJ, 2007. Ventilatory responses to isocapnic and poikilocapnic hypoxia in humans. Respir Physiol Neurobiol 155, 104–113. [DOI] [PubMed] [Google Scholar]
- Taylor A, Hidaka O, Durbaba R, Ellaway PH, 1997. Fusimotor influence on jaw muscle spindle activity during swallowing-related movements in the cat. J Physiol 503 ( Pt 1), 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche MS, Huebner I, Rosenbek JC, Okun MS, Sapienza CM, 2011. Respiratory-Swallowing Coordination and Swallowing Safety in Patients with Parkinson’s Disease. Dysphagia 26, 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oostdam J, Walker D, Knudson K, Dirks P, Dahlby R, Hogg J, 1986. Effect of breathing dry air on structure and function of airways. Journal of Applied Physiology 61, 312–317. [DOI] [PubMed] [Google Scholar]
- Weil JV, 1986. Ventilatory control at high altitude. Comprehensive Physiology. [Google Scholar]
- Wheeler Hegland K, Huber JE, Pitts T, Davenport PW, Sapienza CM, 2011. Lung Volume Measured During Sequential Swallowing in Healthy Young Adults. Journal of Speech, Language, and Hearing Research 54, 777. [DOI] [PubMed] [Google Scholar]
- Wheeler Hegland KM, Huber JE, Pitts T, Sapienza CM, 2009. Lung volume during swallowing: single bolus swallows in healthy young adults. Journal of Speech, Language, and Hearing Research 52, 178. [DOI] [PubMed] [Google Scholar]


